Abstract
The prognosis for stage IIIa non-small cell lung cancer (NSCLC) remains poor, necessitating innovative treatment strategies to improve patient outcomes. This study evaluates the efficacy of neoadjuvant immunotherapy combined with chemotherapy in enhancing resection rates, survival, and progression-free survival in patients with stage IIIa NSCLC. A retrospective analysis was conducted on 82 patients treated at The Third People’s Hospital of Zhengzhou, Henan, China, between October 2018 and April 2019. Patients were divided into two groups: an experimental group (n = 41) treated with a combination of platinum-based chemotherapy (pemetrexed and nedaplatin) and the PD-1 inhibitor cindilizumab, and a control group (n = 41) treated with conventional platinum-based chemotherapy. The experimental group demonstrated a significantly higher objective response rate, superior R0 resection rates, and fewer metastatic lymph nodes compared to the control group. Postoperative complications were significantly reduced in the experimental group, accompanied by substantial reductions in tumor markers, including cytokeratin 19 fragments and carbohydrate antigen 125. Median overall survival and progression-free survival were longer in the experimental group than in the control group. Hematological safety was confirmed with no significant differences in routine blood counts between the two groups before or after treatment. These findings support the integration of neoadjuvant immunotherapy and chemotherapy as a promising strategy for treating stage IIIa NSCLC, offering improved resection rates, survival, and reduced complications. Further research is warranted to optimize treatment protocols and validate these results in larger, multicentre studies.
Similar content being viewed by others
Introduction
Lung cancer, the second leading cause of death globally after breast cancer, is classified into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). According to recent global estimates, over 2.2 million new cases of lung cancer (LC) were reported in 2022, with approximately 1.8 million deaths, highlighting its continued burden on global health1,2,3,4. SCLC, known for its rapid metastasis, accounts for 10–15% of cases, while NSCLC represents approximately 85%4. According to the 2022 GLOBOCAN report, approximately 2.2 million individuals were diagnosed with LC, with nearly 2 million deaths1. SCLC, characterized by rapid metastasis, makes up 10–15% of cases, while NSCLC represents around 85%. Roughly 20% of NSCLC patients are diagnosed with potentially resectable T4N1-2 or T1-3N2 (stage IIIA or IIIB) disease due to direct organ invasion (T4) or mediastinal lymph node spread (N2). Managing these cases remains challenging, as 5-year overall survival rates are only 41% for stage IIIA and 24% for stage IIIB1,4.
Over recent decades, approaches such as neoadjuvant chemotherapy, concurrent or sequential chemoradiotherapy (CRT/SRT), neoadjuvant or adjuvant radiotherapy, and surgery following CRT have been employed for potentially resectable stage IIIA NSCLC patients. However, these strategies have yielded only modest improvements, emphasizing the need for alternative treatments5,6,7. Moreover, NSCLC patients in stage IIIa often face significant challenges during surgical treatment due to peripheral invasion and elevated rates of postoperative recurrence and metastasis. These complications contribute to a poor prognosis, necessitating more effective treatment strategies8. The advent of neoadjuvant therapy has improved survival outcomes for patients with high tumour burden by facilitating earlier treatment and potentially reducing tumour size prior to surgery8. In recent years, advancements in understanding programmed death receptor-ligand 1 (PD-L1) and the emergence of immunotherapy have transformed the landscape of cancer treatment, leading to significant improvements in survival rates9. Immunotherapeutic agents, particularly those targeting PD-L1, have been shown to enhance the body’s immune response against tumours, offering renewed hope for patients with advanced lung cancer9,10. A recent study reported a pathological remission rate of 45% in stage IIIa NSCLC patients treated with nivolumab monotherapy, with 10% achieving pathological complete remission11,12. These findings indicate the potential of immunotherapy as a pivotal component of treatment regimens.
