Abstract
A composite antimicrobial film (Carrageenan/Polyoxometalate, Carr/POM) was prepared by doping Keggin-type polyoxometalate Na3PW12O40 into carrageenan using the casting method. The mechanical properties, thermal stability, surface morphology, structure, antimicrobial activity and biodegradability of the films were systematically investigated. The results showed that the incorporation of Na3PW12O40 enhanced the water resistance of the carrageenan film and significantly impacted the surface morphology and crystal structure of the film. In antibacterial tests, the Carr/POM film exhibited strong bactericidal activity against Staphylococcus aureus(S.aureus), with the Carr/POM-4 film demonstrating the strongest inhibitory effect, corresponding to an inhibition zone diameter of 10.58 mm.In addition, the Carr/POM films showed excellent biodegradability, with complete decomposition occurring in soil within approximately one week.These findings suggest that the prepared films possess excellent antimicrobial properties against foodborne pathogenic microorganisms, making them promising candidates for further application in the field of food packaging.
Similar content being viewed by others
Introduction
Foodborne pathogenic bacteria are one of the main factors affecting food safety, particularly in areas such as food processing, storage, transportation, and sales. To ensure food hygiene and safety, the role of food packaging is crucial. Antimicrobial cling films can inhibit the growth of foodborne pathogens, improving food quality and safety, while extending the shelf life of food. As a result, these films have garnered significant attention from the food packaging industry. However, the environmental pollution caused by traditional plastic cling films has become an increasingly prominent issue, and the search for biodegradable alternative materials has become a major focus for researchers. Natural biopolymers have attracted considerable interest due to their abundant availability, biodegradability, and ease of processing1.Common natural biopolymers include proteins (such as collagen, casein, gelatin, and zein)2,3,4, polysaccharides (such as starch, chitosan, carrageenan, and cellulose)5,6,7,8, and lipids (such as fatty acids, stearic acid, and beeswax)9,10,11.
Carrageenan is a natural, linear sulfated polysaccharide obtained from red seaweeds. It is composed of alternate units of D-galactose and 3,6-anhydro-galactose joined by α−1,3 and β−1,4-glycosidic linkages12,78,79,80. Based on the position and number of ester sulfate groups, these polysaccharides are classified into κ-carrageenan, i-carrageenan, and λ-carrageenan et al.13. Among them, κ-carrageenan possesses excellent film-forming properties and has been widely developed as a food packaging material14. Carrageenan is commonly used as a multifunctional packaging film to extend the shelf life of food products due to its outstanding film-forming ability, biocompatibility, biodegradability, high gelling ability, and barrier properties15. Studies have shown that the incorporation of antimicrobial agents into carrageenan films is a promising method to enhance their antimicrobial effect16,17,18.
Polyoxometalates (POMs) are metal-oxygen cluster compounds formed by the coordination of transition metals with oxygen, including various structures such as Keggin, Well-Dawson, Anderson, Waugh, and others.Due to their unique structure and excellent antimicrobial19,20and enzyme inhibition21,22properties, POMs, particularly the Keggin and Well-Dawson types, have important applications in food preservation23,81,82,83,84,85,86. Fang JQ et al.24 found that Dawson P2Mo17Ni could effectively inhibit the activity of polyphenol oxidase, slowing down the blackening and spoilage processes of South American white shrimp, thereby prolonging its shelf life. Xing R et al.25 found that manganese-substituted PMo11Mn was able to better maintain the flavor and taste quality of grape berries, reduce the weight loss and browning rates of fresh grapes, inhibit the degradation of titratable acid content, and effectively prolong the shelf life of grapes.
The Keggin-structured Na3PW12O40 exhibits excellent antibacterial properties.Lv BL et al.26studied the antibacterial activity of POMs (PW12, H3PW12) against Escherichia coli (E.coli) and S.aureus, and found that PW12 exhibited the strongest antibacterial activity against both bacteria.Wang L et al.27found that Na3PW12O40 demonstrated certain antibacterial effects against E.coli, S.aureus, yeast, and Aspergillus niger, with the most notable effect against S.aureu.
Based on the excellent properties of POMs, researchers have incorporated POM as a functional additive into film-forming substrates to prepare functionalized antibacterial composite films28.Xu L et al.29used silver phosphotungstate as an antimicrobial agent and polyvinyl alcohol (PVA) as a film-forming substrate to prepare silver phosphotungstate/PVA composite films via the casting method. They studied the mechanical properties, WVP, and antimicrobial properties of the composite films. The results showed that with an increase in the silver phosphotungstate content, the mechanical properties of the composite film gradually improved, and the film exhibited enhanced antimicrobial effects against S.aureus and E. coli.
When preparing composite films, compatibility between POMs and bio-based matrices is essential30,93,94,95,96. The good water solubility of Na3PW12O40 facilitates its uniform dispersion within the carrageenan matrix, ensuring homogeneous distribution of the antimicrobial agent and enhancing the overall functional performance of the packaging material. Additionally, Na3PW12O40 exhibits high thermal stability, commercial availability31,32,86,87,88,89,90, and ease of synthesis, with inexpensive and readily accessible raw materials. Based on its outstanding antimicrobial efficacy, stability, and compatibility, Na3PW12O40 was selected as a functional additive for the fabrication of carrageenan-based composite films with enhanced antimicrobial activity.
Research on the direct application of Keggin-type POMs in food packaging remains in its early stages. Enderle A G and colleagues33,97,98,99developed hybrid films by integrating POM-IL with poly(methyl methacrylate) which can function as surface coatings or packaging materials for ready-to-eat foods. These hybrid films effectively limit the proliferation and transmission of pathogenic microorganisms, thereby reducing the risk of microbial spread through surface contact. Multilayer films combining POMs with methylene blue have also demonstrated antibacterial activity against E.coli, whereas methylene blue alone exhibits limited efficacy, further underscoring the potential application of POM-based systems in food packaging34,91,92.
This study incorporated Keggin-type POM into κ-carrageenan and prepared Carr/POM films via the casting method. The effects of the POM content on the mechanical properties, physical properties, thermal stability, and microstructure of the films were thoroughly investigated.Additionally, antibacterial tests were conducted to further investigate the antibacterial properties of the films.
