Abstract
A growing body of literature supports the association between ambient particulate pollution and the risk of type 2 diabetes (T2DM). Both issues are particularly relevant in Italy. This study investigates the relationship between T2DM and exposure to PM2.5 and PM10 in Italian municipalities from 2013 to 2021. Data on T2DM were provided by the Italian Association of Diabetologists (AMD), representing the only national outpatient dataset not based on self-reported information. Air pollution data, sourced from the Italian Institute for Environmental Protection and Research, ISPRA, were summarized using the population-weighted exposure (PWE) indicator. Both datasets were made available through a dedicated research agreement. Random effects models and non-parametric methods were applied to assess the association between air pollution and T2DM. Results indicate a statistically significant relationship, particularly between T2DM and PM2.5. T2DM incidence rates were significantly negatively associated with time (coefficient = − 0.07961, p < 0.01), indicating a decreasing trend over time. After adjusting for other covariates, PM10 population-weighted exposure was not significantly associated with incidence rates (coefficient = − 0.00057, p = 0.58). On the other hand, increases in the ratio of PM2.5 to PM10 (pwratio) were significantly positively associated with increases in T2DM incidence rates (coefficient = 0.52304, p < 0.01) at the municipal level. T2DM prevalence proportions were significantly positively associated with time (coefficient = 0.01749, p < 0.01), suggesting an increasing trend over time. PM10 was significantly negatively associated with prevalence proportions (coefficient = − 0.00298, p = 0.03), while increases in pwratio were significantly positively associated with increases in prevalence proportions (coefficient = 0.18724, p < 0.01). Thus, municipalities with a higher share of PM2.5 within the same level of PM10, tended to show higher T2DM prevalence proportions and incidence rates, consistent with the spatial distribution of air pollution and disease burden observed across Italy.
Similar content being viewed by others
Introduction
The association between particulate air pollution and an increased risk of type II Diabetes (T2DM) is well supported in the literature1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18.
Both air pollution and T2DM are critical issues for Italy. According to the European Environmental Agency, Italy “reported the highest concentrations of particulate matter” among Western Europe countries19. Since 2010, thanks to a time of greater energy efficiency, the spread of renewable sources, and more stringent emission limits in the use of energy for industrial use, particulate pollution, specifically PM2.5 and PM10, with the former being a subcategory of the latter due to its smaller diameter, have shown a decreasing trend not sufficient, however, to substantially reduce further the level of two pollutants, especially in certain areas of the country20. Concerning T2DM, prevalence is 6.3% of the population, greater for men than women: 6.6% and 6.1%, apiece, with a progressively increasing trend in the last fifteen years21 and a continuous growth trajectory up to 20505. This is mainly due to the aging population and worsening lifestyle: Italy has the oldest population in Europe, with an average citizen age of 48 years, 34.2% of adults are overweight, 12.0% are obese, and 33.7% are physically inactive21. Italy exhibits other peculiar traits. It suffers from a historical development gap characterized by a North-South dualism22 that carries huge differences between regions regarding industrialization, urbanization, and population. In general, almost half of the entrepreneurial fabric is concentrated in northern Italy, which also has the highest population density23.
Considering that, and well aware that air pollution has long-term negative health effects, this study aims to verify the possible association between T2DM and exposure to PM2.5 and PM10 in all Italian regions at the municipality level, over the 2013–2021 time period. T2DM data comes from the Italian Diabetologists Association, AMD, a member of the International Diabetes Federation. The AMD longitudinal dataset is the only existing outpatient national dataset for Italy in which diabetes is not self-reported. Data on exposure to PM2.5 and PM10, comes from the Italian Institute for Environmental Protection and Research, ISPRA. All data used are original and not publicly available. They were provided to us through exclusive research collaboration agreements.
To the best of our knowledge, this study is the first attempt to calculate the association between exposure to air pollution and T2DM incidence and prevalence in Italy based on original data. Additionally, there are several other important research innovations outlined below. The first relates to the use of clinical data to detect T2DM cases, rather than surveys where diabetes is self-reported17. Employing longitudinal, clinical-level data allows for higher diagnostic accuracy compared to ecological or self-reported data. The second relates to T2DM patients who do not experience hospitalization24 and for whom there is almost no public data on clinical follow-up.
The third is that our study focuses on municipalities distributed throughout the entire Italian territory, as opposed to being concentrated in a few large cities, such as Milan25,26, or aggregated at the regional level, as in Lombardy27. In contrast to the data presented in27, our study utilizes data at the municipality level for both T2DM prevalence and incidence as well as population-weighted exposure (PWE). The latter derived from a spatiotemporal modeling framework28, which allows us to assess particulate matter (PM2.5 and PM10) exposure risk for individuals residing in all Italian municipalities. The data presented herein are original and were explicitly computed and estimated for the present research study. Analyzing municipality-level data for the entire Italian territory facilitates a more complete depiction of the phenomenon.