To further investigate the efficacy of neoadjuvant approaches, a novel strategy combining adjuvant immunotherapy with neoadjuvant chemotherapy has been proposed. This combined approach aims to enhance the therapeutic impact on tumour reduction and improve surgical outcomes. This paper examines the therapeutic value of immunotherapy when paired with neoadjuvant chemotherapy for patients with stage IIIa NSCLC, seeking to provide a scientific basis for clinical decision-making and optimise treatment pathways for improved patient outcomes. While several recent randomized controlled trials have investigated neoadjuvant immunochemotherapy, this study offers complementary insights by evaluating real-world clinical outcomes in a Chinese population using retrospective data. Despite a limited sample size, its significance lies in demonstrating the feasibility, safety, and potential survival benefits of integrating cindilizumab-based neoadjuvant immunochemotherapy into clinical practice, providing a foundation for future prospective studies.
Results
General condition of patients
The demographic analysis revealed no significant differences between the experimental group (n = 41) and the control group (n = 41) regarding family history, sex, age, alcohol consumption, or smoking history (P > 0.05). This homogeneity suggests that the groups were well-matched, ensuring that the observed effects could be attributed to the interventions rather than demographic variables (Table 1). The educational background of patients was also similar between the groups, with no statistically significant differences observed (P = 0.651).
Comparison of blood routine
Blood routine tests indicated no significant differences in white blood cell count or hemoglobin count between the two groups before and after treatment (P > 0.05). However, a significant decrease in platelet count was observed within each group post-treatment, with the experimental group showing a slightly greater reduction (P = 0.007) compared to the control group (P = 0.029) (Table 2). This stability across other hematological parameters suggests that both treatment regimens did not induce major adverse reactions, indicating their relative safety.
Evaluation of curative effect and comparison of objective effective rate
The analysis of treatment outcomes revealed a statistically significant difference in the objective effective rate post-treatment (P < 0.01). Specifically, the experimental group exhibited a remarkable objective effective rate of 95.12%, compared to just 34.15% in the control group (Table 3). Complete remission was observed in 51.22% of the experimental group, while only 12.20% of the control group achieved this outcome. Additionally, no cases of disease progression were noted in the experimental group compared to 31.71% in the control group, further emphasizing the efficacy of the treatment.
Results of surgical treatment
In surgical outcomes, the experimental group achieved a notably higher R0 resection rate of 82.93% compared to 58.54% in the control group (P = 0.015), indicating more complete tumor removal (Table 4). Additionally, the experimental group had fewer instances of lymph node metastasis (3.27 vs. 4.80, P < 0.01) and a lower lymph node metastasis rate (0.17 ± 0.08 vs. 0.23 ± 0.12, P = 0.008). Postoperative complications were also significantly reduced in the experimental group (19.51% vs. 46.34%, P = 0.010). These findings suggest that the experimental treatment not only enhances the likelihood of successful surgical outcomes but also reduces postoperative complications.
Comparative analysis of tumor marker levels
Tumor marker levels, which are crucial for monitoring disease progression, were comparable between groups before treatment (P > 0.05). However, post-treatment results showed significant reductions in levels of CEA, CA125, and CYFRA21-1 in the experimental group (P < 0.01) (Table 5). For instance, the experimental group’s CEA level decreased from 37.63 ± 2.68 before treatment to 20.30 ± 1.31 after treatment, indicating a robust response to the experimental therapy. Similarly, CA125 and CYFRA21-1 levels were significantly reduced in the experimental group compared to the control group, demonstrating the therapy’s efficacy in controlling tumor progression.
Overall survival rate
Survival analysis demonstrated that the experimental group had significantly better overall survival rates than the control group (P = 0.028, Fig. 1) (Table 6). The median overall survival time was 30.60 months for the experimental group compared to 22.30 months for the control group. The three-year follow-up data indicated that 39.02% of the experimental group remained alive, contrasting sharply with only 17.07% in the control group. These findings suggest a substantial survival benefit associated with the experimental treatment.
