Abstract
The use of microbial loaded biochar in soil remediation is becoming popular worldwide. In the current research, a maize straw biochar (MB) and Trichoderma harzianum loaded biochar (MBT) were used at various rates in Cd–Cu dual polluted soil, to see their effect on Cd–Cu removal and to explore vigorous changes of metals bio-accessibility. Throughout the 90 days of remediation experiment, the dynamic impacts on the soil’s physiochemical characteristics were noted. According to the current study’s findings, applying 5% MBT to the soil early in the incubation period significantly raised its pH, which eventually dropped to a neutral-alkaline level. The application of MBT promoted residual bound Cu–Cd fraction and decreased carbonate and exchangeable bound fraction in the treated soil. The exchangeable portion of Cd highly decreased by 15.33% in the MBT5 amendments. Similarly, the DTPA extractable Cu and Cd concentration (i.e., DTPA-Cu, DTPA-Cd) in the amended soil gradually decreased with time. After 90 days, the DTPA-Cd contents lowered from 45 mg kg− 1 in CT to 25.3 mg kg− 1 in MBT5 whereas the DTPA-Cu concentration was 62.3 mg kg− 1 in MBT5, respectively. The Cd bio-accessibility of gastric juice was reduced in all treatments including 68.1% of MBT5. Gastrointestinal fractions at the various treatments were recorded to be 73% (MB1), 71.1% (MBT1), 67.4% (MB5), and 63.5% (MBT5). In comparison, an obvious reduction in Cu bio-accessibility was perceived in all four amendments, viz. MB1 (72.5%), MBT1 (69.6%), MB5 (65.1%), and MB5 (61.4%) in gastric solution. The addition of MBT also enhanced soil enzymatic activity particularly urease and catalase, in the later phase of the experiment, showing the retrieval function of soil for the post-stabilization of metal. The present study confirmed that MBT effectively reduces Cd and Cu bio-accessibility and mobility, supporting long term soil stabilization. It also restores soil enzymatic activity, making it a sustainable and ecofriendly approach for heavy metal remediation.
Similar content being viewed by others
Introduction
Contamination of soil, especially with heavy metals (HMs), is a concerning worldwide ecological issue1. The ecosystem and human health are significantly impacted by heavy metals2. Because of the heavy metals’ high toxicity, long-lasting impacts, irreversible nature of contamination, and storage in the food chain, their presence in soil poses serious issues. According to risk assessment, HMs pose a high risk of cancer. The list of HMs includes Cd, Al, Pb, Cu, Br, and Ar. Higher concentrations of these metal ions and their water-soluble nature make them dangerous to living organisms including humans, animals, and plants3. Various metal-contaminated localities have been recognized in various countries, across the globe. In the USA, around 100,000 polluted localities have been recognized4. In the European Union nations, about 250,000 locations have been reported to be contaminated and need remediation, urgently5. In a random survey, the Chinese Ministry of Environment Protection reported 16.2% of locations in China as contaminated while 11.3% of sites were registered as minor contaminated. It has been reported that inorganic pollutants account for most of the pollution (82.8%) (MEP, 2014).
Several methods have been utilized for the removal of heavy metals in contaminated soil. Phytoremediation, soil washing, and stabilization are among the extensively utilized techniques6. Soil metal remediation has typically made use of biochar, a high-carbon mass employed in the thermal transformation of biomass in anaerobic environment. Biochar has a porous shape, a larger surface area, and several functional groups7. Biochar can adsorb harmful metals and decrease their mobility, liability, and therefore harmfulness in soil8. Numerous researchers have examined the main technique for immobilizing the HMs in contaminated soil with the help of various forms of biochar9. Biochar primarily removes heavy metals from the environment by precipitation, reduction, cation exchange, complexation, and surface contact. Additionally, the addition of charcoal to the soil may alter its pH, texture, CEC, and soil organic matter, all of which could increase the soil’s potential to store metals10.
The heavy metals immobilization in polluted soil with biochar treatments is mostly dependent on the attributes of the biochar, such as the kind of feedstock, temperature during pyrolysis, and duration of heating. Various researchers have documented the grass-based biochar (e.g., maize straw, rice husk, and wheat husk biochar) as more nominal than wood straw biochar in declining Cd leakage potential and accumulation in plant cells11. It has been documented that biochar prepared at lower temperatures is more effective for metal immobilization in comparison to biochar prepared at high temperatures12. Furthermore, the control of HMs in the polluted soil is also affected by a few other factors, including the amount of biochar present, the mixing depth, soil characteristics, climate, and soil microbial diversity13.
The most effective and recently developed method for the restoration of contaminated soil is thought to be the loading of biochar by beneficial microbial communities (bacteria, fungi, etc.) and its application in bioremediation14. Several microbe species with robust metal adsorption and tolerance capability have been identified and added to the polluted soil as microbial agents to immobilize HMs in the contaminated soil. Typically, synthetic macromolecules and composite materials are used as carrier substances to immobilize microbial cells15. As of right now, biochar is the most effective carrier of microorganisms that promote growth16. Various authentic studies prove that the possible relations strategies between soil native microorganisms and biochar reported by many scientists, comprising (i) biochar provides accommodation for microbes; (ii) biochar works as a nutrient source for microorganisms; (iii) biochar can alter soil basic properties, soil microbial population, and enzyme activity of soil; (iv) biochar increase the conversion and degradation of pollutants with the help of sorption, free radicals, as well as electron transport17. Although several studies have examined the individual roles of biochar and soil microorganisms, but their combined effects and mechanism is still not well understood. In general, the investigation of metal speciation which is inadequate in the systematic analysis of metal availability depending on various receptors at different scales is required to assess the stability potential of HMs in soil. The whole process of biochar and microbes working together to recover heavy metals from polluted soil needs to be explained.
Several researchers have reported that, filamentous fungi can be handled easily, and used widely18. Different strains of Trichoderma genus have an extensive functional behavior required in soil remediation, such as effective soil colonization, greater biodegradation potential, and ability to establish symbiotic relation with plant18. Trichoderma can eradicate and concentrate different metals, like Cd, Pb, Cu, Zn, and Ni19. Similarly, biosorption, the fraternization of metals to the surface of cell, has also been pronounced concerning Trichoderma genus in case of both Cd and Cu metals20. Additionally, different researchers have proved that Trichoderma rises the absorption of Cd21, which leads to decrease of Cd uptake by plants and animals, and in contaminated soil, T. harzianum contested effectually with soil minerals to accrue Cd22. It is hypothesized that the use of MBT to Cd–Cu dual-polluted soil will effectively reduce the bio-accessibility and mobility of cadmium and copper by transforming them into more stable residual fractions, thereby minimizing their environmental risk. Additionally, it is expected that MBT will improve soil physicochemical properties, such as pH, and enhance soil enzymatic activities, contributing to the restoration of soil health and promoting long-term stabilization through a sustainable and eco-friendly remediation approach.