Materials and methods
Materials and reagents
κ-Carrageenan was purchased from Aladdin, glycerol was purchased from GENERAL-REAGENT, Shanghai Titan Technology Co. and sodium phosphotungstate hydrate (Na3PW12O40.xH2O) was purchased from Sinopharm Chemical Reagent Co. E.coli (ATCC 25922) and S.aureus (ATCC 6538) were purchased from Beijing Preservation Biotechnology Co. Nutrient broth medium (NB, CN120054-250 g) and nutrient agar medium (NA, CN230275-250 g) were purchased from Qingdao Haibo.
Synthesis of carrageenan-based keggin polyoxometalate (Na3PW12O40) antibacterial films (carrageenan/Na3PW12O40 films)
κ-Carrageenan powder (1%, W/V) and glycerol (1%, V/V) were dissolved in sterile water and magnetically stirred at 80 °C for 30 min to obtain a transparent and homogeneous film-forming solution. Subsequently, Na3PW12O40 was dissolved in 5 mL of sterile water to produce a yellow transparent solution. This solution was then magnetically stirred with the κ-Carrageenan film-forming solution at 60 °C for 20 min to obtain Carrageenan/Na3PW12O40 film solutions of different concentrations (0, 1, 2, 4, 8 mg/mL), labeled as Carr, Carr/POM-1, Carr/POM-2, Carr/POM-3, and Carr/POM-4, respectively. The films were prepared using the tape-casting method. Fifteen milliliters of the film-forming solution were poured into a sterile petri dish with a diameter of 90 mm and subjected to ultrasonic treatment for 10 min to remove air bubbles. Finally, the films were dried in a hot-air circulation oven at 35 °C for 24 h. The prepared films were stored at room temperature (25 ± 1 °C) and 50% relative humidity.
Characterizations
The film thickness was measured using a micrometer (DL9325). Five random points were selected on each film, and the average value of the measurements was used as the film thickness. The mechanical properties of the films were tested using a universal testing machine (MTS E43.104) manufactured by Shenzhen Yinfei Electronic Technology Co., Ltd. The film samples were cut into dimensions of 1 cm×3 cm and tested according to GB/T453-2002 standards. The WVP of the films was measured using the W3-031 WVP testing system (W010B) from Jinan Languang Electromechanical Technology Co., Ltd. The films were placed in the device under conditions of 38 ± 0.6 °C and 90 ± 2% relative humidity (RH) for 24 h to obtain the WVP, which was used to evaluate the films’ barrier properties against water vapor. The surface color indices (L, a, b values) of the films were measured using a colorimeter (NR110) manufactured by Guangdong Sanenshi Technology Co., Ltd. The device was calibrated with white paper, and measurements were taken at three different locations on each film sample. The film color was represented using the CIE Lab color system, which evaluates its appearance in terms of brightness (L), red/green (a), and yellow/blue (b) values. The color difference (∆E) was calculated using the corresponding formula.
The infrared spectra of the films were recorded using a Thermo Nicolet iS10 Fourier Transform Infrared (FT-IR) spectrometer (USA). The scanning wavenumber range was 4000–400 cm−1, with a resolution of 4 cm−1. The surface microstructure of the films was examined using a Zeiss Gemini Sigma 300 scanning electron microscope (SEM) equipped with an Oxford Xplore30 energy dispersive spectroscopy system. The films were cut, sputter-coated with gold, and analyzed under the SEM. The thermal stability of the films was assessed using a TGA55 thermogravimetric(TG) analyzer (TA Instruments, USA). The samples underwent TG analysis under a nitrogen atmosphere, heated from 30 °C to 800 °C at a rate of 10 °C/min. The changes in sample mass with increasing temperature were recorded to obtain the TG and derivative thermogravimetric (DTG) curves. The crystalline characteristics of the films were analyzed using an XRD-3X X-ray diffractometer (Beijing Purkinje General Instrument Co., Ltd.). The film samples were cut to appropriate sizes, placed into the circular grooves of a glass slide, flattened, and analyzed using Cu-Kα radiation. Following UV irradiation for 30 min, the films were punched into circular discs with a diameter of 6 mm. These discs were placed on agar plates inoculated with E. coli and S. aureus, incubated at 37 °C for 24 h, and then removed to record and photograph the inhibition zone diameters.Biodegradation of Carr and Carr/POM-4 films was assessed using a protocol adapted from Rech et al.35. Each film sample was wrapped in gauze and buried 10 cm deep in soil maintained at 25 °C and 80% relative humidity. The samples were retrieved daily, photographed, and examined to assess the extent of degradation.
Results and discussion
Analysis of thickness measurement results
The effect of Na3PW12O40 incorporation on the thickness of Carr/POM films was systematically investigated. As shown in Fig. 1, the thickness of pure carrageenan films without POMs was 0.038 ± 0.001 mm. The addition of a low concentration of POMs (1 mg/mL) had negligible effects on film thickness. However, as the concentration of POMs increased, the film thickness increased significantly (p < 0.05), likely due to the incorporation of POMs, which increased the solid content or the internal gaps between polymer chains and glycerol in the film matrix28,36. The incorporation of bee pollen extract and honey extract into κ-carrageenan-based films also resulted in the increase in film thickness37.
An increase in film thickness typically influences its flexibility, particularly affecting tensile strength and deformability. While increased thickness may enhance the structural integrity of the film, excessive thickness may compromise its flexibility under certain conditions. Incorporating small amounts of additives can improve the ductility and flexibility of the film by promoting intermolecular cross-linking, such as hydrogen bonding38. However, the excessive incorporation of additives may cause aggregation and the formation of stress concentration points, leading to brittle fracture and reduced flexibility of the film39.
The barrier properties of films, particularly their WVP, are critical for a wide range of practical applications, particularly in food packaging systems. An increase in film thickness generally leads to enhanced barrier properties. Although increased thickness extends the diffusion path of water molecules, studies suggest that the densification of the film structure is the primary factor responsible for the reduced WVP40,41. In the design of food packaging materials, a careful balance between thickness and flexibility must be achieved to meet practical performance requirements.