Without having an inferential or representative aim, this study provides a comprehensive description of the potential association between T2DM and PM2.5 and PM10 exposure, both using parametric statistics and machine learning approaches. Our contribution fills a gap in the literature by estimating this association. Via mixed-effects models, we provide insights into the effects of particulate matter on non-communicable diseases. The remainder of this work is organized as follows. The next two sections describe the two datasets and methods. Section “Results” presents descriptive statistics and results. Section “Discussion” discusses our findings, while Section “Conclusion and policy implications” concludes. Every step of our research adhered to the GATHER (Guidelines for Accurate and Transparent Health Estimates Reporting) statement.
Data and methods
AMD dataset
The AMD dataset is an outpatient national longitudinal dataset collected for the 2004–2021 time period in almost 300 diabetological centers adherent to the network of the AMD, Italian Diabetologists Association, a member of the International Diabetes Federation. The AMD dataset covers approximately half of all diabetes outpatients in Italy29. AMD centers generally treat more complex patients who seldom require insulin but do not need hospitalization. Less severe cases, as well as elderly and bedridden patients, are treated by general practitioners or diabetes centers that are not part of the AMD network. Distributed across all 20 Italian regions, the AMD centers collect clinical and demographic information thanks to a computerized medical record for patient management. The dataset enables the extraction of a standardized set of clinical information and is employed to calculate the quality of care indicators at both centralized and local levels. It is structured to draw information on different types of Diabetes and all their complications. It also represents a valuable source of observational research data. In the absence of a national diabetes registry, the AMD dataset is the only tool comparable to a registry in Italy 30. All patients enrolled provided written informed consent prior to partecipating. The AMD dataset collection was conducted following the Declaration of Helsinki. Patients’ data are anonymized using an ID and have been aggregated at the municipality level.
ISPRA dataset
The ISPRA dataset on annual population-weighted exposure refers to 8100 Italian municipalities. Data are collected from 2013 to 2021 and obtained through a methodology reported in the following section. Primary data come from the ISPRA which supports the Italian Ministry of Environment on the collection, control, management, processing, and communication at the European level of information on air quality (data and metadata), per the provisions of Legislative Decree 155/2010. Monitoring data come from the National System for Environmental Protection (bringing together the Regional Environmental Protection agencies and ISPRA). Long-term monitoring data and statistics are thus available, as well as indicators and periodic reports on air quality status and trends.
Exposure assessment
In order to assess the exposure of the Italian population to particulate matter, we employed Bayesian hierarchical models 31 incorporating 11 spatial and spatio-temporal predictors, including meteorological variables and aerosol optical depth. The residual dependence described by the covariates was represented through the stochastic partial differential equation approach with a lag-1 temporal autoregressive component 32 based on 12 monthly models with the same set of covariates. The integrated nested Laplace approximation was used to perform the inference 28,33. The final calibrated estimates were a collection of high-resolution (1 Km²) daily maps of PM10 and PM2.5 ground-level concentrations over the same spatial domain, within the country boundary, including the islands.
The daily gridded concentrations are used to detect the annual mean PM10 and PM2.5 values at each square kilometer grid cell. The annual population-weighted exposure within all the cells of the one-square-kilometer grid that intersects each municipality’s Italian communal border was estimated in 8100 Italian municipalities from 2013 to 2021. The processing of all data was executed through the integrated utilization of the Climate Data Operator (CDO) software and the R version 4.3.1. statistical package. Supplementary materials provide further details on the implemented models and the PWE calculation.
T2DM assessment
For each AMD center, prevalence and incidence counts of T2DM were computed separately for males and females. Specifically, for the purpose of this study, prevalence at the municipality level was defined as the count of patients in the dataset at each specific year. The prevalence proportions every 100,000 patients were calculated as the proportion of individuals accessing the centers in each municipality, in a specific year, relative to the total population of patients within the geographical region where the center is located. Incidence rates were determined as the rate of new cases of T2DM cases within a year over the total population of patients in the region where the center is located. In order to account for potential heterogeneity in the distribution of the AMD centers, we have chosen to use the number of patients instead of the number of people at risk in each region, therefore using a slightly different definition of incidence rates, which uses at the denominator the number of people at risk. Despite data being available at the municipality level, for privacy reasons, they will be displayed here only for Italian regions grouped namely: North-West (NW), North-East (NE), Center (CE), South (SO), and Islands (IS).