The objectives of the current study was to evaluate the effectiveness of MBT in remediating Cu and Cd contaminated soils. Specifically, the study sought to explore the impact of MBT, applied at different rates, on the removal and stabilization of these HMs in a dual-contaminated soil system. The objectives included investigating the changes in metal bio-accessibility and mobility, assessing the temporal variations in soil physicochemical attributes like pH, and analyzing the transformation of HMs into more stabilized forms, particularly the shift from exchangeable and carbonate-bound fractions to residual fractions. The study also aimed to determine the effectiveness of MBT in reducing DTPA-extractable and gastric/gastrointestinal bio-accessible fractions of Cd and Cu, which are critical indicators of metal availability and risk. Additionally, it evaluated the enhancement of soil enzymatic activities, notably urease and catalase, as indicators of soil biological recovery and functionality during and after the remediation process. Through a 90-day incubation experiment, the study ultimately aimed to establish MBT loaded biochar as a sustainable, eco-friendly, and efficient strategy for the long-term stabilization of HMs in contaminated soils, contributing to environmental safety and improved soil health.
Materials and methods
Production of biochar
As previously stated, the maize straw biochar (MB) was utilized for biochar synthesis due to its high creation and exceptional potential in the immobilization of metals18. The Ref.23 procedure was followed in the preparation of biochar. For five hours, maize straw was subjected to a gradual pyrolysis process in a vacuum furnace without oxygen at 600 °C. The fundamental features of the MB were noted (Table 1). The prepared biochar was crushed and run through a 0.30 mm pore-size sieve before being used.
Preparation of biochar-loaded Trichoderma Harzianum
For the preparation of T. harzianum loaded biochar, T. harzianum was cultured on potato dextrose agar media and blended with autoclaved seeds of Sorghum, for 10 days. The T. harzianum was mixed with Sorgham seeds because it provides nutrients to the fungus prior to the attachment with biochar’s surface. Later on, fungus-coated sorghum seeds were amalgamated with synthesized biochar in a 1:1 ratio (w/w). The mixture was placed for 7 days in an air tight close container at 4 °C in the dark, with 10% moisture, to maintain fungal viability and prevent contamination before their further use. The sorghum seeds were separated from the biochar after 7 days of incubation and the prepared MBT was observed via scanning electron microscope (SEM) (JEOLJSM, 25910). The pure biochar was also monitored through SEM.
Soil incubation testing
The samples of the soil were collected from the local farmland in Islamabad (33.6996° N, 73.0362° E). Measurements were made of the elemental composition (C, K, P, and Na), pH, soil texture, electrical conductivity (EC), organic materials, cation exchange capacity (CEC), and other fundamental soil parameters (RK, 1999). To get rid of various contaminants including nuggets and organic remnants, the soil was shed-dried and sieved. Subsequently, the Cu (NO3)2 and Cd (NO3)2 solutions were carefully mixed with the soil and allowed to sit at 25 °C for two weeks (14 days), so that the applied metal dispersed uniformly throughout the soil. The amounts of Cd and Cu in the treated soil were calculated 60 mg kg− 1 and 258 mg kg− 1, correspondingly.
The 90-day incubation trial was performed at 25 °C in a thermostatic chamber. The Cd and Cu-modified soils were given the designations MB1, MB5, MBT1, and MBT5, respectively, after being treated with MB and MBT at 1% and 5% concentrations. The soil was kept at 35% humidity for the duration of the incubation period. The soil samples were shed-dried and sieved through the sieve for further evaluation following the 90-day incubation period.
Heavy metal extraction from soil using the Tessier sequential approach
Fluctuations in Cd and Cu speciation and redistribution in contaminated soil samples were examined using the “Tessier sequential extraction method”24. Five varied components nominated through this method were exchangeable (EX), carbonate bound (CB), matter bound (OM), Fe–Mn oxide bound (OX), and residual fraction (RS). Using 1.0 mol L− 1 MgCl2, 1.0 mol L− 1 NaOAc (pH 5), 0.04 mol L− 1 NH2OH·HCl, and 0.04 mol L− 1 HNO3 (in 30% H2O2), respectively, the first four of them were isolated. By using a combination of HNO3-HF-HClO4 to digest the leftover material, the fifth fraction was obtained.
Phyto-availability test of heavy metals
Diethylenetriamine Penta acetic acid (DTPA) extraction of soil heavy metals is recognized as the readily assimilated fraction for plant consumption25. In polluted soils, DTPA extraction assessment is also used to identify bioavailability, ecotoxicity, and heavy metal concentrations26. As a result, the effects of MB and MBT amendments on the phyto-availability of Cu and Cd were assessed using the DTPA extraction procedure. The extract suspension containing 0.005 mol L− 1 DTPA, 0.1 mol L− 1 triethanolamine (pH 7.3), and 0.01 mol L− 1 CaCl2 was combined with soil samples at a 1:2 (m/v) solid-to-water ratio. The solutions were centrifuged, filtered through 0.45 μm polyethersulfone filter paper, and spun on a shaker at 25 °C for two hours at 180 rpm. Using an atomic absorption spectrophotometer, the amounts of Cu and Cd in the filtrates of the aforesaid sequential extraction were determined.
HMs bioavailability in soil
The analysis of Cd and Cu intake by the human body through oral intake was conducted using the Unified Bio-Accessibility Method (UBM)27. According to Zhong and Jiang (2017), gastric juice often contains a larger amount of Cd and Cu that is bio-accessible than intestinal juice. Briefly stated each 2 g amended soil sample was assorted using 45 mL of an artificial stomach solution that was made using a modified UBM technique28. The solution’s pH was raised to 1.1.
For the assessment of Cd and Cu in the intestine of humans, the intestinal juice was prepared according to the U.S. Pharmacopeia procedure. Briefly, the intestinal solution was synthesized from the gastric solution through the addition of pancreatin and neutralization with solid sodium hydrogen carbonate to gain pH 7. Then 2 g of soil from every treatment was blended with the simulated intestinal solution (45 mL) and the mixture was agitated slowly for 60 min at 37 °C. After that, the suspensions were centrifuged, passed through a filter, and kept at 4 °C. Cu and Cd contents in the filtrates of the gastric and intestine phases were quantified through an atomic absorption spectrophotometer. The ratio of the content of Cu and Cd in the simulated extract to their total concentration in the contaminated soil was used to display the bio-accessibility.
