Abstract
The glucose-potassium ratio (GPR) index has been recognized as an early prognostic marker for central nervous system injuries, including aneurysmal subarachnoid hemorrhage and acute intracerebral hemorrhage. Nevertheless, its prognostic significance in critically ill patients suffering from acute myocardial infarction (AMI) who are admitted to the intensive care unit (ICU) has not been thoroughly examined. This study sought to explore the relationship between the GPR index and clinical outcomes in this group of patients. This retrospective cohort study employed data from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database to identify critically ill patients with AMI who necessitated admission to the ICU. The patients were categorized into quartiles according to their GPR index levels. The primary outcomes assessed were all-cause mortality at 1 year and at 180 days. To compare survival rates across the four groups, Kaplan–Meier analysis was utilized. Additionally, Cox proportional hazards regression models and restricted cubic spline analyses were performed to investigate the association between the GPR index and clinical outcomes. The study comprised a total of 3811 patients diagnosed with acute myocardial infarction (AMI), of which 70.40% were male. Within this cohort, 999 patients, representing 26.21%, succumbed within one year. Kaplan–Meier analysis indicated a statistically significant elevation in mortality risk among patients exhibiting increased GPR indices (log-rank P < 0.001). Furthermore, restricted cubic spline analysis demonstrated a non-linear escalation in the risk of all-cause mortality corresponding to rising GPR indices (P for nonlinearity < 0.001). Multivariate Cox proportional hazards analysis revealed that individuals in the highest quartile of GPR indices faced a markedly increased risk of 1-year all-cause mortality when compared to those in the lowest quartile [HR 1.70; 95% CI (1.40–2.07); P < 0.001]. Consistent patterns were noted in the assessment of 180-day all-cause mortality. In patients with acute myocardial infarction (AMI) who are critically ill, an elevated GPR index correlates with a heightened risk of all-cause mortality at both 1 year and 180 days. These results indicate that the GPR index could be an important instrument for the identification of high-risk individuals with AMI.
Similar content being viewed by others
Introduction
Currently, the global incidence of AMI is increasing, with a notable trend towards younger individuals being affected1,2. Cardiovascular diseases have consistently ranked as a leading cause of mortality worldwide, with AMI presenting a significant risk and frequently resulting in severe complications such as arrhythmias, heart failure, cardiac rupture, and shock3,4. These complications contribute to substantial economic and social burdens for nations across the globe5. Despite considerable advancements in treatment modalities as outlined in recent clinical guidelines, the occurrence of adverse cardiovascular events remains elevated among critically ill patients with AMI6,7. Consequently, the early identification of high-risk individuals and the implementation of stratified management strategies are essential for enhancing patient outcomes.
Glucose and potassium levels are integral to cardiovascular health. Glucose functions as a crucial substrate for energy production, facilitating the heart’s pumping mechanism and engaging in various metabolic activities8. Potassium is essential for sustaining the normal excitability, automaticity, and conductivity of myocardial cells9. Controlling blood glucose has always been a key point in cardiovascular management. Hyperglycemia is associated with the development of heart failure in patients with coronary heart disease10. Keskin et al. 11believe that ST—segment elevation myocardial infarction patients with a serum potassium level of ≤ 3.5 mEq/L have a higher risk of in—hospital and long-term mortality, and those with a potassium level of 4 to < 4.5 mEq/L have the lowest mortality risk. Abnormal potassium levels can lead to various arrhythmias, including atrial fibrillation12. The intricate relationship between glucose and potassium has led to the development of the glucose-potassium ratio (GPR)13. The GPR index has been recognized as an early prognostic indicator for various conditions, including aneurysmal subarachnoid hemorrhage, acute intracerebral hemorrhage, severe traumatic brain injury, and ischemic stroke14,15,16,17. Within the cardiovascular field, Demir et al.18 established that the GPR index is a significant predictor of in-hospital mortality among patients admitted to coronary care units. Furthermore, in the context of cardiovascular surgery, the GPR index has been identified as a reliable predictor of postoperative mortality risk in patients with type A aortic dissection19.