To investigate a potential association between particulate matter exposure, demographic factors, and T2DM prevalence proportions and incidence rates, regression trees were employed. Prevalence and incidence were used as outcome variables, whereas sex, age, PM10 (i.e., population-weighted exposure to PM10), and the ratio between the population-weighted exposure of PM2.5 and PM10 (pwratio = PM2.5/PM10) were included as covariates. This last covariate (pwratio) has been included to take into account the different composition of PM without incurring in multicollinearity: pwratio represents the proportion of PM10 which is due to PM2.5 particles. The non-parametric nature of the machine learning technique allows for exploration of complex interactions and nonlinear relationships within datasets. The available data are recursively partitioned into subsets. This process minimizes the residual sum of squares (RSS) within the end nodes, allowing the identification of complex relationships and interactions between variables that may not be captured by traditional linear regression models.
To investigate at the municipal level the relationship between T2DM prevalence and incidence counts, PM10 population-weighted exposure and pwratio, a Poisson mixed-effects model was employed, including as fixed effects:
-
year, (to capture possible trend in the data)
-
PM10,
-
pwratio (to take into consideration the proportion of PM10 due to PM2.5).
Random effects for both municipality and region, with municipality serving as a nested random effect within the region to accommodate the hierarchical structure of the data and account for potential clustering and correlation within municipalities, have been included in the models.
where:
-
\(\:{\beta\:}_{k}\) and \(\:{\gamma\:}_{k}\) are the coefficients for the fixed effects for prevalence and incidence counts, respectively;
-
\(\:Yea{r}_{\left\{ij\right\}},\:PM{10}_{\left\{ij\right\}},\:pwrati{o}_{\left\{ij\right\}}\) are the values for the predictor variables for municipality i in region j;
-
\(\:{u}_{\left\{0j\right\}},\:{v}_{\left\{ij\right\}}\:\) are the random effects for region j for prevalence and incidence, respectively;
-
\(\:{w}_{\left\{0j\right\}},\:\:{z}_{\left\{ij\right\}}\) are the random effects for municipality i within region j for prevalence and incidence, respectively.
The relationship between incidence rates and prevalence proportions at the municipal level and the covariates has been assessed with a mixed-effects model using the logarithm of the incidence rates (IncRate) or the prevalence proportions (PreProp) as outcomes, while the predictor variables used were year, population-weighted exposure to PM10, pwratio, and the interaction between age and sex. Random effects for municipalities nested within regions to allow for the inclusion of the hierarchical structure of the data into the model were used.
where:
-
\(\:{\delta\:}_{k},\:{\eta\:}_{k}\) are the coefficients for the fixed effects for prevalence proportions and incidence rates, respectively;
-
\(\:{t}_{\left\{0j\right\}},\:{p}_{\left\{0j\right\}}\:\) are the random effects for region j for prevalence proportions and incidence rates, respectively;
-
\(\:{e}_{\left\{ij\right\}},\:{m}_{\left\{ij\right\}}\) are the random effects for municipality i within region j for prevalence proportions and incidence rates, respectively.
Categorical variables will be presented as frequencies, while continuous variables will be summarized using either mean values with their respective standard deviations or median values with quartiles, depending on the data’s distributional characteristics. Incidence rates and prevalence proportions will be reported per 100,000 subjects, along with the one-standard-deviation interval. Differences in quantitative variables across different groups or conditions have been evaluated using either the Mann-Whitney test or the Kruskal-Wallis test, as appropriate. Post-hoc comparisons were performed using Dunn’s test with Bonferroni correction for multiple comparisons. The significance level for the tests was set at α=0.05. All statistical analyses were performed using R version 4.3.1.
Results
Descriptive statistics
Tables 1 and 2 show the population-weighted exposure mean and percentiles to PM2.5 and PM10, over the 2013–2021 period.
In 2021, as shown in Figures 1 and 2, PWE for both pollutants at the municipality levelexhibits a large variability across the country. The most critical area is the Po Valley, spanning the northern side and bordered by the Alps and the Apennines. High exposure, though limited to a smaller territory portion, is in the Sacco Valley, the Venafro plan, and the inland Naples and Caserta provinces. PWE variability is mostly due to different burdens of both particles and precursor pollutants emission (i.e., nitrogen oxides, ammonia, volatile organic compounds), the meteo-climatic and orographic peculiarity of the different zones.
Concerning T2DM, in 2013, there were 543,898 patients, increased to 694,000 in 2021, with the median duration in the study equal to 4 years (see Supplementary Material Table S1).
In 2021, (cfr Table 3) the median age for men was 71 years (Q1 = 63, Q3 = 78) while women were slightly older (p-value < 0.001) with a median age of 73 years (Q1 = 65, Q3 = 81). Both ages slightly increased from 2013 to 2021 due to the longitudinal nature of the study and the aging of the population (p-value < 0.001).
The median age across macro-areas is quite similar (Table 4), and differences for each single year are significant (p < 0.001).
In 2013, post hoc tests showed that, after adjusting for Bonferroni’s correction, only the differences between Center and Islands (p = 0.7979) and North-East and North-West (p = 1.0000) are not significant. In 2021, the only non-significant difference in age is between Center and North-West (p = 1.0000) (see Supplementary Material Table S2).