Study of soil enzymatic activity
The catalase (CAT) activity was evaluated according to the29 standard methodology. Briefly said, 40 mL of distilled water, 5 mL of 0.3% H2O2, and 2 g of dirt were combined. After 20 min of stirring at 25 °C, 5 mL (1.5 mL) of H2SO4 were added to the mixture. 0.1 M KMnO4 was used to titrate the suspensions. The Guan (1986) methodology was used in measuring the urease potential. 5 g of moist soil was incubated in 20 ml of borate buffer at 37 °C for 2 h to measure the urease activity. 50 mL of a 1 M KCl solution was combined and shaken for 30 min following incubation. The mixture’s absorbance was calculated using a UV-visible spectrophotometer at 690 nm.
Data analysis
Three duplicates of the entire trial were conducted. The means and standard deviations of the results were displayed. The statistical software SPSS Statistics 19.0 was employed for the analyses. ANOVA was used to quantify the effects of different interventions on the components under consideration.
Results and discussion
Description of T. harzianum loaded biochar
Scanning electron microscopy of MB confirmed cracked and porous surface appearance (Fig. 1A), which may assist the growth and dissemination of the T. harzianum. The T. harzianum (Fig. 1B) stuck well to the surface of biochar and the majority of hyphae colonized or dispersed on the biochar’s surface, though, it is equitable that some hyphae pass in via the holes in the biochar’s surface (Fig. 1C). Biochar contains highly activated carbon and has a large surface area. Biochar has a diverse pore structure, including micropores, mesopores, and macropores. This surface diversity increases its efficacy to adsorb a wide range of molecules and substances, in comparison to char and other porous materials. Based on its morphological features, the rough surface of biochar makes it suitable for the attachment of microbial strain, which aligns with the previous study30,31. These investigations concluded that the existence of polycyclic aromatic hydrocarbons and a significant number of minerals might impede the attachment of microbe’s biochar’s surface.
Fluctuations in soil pH
Application of both MB and MBT raised the pH of treated soil at the initial phases of incubation (day 7), particularly with a dosage rate of 5%. During 90-day incubation, the soil pH changed in all treatments (Fig. 2). Less variation in the pH of all the amendments was perceived with the increasing incubation time. Both MB and MBT changed the soil pH at 1% and 5% concentrations. After the addition of 1% MB1 and MBT1, the pH was changed from 7.2 (control soil pH) to 7.8 and 8.0, respectively. After the addition of 5% MB5 and MBT5 biochar, the pH values in the treated soil samples increased to 8.3 and 8.7, respectively. These investigations have shown that the breakdown of alkaline materials (like carbonate) in the biochar was the base of the quick rise in soil pH32. Later on, as alkaline materials are depleted, the pH of the soil drops. In particular, after 42 days of incubation, the soil pH was alarmingly lower with the MBT5 treatment. In the current study, the pH decreased more during MBT treatments than MB treatments. The microbial activity of T. harzianum, which generates organic acids during metabolism, is responsible for the greater reduction in pH in MBT treatments in comparison to MB. These findings suggest that higher concentrations of MBT can effectively enhance soil properties. Nevertheless, following the MBT treatment, the soil’s pH in this experiment was in the neutral-alkaline range. The interesting story of MBT’s pH buffering abilities calls for more investigation into the speciation and liability of Cu and Cd in contaminated soil, particularly in a soil of acidic nature. These results are exactly aligned with the work of previous researchers3.
Cd fractionation in a treated soil
Cd proportion in contaminated soil were interrupted by the addition of biochar treatments (Fig. 3A). Exchangeable Cd (59.10%) was the predominant Cd fraction at 0 days in the control sample (untreated soil), followed by carbonate bound Cd (27.2%), residual bound Cd (8.10%), Fe–Mn oxide Cd (4.80%), and organic matter bound Cd (> 1.0%). The fraction of exchangeable Cd in treated soil reduces to different values and transforms into additional components after 90 days of incubation. The Fe–Mn oxide portions of Cd augmented by 1.90% and 2.11%, respectively, in the MB1 and MBT1 amendment compared to the CT sample, while the residual binding Cd improved by 1.1% and 1.9%, individually. The exchangeable portion of Cd decreased by 6.73% and 15.33% in the MB5 and MBT5 amendments, respectively; in the meantime, the Fe–Mn oxide Cd portion increased by 5.23% and 9.30%, respectively, and the residual bound Cd increased by 2.0% and 6.31%, respectively. The increase in the Fe–Mn oxide Cd fraction after MBT and MB may be due to the availability of Fe and Mn oxides on the biochar surface and in soil. These oxides provide strong adsorption sites that can immobilize Cd by forming stable complexes, thus shifting Cd from more mobile forms to less bioavailable Fe–Mn oxide–bound fractions. Additionally, microbial activity may stimulate redox reactions that favor Cd binding to these oxides.
The greatest quantity of organic matter-bound fraction of Cd was found in MB5 (0.65%) and MBT5 (0.75%), with very slight variations in the organic matter binding fraction seen in all treatments. In all amendments, no discernible variations in the carbonates bound Cd percentage were found. The incorporation of biochar was found to significantly alter the heavy metal speciation in the soil. In this investigation, the amount of Cd exchangeable content decreased gradually as the dosage rate of biochar increased. Previous studies also stated that the exchangeable fraction of Cd is the primary proportion of the total Cd content in the soil33. In our findings, no reduction was detected in the carbonate bound fraction which can be attributed to the higher pH of biochar additions because the change in pH has been reported to promote carbonate precipitation like CdCO334. The present investigation revealed an increase in both the Fe–Mn bound fraction and the residual bound fraction. Applying biochar to the soil has been shown by some earlier researchers to significantly reduce the reducible Cd concentration and increase the oxidizable and residual Cd concentration.