Progression-free survival rate
Progression-free survival rates at 1, 2, and 3 years were also favorably higher in the experimental group (Table 7; Fig. 2). While the 1- and 2-year differences were not statistically significant (P > 0.05), the three-year progression-free survival rate in the experimental group was higher (24.39% vs. 7.32%, P = 0.07). Although the P-value was marginal, the data suggest a trend favoring the experimental treatment in prolonging progression-free survival.
Discussion
Most patients with early lung cancer exhibit no obvious clinical symptoms and do not seek medical attention until symptoms arise. By this time, the disease often progresses to a more advanced stage, making it challenging to achieve ideal therapeutic effects through surgery alone. Neoadjuvant therapy has shown significant efficacy in clinical practice, effectively reducing tumor load and the clinical stage of patients with stage III lung cancer13,14. Studies indicate that the effective rate ranges from 25 to 74%, with pathological remission rates between 19% and 67%, thus increasing the resectability rate and improving patient survival times15. With the success of immunotherapy in treating advanced tumours, immune checkpoint inhibitors have been explored in lung cancer patients16. Oncologists refer to immunotherapy as the fourth method of cancer treatment. This approach has quickly become a mainstay for treating non-small cell lung cancer (NSCLC) due to its higher efficacy and lower side effects compared to traditional chemotherapy17. Research indicates that patients with high PD-L1 expression demonstrate improved survival rates when treated with the PD-L1 inhibitor pembrolizumab. Additionally, those with lower PD-L1 expression benefit from the combination of chemotherapy and immunotherapy, achieving higher survival rates than with chemotherapy alone18,19,20. The mechanism of action of PD-L1 inhibitors involves blocking the PD-1/PD-L1 axis, reactivating cytotoxic T cells in the tumor microenvironment. Other immune checkpoints such as CTLA-4, LAG-3, and TIGIT are also under investigation and may serve as future therapeutic targets to enhance immune responses21. A previous study on resectable lung cancer published in 2018 reported only a 10% objective response rate (ORR) with neoadjuvant therapy alone12. However, a recent study demonstrated improved treatment outcomes with the combination of dual immune therapies (neoadjuvant therapy plus ipilimumab)22. In a phase III NSCLC clinical trial, it was observed that combined immunochemotherapy resulted in a major pathological response (MPR) of 83% and an ORR of 78%, with reduced drug toxicity23. In this study, significant reductions in platelet count were observed within both groups post-treatment, with the experimental group showing a greater reduction (P = 0.007) than the control group (P = 0.029). These findings suggest that neoadjuvant regimens were safe overall, with no significant adverse effects on white blood cell count or hemoglobin levels. Notably, a higher incidence of grade 3–4 adverse reactions was observed in melanoma trials with dual therapies compared to single-agent therapies during the same period, indicating a need for more clinical evidence to assess the toxicity of neoadjuvant immunotherapy24.
The experimental group exhibited a significantly higher objective response rate (ORR) compared to the control group (95.12% vs. 34.15%, P < 0.01). Among the experimental group, 34 patients underwent R0 resection, significantly higher than the 24 in the control group (82.93% vs. 58.54%, P = 0.015) (Table 4). Additionally, the experimental group had a lower lymph node metastasis rate (0.17 vs. 0.23, P = 0.008), reflecting reduced tumor spread. Postoperative complications were also significantly lower in the experimental group (19.51% vs. 46.34%, P = 0.010). Notably, a subset of patients with stage III NSCLC may require pneumonectomy, particularly when tumors involve central structures or extensive disease burden. Traditionally, pneumonectomy carries higher perioperative risks compared to lobectomy. However, recent studies have demonstrated that pneumonectomy following neoadjuvant immunotherapy can be performed safely and effectively. For example, Li et al.25 reported that patients undergoing pneumonectomy after chemo-immunotherapy experienced higher pathological complete response rates and improved 3-year overall survival compared to those receiving chemotherapy alone, without a significant increase in postoperative complication. Similarly, Cheng et al.26 found pneumonectomy feasible and beneficial in a real-world setting. These findings support the feasibility of integrating immunotherapy even in patients requiring more extensive surgery.