Average incidence rates by sex and geographical area have been reported in Table 5.
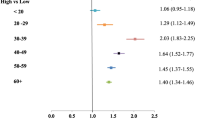
The rates and proportions are expressed as per 100,000 patients in the region, together with the one standard deviation interval around the mean (lo, up). Incidence is consistently higher for men and tends to decrease from the South to the North (North comprises both North-West and North-East together). In 2013, the South showed an average incidence rate, at the municipality level, equal to 26.56 for women and 33.73 for men, both decreased in 2021 to 16.72 and 21.99, respectively. North-East shows the smallest average incidence in 2013 (women = 5.92, men = 7.94) and 2021 (women = 3.41, men = 4.82).
Regarding prevalence proportions, they are consistently greater for men than women.
In 2013, the South showed an average prevalence proportion at the municipality level of 132.9 for women and 155.5 for men. Values show an increase from 2013 to 2021 (women = 143.5, men = 172.9 in 2021). From 2013 to 2021, the Center and the North-West showed a slight decrease in the average prevalence rates for both sexes, while the other areas showed an increase (Table 6).
Findings
Regression Trees (CARTs) rank covariates in order of importance in explaining the variability in the outcome, based on the ability to reduce the residual sum of squares (RSS) within each node (Fig. 3).
In 2021, according to the prevalence proportion, geographical area is the first covariate used to partition the dataset: the South accounts for 12% of the observations and shows an average prevalence proportion of 159, while the rest of the data (left-hand side of the tree) presents an average prevalence proportion of 49. The most influential variable in reducing the RSS and, therefore, in explaining the variability of the prevalence proportion is PM10, followed by pwratio, age, area, and sex. Figure 4 shows the regression tree for incidence rates.
The initial split is once again performed using geographical location: in 2021, the South showed an average incidence rate of 19, while the rest of the macro-areas displayed an average incidence rate of 6.3. After the initial split, the following one is performed based on PM10, both for the South and the rest of the macro-areas. The most influential variable in reducing the RSS is PM10, followed by pwratio, area, age, and sex.
To investigate the relationship between PM10, pwratio, and the counts of incidence and prevalence, a Poisson mixed-effects model was employed, including random effects for both municipality and region, with municipality serving as a nested random effect within the region to accommodate the hierarchical structure of the data and account for potential clustering and correlation within municipalities (Table 7).
Year shows a positive significant effect on prevalence (p < 0.001), all other things being equal, suggesting an increase over time in the prevalence of T2DM. PM10 also shows a positive effect on the expected prevalence (p < 0.001) with higher values of PM10 being associated with higher prevalence counts, keeping pwratio and year constant. The coefficient associated with pwratio is negative (log(PR)=−0.04, p < 0.001), indicating a negative association between expected counts for prevalence and pwratio, all other things being equal.
The Poisson mixed effect model for incidence (Table 7) shows a negative trend of the incidence of T2DM, all other things being equal (log(IRR)=−0.18, p < 0.001). PM10 shows a positive effect on incidence, with higher values of PM10 being associated with higher expected incidence counts (p = 0.003). An analogous effect can be observed for pwratio (log(IRR) = 0.03, p < 0.001), with higher values of pwratio, all other things being equal, being associated with higher expected counts of incidence.
The effects of PM10, pwratio, age, and sex on the log prevalence proportions and log incidence rates have been assessed via a linear mixed-effects model (Table 8).
All other things being equal, prevalence proportions at the municipality level tend to increase with time (coeff = 0.01749, p < 0.001). PM10 shows a negative association, controlling for year, pwratio, sex, and age, on the log prevalence proportion (coeff=−0.00298, p = 0.026) while an increase in the pwratio, keeping PM10 fixed and all other things being equal, is on average associated with an increase (coeff = 0.18724, p < 0.001) in prevalence rates at the municipality level. Prevalence rates are on average higher for men (coeff = 0.17758, p < 0.001) and age (coeff = 0.03088, p < 0.001), with a stronger increment with age for men compared to women (interaction coeff = 0.00198, p < 0.001), at the municipality level.
Incidence rates, at the municipality level, show a negative trend with time, all other things being equal, (coeff=−0.07961, p < 0.001). No significant association with PM10 is observed controlling for all the other variables (coeff=−0.00057, p = 0.579). On average, an increase in pwratio is associated with an increase in the incidence rates at the municipality level (coeff = 0.52304, p < 0.001). Men show on average higher incidence rates (coeff = 0.43196, p < 0.001) than women. Increases in age tend to be associated with higher incidence rates (coeff = 0.01098, p < 0.001). The association with age is dampened for men (interaction coeff=−0.00297, p < 0.001).