Cu fractionation in a treated soil
Cu proportion in polluted soil were interrupted by the addition of biochar treatments (Fig. 3B). In contrast to cadmium, copper was predominantly found in the carbonate-bound Cu (50.70%) ratio in the control sample (CT, 0 days), with residual Cu (27.33%), Fe–Mn oxide Cu (16.50%), organic matter Cu (3.30%), and exchangeable Cu (2.44%) coming in second and third, respectively. On the 90th day of the experiment, the exchangeable Cu fraction decreased up to 2.0% in MB1, 1.96 in MBT1, 1.70% in MB5, and 1.20% in MBT5. In comparison to the CT amendment, the carbonate-bound Cu portions of MB5 and MBT5 amendments were notably decreased to 38.80% and 34.64%, respectively. The residual Cu fraction was increased by 7. 47%, 9.37%, 13.63%, and 19.65% in MB1, MBT1, MB5, and MBT5 respectively. In this study, the Fe–Mn oxide Cu portion was reduced which may be changed to organic matter Cu portion within 90 days of incubation, as it increased in all treatments (MB1, MBT1, MB5, and MBT5). Many researchers indicated that the use of biochar increases the fraction of organically bound Cu35. The biochar surface comprises multiple oxygen-containing functional groups that may result in enhanced oxidizable fractions to create organic-bound Cu by interacting with Cu36. According to37, biochar can increase the organic matter-bound Cu ratio. The soil organic matter Cu fraction was not increased correspondingly, even if the current data showed improved organic bound fraction with biochar treatments. In the current work, the carbonate-bound Cu fraction was enhanced to become the residual bound Cu fractions, which are the most stubborn. The component that is most readily available is indicated by the carbonate and exchangeable bound content, whereas the resistant component is identified by the Fe-Mn oxide, residual fractions, and organic matter bound. Both the recalcitrant and liable proportions can be employed to estimate the efficacy of in-situ stabilization38.
In the current work, we find out that both bioavailable Cd and Cu contents of the MB5 amendment were considerably lesser than the MB1 amendment (Fig. 3A,B). Furthermore, MBT may effectively reduce the bioavailable Cu and Cd in the contaminated soil compared to MB treatment, increasing the immobility of Cu and Cd, especially at a 5% application rate. Cu and Cd might be changed from bioavailable portions to less bioavailable ones by both MB and MBT. Nonetheless, there are differences in the fraction dissemination of Cd and Cu in the modified soils. Particularly, the soil exchangeable Cu fraction is lesser than that of Cd, whereas the soil organic matter bond Cu portion is distinctly greater than that of Cd. Cu higher sorption potential and affinity sequence for organic materials (such humic acid and biochar) as well as soil inorganic matter may be the source of this39. Therefore, applying biochar to the soil can reduce the environmental harm posed by heavy metals by condensing them into a more stable state.
Phyto-availability of Cu and Cd
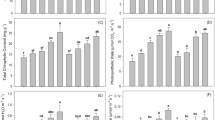
DTPA extractable Cd and Cu concentration (i.e., DTPA-Cu, DTPA-Cd) in the amended soil gradually decreased with an increase in incubation duration (Fig. 4A,B). After 90 days, the DTPA-Cd contents lowered from 45 mg kg− 1 in CT to 38 mg kg− 1 in MB1, 36 mg kg− 1 in MBT1, 31.1 mg kg− 1 in MB5 and 25.3 mg kg− 1 in MBT5. In comparison to control, the declination in DTPA-Cu concentration was observed in all treatments. Though, different results have been reported in some other studies40. Some other workers have also perceived an escalation in the phyto-availability of Cu41. This increase might be because of an amplified proportion of dissolved organic matter present in biochar. Dissolved organic matter produced from biochar has various hydroxyl and carboxylate functional groups that can interact with Cu cations and elevate their mobility and bioavailability42. Biochar has been reported to produce a minimum amount of dissolved organic matter43. In comparison to the individual MB amendment, the MBT amendment with an equal dose rate had a lesser concentration of DTPA-Cu and DTPA-Cd. In specific, on the 90th day, the DTPA-Cd contents were 23.2 and 16.7 mg kg− 1 in MBT1 and MBT5; whereas the DTPA-Cu concentration was 104.2 and 62.3 mg kg− 1 in MBT1 and MBT5, respectively. These findings showed that MBT creates a momentous impact in declining the phyto-availability of Cu and Cd in soil, particularly at a treatment dose of 5%. The significant reduces the phyto-availability of Cu and Cd because the higher application rate provides more surface area, functional groups, and microbial biomass. This enhances metal adsorption, microbial immobilization processes, and transformation of metals into less bioavailable forms, which is less effective at the lower 1% dose.
Bio-accessibility of Cd and Cu
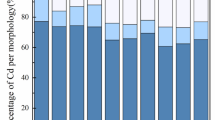
In this study, a unified bio-accessibility test was successfully implemented to see bio-accessible Cu and Cd proportions from the contaminated soil. This test is cost-effective and quick, while other animal studies are costly, difficult in management and care, and time-consuming. It is always desirable to shift from in vivo studies approaches to in vitro approaches44; though, a consistently reproducible extraction protocol for several soils is still needed. Additionally, this procedure will be easily applied by various research laboratories. Artificial biofluids have been applied in different physiological tests, for the expectation of drug uptake, nutritional analysis45, and metal solubility of orthodontic applications46. Gastrointestinal fractions at the various treatments were recorded to be 73% (MB1), 71.1% (MBT1), 67.4% (MB5), and 63.5% (MBT5) (Fig. 5A). In comparison, an obvious reduction in Cu bio-accessibility was perceived in all four amendments, viz. MB1 (72.5%), MBT1 (69.6%), MB5 (65.1%), and FB5 (61.4%) in the intestinal juice for all four amendments, including MB1 (66.4%), MBT1 (60.1%).
MB5 (58%), and MBT5 (54.4%) (Fig. 5B). Different studies have reported the potential of biochar to decrease metal bioavailability, even at small doses47. Though the majority of researchers used acidic soils48, some studies have assessed the effect of three types of biochar on the oral bioavailability of Cd, Zn, and Pb in basic soil (pH 7.6). The addition of acid biochar (pH 3.2) had no impact on the bioavailability of Cd in two different basic soils (pH 7.91 and 8.5), though, the neutral biochar was proficient in declining the bioavailability of Cd49.
Compared to stomach fractions, the intestinal juice in amended soil had significantly lower levels of Cd and Cu bioavailability (Fig. 5C,D), which is consistent with another research that have been published50. This might be caused due to the high pH and the use of organic substances in the intestinal juice, such as pancreatin and bile extract. A portion of the solubilized Cd may precipitate as a result of the pH change (from 1.5 to 7.0 in the intestinal phase)51. Additional research has demonstrated that Cd may form insoluble complexes with the phytate found in human diets52.
Effect of biochar treatment on CAT and urease activity
Considerable fluctuations in the activities of soil urease and CAT were noted during the incubation period. The biological activity of soil microorganisms may be negatively impacted by heavy metal contamination53. The biological activities of soil are primarily examined to determine how soil treatment affects soil enzymes, particularly urease and catalase activities, which function as sensitive bioindicators to heavy metals54. Some studies described that biochar affects soil enzymatic activities in contaminated soil adversely, which may be due to biochar properties and soil ecology55.