Analysis of tensile strength and elongation at break results
The mechanical properties of the films are commonly represented by tensile strength and elongation at break. Tensile strength reflects the mechanical resistance of the films, while elongation at break indicates their flexibility. The mechanical properties of the films are summarized in Table 1. As shown in the table, the elongation at break increased significantly following the addition of POM, thereby enhancing the flexibility of the films. This improvement is attributed to strong intermolecular interactions between an optimal amount of POM and carrageenan42.The tensile strength of the films varied with the POM content. As the POM content increased, both the tensile strength and elongation at break of the composite films initially increased and then decreased. Carr/POM-2 exhibited the highest tensile strength and elongation at break, which is due to the fact that when an appropriate amount of POM is added, the crosslinking degree between POM and carrageenan increases, resulting in higher membrane tensile strength. However, as the amount of POM exceeds the optimal level, the intermolecular and intramolecular interactions of carrageenan decrease43, leading to a reduction in membrane tensile strength. The tensile strength of Carr/POM-4 is markedly lower than that of pure carrageenan, mainly due to the agglomeration of POM within the carrageenan matrix at elevated POM concentrations, resulting in a diminished tensile strength of the composite film29.This finding is corroborated by SEM images of Carr/POM-4, which reveal POM aggregation on the film surface at elevated concentrations. Compared with other carrageenan-based films or films containing POM, Carr/POM has higher mechanical properties, as shown in Table 2.
Analysis of WVP results
WVP is commonly used to assess the water resistance of films, with lower WVP values indicating better water resistance. The WVP results of the films are presented in Table 3. The WVP of Carr/POM films was lower than that of pure carrageenan films. As the POM content increased, the WVP of the composite films decreased, suggesting that POM significantly enhanced the water vapor barrier properties of carrageenan films. Hydrogen bonding interactions between Na3PW12O40 and κ-carrageenan result in the formation of a dense intermolecular network. This network structure significantly reduces the availability of hydrophilic groups for water vapor adsorption, thereby decreasing the WVP of the film48. Another contributing factor is the increased surface roughness of the film, which becomes more pronounced as higher amounts of Na3PW12O40 are incorporated into the matrix. The aggregation of Na3PW12O40 particles obstructs the microchannels for water vapor diffusion within the film matrix49, further reducing the WVP. These observations are consistent with the results obtained from SEM characterization.
Analysis of color measurement results
Table 4 presents the L, a, b, and ΔE values of carrageenan and Carr/POM films. As shown in Fig. 2, pure carrageenan films without POM were nearly colorless and transparent. Upon the incorporation of POM, Carr/POM films exhibited blue and green hues, as indicated by the negative values of a and b. Furthermore, as the concentration of POM increased, the color of the composite films darkened, as evidenced by the decrease in L values. With increasing POM content, the absolute value of a increased, signifying a more pronounced green color in the composite films. The ΔE value of Carr/POM films was higher than that of pure carrageenan films (p < 0.05), suggesting that Carr/POM films were darker in color50. The absolute value of b for the composite films was greater than that for pure carrageenan films, indicating that the composite films were bluer, as shown in Fig. 2.
Analysis of fourier transform infrared (FT-IR) spectroscopy results
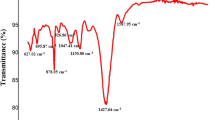
The intermolecular interactions between the carrageenan matrix and POM were investigated using FT-IR spectroscopy, as illustrated in Fig. 3. The FT-IR spectrum of pure carrageenan exhibited several characteristic peaks in the range of 500–3700 cm−1. A broad absorption peak centered at 3330 cm−1, within the range of 3000–3600 cm−1, corresponded to the O-H stretching vibration. The peak at 1643 cm−1 was attributed to the bending vibration of water molecules. Peaks at 2942 cm−1 and 2880 cm−1 corresponded to the asymmetric and symmetric stretching vibrations of methylene (− CH2) groups. The peak at 916 cm−1 represented the C − O stretching vibration of 3,6-anhydro-D-galactose. The peaks at 842 cm−1 and 1217 cm−1 were attributed to the C-O-S stretching vibration and the S-O asymmetric stretching vibration, respectively13. Additionally, carrageenan displayed characteristic sulfate-related vibrations, with the peak at 842 cm−1 corresponding to the vibration of D-galactose-4-sulfate, and the peak at 1217 cm−1 representing the asymmetric stretching vibration of sulfate (O = S = O). Peaks at 1030 cm−1 and 1156 cm−1 were assigned to the C-O stretching vibration in glycosidic bonds and the asymmetric stretching vibration of C − O−C, respectively8,51.
The FT-IR absorption peaks of Na3PW12O40 included P-Oa, W-Od, W-Ob-W, and W-Oc-W, which are characteristic of typical Keggin-type POMs52. Among these, the peak at 793 cm−1 corresponded to the asymmetric stretching vibration of W-Oc-W, while the peak at 968 cm−1 was attributed to the W-Od stretching vibration. The P-Oa and W-Ob-W stretching vibration peaks of Na3PW12O40 may overlap with the vibration peaks of carrageenan53,54.
When Na3PW12O40 was incorporated into the films, changes in the position and intensity of FT-IR peaks were observed. As the Na3PW12O40 content increased in the composite films, the absorption peaks at 968 cm−1 and 793 cm−1 became more pronounced, while the peak at 842 cm−1 weakened. The absorption peaks at 968 cm−1 and 793 cm−1 are characteristic of Na3PW12O40, further confirming the interaction between Na3PW12O40 and carrageenan. This interaction is likely attributed to the terminal and bridging oxygen atoms on the POM surface forming hydrogen bonds with the –OH groups of carrageenan30. Additionally, as the POM content increased, the intensity of the peak at 842 cm−1, a characteristic peak of carrageenan, decreased. This is primarily because the relative amount of carrageenan in the composite films decreased with the increasing POM content.
SEM analysis
The surface morphology of the films was examined using scanning electron microscopy (SEM). As shown in Fig. 4, the surface of the pure carrageenan film was smooth and uniform, indicating good compatibility between κ-carrageenan and glycerol. The surfaces of Carr/POM-1, Carr/POM-2, and Carr/POM-3 films exhibited no significant differences compared to the pure carrageenan film, suggesting that, at low concentrations (≤ 4 mg/mL), POM was well integrated into the κ-carrageenan film matrix. However, as the POM concentration increased, the surface roughness of the films gradually intensified, and cracks began to form. This phenomenon was particularly noticeable at high POM concentrations (8 mg/mL) and attributed to the aggregation of POM, which led to a less smooth film surface. Similar changes in surface smoothness were observed when Dawson-type K6[Mo18O62P2] was incorporated into κ-carrageenan films28.