Post-treatment, levels of carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), and cytokeratin 19 fragment (CYFRA21-1) were significantly lower in the experimental group (P < 0.01). For instance, CEA levels in the experimental group decreased from 37.63 ± 2.68 to 20.30 ± 1.31 post-treatment, highlighting the therapy’s effectiveness. The median overall survival time for the experimental group was 30.6 months, compared to 22.3 months for the control group, indicating a substantial survival benefit associated with the experimental treatment. Similarly, the median progression-free survival (PFS) was 25.4 months for the experimental group compared to 22.3 months for the control group, with trends favoring the experimental therapy (P = 0.07 for 3-year progression-free survival). Nonetheless, the follow-up duration in this study remains relatively short. Longer-term follow-up is necessary to determine the durability of survival benefits and to identify any delayed treatment-related toxicities. Moreover, there were no significant differences before and after treatment regarding operation time, intraoperative blood loss, or total lymph node counts between the groups (P > 0.05). While patients with lung cancer benefit significantly from neoadjuvant immunotherapy, variations exist among different treatment modalities. Current evidence indicates that the application of immunoneoadjuvant therapy in earlier NSCLC stages is not markedly beneficial, and the efficacy of single-agent treatments is inferior to that of combination therapies27,28. Although the experimental group showed significantly better lymph node metastasis rates and progression-free survival, these findings underline the importance of carefully selecting treatment regimens tailored to the patient’s tumor burden and clinical stage. Although combination therapies yield better outcomes, the specific combinations and timing of drug administration remain undefined. Effective tumor treatment must be tailored to the patient’s clinical stage and tumor burden, necessitating ongoing clinical studies to explore these variables further. Other retrospective studies have reported consistent benefits with neoadjuvant immunochemotherapy. For instance, Wang et al.29, Fujimoto et al.30 and Lu et al.31 reported improved R0 resection rates and nodal downstaging with nivolumab- or pembrolizumab-based regimens. Our study extends this evidence by assessing the PD-1 inhibitor cindilizumab in a homogeneous surgical cohort from a real-world Chinese setting. Several Phase III trials provide important context. The CheckMate 816 trial showed that neoadjuvant nivolumab plus chemotherapy significantly improved pathological complete response (24% vs. 2.2%) and event-free survival versus chemotherapy alone in resectable stage IB–IIIA NSCLC32. Similarly, KEYNOTE-671 demonstrated that pembrolizumab combined with chemotherapy improved event-free survival and pCR in stage II–IIIB NSCLC patients33. Although our study is retrospective and has a smaller sample size, it complements these trials by showing real-world feasibility and survival benefit in a population treated with cindilizumab. In the adjuvant setting, IMpower010 highlighted the benefit of atezolizumab following platinum-based chemotherapy in resected stage II–IIIA NSCLC34. These studies reinforce the value of immune checkpoint inhibition across the perioperative continuum.
This study also has limitations. Being retrospective in nature, it is subject to selection bias and unmeasured confounding variables. The lack of randomization limits causal inference, and factors such as nutritional status, comorbidities, or molecular tumor heterogeneity may have influenced the outcomes. Furthermore, the relatively small sample size limits statistical power and generalizability. Additionally, the absence of stratified subgroup analyses—particularly for PD-L1 expression and other predictive biomarkers—limits the ability to assess differential treatment effects. Future multicenter prospective studies with integrated biomarker analysis are warranted to validate these findings and refine patient selection. Future research should focus on stratified treatment strategies based on PD-L1 expression, tumor mutational burden (TMB), and co-alterations. Prospective trials should include predefined subgroup analyses to evaluate which patients derive the greatest benefit from neoadjuvant immunochemotherapy. Additionally, novel combinations involving immune checkpoint inhibitors (e.g., CTLA-4, LAG-3) and anti-angiogenic agents should be explored. Incorporating circulating tumor DNA (ctDNA) or other minimally invasive biomarkers may also improve real-time response monitoring and patient selection. These approaches should be validated through large-scale, multicenter clinical trials with extended follow-up to assess long-term outcomes.