At the start of the incubation period, all amendments’ urease and catalase activities progressively dropped before rising after the trial. Due to their inhibitory effects, the large amounts of Cd (60 mg kg− 1) and Cu (258 mg kg− 1) in the contaminated soil may have caused the initial phase of the drop-in enzyme activity. A similar pattern for urease assay in sediment contaminated with Cd after applying biochar55. Remarkably, within the first 2 weeks of treatment, soil treated with MBT5 exhibited the highest catalase activity among all amendments (Fig. 6A). Fungal survival in the porous structure of biochar is most likely the cause of this56. Catalase activity was roughly the same in all modifications.
A significant portion of soil nitrogen conversion is carried out by urease. In our investigation, the addition of biochar decreased the amount of soil urease activity for the first forty-two days of incubation. This activity then increased and peaked on the ninetieth day (Fig. 6B). Previous research57 has also shown a similar trend of outcomes following the application of high doses of biochar, most likely due to the detrimental effects of biochar on soil microbes. The application of MBT initially caused a decline in the activity of soil urease, but after 42 days of incubation, this activity increased. These results showed a quick and greater self-restoring ability of soil activities in polluted soil. Former studies have also proposed the effect of HMs on soil enzymatic activities58, which ultimately reveals the capability of polluted soil to self-purify59. MBT-treated. According to60, polluted soil can self-purify. Previous research has also suggested that heavy metals have an impact on soil enzymatic activity61.
Mechanisms and effects
According to the results of the current work, the soil treated with MBT and MB showed a drop in the bioavailable parts of Cd and Cu. Out of all these different amendments, the MBT5 application produced the best activity for stabilizing heavy metals and restoring the soil. The main technique for rendering heavy metals in contaminated soil immobile was elucidated in the current work. Due to the biochar surface’s ability to serve as a growth-enhancing microbe carrier. Different microbes were introduced into the contaminated soil to stabilize the heavy metals in the contaminated soil. Trichoderma harzianum grows on the biochar surface by colonizing its porous structure, which offers shelter. The surface also retains moisture and nutrients, creating a favorable environment that supports fungal hyphal growth. These results are align with the work of previous researchers62,63.
Metal immobility in a fungus-loaded biochar has been shown to be controlled by a number of processes, including precipitation, complexion, ion exchange, bio-sorption, bioaccumulation and mass transfer of metals. The oxygen-containing functional groups in biochar help in the ion exchange. The functional groups in biochar, such as –OH, –COOH, –C O–, and –C N, offer HMs binding sites to form complexes, which raise the metals’ specific adsorption.When T. harzianum is present, a range of extracellular polymeric substances (EPS) is released, including polysaccharides, aromatic amino acids, and cationic and anionic functional groups (NH3 +, COO−, HPO4−, etc.). These interactions cause different physical and chemical reactions between the metals and the biochar surface, creating a strong. Additionally, hyphae growth increases surface area, which is another important element that might directly induce considerable retention of metals64,65,66. Insoluble precipitates may be formed when the mineral components in biochar precipitate with metals67,68. According to studies done so far, utilizing biochar alone does not result in the same level of heavy metal immobilization as when employing fungus69,70. The long-term stability of fungal loadedd biochar depends on soil conditions such as moisture, pH, nutrient availability and temperature.
Biosorption avoids the endosmosis of heavy metal into cells. The cell surface of fungi is having negative charge because of the existence of different biomolecules such as polysaccharides, phenolic acids, and proteins which possess various functional groups such as hydroxyl, amine, carbonyl, and phosphoryl groups, thus supporting interactions with the positive charge containing metal ions71,72. Fungus is considered to be an excellent biosorbent organism, because its cell wall contains almost 90% of polysaccharides. Studies propose that specific microbial species may be specific toward heavy metals because of the variation in cellular compositions and the functional sites involved in the metal binding73,74. Cd adsorption needs the participation of –OH and –C–O groups. Though, the amino groups of chitosan in the fungus are the primary sites responsible for the adsorption of heavy metals. A similar pattern of results have been described previously by researchers75,76,77. Physicochemical constraints of heavy metal play a noteworthy role in the adsorption of metal78. Usually, a reduction in the biosorption potential was determined with a rise in the heavy metal concentration may because of the saturation of metal-binding sites. In bioaccumulation, the heavy metal are precipitated in different cellular organelles, thus creating the non-toxic form of metal. In comparison to biosorption, bioaccumulation is viable only in live cells. Bioaccumulation in fungi relies on different parameters like concentration of metals, pH, and temperature. Variation in temperature changes the configuration and stability of the cell wall and even ionize the chemical medieties, disturbing the uptake of metal. Several researchers propose that biosorption is followed by bioaccumulation, where its accumulation follows the non-metabolic uptake of metal79.
Application of both MB and MBT raised the pH of treated soil at the initial phases of incubation. The initial rise in the pH of the soil after applying MBT and MB was due to the alkaline nature and ash content of biochar. With the passage of time, the pH gradually decreases as microbial activity, especially from T. harzianum, produces organic acids. These acids neutralize the alkalinity, leading to a more stable, neutral to slightly alkaline soil environment. Similar variations in soil pH during different incubation periods have been reported earlier80. In the current work, we find out that both bioavailable Cd and Cu contents of the MB5 amendment were considerably lesser than the MB1 amendment. Additionally, in comparison to MB treatment, MBT could successfully lower the bioavailable Cu and Cd in the contaminated soil; thereby enhancing the immobility of Cu and Cd, particularly with a 5% application rate. Cu and Cd might be changed from bioavailable portions to less bioavailable ones by both MB and MBT. MBT showed a greater decline in metal availability compared to MB, due to the synergistic effect of biochar and fungal activity. Similar pattern of results was noted that the amount of biochar added to contaminated soil increases, the proportion of calcitrant Cu and Cd increases while the bioavailable portions of these metals decrease81,82.
As the length of incubation increased, the amounts of DTPA-Cu and DTPA-Cd in the modified soil steadily reduced. These results point to MBT’s potential to decrease the danger of Cu and Cd on human health. When DTPA-Cu and DTPA-Cd were added to polluted soil at a dosage rate of 5% using bamboo biochar, previous studies discovered comparable patterns83, Similar to DTPA-Phyto-availability, applying two treatments of biochar at varying concentrations lowered the Cu and Cd bioavailability of the altered soil to varying percentages. The study’s observations of altered soil enzyme activity have consequences for a number of soil processes, including basal respiration, nutrient cycling, litter decomposition, and N2O emissions84. Biochar can improve water-holding capacity and promote soil porosity and texture, which can have an effect on enzymatic activities85.