TG-DTG analysis
TG analysis was conducted to investigate the effect of polyoxometalate (POM) concentration on the thermal stability of the films. The TG and DTG curves of pure carrageenan films and Carr/POM films are presented in Fig. 5. These curves revealed three stages of weight loss for all film samples. The first stage (60–120 °C) was attributed to the evaporation of free water, which can be ascribed to the hydrophilic nature of carrageenan55. The second stage (215–235 °C) was primarily due to the degradation of glycerol in the films56,57. Previous studies58have shown that potassium tungstophosphate and similar POMs exhibit excellent thermal stability, remaining stable without decomposition below 800 °C. The third stage (230–800 °C) was associated with the decomposition of carrageenan59,60. Compared to pure carrageenan films, the third decomposition stage of Carr/POM composite films occurred at a relatively lower temperature, indicating that Keggin-type POMs reduce the thermal stability of carrageenan films, likely due to diminished interactions among POM, glycerol, and the κ-carrageenan matrix61. In the first decomposition stage, the weight loss of pure carrageenan films was lower than that of Carr/POM composite films, possibly due to the presence of POM in the composite films. In the second decomposition stage, pure carrageenan films experienced greater weight loss than Carr/POM composite films. As the POM content increased, the weight loss gradually decreased, likely because the incorporation of POM reduced the glycerol mass fraction in the films, leading to less weight loss in the second stage.
XRD analysis results
X-ray diffraction (XRD) analysis was conducted to examine the crystalline characteristics of carrageenan-POM films, as illustrated in Fig. 6. Due to the amorphous nature of carrageenan, pure carrageenan films did not exhibit any distinct crystalline peaks, as previously reported62. The characteristic diffraction peak of Keggin-structured POMs was observed at 2θ = 26.4 °63,64. The XRD patterns of all film samples showed similar diffraction features, with a broad peak around 2θ = 20 °, which can be attributed to the semi-crystalline nature of the films65,66. Similar diffraction peaks of pure carrageenan films were observed in previous studies67.
Furthermore, as the POM content increased, the intensity of the diffraction peaks diminished, likely due to interactions between POM, carrageenan, and glycerol, which disrupted the original crystalline structure of carrageenan and reduced its crystallinity. A similar phenomenon was observed upon the addition of rutin essential oil (RGEO) to chitosan68.The interaction between chitosan and RGEO slightly disrupted the original crystalline structure of chitosan, resulting in a decrease in the crystallinity of the chitosan film69,70. XRD analysis indicated good compatibility between carrageenan, POM, and glycerol.
Antibacterial performance of carr/pom films
As shown in Fig. 7, in the investigation of the antibacterial performance of κ-carrageenan and its composite films with Na3PW12O40 against E.coli and S.aureus, the samples were incubated at 37 °C for 24 h.The results indicated that neither the κ-carrageenan film nor the Carr/POM film exhibited significant antibacterial activity against E.coli, as shown in Table 5. Under identical incubation conditions, neither the κ-carrageenan film nor the Carr/POM film containing low concentrations of Na3PW12O40 demonstrated significant antibacterial activity against S.aureus. However, when the concentration of Na3PW12O40 reached 4 mg/mL, a clear inhibition zone formed around the Carr/POM composite film, suggesting a marked enhancement in its antibacterial activity.Furthermore, as the Na3PW12O40 concentration increased to 8 mg/mL, the diameter of the inhibition zone further expanded, demonstrating that the antibacterial effect of the composite film is strongly correlated with the concentration of Na3PW12O40 in the film. Following antibacterial testing, the films underwent dissolution. Future research will aim to enhance the stability of the films under high-humidity conditions, thereby broadening their range of applications.
The antibacterial experiment demonstrated that the Carr/POM film exhibited a significantly stronger antibacterial effect against S.aureus compared to E.coli. This difference is speculated to arise from the fact that E.coli is a Gram-negative bacterium with a complex cell wall structure that is difficult to disrupt, whereas S.aureus is a Gram-positive bacterium with a relatively weaker and more susceptible cell wall structure71.
The antimicrobial mechanism of Keggin-type POMs against S.aureus is believed to involve several pathways, including disruption of the cell membrane, interference with the bacterial respiratory chain, and the generation of reactive oxygen species (ROS)19,72. POMs exhibit antibacterial activity primarily by interacting electrostatically with the oppositely charged components of microbial cell walls, which leads to the leakage of intracellular contents73,79,93. Furthermore, Keggin-structured POMs possess redox activity, allowing them to oxidize key electron carriers, thereby disrupting the bacterial respiratory system, impairing ATP synthesis, and inflicting irreversible damage to the cells. POMs can also directly oxidize bacterial proteins, lipids, and other biomolecules, generating ROS, or indirectly increase ROS levels by depleting glutathione, thereby leading to cellular oxidative damage19.
In Carr/POM films, carrageenan itself typically does not possess significant antibacterial properties and mainly serves as the film matrix74, whereas POM is incorporated as the antibacterial agent. This observation aligns with previous studies and is further evidenced by the absence of antimicrobial activity in pure carrageenan films, as demonstrated in antibacterial assays28,75. In the chitosan/POM system, given that chitosan exhibits intrinsic antibacterial properties, the antibacterial efficacy of the chitosan/POM composite is predominantly attributed to the synergistic interaction between chitosan and POM. The electrostatic attraction between positively charged chitosan (CS) and negatively charged bacterial membrane components not only enhances membrane permeability but also promotes contact between POM and the bacterial membrane, thereby augmenting the antibacterial efficacy of the composite30,100,101,102,103,104,105.