Discussion
In Italy, several regions are identified as hotspots for particulate matter air pollution. Exposure spatial variability across the country, mostly due to different burdens of both particles and precursor pollutants emission (i.e., nitrogen oxides, ammonia, volatile organic compounds) and meteo-climatic and orographic peculiarity of the different zones, can be visually appreciated in Figures. 1 and 2. The most critical area is the Po valley, spanning the northern side of the country, bordered by the Alps and Apennines (regions Piedmont, Lombardy, Veneto, Emilia-Romagna). A similar pattern of high exposure, though limited to a smaller territory portion, regards the Sacco valley (in the Lazio region) and the Venafro plan (Molise region), as well as the inland of Naples and Caserta provinces (Campania region). These regions experience high levels of anthropogenic emissions and unfavorable climatic conditions that exacerbate air quality issues. During the period under investigation, the particulate matter levels recorded in these areas consistently exceeded the limit values established by the European Union, as well as the more stringent guidelines set forth by the World Health Organization. Within the European context, these areas, along with certain Eastern European countries, rank among those with the highest levels of population exposure to air pollution. The patterns of particulate matter exposure generated by the implemented exposure models align closely with the levels observed at fixed measurement stations.
Air pollution has a major impact on T2DM in Italy 34. Our findings show an association between T2DM prevalence and incidence, and population-weighted exposure to PM10 and pwratio. In particular, the share of the PM2.5 fraction on the total pollutants (pwratio) constitutes a significant toll on T2DM. It follows that the most polluted areas of Italy are more at risk of suffering from T2DM than the least polluted ones: at the municipal level, both amounts of the two pollutants can significantly explain the variability of prevalence and incidence of T2DM, in line with the variability of PWE across the country.
Our results are entirely consistent with the findings of previous studies, particularly those conducted in high-income countries. Well-established research 4 has indicated a positive correlation between air pollution and T2DM risk. Furthermore, a recent meta-analysis confirmed that exposure to PM2.5 and PM10 particulate matter substantially raises the risk of T2DM 9. Our results are also consistent with the limited research relating to Italy, focusing particularly on the two largest and most populous Italian cities, Rome and Milan 25,26. In addition, the same results have been demonstrated in a study based on surveys conducted throughout the country, but based on self-reported diabetes 17. Finally, a more recent study has explored the association between air pollution and T2DM 27, demonstrating a preliminary complex link, probably mediated by socio-economic factors, between pollutants and incidence. Taken together, these findings support our hypothesis that air pollution plays a significant role in the development of T2DM.
While the positive association of PM10 with T2DM incidence counts is expected, the results for pwratio and prevalence counts are surprising: for the same amount of PM10, increases in pwratio, i.e. and increase in the fraction of pollutant due to PM2.5, all other things being equal, are associated with decreases in prevalence, probably due to the effect of increased mortality caused by this more aggressive pollutant 3,35.
Considering incidence rates and prevalence proportions, our findings show a significant positive effect of pwratio on T2DM incidence rates and prevalence proportions, when considering the same amount of total pollution and while controlling for sex, age, and the interaction between sex and age.
The same conclusion can be drawn by observing the results of the regression trees, where higher values of pwratio are always associated with higher incidence rates and prevalence proportions. Looking at sex, both incidence rates and prevalence proportions are higher for men compared to women, confirming what is already known in the literature 7,36. As expected, age shows a significant positive effect on T2DM prevalence proportions and incidence rate, confirming the association between age and that disease 27,37. The same conclusion is also confirmed by the regression trees, where age appears at various levels of the splitting process, and therefore suggests a possible complex relation between age, the other covariates, and the outcome. Finally, our results show a reversed dualism between the Northern and Southern parts of the country, to the advantage of the latter, this time likely benefiting from lower pollutant levels and less urbanization.
This study has important strengths that contribute to its relevance. Firstly, it fills a gap in the literature on this topic and represents the first investigation ever conducted in Italy on such a large dataset, providing new insights into the association between exposure to air pollution and the incidence and prevalence of T2DM. Secondly, the use of the exclusive AMD dataset allows us to calculate incidence and prevalence at the municipality level directly from clinical data, providing a valuable contribution to understanding the impact of the disease. Unlike other available national sources in Italy, which rely on self-reported diagnoses through sample surveys, the AMD dataset provides longitudinal and clinically diagnosed information on T2DM. Thirdly, the study uses a purpose-built dataset provided by ISPRA, the national governmental body tasked with monitoring, protecting, and conducting research on environmental issues, and based on the annual population-weighted exposure to PM2.5 and PM10 within all the cells of the one square kilometer, which allows us to associate T2DM data at a municipality level.