This study showed a significant increase in urease and catalase activities. The effect of biochar treatment on urease activity is important since this enzyme is critically implicated in N cycling and availability, and N2O releases from agricultural arenas with consequential implications on the environment86. Generally, the higher urease activities were noted at the highest biochar application rate suggesting that biochar has a significant influence. Similar results attesting to elevated urease and catalase activity following charcoal amendment86. The usage of biochar has been linked to a rise in soil organic matter, a decrease in nutrient loss, and the stability of heavy metals, all of which have been linked to an increase in enzyme activity87,88. Overall, this study’s positive findings about biochar’s impact on soil enzyme activity are consistent with its advantageous effects on soil biota and nutrient cycling (Fig. 7). Similarly, the role of the fungus in metal immobilization is depicted in Fig. 8.
Conclusions
Applying biochar to the soil is an effective way to remediate it when heavy metals are present. The 5% MBT treatment significantly reduced the bio-available and mobile forms of cadmium and copper by promoting their transformation into more stable residual fractions, thereby minimizing environmental risks. Moreover, MBT stabilizes the pH, raised the enzymatic activity, improved quality of the soil, decreased metal bio-accessibility in simulated gastric and gastrointestinal phases, and had a greater financial benefit than the individual biochar (MB) addition. The results of this study indicate that using functional microbial pathogens in conjunction with a biochar treatment may prove to be a beneficial method for cleaning up polluted soil. Collaborate with farmers, land managers, and policymakers to implement large-scale field trials and evaluate the practicality of integrating biochar into agricultural and environmental management practices.
Data availability
Data is available on the request from corresponding author.
References
Qadir, A. & Malik, R-N. Heavy metals in eight edible fish species from two polluted tributaries (Aik and Palkhu) of the river chenab, Pakistan. Bio Trace Elem. Res. 143, 1524–1540 (2011).
Zhao, J., Csetenyi, L. & Gadd, G. M. Biocorrosion of copper metal by Aspergillus Niger. Int. Biodeterior. Biodegrad. 154, 105081 (2020).
Azizullah, A., Khattak, M-N-K., Richter, P. & Häder, D-P. Water pollution in Pakistan and its impact on public health. A review. Environ. Int. 37, 479–497 (2011).
Wei, B. & Yang, L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 94, 99–107 (2010).
Colin, V. L., Villegas, L. B. & Abate, C. M. Indigenous microorganisms as potential bioremediators for environments contaminated with heavy metals. Int. Biodeterior. Biodegrad. 69, 28–37 (2012).
Oyetibo, G. O. et al. Biodegradation of crude oil and phenanthrene by heavy metal resistant Bacillus subtilis isolated from a multi-polluted industrial wastewater creek. Int. Biodeterior. Biodegrad. 120, 143–151 (2017).
Li, H. et al. Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere. 178, 466–478 (2017).
Shen, Z., Jin, F., O’Connor, D. & Hou, D. Solidification/stabilization for soil remediation: an old technology with new vitality. ACS Publ. (2019).
Godlewska, P., Schmidt, H-P., Ok, Y-S. & Oleszczuk, P. Biochar for composting improvement and contaminants reduction. A review. Bioresour. Technol. 246, 193–202 (2017).
Nie, C. et al. Impact of sugarcane bagasse-derived Biochar on heavy metal availability and microbial activity: a field study. Chemosphere. 200, 274–282 (2018).
Qian, L. & Chen, B. Dual role of biochars as adsorbents for aluminum: the effects of oxygen-containing organic components and the scattering of silicate particles. Environ. Sci. Technol. 47, 8759–8768 (2013).
Wei, J. et al. Pyrolysis temperature-dependent changes in the characteristics of biochar-borne dissolved organic matter and its copper binding properties. Bull. Environ. Contam. Toxicol. 103, 169–174 (2019a).
Fang, Z. et al. A critical review on remediation of bisphenol S (BPS) contaminated water: efficacy and mechanisms. Crit. Rev. Environ. Sci. Technol. 50, 476–522 (2020).
Guirado, M. et al. Effectiveness of biochar application and bioaugmentation techniques for the remediation of freshly and aged diesel-polluted soils. Int. Biodeterior. Biodegrad. 163, 105259 (2021).
Liu, J., Ding, Y., Ma, L., Gao, G. & Wang, Y. Combination of biochar and immobilized bacteria in cypermethrin-contaminated soil remediation. Int. Biodeterior. Biodegrad. 120, 15–20 (2017).
He, L. et al. Remediation of heavy metal contaminated soils by biochar: mechanisms, potential risks and applications in China. Environ. Pollut. 252, 846–855 (2019).
Hou, D. et al. A sustainability assessment framework for agricultural land remediation in China. Land. Degrad. Deve. 29, 1005–1018 (2018).
Fiorentino, N. et al. Assisted phytoextraction of heavy metals: compost and trichoderma effects on giant Reed (Arundo donax L.) uptake and soil N-cycle microflora. Ital. J. Agron. 8, 244–254 (2013).
Kacprzak, M. J., Rosikon, K., Fijalkowski, K. & Grobelak, A. The effect of Trichoderma on heavy metal mobility and uptake by miscanthus giganteus, Salix sp., Phalaris arundinacea, and Panicum virgatum. Appl. Environ. Soil Sci. 2014 (2014).
Kavamura, V. N. & Esposito, E. Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnol. Adv. 28, 61–69 (2010).
Bazrafshan, E., Zarei, A. A. & Mostafapour, F. K. Biosorption of cadmium from aqueous solutions by trichoderma fungus: kinetic, thermodynamic, and equilibrium study. Desalin. Water Treat. 57, 14598–14608 (2016).
Al-Rajhi, A. M. H. Impact of biofertilizer trichoderma Harzianum Rifai and the biomarker changes in Eruca sativa L. plant grown in metal-polluted soils. World Appl. Sci. J. 22, 171–180 (2013).
Kavitha, B. et al. Benefits and limitations of Biochar amendment in agricultural soils: A review. J. Environ. Manag. 227, 146–154 (2018).
Shu, R., Wang, Y. & Zhong, H. Biochar amendment reduced Methylmercury accumulation in rice plants. J. Hazard. Mater. 313, 1–8 (2016).
Wei, J. et al. Assessing the effect of pyrolysis temperature on the molecular properties and copper sorption capacity of a halophyte biochar. Environ. Pollut. 251, 56–65 (2019b).