Future research will further explore the synergistic effects between POMs and other antibacterial agents. Silver nanoparticles modified with H3PW12O40 induce severe physical damage to both Gram-negative (E.coli) and Gram-positive (S.aureus) bacterial cells. However, their antibacterial efficacy is notably stronger against E. coli. The antibiotic activity of silver ions (Ag+) appears to potentiate the effect of H3PW12O40, with their synergistic interaction markedly enhancing antibacterial efficacy. Silver nanoparticles disrupt bacterial cell walls and generate high concentrations of reactive oxygen species (ROS), thereby stabilizing and facilitating the delivery of POMs into bacterial cells19. Additionally, chitosan–polyoxometalate (CS–POM) nanocomposites, fabricated with POMs and chitosan as the primary matrix, exhibit robust synergistic antibacterial activity against E. coli. The synergistic antibacterial mechanism between CS and H5PMo10V2O40 involves CS-mediated membrane destabilization and POM-induced oxidation of electron transport chain substrates30. Chen D et al. employed layer-by-layer self-assembly to construct two multilayer films incorporating Keggin-type POMs (α-[SiW12O40]4−/α-[PMo12O40]3−) and methylene blue, both exhibiting substantial antibacterial activity against E. coli34. This study establishes a foundation for the development of multilayer composite film architectures, which may be further optimized to improve antibacterial performance.
Biodegradability of carr/pom anti-bacterial film in soil
Biodegradation analysis of the Carr/POM antimicrobial films is presented in Fig. 8. As shown in Fig. 8A, the pure carrageenan film remained largely intact during the first two days, exhibiting no significant structural damage. However, degradation began on the third day, with visible fragmentation occurring and progressing to complete disintegration by day seven. Similarly, Fig. 8B shows that the Carr/POM-4 film maintained its structural integrity on the first day, with no apparent damage, though a noticeable fading in color was observed. From the second day onward, the film developed significant cracks and ultimately underwent full degradation by the seventh day. These results demonstrate that Carr/POM films possess excellent biodegradability in soil, highlighting their potential as environmentally friendly antimicrobial materials. This observation is consistent with previous studies, which have reported favorable biodegradability of carrageenan-based films76,77.
Conclusion
In the present study, Carr/POM films were fabricated by incorporating the Keggin-type polyoxometalate Na3PW12O40 into κ-carrageenan via the solution casting method. The mechanical strength, thermal stability, microstructure, and antibacterial properties of the composite films were thoroughly investigated. The results demonstrated interactions between Na3PW12O40 and carrageenan. The incorporation of Na3PW12O40 enhanced the water resistance of the carrageenan films and modified their microstructure. In antibacterial assays, Carr/POM films exhibited robust antibacterial activity against S.aureus, especially when the Na3PW12O40 concentration reached 8 mg/mL, resulting in the most significant inhibition. The incorporation of an appropriate amount of POMs into κ-carrageenan to prepare composite antibacterial materials can significantly enhance its inhibitory effect against common pathogenic microorganisms in food.This composite material demonstrates significant potential for the development of antibacterial materials and holds broad application prospects in the field of food packaging.
However, large-scale production of Na3PW12O40 may encounter challenges related to raw material availability and process optimization, particularly in maintaining product consistency and controlling production costs. Owing to the limited hydrophilicity of the prepared Carr/POM films, future research will aim to develop preservative films with improved stability. Furthermore, identifying POMs with superior antibacterial properties will be central to developing preservative films exhibiting broad-spectrum antibacterial activity. The long-term effects of POMs on food safety warrant further investigation to validate their feasibility in food packaging applications. Moreover, research on the in vivo activity of POMs remains insufficient. Whether POMs generate harmful metabolites during bodily metabolism and their subsequent impact on the internal environment remains unclear. The degradation behavior and environmental impact of POMs also necessitate further study, particularly concerning their potential ecological toxicity in discarded packaging materials.
Data availability
All data generated or analysed during this study are included in this published article.
References
Cheng, H. et al. Research progress on natural biodegradable materials [J]. J. Food Saf. Qual. Inspection. 14 (11), 205–213 (2023).
Vimala Bharathi, S. K. et al. Zein-based anti‐browning cling wraps for fresh‐cut Apple slices[J]. Int. J. Food Sci. Technol. 55 (03), 1238–1245 (2020).
Calva-Estrada, S. J., Jiménez-Fernández, M. & Lugo-Cervantes, E. Protein-based films: advances in the development of biomaterials applicable to food packaging[J]. Food Eng. Rev. 11, 78–92 (2019).
Bhatia, S. et al. Gallic acid crosslinked gelatin and casein based composite films for food packaging applications[J]. Polymers 14 (19), 4065 (2022).
Zhou, W. et al. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: characterization and application in Mango preservation[J]. Carbohydr. Polym. 256, 117579 (2021).
Hasan, M. I., Wang, J. & Tajvidi, M. Controlled shrinkage of cellulose nanofibril films to enhance mechanical and barrier properties[J]. Carbohydr. Polym. 342, 122390 (2024).
Wang, C. et al. Preparation and characterization of antioxidant and pH-sensitive films based on arrowhead (Sagittaria sagittifolia) starch, κ-carrageenan and black chokeberry (Aronia melanocarpa) extract for monitoring spoilage of chicken wings[J]. Int. J. Biol. Macromol. 224, 544–555 (2023).
Simona, J. et al. Edible films from carrageenan/orange essential oil/trehalose—structure, optical properties, and antimicrobial activity[J]. Polymers 13 (03), 332 (2021).
Syahida, S. N. et al. Effects of palm wax on the physical, mechanical and water barrier properties of fish gelatin films for food packaging application[J]. Food Packaging Shelf Life. 23, 100437 (2020).
Amin, U. et al. Potentials of polysaccharides, lipids and proteins in biodegradable food packaging applications[J]. Int. J. Biol. Macromol. 183, 2184–2198 (2021).
Romani, V. P. et al. Cold plasma and Carnauba wax as strategies to produce improved bi-layer films for sustainable food packaging[J]. Food Hydrocoll. 108, 106087 (2020).
Zia, K. M. et al. A review on synthesis, properties and applications of natural polymer based Carrageenan blends and composites[J]. Int. J. Biol. Macromol. 96, 282–301 (2017).
Bhatia, S. et al. Enhancing tensile strength, thermal stability, and antioxidant characteristics of transparent kappa Carrageenan films using grapefruit essential oil for food packaging Applications[J]. ACS Omega. 9 (08), 9003–9012 (2024).
Shahbazi, M. et al. Kinetic study of κ-carrageenan degradation and its impact on mechanical and structural properties of chitosan/κ-carrageenan film[J]. Carbohydr. Polym. 142, 167–176 (2016).
Chi, W. et al. Simultaneously realizing intelligent color change and high haze of κ-carrageenan film by incorporating black corn seed powder for visually monitoring pork freshness[J]. Food Chem. 402, 134257 (2023).