Nonetheless, it also has some limitations. This study relies exclusively on administrative data for T2DM and has an observational nature, which may introduce biases and limit the establishment of causal relationships. The AMD dataset is not representative of the entire Italian diabetic population since it tends not to include patients with less severe T2DM complications that are usually cared for by general practitioners, and finally, the population exposure to particulate matter dataset does not take into account the exact residence of the individuals used in the study as a population. Data from the AMD dataset have been aggregated at the municipality level: any implications and comments at individual patient level may suffer from ecological fallacy. In this study, all associations and results have been commented at the municipal level. Another limitation is associated with the absence of individual-level confounders (e.g., smoking, obesity) and socioeconomic factors (e.g., education, income). Although these variables are nominally included in the AMD dataset, the data were originally collected for clinical and administrative purposes. While the dataset provides high-quality and reliable information about diagnosis, treatment, and disease progression, the other variables are less systematically recorded and are not uniformly available across municipalities. To avoid introducing potential bias due to incomplete or inconsistently collected data, these factors were not taken into account in the analysis.
Conclusion and policy implications
This study aims to verify in Italy the association between air pollution and T2DM incidence and prevalence, taking into account age, sex, and geographical area. Italy bears attention because both T2DM and air pollution are major issues. Our investigation represents the first attempt to study the association between PM10 and PM2.5 and T2DM, measuring incidence and prevalence directly from a longitudinal dataset in which the diagnosis of diabetes is not self-reported. Moreover, the PWE indicator depicts a granular representation of exposure and captures the insidious role of particulate matter, especially PM2.5, on non-communicable diseases.
Our results constitute an important contribution by providing new insights for Italy. The implication of our findings emphasizes the need to conduct more analyses on the association between air pollution and T2DM. Both factors take a heavy toll on the Italian healthcare system, society, economy, and environment. This study highlights the possible role of environmental determinants of T2DM at the municipal level, suggesting the need to understand the mechanism that governs the propensity to develop the disease, which, to date, is still largely focused on genetic factors, lifestyles, and the socio-economic context 38. The results of the machine learning analyses point to the presence of complex and nonlinear relations between the factors that have been considered in this study and T2DM, which denotes the need for a thorough and deep analysis of the phenomenon. Finally, this paper suggests the importance of air pollution in healthcare settings, which could be turned into a barrier to achieving health equity. Indeed, equity issues arise as the disease tends to affect vulnerable populations disproportionately, such as low-income groups, people living in densely populated urban areas, and workers exposed to environmental pollutants, thereby reinforcing health and economic inequalities 39. Furthermore, the investigated issue has a double negative economic impact. Diabetes is costly for both the healthcare system and patients in terms of direct healthcare costs and indirect costs such as loss of productivity, absenteeism, and disability, which are at least partially avoidable if they are linked to environmental pollution.
The implications for economic policy are relevant and wide-reaching. The matter concerns the environment and health from a ‘One Health’ perspective 40. The environmental issue is already known and debated in terms of policy implications, such as the correct approach to the negative externalities associated with pollution. As has already been implemented, the advantages of environmental taxation are evident and should be pursued 41. Nevertheless, an ex-ante health impact assessment of environmental policies, with the explicit inclusion of metabolic health indicators, including diabetes, in the cost-benefit analysis of infrastructure, urban planning, and industrial projects, could be helpful 42. Redistributive and compensatory policies for population groups exposed to greater environmental and health risks, such as providing subsidies and free access to screening and treatment for diabetes, are also important 43,44. This should be accompanied by strengthening healthcare welfare in areas with high environmental exposure 45. Lastly, the subject addressed in this study underscores the persistent fragmentation, often referred to as “silos”, that continues to characterize the health sector, despite frequent calls for more integrated approaches. This compartmentalization persists as a significant impediment to effective policy coordination. The findings of this study further underscore the pressing need to enhance intersectorial collaboration, particularly among the Ministries of health, environment, and economy 46. Future developments of this investigation may research the presence of structural changes in the connection between exposure to pollutants and T2DM before and after the COVID-19 pandemic and the inclusion of individual-level confounders not available at the moment such as smoking, obesity, and occupational exposures, trying to mitigate the effect of aggregating data at the municipal level conducting the analyses on a restricted dataset but at individual level.
Data availability
The data supporting the findings of this study can be accessed through AMD and ISPRA; however, there are restrictions on its availability, as it was used under license for this specific study and is not publicly accessible. However, the authors can provide the data upon reasonable request, but only with the permission of AMD and ISPRA.
References
Burkart, K. et al. Estimates, trends, and drivers of the global burden of type 2 diabetes attributable to PM₂.₅ air pollution, 1990–2019: analysis from the global burden of disease study 2019. Lancet Planet. Health. https://doi.org/10.1016/S2542-5196(22)00122-X (2022).
Wang, B. et al. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: a systematic review and meta-analysis of cohort studies. Eur. J. Endocrinol. 171, R7–R13. https://doi.org/10.1530/EJE-14-0365 (2014).
Li, Y. et al. Association between air pollution and type 2 diabetes: an updated review of the literature. Ther. Adv. Endocrinol. Metab. https://doi.org/10.1177/2042018819897046 (2019).