O’Connor, D. et al. Biochar application for the remediation of heavy metal polluted land: a review of in situ field trials. Sci. Total Environ. 619, 815–826 (2018).
Chen, H. et al. Enhanced Pb immobilization via the combination of Biochar and phosphate solubilizing bacteria. Environ. Int. 127, 395–401 (2019).
Bouabidi, Z-B., El-Naas, M-H. & Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: a review. Environ. Chem. Lett. 17, 241–257 (2019).
Partovinia, A. & Rasekh, B. Review of the immobilized microbial cell systems for bioremediation of petroleum hydrocarbons polluted environments. Crit. Rev. Environ. Sci. Technol. 48, 1–38 (2018).
Wu, B. et al. The performance of biochar-microbe multiple biochemical material on bioremediation and soil micro-ecology in the cadmium aged soil. Sci. Total Environ. 686, 719–728 (2019).
Zhu, X., Chen, B., Zhu, L. & Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environ. Pollut. 227, 98–115 (2017).
Bandara, T. et al. Chemical and biological immobilization mechanisms of potentially toxic elements in biochar-amended soils. Crit. Rev. Environ. Sci. Technol. 50, 903–978 (2020).
Yuan, J. H., Xu, R. K. & Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 102 (3), 3488–3497 (2011).
Liu, Q. et al. Can Biochar alleviate soil compaction stress on wheat growth and mitigate soil N2O emissions. Soil. Biol. Biochem. 104, 8–17 (2017).
R-K L. Analysis method of soil agricultural chemistry ML Beijing. CASTP. (1999).
Tessier, A., Campbell, P-G. & Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 51, 844–851 (1979).
Fellet, G., Marmiroli, M. & Marchiol, L. Elements uptake by metal accumulator species grown on mine tailings amended with three types of Biochar. Sci. Total Environ. 468, 598–608 (2014).
Conder, J-M., Lanno, R-P. & Basta, N-T. Assessment of metal availability in smelter soil using earthworms and chemical extractions. J. Environ. Qual. 30, 1231–1237 (2001).
Otomo, P-V., Owojori, O., Reinecke, S., Daniels, S. & Reinecke, A. Using estimates of metal bioavailability in the soil and genetic variation of allozymes to investigate heavy metal tolerance in the earthworm Eisenia fetida (Oligochaeta). Ecotoxicol. Environ. Saf. 74, 2070–2074 (2011).
Wragg, J. et al. An inter-laboratory trial of the unified BARGE bioaccessibility method for arsenic, cadmium and lead in soil. Sci. Total Environ. 409, 4016–4030 (2011).
Zhong, M-S. & Jiang, L. Refining health risk assessment by incorporating site-specific background concentration and bioaccessibility data of nickel in soil. Sci. Total Environ. 581, 866–873 (2017).
Kumar, V., Sharma, A., Thukral, A. & Bhardwaj, R. Assessment of soil enzyme activities based on soil samples from the Beas river bed, India using multivariate techniques. Malaysian J. Soil. Sci. 20, 135–145 (2016).
Quilliam, R-S., Glanville, H-C., Wade, S-C. & Jones, D-L. Life in the ‘charosphere’—Does Biochar in agricultural soil provide a significant habitat for microorganisms. Soil. Biol. Biochem. 65, 287–293 (2013).
Chen, S. et al. Promoting interspecies electron transfer with Biochar. Sci. Rep. 4, 1–7 (2014).
Tu, C. et al. Biochar and bacteria inoculated Biochar enhanced cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 137, 105576 (2020).
Huang, Y. et al. Current status of agricultural soil pollution by heavy metals in china: A meta-analysis. Sci. Total Environ. 651, 3034–3042 (2019).
Lu, K. et al. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, cu, Pb and Zn) in contaminated soil. J. Environ. Manage. 186, 285–292 (2017).
Yang, Q. et al. A review of soil heavy metal pollution from industrial and agricultural regions in China. Pollut. Risk Asses. 642, 690–700 (2018).
Park, J-H., Choppala, G-K., Bolan, N-S., Chung, J-W. & Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant. Soil. 348, 439–451 (2011).
Rinklebe, J., Shaheen, S-M., Schröter, F. & Rennert, T. Exploiting biogeochemical and spectroscopic techniques to assess the geochemical distribution and release dynamics of chromium and lead in a contaminated floodplain soil. Chemosphere. 150, 390–397 (2016).
Gholami, L. & Rahimi, G. Chemical fractionation of copper and zinc after addition of Carrot pulp Biochar and thiourea–modified Biochar to a contaminated soil. Environ. Technol. 42, 3523–3532 (2021).
He, D. et al. Effects of soil amendments applied on cadmium availability, soil enzyme activity, and plant uptake in contaminated purple soil. Sci. Total Environ. 654, 1364–1371 (2019).
Jiang, J., Xu, R-K., Jiang, T-Y. & Li, Z. Immobilization of Cu (II), Pb (II) and cd (II) by the addition of rice straw derived Biochar to a simulated polluted ultisol. J. Hazard. Mater. 229, 145–150 (2012).
Dindar, E., Şağban, F. O. T. & Başkaya, H. S. Variations of soil enzyme activities in petroleum-hydrocarbon contaminated soil. Int. Biodeterior. Biodegrad. 105, 268–275 (2015).
Ding, Y. et al. Binding characteristics of heavy metals to humic acid before and after fractionation by ferrihydrite. Chemosphere. 226, 140–148 (2019).
Sellaoui, L. et al. A new statistical physics model for the ternary adsorption of Cu2+, Cd2 + and Zn2 + ions on bone char: experimental investigation and simulations. J. Chem. Eng. 343, 544–553 (2018).
Sipos, P. et al. Partition of cd, cu, Pb and Zn among mineral particles during their sorption in soils. J. Soils Sediments. 19 (4), 1775–1787 (2019).
Mendez, A., Gomez, A., Paz-Ferreiro, J. & Gasco, G. Effects of sewage sludge biochar on plant metal availability after application to a mediterranean soil. Chemosphere 89, 1354–1359 (2012).
Ruby, M-V., Davis, A., Schoof, R., Eberle, S. & Sellstone, C-M. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ. Sci. Technol. 30, 422–430 (1996).
Mustafa, Y-L., Keirouz, A. & Leese, H-S. Molecularly imprinted polymers in diagnostics: accessing analytes in biofluids. J. Mater. Chem. B. 10 (37), 7418–7449 (2022).
Petković Didović, M. et al. Cytotoxicity of metal ions released from NiTi and stainless steel orthodontic appliances, part 1: surface morphology and ion release variations. Materials. 16 (11), 4156 (2023).