In conclusion, this study demonstrates that neoadjuvant immunotherapy combining pemetrexed, nedaplatin, and cindilizumab significantly enhances outcomes for stage IIIa NSCLC patients. The experimental group exhibited a markedly higher objective response rate and R0 resection rate compared to the control group, along with reduced lymph node metastasis and postoperative complications. Additionally, the overall survival and progression-free survival rates were improved in the experimental group. Further research is warranted to optimize treatment protocols, validate these results, and explore the most effective combinations and timing of therapies through multicenter, prospective clinical trials.
Materials and methods
Clinical data
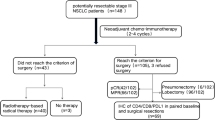
Clinical data from 82 patients with stage IIIa non-small-cell lung cancer (NSCLC) treated at The Third People’s Hospital of Zhengzhou, Zhengzhou, Henan, China were retrospectively analysed from October 2018 to April 2019. Inclusion criteria were as follows: (1) diagnosis of NSCLC stage IIIa confirmed by preoperative evaluation; (2) all patients were newly treated at our centre and had not received any prior tumour-related treatments; (3) no history of malignant tumours, diabetes, immune system disorders, or other serious internal diseases; (4) patients able to tolerate chemotherapy based on the Karnofsky Performance Status (KPS) score. Aside from high tumour burden, there were no contraindications for chemotherapy or surgery. Patients were excluded if they met any of the following criteria: (1) history of other malignancies; (2) presence of uncontrolled comorbid conditions, such as severe cardiovascular or pulmonary disease; (3) known hypersensitivity to any of the drugs used in the study; (4) significant psychiatric disorders affecting compliance; (5) concurrent participation in other clinical trials. The research adheres to the Declaration of Helsinki. The study was approved by the research ethics committee of The Third People’s Hospital of Zhengzhou, Zhengzhou, Henan, China under approval number 2024-04-041-K01. The review board waived the need for patient informed consent, as this was a retrospective study, and all data had been deidentified. Although national guidelines in China had not formally endorsed neoadjuvant immunotherapy for stage IIIA NSCLC at the time of treatment (2018–2019), all patients received therapy based on the latest international clinical evidence and multidisciplinary team recommendations. The therapeutic decisions prioritized patient safety, clinical benefit, and informed judgment. Ethical approval was obtained to ensure compliance with institutional and international ethical standards, thereby safeguarding patient rights and interests. The flow chart of the study is included in Fig. 3.
Demographic information
Demographic data, including gender, age, medication history, smoking and alcohol consumption, history of type 2 diabetes mellitus (T2DM), past medical conditions, and family history, were recorded by designated personnel.
Treatment groups
The patients were assigned to two groups: an experimental group (n = 41) and a control group (n = 41).The experimental group received a combination of platinum-based chemotherapy (pemetrexed + nedaplatin) with the PD-1 inhibitor cindilizumab. Pemetrexed (500 mg/m2) was mixed with 500 ml saline and administered intravenously every 21 days. Injectable nedaplatin (75 mg/m2) was added to 500 ml of 5% glucose for weekly intravenous infusion. cindilizumab (200 mg) was administered via intravenous drip every 21 days alongside the other treatments. The control group received conventional platinum-based chemotherapy (pemetrexed + nedaplatin) with identical dosages and schedules. All patients underwent three treatment cycles, each lasting 21 days, followed by surgery performed by the same surgeon. Pemetrexed was sourced from Nanjing Xiansheng Dongyuan Pharmaceutical Co., Ltd. (approval number H20133216), nedaplatin from Jiangsu Osaikang Pharmaceutical Co., Ltd. (approval number H20051987), and cindilizumab from Xinda Biopharmaceutical Co., Ltd. (approval number S20180016).