Eze, I. C., Hemkens, L. G., Bucher, H. C., Hoffmann, B. & Probst-Hensch, N. M. Association between ambient air pollution and diabetes mellitus in Europe and North america: systematic review and meta-analysis. Environ. Health Perspect. 123, 381–389. https://doi.org/10.1289/ehp.1307823 (2015).
Meo, S. A. et al. Effect of environmental air pollution on type 2 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 19, 1– (2015).
Park, S. K. & Wang, W. Ambient air pollution and type 2 diabetes mellitus: a systematic review of epidemiologic research. Curr. Environ. Health Rep. 1, 201–210 . https://doi.org/0.1007/s40572-014-0017-9 (2014).
Balti, E. V., Echouffo-Tcheugui, J. B., Yako, Y. Y. & Kengne, A. P. Air pollution and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res. Clin. Pract. 106, 161–172. https://doi.org/10.1016/j.diabres.2014.08.010 (2014).
Patel, C. J., Bhattacharya, J. & Butte, A. J. An environment-wide association study (EWAS) on type 2 diabetes mellitus. PLoS One. 5, e10746. https://doi.org/10.1371/journal.pone.0010746 (2010).
Yang, B. Y. et al. Ambient air pollution and diabetes: a systematic review and meta-analysis. Environ. Res. 180, 108817. https://doi.org/10.1016/j.envres.2019.108817 (2020).
Thiering, E. & Heinrich, J. Epidemiology of air pollution and diabetes. Trends Endocrinol. Metab. 26, 384–394. https://doi.org/10.1016/j.tem.2015.04.004 (2015).
Rajagopalan, S. & Brook, R. D. Air pollution and type 2 diabetes: mechanistic insights. Diabetes 61, 3037–3045 https://doi.org/0.2337/db12-0190 PMID: 23172950 (2012).
Janghorbani, M., Momeni, F. & Mansourian, M. Systematic review and meta-analysis of air pollution exposure and risk of diabetes. Eur. J. Epidemiol. 29, 231–242. https://doi.org/10.1007/s10654-014-9907-2 (2014).
Liang, W. et al. Ambient air pollution and gestational diabetes mellitus: an updated systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 255, 114802. https://doi.org/10.1016/j.ecoenv.2023.114802 (2023).
Wu, Y. et al. Ambient air pollution associated with incidence and dynamic progression of type 2 diabetes: a trajectory analysis of a population-based cohort. BMC Med. 20, 375. https://doi.org/10.1186/s12916-022-02558-1 (2022).
Shin, M. K. & Kim, K. N. Association between long-term air pollution exposure and development of diabetes among community-dwelling adults: modification by dietary nutrients. Environ. Int. 174, 107908. https://doi.org/10.1016/j.envint.2023.107908 (2023).
Paul, L. A. et al. The impact of air pollution on the incidence of diabetes and survival among prevalent diabetes cases. Environ. Int. 134, 105333. https://doi.org/10.1016/j.envint.2019.105333 (2020).
Orioli, R., Cremona, G., Ciancarella, L. & Solimini, A. G. Association between PM₁₀, PM₂.₅, NO₂, O₃ and self-reported diabetes in italy: a cross-sectional, ecological study. PLoS One. 13, e0191112. https://doi.org/10.1371/journal.pone.0191112 (2018).
Magnoni, P., Murtas, R. & Russo, A. G. Traffic noise, air pollutants and incidence of diabetes mellitus: a population cohort study in Milan. Eur. J. Public. Health. 30, ckaa166–ckaa165. https://doi.org/10.1093/eurpub/ckaa166.165 (2020).
EEA & Europe’s Air Quality Status (2023). Accessed 24 Apr 2024. (2023).
ISPRA. Eco Atlante – inquinamento atmosferico. Accessed 24 Apr 2024. (2024).
ISTAT. Aspetti della vita quotidiana. Fattori di rischio per la salute: fumo, obesità, alcol e sedentarietà. (2023).
Schachter, G. & Engelbourg, S. The steadfastness of economic dualism in Italy. J. Dev. Areas. 22, 4 (1988).
ISTAT. Statistical Yearbook of Italy, Territories. (2020).
Solimini, A. et al. Ecological correlation between diabetes hospitalizations and fine particulate matter in Italian provinces. BMC Public. Health. 15, 1. https://doi.org/10.1186/s12889-015-2018-5 (2015).
Meroni, G. et al. The relationship between air pollution and diabetes: a study on the municipalities of the metropolitan City of Milan. Diabetes Res. Clin. Pract. 174, 108748. https://doi.org/10.1016/j.diabres.2021.108748 (2021).
Renzi, M. et al. Air pollution and occurrence of type 2 diabetes in a large cohort study. Environ. Int. 112, 68–76. https://doi.org/10.1016/j.envint.2017.12.007 (2018).