Jiang, G. et al. Preparation and characterization of κ-carrageenan/dextran films blended with nano-ZnO and anthocyanin for intelligent food packaging[J]. Int. J. Biol. Macromol. 282, 137203 (2024).
You, S. et al. Development of highly stable color indicator films based on κ-carrageenan, silver nanoparticle and red grape skin anthocyanin for marine fish freshness assessment[J]. Int. J. Biol. Macromol. 216, 655–669 (2022).
Abbas Shah, Y. et al. The Applications of Lime (Citrus aurantifolia) Essential Oil as a Functional Ingredient in Gelatin/Kappa-Carrageenan Composite Films for Active Packaging[J]41199–1208 (ACS Food Science & Technology, 2024). 05.
Bijelic, A., Aureliano, M. & Rompel, A. The antibacterial activity of polyoxometalates: structures, antibiotic effects and future perspectives[J]. Chem. Commun. 54 (10), 1153–1169 (2018).
Özbek, H. A. Synthesis and characterization of Anderson-Evans type polyoxometalates, antibacterial properties[J]. Turk. J. Chem. 47 (04), 742–748 (2023).
Zhao, M. et al. Research progress on the Inhibition of enzymes by polyoxometalates[J]. Inorg. Chem. Front. 7 (22), 4320–4332 (2020).
Wang, A. X., Fang, X. H. & Zhang, Q. Research progress on the Inhibition of tyrosinase activity by polyoxometalates [J]. J. Baoshan Univ. 40 (05), 35–41 (2021).
Zhou, F. et al. Research progress on the biological activity of polyoxometalates and their application in food preservation [J]. Food Mach. 36 (05), 216–220 (2020).
Fang, J. Q. et al. Evaluation of the inhibitory activity of polyoxometalates on polyphenol oxidase and their application in the preservation of South American white shrimp [J]. Food Mach. 39 (03), 114–121 (2023).
Xing, R. et al. Preservation effect of Manganese-Substituted phosphomolybdate on grapes [J]. Food Ind. 44 (06), 95–99 (2023).
Lv, B. L. et al. Synthesis, characterization, and antibacterial activity of polyoxometalates [J]. J. Hubei Normal Univ. (Natural Sci. Edition) 27(04), 5–7 (2007).
Wang, L., Wang, F. & Cai, H. N. Enzyme Inhibition and antibacterial effects of phosphotungstate [J]. Food Sci. 30 (03), 51–53 (2009).
Zhou, F. et al. Preparation and characterization of biodegradable κ-carrageenan based anti-bacterial film functionalized with Wells-Dawson polyoxometalate[J]. Foods 11 (04), 586 (2022).
Xu, L. et al. Preparation and properties of silver phosphotungstate/pva antibacterial packaging films [J]. Plastics 53 (05), 38–41 (2024).
Fiorani, G. et al. Chitosan-polyoxometalate nanocomposites: synthesis, characterization and application as antimicrobial agents[J]. J. Cluster Sci. 25 (03), 839–854 (2014).
Patel, A. et al. Keggin-type lacunary and transition metal substituted polyoxometalates as heterogeneous catalysts: A recent progress[J]. Catal. Reviews. 58 (03), 337–370 (2016).
Sampurnam, S. et al. Synthesis and characterization of Keggin-type polyoxometalate/zirconia nanocomposites—Comparison of its photocatalytic activity towards various organic pollutants[J]. J. Photochem. Photobiol., A. 370, 26–40 (2019).
Enderle, A. G. et al. Hybrid antimicrobial films containing a polyoxometalate-ionic liquid[J]. ACS Appl. Polym. Mater. 4 (06), 4144–4153 (2022).
Chen, D. et al. Fabrication and characterization of Antibacterial-active multilayer films based on keggin polyoxometalates and methylene Blue[J]. Z. Für Naturforschung B. 65 (02), 140–146 (2010).
Rech, C. R. et al. Biodegradation of polyhydroxybuterate films incorporated with Eugenol in different soil types[J]. Case Stud. Chem. Environ. Eng. 2 (04), 100014 (2020).
Roy, S. & Rhim, J. W. Carrageenan-based antimicrobial Bionanocomposite films incorporated with ZnO nanoparticles stabilized by melanin[J]. Food Hydrocoll. 90, 500–507 (2019).
Velásquez, P. et al. k-carrageenan edible films for beef: honey and bee pollen phenolic compounds improve their antioxidant capacity[J]. Food Hydrocoll. 124, 107250 (2022).
Jafarzadeh, S. et al. Preparation and characterization of Bionanocomposite films reinforced with nano kaolin[J]. J. Food Sci. Technol. 53, 1111–1119 (2016).
Rhim, J. W. Effect of clay contents on mechanical and water vapor barrier properties of agar-based nanocomposite films[J]. Carbohydr. Polym. 86 (02), 691–699 (2011).
Lagaron, J. M. et al. Improving packaged food quality and safety. Part 2: Nanocomposites[J]. Food Addit. Contam. 22 (10), 994–998 (2005).
Lange, J. & Wyser, Y. Recent innovations in barrier technologies for plastic packaging—a review[J]. Packaging Technol. Science: Int. J. 16 (04), 149–158 (2003).
Meng, F. et al. Mechanical, hydrophobic and thermal properties of an organic-inorganic hybrid carrageenan-polyvinyl alcohol composite film[J]. Compos. Part. B: Eng. 143, 1–8 (2018).
Jaiswal, L., Shankar, S. & Rhim, J-W. Carrageenan-based functional hydrogel film reinforced with sulfur nanoparticles and grapefruit seed extract for wound healing application[J]. Carbohydr. Polym. 224, 115191 (2019).
Huang, X. et al. Formation mechanism of egg white protein/κ-Carrageenan composite film and its application to oil packaging[J]. Food Hydrocoll. 105, 105780 (2020).
Shahbazi, M., Majzoobi, M. & Farahnaky, A. Physical modification of starch by high-pressure homogenization for improving functional properties of κ-carrageenan/starch blend film[J]. Food Hydrocoll. 85, 204–214 (2018).
Shojaee-Aliabadi, S. et al. Characterization of κ-carrageenan films incorporated plant essential oils with improved antimicrobial activity[J]. Carbohydr. Polym. 101, 582–591 (2014).