Glucose and potassium perturbations are integral to AMI pathophysiology. Stress-induced hyperglycemia and hypokalemia reflect sympathetic activation, potentially exacerbating myocardial injury20. While the GPR index captures these dynamics, its prognostic value in critically ill AMI patients remains underexplored.d. This study focuses on exploring the effectiveness of GPR in assessing the mortality risk of such patients. It is committed to identifying indicators that are closely associated with the all-cause mortality of patients with severe AMI, possess both economic feasibility and detection convenience, and have crucial clinical value in reducing the case fatality rate of patients with AMI, so as to provide a reference for clinical practice. The ultimate goal is to optimize the clinical management system and ensure the rapid and accurate implementation of intervention measures for high-risk patients.
Methods
Study population
This investigation employed a retrospective analysis of data sourced from the MIMIC-IV (https://mimic.mit.edu) database, an open-access repository developed and maintained by the Computational Physiology Laboratory at the Massachusetts Institute of Technology. The MIMIC-IV database contains extensive and detailed medical records of patients who were admitted to the intensive care units (ICUs) at Beth Israel Deaconess Medical Center. Data extraction for this study was performed by Wen Pan, one of the co-authors. To gain access to the database, Wen Pan obtained certification from the Collaborative Institutional Training Initiative (CITI) and subsequently extracted the variables pertinent to this research (Certification Number: 13574487).
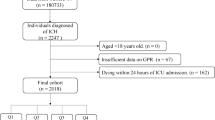
The focus of this study was on the research population in MIMIC-IV, which consisted of 3,811 individuals. Inclusion criteria: Participants were eligible for inclusion in the study if they met the following conditions: (a) diagnosed with acute myocardial infarction according to ICD-9 or ICD-10 codes; (b) aged 18 years or older; (c) admitted to the intensive care unit (ICU) for the first time during their initial hospitalization. Exclusion criteria: Participants were excluded from the study if they met any of the following criteria: (a) died within the initial 24 h of ICU admission, as their clinical trajectories may have been incompletely recorded or had negative survival time values; (b) had missing crucial laboratory data, specifically glucose and potassium levels, on the first day of ICU admission, as these are essential for our analytical purposes. Consequently, a total of 3,811 patients were included in this study (Fig. 1).
Data collection
Data extraction was performed using the pgAdmin PostgreSQL tool (version 6.1) from the MIMIC-IV database. The collected data were consistent with the study’s objectives and encompassed the following categories: 1. Demographic information, which included age, gender, body mass index (BMI), tobacco use status, and racial background. 2. Comorbidities, which consisted of hypertension, diabetes mellitus, heart failure (HF), chronic kidney disease(CKD), acute renal failure (ARF), stroke, Malignant tumor, and cardiogenic shock. 3. Laboratory indicators documented within the first 24 h of admission, including sodium, potassium, total calcium, chloride, blood glucose, anion gap, White Blood Cell Count (WBC), Red Blood Cell Count (RBC), Neutrophil Count (NEUT), Lymphocyte Count (LYM), Platelet Count (PLT), Hemoglobin Concentration (Hb), Red Cell Distribution Width (RDW), Hematocrit (HCT), Albumin Concentration (ALB), Globulin Concentration (GLB), Total Protein (TP), Sodium, Potassium, Total Calcium, Chloride, Blood Glucose (GLU), Glycated Hemoglobin (HbA1c), Anion Gap (AG), Blood Urea Nitrogen (BUN), Creatinine (Cr), Uric Acid (UA), Lactate Dehydrogenase (LDH), Creatine Kinase (CK), Creatine Kinase—MB (CK-MB), Triglycerides (TG), Total Cholesterol (TC), High-Density Lipoprotein (HDL), Low-Density Lipoprotein (LDL), Total