PD-L1 expression
PD-L1, also known as B1-H1, is a protein expressed on the cell surface, encoded by the CD274 gene. Tumour cell PD-L1 expression was confirmed through immunohistochemical staining of tumour tissue obtained from biopsies or surgical resection.
Evaluation criteria
Primary outcomes included: (1) blood counts before the first chemotherapy and after two cycles (anemia, haemoglobin, and platelet levels), intraoperative blood loss, number of lymph node metastases, and follow-up data for three years post-surgery; (2) chest computed tomography (CT) scans evaluated every cycle using solid tumour evaluation criteria26. Response metrics included complete response (CR), partial remission (PR), stable disease (SD), and progressive disease (PD), with the objective response rate (ORR) calculated as \(\:{\text{OPR}} = CR + PR/n \times \:100\) ; (3) postoperative pathological evaluation metrics included major pathological response (MPR) and complete pathological remission (CPR), with MPR defined as pathologically residual tumour ≤ 10%; (4) time from the end of neoadjuvant therapy to surgery (TrSs); (5) fasting venous blood samples (5 ml) were taken after three cycles for analysis of tumour markers CEA, CA125, and CYFRA21-1 via electrochemiluminescence immunoassay; (6) any adverse events occurring within one month post-treatment were documented and assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. The total rate of adverse reactions was calculated based on the number of patients experiencing side effects during treatment13.
Statistical analysis
Data were compiled and analysed using SPSS version 25.0. Continuous variables were expressed as mean ± standard deviation. Independent sample t-tests were conducted for comparisons between groups, while paired sample t-tests were used for within-group comparisons. Categorical data were analysed using chi-squared tests. Kaplan–Meier survival analysis was employed to compare overall survival and progression-free survival rates between the experimental and control groups, with statistical significance set at P < 0.05.
Data availability
Data is associated with this manuscript is available at figshare (https://figshare.com/) https://figshare.com/s/3d09694b6a9668a3392e.
References
Filho, A. M. et al. The GLOBOCAN 2022 cancer estimates: data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer. 156, 1336–1346 (2025).
Liu, X. et al. Targeting T cell exhaustion: emerging strategies in non-small cell lung cancer. Front. Immunol. 15, 1507501 (2024).
Xu, S. & Lu, Z. The role of LMNB2 as a diagnostic and prognostic biomarker in lung adenocarcinoma. Asian J. Surg (2024).
Xu, S. & Lu, Z. Exploring FNDC4 as a biomarker for prognosis and immunotherapy response in lung adenocarcinoma. Asian J. Surg. (2024).
Pless, M. et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet. 386, 1049–1056 (2015).
Albain, K. S. et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 374, 379–386 (2009).
Le Pechoux, C. et al. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): an open-label, randomised, phase 3 trial. Lancet Oncol. 23, 104–114 (2022).
Rosell, R. & Gonzalez-Cao, M. Cemiplimab monotherapy in advanced non-squamous and squamous NSCLC. Lancet. 397, 557–559 (2021).
Chen, X. & Ma, K. Neoadjuvant therapy in lung cancer: what is most important: objective response rate or major pathological response? Curr. Oncol. 28, 4129–4138 (2021).
Assi, H. I. et al. Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer. 124, 248–261 (2018).
Huang, S. et al. Cellular dynamics of tumor microenvironment driving immunotherapy resistance in non-small-cell lung carcinoma. Cancer Lett. 217272 (2024).
Forde, P. M. et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 378, 1976–1986 (2018).
Garon, E. B. et al. Pemetrexed maintenance with or without pembrolizumab in non-squamous NSCLC: A cross-trial comparison of KEYNOTE-189 versus PARAMOUNT, PRONOUNCE, and JVBL. Lung Cancer. 151, 25–29 (2021).
Garon, E. B., Kim, J. S. & Govindan, R. Pemetrexed maintenance with or without pembrolizumab in non-squamous non-small cell lung cancer: a cross-trial comparison of KEYNOTE-189 versus PARAMOUNT, PRONOUNCE, and JVBL. Lung Cancer. 151, 25–29 (2021).