Martiny, T. R. et al. Bio-based active packaging: Carrageenan film with Olive leaf extract for lamb meat preservation[J]. Foods 9 (12), 1759 (2020).
Zhang, X. et al. Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black Plum Peel extract[J]. Food Hydrocoll. 94, 80–92 (2019).
Siripatrawan, U. & Kaewklin, P. Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging[J]. Food Hydrocoll. 84, 125–134 (2018).
Liu, J. et al. Films based on κ-carrageenan incorporated with Curcumin for freshness monitoring[J]. Food Hydrocoll. 83, 134–142 (2018).
Jancikova, S. et al. Chemical and physical characteristics of edible films, based on κ-and ι-carrageenans with the addition of Lapacho tea extract[J]. Foods 9 (03), 357 (2020).
Chen, C. et al. Photodegradation of Dye Pollutants Catalyzed by Porous K3PW12O40 Under Visible irradiation[J]. Environ. Sci. Technol. 12, 403965–403970 (2006).
Kahloul, M. et al. Green complexation for heavy metals removal from wastewater by Keggin-polyoxometalates enhanced ultrafiltration[J]. Water Sci. Technol. 86 (06), 1510–1526 (2022).
Wen, Z. et al. Molybdic Acid-Catalyzed hydrolysis of microcrystalline cellulose for glucose Preparation [J]. J. Changzhou Univ. (Natural Sci. Edition). 34 (05), 30–38 (2022).
Han, Y., Yu, M. & Wang, L. Bio-based films prepared with soybean by-products and pine (Pinus densiflora) bark extract[J]. J. Clean. Prod. 187, 1–8 (2018).
Zhang, C. et al. A green strategy for maintaining intelligent response and improving antioxidant properties of κ-carrageenan-based film via Cork bark extractive addition[J]. Food Hydrocoll. 113, 106470 (2021).
Wang, X. et al. Preparation and characterization of antioxidant and pH-sensitive films based on Chitosan and black soybean seed coat extract[J]. Food Hydrocoll. 89, 56–66 (2019).
Li, F. M. Study on the Performance of Phosphotungstate in Visible Light Catalytic Degradation of Dyes [D] (Donghua University, 2011).
Duan, N. et al. Preparation and characterization of k-carrageenan/konjac glucomannan/TiO2 nanocomposite film with efficient anti-fungal activity and its application in strawberry preservation[J]. Food Chem. 364 (02), 130441 (2021).
Yong, H. et al. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into Chitosan matrix[J]. Food Hydrocoll. 90, 216–224 (2019).
Zhou, X. et al. Emulsified blend film based on Konjac glucomannan/carrageenan/camellia oil: physical, structural, and water barrier properties[J]. Carbohydr. Polym. 251, 117100 (2021).
Liu, Y. et al. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and Peel extracts[J]. Int. J. Biol. Macromol. 147, 1076–1088 (2020).
Guo, D. et al. Phosphotungstate-Catalyzed synthesis of 2-(4’-Ethylbenzoyl)benzoic acid [J]. Appl. Chem. 46 (09), 1665–1668 (2017).
Wang, J., Liu, Y. J. & Cai, X. W. Preparation and characterization of Keggin-Type polyoxometalate particles [J]. Chem. Bull. 71 (11), 868–872 (2008).
Aziz, M. S. A., Salama, H. E. & Sabaa, M. W. Biobased alginate/castor oil edible films for active food packaging[J]. Lwt 96, 455–460 (2018).
Moreno, M. A. et al. Active properties of edible marine polysaccharide-based coatings containing Larrea nitida polyphenols enriched extract[J]. Food Hydrocoll. 102, 105595 (2020).
Liu, Y. et al. Preparation of pH-sensitive and antioxidant packaging films based on κ-carrageenan and mulberry polyphenolic extract[J]. Int. J. Biol. Macromol. 134, 993–1001 (2019).
Grande Tovar, C. D. et al. Synthesis, characterization, and histological evaluation of chitosan-Ruta graveolens essential oil films[J]. Molecules 25 (07), 1688 (2020).
Prateepchanachai, S. et al. Mechanical properties improvement of Chitosan films via the use of plasticizer, charge modifying agent and film solution homogenization[J]. Carbohydr. Polym. 174, 253–261 (2017).
Jahed, E. et al. Physicochemical properties of carum Copticum essential oil loaded Chitosan films containing organic nanoreinforcements[J]. Carbohydr. Polym. 164, 325–338 (2017).
Xie, L. F. et al. Antibacterial activity of six polyoxometalates [J]. Appl. Chem. 34 (06), 700–704 (2017).
Chang, D. et al. Polyoxometalate-based nanocomposites for antitumor and antibacterial applications[J]. Nanoscale Adv. 4 (18), 3689–3706 (2022).
Farzana, R., Iqra, P. & Hunaiza, T. Antioxidant and antimicrobial effects of polyoxometalates[J]. Microbiol. Curr. Res. 2 (01), 7–11 (2018).
Kokkuvayil Ramadas, B., Rhim, J. W. & Roy, S. Recent progress of carrageenan-based composite films in active and intelligent food packaging applications[J]. Polymers 16 (07), 1001 (2024).
Abdelgawad, A. M. et al. Antibacterial carrageenan/cellulose nanocrystal system loaded with silver nanoparticles, prepared via solid-state technique[J]. J. Environ. Chem. Eng. 8 (05), 104276 (2020).
Abdillah, A. A. & Charles, A. L. Characterization of a natural biodegradable edible film obtained from Arrowroot starch and iota-carrageenan and application in food packaging[J]. Int. J. Biol. Macromol. 191, 618–626 (2021).
Lai, D. et al. Comprehensive properties of photodynamic antibacterial film based on κ-Carrageenan and curcumin-β-cyclodextrin complex[J]. Carbohydr. Polym. 282, 119112 (2022).
Zang, D. & Wang, H. Polyoxometalate-based nanostructures for electrocatalytic and photocatalytic CO2 reduction[J]. Polyoxometalates 1 (1), 9140006 (2022).
Lei, S. et al. Study on the regulatory mechanisms of mitochondrial biosynthesis by polyoxometalates[J]. Polyoxometalates 4 (1), 9140074 (2025).