Abbafati, C., Nieddu, L. & Quarto, A. Exploring the link between particulate matter pollution and type II diabetes in Italy and Lombardy using clinical longitudinal data: a comparative analysis (2025). https://doi.org/10.1007/s10389-025-02482-5
Fioravanti, G., Martino, S., Cameletti, M. & Cattani, G. Spatio-temporal modeling of PM₁₀ daily concentrations in Italy using the SPDE approach. Atmos. Environ. 248, 118192. https://doi.org/10.1016/j.atmosenv.2021.118192 (2021).
Ministero della Salute. Stato del Diabete Mellito in Italia, (2022).
WHO. Registries and information systems for diabetes care in the WHO European Region: preliminary findings for consultation. (2021).
Clark, J. S. & Gelfand, A. E. A future for models and data in environmental science. Trends Ecol. Evol. 21, 375–380. https://doi.org/10.1016/j.tree.2006.03.016 (2006).
Lindgren, F., Rue, H. & Lindstrom, J. An explicit link between Gaussian fields and Gaussian Markov random fields: the SPDE approach. J. R Stat. Soc. B. 73, 423–498. https://doi.org/10.1111/j.1467-9868.2011.00777.x (2011).
Rue, H., Martino, S. & Chopin, N. Approximate bayesian inference for latent Gaussian models using integrated nested Laplace approximations. J. R Stat. Soc. B. 71, 319–392. https://doi.org/10.1111/j.1467-9868.2008.00700.x (2009).
Beulens, J. W. J. et al. Environmental risk factors of type 2 diabetes—an exposome approach. Diabetologia 65, 263–274. https://doi.org/10.1007/s00125-021-05618-w (2022).
Conti, S. et al. Time-Trends in air pollution impact on health in italy, 1990–2019: analysis from the global burden of disease study 2019. Int. J. Public. Health. 68 https://doi.org/10.3389/ijph.2023.1605959 (2023).
Kautzky-Willer, A. et al. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 37, 278–316. https://doi.org/10.1210/er.2015-1137 (2016).
Abbafati, C., Nieddu, L. & Monasta, L. Measures of type 2 diabetes burden in Italy assessed using the AMD dataset over twelve years across the great recession. Sci. Rep. 14, 4901 (2024).
Stringhini, G. et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1.7 million men and women. Lancet 389, 1229–1237. https://doi.org/10.1016/S0140-6736(17)30606-2 (2017).
Prüss-Ustün, A. et al. Diseases due to unhealthy environments: an updated estimate of the global burden of disease attributable to environmental determinants of health. J. Public. Health. 39, 464–475. https://doi.org/10.1093/pubmed/fdw085 (2017).
Cook, R. A., Karesh, W. B. & Osofsky, S. A. The Manhattan Principles on One World, One Health – building interdisciplinary bridges to health in a globalized world. Wildl. Conserv. Soc. Symp. Rep. (2004).
Bovenberg, A. L. & Goulder, L. H. Elsevier,. Environmental taxation and regulation. In Handbook of Public Economics 3, 1471–1545 (2002).
Williams, J. T. W., Bell, K. J. L., Morton, R. L. & Dieng, M. Methods to include environmental impacts in health economic evaluations and health technology assessments: a scoping review. Value Health. 27, 794–804. https://doi.org/10.1016/j.jval.2024.02.019 (2024).
Clark, M. L. & Utz, S. W. Social determinants of type 2 diabetes and health in the united States. World J. Diabetes. 5, 296–304. https://doi.org/10.4239/wjd.v5.i3.296 (2014).
Hill-Briggs, F. et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 44, 258–279. https://doi.org/10.2337/dci20-0053 (2020).
Olden, K., Ramos, R. M. & Freudenberg, N. To reduce urban disparities in health, strengthen and enforce equitably environmental and consumer laws. J. Urban Health. 86, 819–824. https://doi.org/10.1007/s11524-009-9380-5 (2009).
Marmot, M. & Bell, R. Social determinants and non-communicable diseases: time for integrated action. BMJ 364, l251. https://doi.org/10.1136/bmj.l251 (2019).
Acknowledgements
We would like to express our gratitude to the Italian Diabetologist Association (AMD) for their exclusive collaboration research agreement and for providing the data used in this study. A special thanks goes to the president of AMD, Riccardo Candido.
Author information
Authors and Affiliations
Contributions
C.A. and L.N. conceptualized the study. C.A., L.N. G.C., and M.R. analyzed the data and drafted the manuscript. C.A. and L.N. finalized and edited the manuscript. P.P. contributed to reviewing the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no conflict of interest, financial or otherwise.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Abbafati, C., Nieddu, L., Cattani, G. et al. Association between air pollution and type II diabetes in Italy from clinical data and population-weighted exposure at the municipality level. Sci Rep 15, 28326 (2025). https://doi.org/10.1038/s41598-025-13733-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13733-6