Hou, J., Pugazhendhi, A., Phuong, T. N., Thanh, N. C., Brindhadevi, K., Velu, G. et al. Plant resistance to disease: Using biochar to inhibit harmful microbes and absorb nutrients. Environ. Res. 214, 113883 (2022).
Bashir, S., Hussain, Q., Shaaban, M. & Hu, H. Efficiency and surface characterization of different plant derived Biochar for cadmium (Cd) mobility, bioaccessibility and bioavailability to Chinese cabbage in highly contaminated soil. Chemosphere. 211, 632–639 (2018).
Lin, Q. et al. The speciation, leachability and bioaccessibility of Cu and Zn in animal manure-derived biochar: effect of feedstock and pyrolysis temperature. Front. Environ. Sci. Eng. 11 (3), 1–12 (2017).
Yoo, J-C. et al. A combination of ferric nitrate/EDDS-enhanced washing and sludge-derived Biochar stabilization of metal-contaminated soils. Sci. Total Environ. 616, 572–582 (2018).
Janus, A. et al. Do biochars influence the availability and human oral bioaccessibility of cd, pb, and Zn in a contaminated slightly alkaline soil. Environ. Monit. Assess. 190, 1–13 (2018).
Qi, F. et al. Cadmium solubility and bioavailability in soils amended with acidic and neutral biochar. Sci. Total Environ. 610, 1457–1466 (2018).
Fu, J. & Cui, Y. In vitro digestion/Caco-2 cell model to estimate cadmium and lead bioaccessibility/bioavailability in two vegetables: the influence of cooking and additives. Food Chem. Toxicol. 59, 215–221 (2013).
Pelfrêne, A. et al. Use of an in vitro digestion method to estimate human bioaccessibility of cd in vegetables grown in smelter-impacted soils: the influence of cooking. Environ. Geochem. Health. 37, 767–778 (2015).
Mounicou, S., Szpunar, J., Andrey, D., Blake, C. & Lobinski, R. Development of a sequential enzymolysis approach for the evaluation of the bioaccessibility of cd and Pb from cocoa. Analyst. 127, 1638–1641 (2002).
Versantvoort, C-H., Oomen, A-G., Van de Kamp, E., Rompelberg, C-J. & Sips, A-J. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 43, 31–40 (2005).
Jiang, J. et al. Effects of multiple heavy metal contamination and repeated phytoextraction by Sedum plumbizincicola on soil microbial properties. Eur. J. Soil. Biol. 46, 18–26 (2010).
Dhankhar, R. & Hooda, A. Fungal biosorption—an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ. Technol. 32 (5), 467–491 (2011).
Ramya, D., Kiruba, N. J. M. & Thatheyus, A. J. Biosorption of heavy metals using fungal biosorbents—A review. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-technology, 331–352 (2021).
Barros Júnior, L. M., Macedo, G. R., Duarte, M. M. L., Silva, E. P., Lobato, A. K. & C. L Biosorption of cadmium using the fungus Aspergillus niger. Braz. J. Chem. Eng. 20, 229–239 (2003).
Awasthi, A. K., Pandey, A. K. & Khan, J. Biosorption an innovative tool for bioremediation of metal-contaminated municipal solid waste leachate: optimization and mechanisms exploration. Int. J. Environ. Sci. Technol. 14, 729–742 (2017).
Priyadarshini, E., Priyadarshini, S. S., Cousins, B. G. & Pradhan, N. Metal-fungus interaction: review on cellular processes underlying heavy metal detoxification and synthesis of metal nanoparticles. Chemosphere. 274, 129976 (2021).
Ramrakhiani, L., Ghosh, S. & Majumdar, S. Surface modification of naturally available biomass for enhancement of heavy metal removal efficiency, upscaling prospects, and management aspects of spent biosorbents: a review. Appl. Biochem. Biotechnol. 180, 41–78 (2016).
Ghaed, S., Shirazi, E. K. & Marandi, R. Biosorption of copper ions by Bacillus and Aspergillus species. Adsorpt. Sci. Technol. 31 (10), 869–890 (2013).
Pan, J. & Yu, L. Effects of cd or/and Pb on soil enzyme activities and microbial community structure. Ecol. Eng. 37, 1889–1894 (2011).
Tu, C. et al. Stabilizing effects on a cd polluted coastal wetland soil using calcium polysulphide. Geoderma. 332, 190–197 (2018).
Bashir, S. et al. Sugarcane bagasse-derived Biochar reduces the cadmium and chromium bioavailability to Mash bean and enhances the microbial activity in contaminated soil. J. Soils Sediments. 18, 874–886 (2018a).
Gong, X. et al. Biochar facilitated the phytoremediation of cadmium contaminated sediments: metal behavior, plant toxicity, and microbial activity. Sci. Total Environ. 666, 1126–1133 (2019).
Bhaduri, D., Saha, A., Desai, D. & Meena, H. Restoration of carbon and microbial activity in salt-induced soil by application of peanut shell Biochar during short-term incubation study. Chemosphere. 148, 86–98 (2016).
Huang, D. et al. The effects of rice straw Biochar on Indigenous microbial community and enzymes activity in heavy metal-contaminated sediment. Chemosphere. 174, 545–553 (2017).
Harris, E-S. et al. Heavy metal and pesticide content in commonly prescribed individual Raw Chinese herbal medicines. Sci. Total Environ. 409 (20), 4297–4305 (2011).
Cui, Y. et al. Ecoenzymatic stoichiometry reveals microbial phosphorus limitation decreases the nitrogen cycling potential of soils in semi-arid agricultural ecosystems. Soil Tillage. Res. 197, 104463 (2020).
Yang, X. et al. Effect of biochar on the extractability of heavy metals (Cd, cu, pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 23, 974–984 (2016).
Acknowledgements
The authors extend their appreciation to the ongoing research funding program (ORF-2025-95), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
A.K.: Experiments, formal analysis, writing. G.A.: Investigation, resources, formal analysis. F.A.: Conceptualization, data curation, formal analysis. M.A.: writing—review and editing, investigation. N.A.: Characterization of fungus, data curation, conceptualization. M.N.: Conceptualization, formal analysis. M.S.R.: conceptualization, review and editing. K.K.: Conceptualization, data curation. M.F.H.M.: Designed experiments, resources, and supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kamal, A., Nazish, M., Kamal, K. et al. Trichoderma harzianum-loaded maize biochar enhances Cd–Cu immobilization and reduces bio-accessibility in contaminated soil. Sci Rep 15, 28099 (2025). https://doi.org/10.1038/s41598-025-13759-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13759-w