Wu, S. et al. Cyclohexanone hydrogenation to cyclohexanol on phosphomolybdate supported Pt single-atom catalyst: A density functional theory study[J]. Polyoxometalates 3 (4), 9140070 (2024).
Li, S. et al. New two fold interpenetrating 3D polyoxovanadate-based metal–organic framework as bifunctional catalyst for the removal of 2-chloroethyl Ethyl sulfide and phenolic compounds[J]. Polyoxometalates 3 (3), 9140061 (2024).
Wang, Q. et al. Fabrication of macroporous poms/biochar materials for fast degradation of phthalic acid esters through adsorption coupled with aerobic oxidation[J]. Polyoxometalates 3 (3), 9140064 (2024).
Zang, D. J. et al. Confined interface engineering of self-supported Cu@N-doped graphene for electrocatalytic CO2 reduction with enhanced selectivity towards ethanol[J]. Nano Res. 15, 8872–8879 (2022).
Liu, G. et al. Indirect electrocatalysis SN/SS bond construction by robust polyoxometalate based Foams[J]. Adv. Mater. 35, 2304716 (2023).
Zang, D. et al. Interface engineering of Mo8/Cu heterostructures toward highly selective electrochemical reduction of carbon dioxide into acetate[J]. Appl. Catal. B Environ. 281, 119426 (2021).
Huang, X. et al. An unprecedented 2-fold interpenetrated Lvt open framework built from Zn ring seamed trivacant polyoxotungstates used for photocatalytic synthesis of pyridine derivatives[J]. Appl. Catal. B: Environ. 323, 122134–122144 (2023).
Qin, K. et al. Polyoxometalates based compounds for green synthesis of aldehydes and ketones[J]. Chin. Chem. Lett. 34, 107999 (2023).
Liu, Y. et al. Recent advances in polyoxometalates acid-catalyzed organic reactions[J]. Chin. Chem. Lett. 34, 108097 (2023).
Cheng, Y., Qin, K. & Zang, D. Polyoxometalates based nanocomposites for bioapplications[J]. Rare Met. 42 (11), 3570–3600 (2023).
Cheng, Y., Qin, K. & Zang, D. Polyoxometalates based nanocomposites for bioapplications[J]. Rare Met. 42, 3570 (2023).
Yang, G. et al. Assembly of Y(III)-containing antimonotungstates induced by malic acid with catalytic activity for the synthesis of imidazoles[J]. Chin. Chem. Lett. 35 (12), 110274 (2024).
Wang, P. et al. Structural transformation from Waugh-type to Keggin-type polyoxomolybdate-based crystalline material for photo/electrocatalysis[J]. Rare Met. 43, 2241 (2024).
Li, L. et al. Self-assembled vesicles containing Podophyllotoxin covalently modified with polyoxometalates for antitumor therapy[J]. Polyoxometalates 4 (2), 9140085 (2025).
Yang, G. et al. A wavy multiple Tm(III)-Containing open Wells–Dawson silicotungstate: synthesis, structure, and catalytic Application[J]. Inorg. Chem. 64, 1041 (2025).
Yang, G. et al. Dy/Ho-encapsulated tartaric acid-functionalized tungstoantimonates: heterogeneous catalysts for isoindolinone synthesis[J]. Chem. Commun. 60, 10934 (2024).
Hu, Q. et al. Polyoxometalate catalysts for the synthesis of N-heterocycles[J]. Polyoxometalates 3 (1), 9140048 (2024).
Liu, Y. et al. Two sandwich-type uranyl-containing polytungstates catalyze aerobic synthesis of benzimidazoles[J]. Rare Met. 43, 1316 (2024).
Gao, Z. et al. Rational fabrication of three imidazole-functionalized ionic-type Pd-Polyoxovanadates for aerobic oxidation of 5-Hydroxymethulfurfural to 2,5-Diformylfuran[J]. J. Catal. 429, 115261 (2024).
Dong, C. et al. Boosting electrocatalytic performance of sulfide oxidation on polyoxomolybdates with synergistic effects of CNT-Doped aerogel Foams[J]. Adv. Funct. Mater. 35(14), 2418410 (2025).
Zhao, H. et al. Transition metal substituted sandwich-type polyoxometalates with a strong metal-C (imidazole) bond as anticancer agents[J]. Chem. Commun. 55, 1096–1099 (2019).
Zhang, H. et al. Hetero-bimetallic transition metal-substituted Krebs-type polyoxometalate with N-chelating ligand as anticancer agents[J]. Tungsten 5, 225–234 (2023).
Lu, Z. J. et al. Free-standing hierarchical Co@CoO/CNFs/Cu-foam composite based on electrochemical deposition as High-performance supercapacitor electrode. J. Alloys Compd. 856, 158075 (2021).
Lu, Z. J. et al. Bifunctional oxygen electrocatalysis at Co-B,N,S-Graphene composite investigated by scanning electrochemical microscopy at variable temperatures and its application in Zn-Air battery. Electrochim. Acta. 389, 138751 (2021).
Liu, X. L. et al. Investigation of functionalization effect of carbon nanotubes as supercapacitor electrode material on hydrogen evolution Side-reaction by scanning electrochemical microscopy. Electrochim. Acta. 429, 141056 (2022).
Yuan, G. J., Bai#, J. L., Chen, X., Zhang, L. & Ren, L. L. The effect of P vacancies on the activity of Cobalt phosphide nanorods as oxygen evolution electrocatalyst in alkali. Appl. Catal. B-Environmental. 284, 119693 (2021).
Acknowledgements
The authors express their gratitude to all the funding sources and the journal staff who contributed to this review.
Funding
This work was funded by Scientific research planning project in Anhui Province [2022AH052061, 2022AH040283, 2022AH052075]; 2024 Domestic Visiting Scholar Training Program for Young Key Teachers (JNFX2024137); School-level scientific research and innovation team project [2023xjkytd4]; School-level excellent youth project[2023xjyq1, 2023xjyq3].
Author information
Authors and Affiliations
Contributions
Anxing Wang and Mengqi Wang wrote the main manuscript text Yu Zhang and Qing Zhang prepared figures.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, A., Wang, M., Zhang, Y. et al. Study on the preparation, characterization, and antibacterial properties of keggin POMs functionalized carrageenan composite films. Sci Rep 15, 29935 (2025). https://doi.org/10.1038/s41598-025-13936-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13936-x