Zhang, Q. & Wang, W. Efficacy of bevacizumab combined with PC chemotherapy in advanced NSCLC. Henan Med. Res. 29, 2416–2417 (2020).
Hao, X. et al. Nab-paclitaxel in combination with bevacizumab in patients with non-squamous NSCLC after failure of at least one prior systemic regimen. J. Cancer. 11, 6421–6428 (2020).
Jimenez Aguilar, E. et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann. Oncol. 30, 1653–1659 (2019).
Zhou, Y. et al. First-line treatment for patients with advanced non-small cell carcinoma and high PD-L1 expression: pembrolizumab or pembrolizumab plus chemotherapy. J. Immunother. Cancer. 7, 1–6 (2019).
Yi, M. et al. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol. Cancer. 21, 28 (2022).
Hayashi, H. & Nakagawa, K. Combination therapy with PD-1 or PD-L1 inhibitors for cancer. Int. J. Clin. Oncol. 25, 818–830 (2020).
Andrews, L. P., Yano, H. & Vignali, D. A. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat. Immunol. 20, 1425–1434 (2019).
Cascone, T. et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat. Med. 29, 593–604 (2023).
Han, B. et al. Penpulimab in combination with anlotinib as first-line treatment in advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 39 (15_suppl), e21072 (2021).
Chae, Y. K. et al. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer: lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J. Immunother. Cancer. 6, 1–27 (2018).
Li, Z. et al. Outcomes after neoadjuvant therapy with or without immunotherapy followed by pneumonectomy in non-small cell lung cancer patients. Ann. Thorac. Surg. 120, 118–128 (2024).
Cheng, T. et al. Pneumonectomy after neoadjuvant immunotherapy combined with chemotherapy is a safe and effective option for central non-small-cell lung cancer patients, a single-center experience. J. Thorac. Dis. 17, 3085 (2025).
Ramalingam, S. S. et al. A phase I study of ADXS-503 alone and in combination with pembrolizumab in subjects with metastatic squamous or non-squamous non-small cell lung cancer (NSCLC). J. Clin. Oncol. 38, e21682 (2020).
Khaddour, L. et al. Immunotherapy in patients with advanced stage non-squamous NSCLC with activating genetic mutations: single institution retrospective study. J. Clin. Oncol. 38, e21577 (2020).
Wang, X. et al. Efficacy of neoadjuvant chemo-immunotherapy in non-small cell lung cancer: a real-world, multicenter, retrospective study. Transl. Lung Cancer Res. 13, 849 (2024).
Fujimoto, D. et al. A real-world study on the effectiveness and safety of pembrolizumab plus chemotherapy for nonsquamous NSCLC. JTO Clin. Res. Rep. 3, 100265 (2022).
Lu, Z. et al. Comparison of pembrolizumab plus chemotherapy versus concurrent or sequential radiochemotherapy in patients with driver mutation-lacking lung adenocarcinoma presenting with recurrent laryngeal nerve invasion leading to hoarseness. J. Clin. Oncol. 42 (16_suppl), e14635 (2024).
Forde, P. M. et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 386, 1973–1985 (2022).
Goodman, A. KEYNOTE-671 trial: perioperative pembrolizumab plus chemotherapy improves outcomes in stage II and III NSCLC. ASCO Highlights (2023).
Felip, E. et al. Adjuvant Atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 398, 1344–1357 (2021).
Author information
Authors and Affiliations
Contributions
R.S., and Y.C. designed the study and wrote the manuscript. R.S., Z.C. and T.Z. collected clinical information. Z.C. and J.H. assisted the statistical analyses. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, R., Chen, Z., Zheng, T. et al. Evaluating the efficacy of neoadjuvant immunotherapy combined with chemotherapy for stage IIIa non-small cell lung cancer. Sci Rep 15, 28003 (2025). https://doi.org/10.1038/s41598-025-13917-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13917-0