Bilirubin (TBIL), Direct Bilirubin (DBIL), Indirect Bilirubin (IBIL), Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Thrombin Time (TT), Plasma Prothrombin Time (PT), Fibrinogen Concentration (FIB), Activated Partial Thromboplastin Time (APTT), Prothrombin International Normalized Ratio (INR), D-dimer, pH, Partial Pressure of Carbon Dioxide (PCO2), Partial Pressure of Oxygen (PO2), Blood Gas Lactate (Lac), Total Carbon Dioxide (TCO2), Ionized Calcium (iCa2+), B-type Natriuretic Peptide (BNP), Troponin T (cTnT), Troponin I (cTnI), Urine Protein (U-PRO), Urine Glucose (U-GLU), Urine White Blood Cell (U-WBC), Urine Nitrite (U-NIT), Urine Bilirubin (U-BIL), Urine Albumin (U-ALB), and Urine Albumin-to-Creatinine Ratio (UACR). In cases where multiple results were available, the mean value was calculated. 4. Reconstruction (including Percutaneous Coronary Intervention and Coronary Artery Bypass Grafting). 5. Survival status and follow-up duration within 1 year post-admission. To reduce potential bias, variables with missing values exceeding 10% were excluded from the analysis. For variables with missing data below the 10% threshold, multiple imputation techniques were utilized to maintain the maximum possible sample size.
The GPR was determined utilizing the following formula
Outcome
The principal outcome of the study was all-cause mortality occurring within one year following hospitalization, while the secondary outcome was all-cause mortality within a 180-day period post-hospitalization.
Statistical analysis
Continuous variables were reported as mean ± standard deviation (SD) or median with interquartile range (IQR), depending on the distribution of the data. Comparisons of continuous variables across groups were conducted using t-tests or analysis of variance (ANOVA) for data that followed a normal distribution, while the Mann–Whitney U test or Kruskal–Wallis test were utilized for data that did not conform to a normal distribution. Categorical variables were presented as counts (n) or percentages (%) and were compared using the Pearson chi-square test or Fisher’s exact test, as deemed appropriate.
The GPR index was categorized into four quartiles to assess the occurrence of outcome events over time in relation to varying levels of the GPR index. Kaplan–Meier survival analysis was utilized, with group differences evaluated through the log-rank test. To estimate hazard ratios (HRs) and their associated 95% confidence intervals (95% CIs), Cox proportional hazards regression models were applied, accounting for multiple confounding variables. Three distinct models were developed: Model 1 was unadjusted; Model 2 was adjusted for age, gender, BMI, race, tobacco use status, and the presence of hypertension, HF, stroke, ARF, CKD, diabetes, cardiogenic shock, as well as revascularization status; and Model 3 included additional adjustments for potassium, chloride, GLU, AG, WBC, RBC, PLT, Hb, RDW, HCT, PT, APTT, BUN, and Cr, based on Model 2. The predictive capability, sensitivity, and specificity of the GPR index for forecasting the primary outcome were evaluated through receiver-operating characteristic (ROC) curve analysis. A restricted cubic spline analysis was conducted to elucidate the dose–response relationship between the GPR index and the risk of the primary outcome. Furthermore, subgroup analyses were performed, stratified by age (< 65 years vs. ≥ 65 years), gender (male vs. female), body mass index (BMI < 24 kg/m2 vs. BMI ≥ 24 kg/m2), cardiogenic shock (absent vs. present) and diabetes history (absent vs. present). Interactions between the GPR index and the stratification variables were assessed using likelihood ratio tests. All statistical analyses were executed using R version 4.1.2 (R Foundation), with statistical significance established at a two-sided P-value of < 0.05.
Results
Baseline characteristics
After carefully reviewing the MIMIC-IV database, we found that there were a total of 5726 patients with acute myocardial infarction who were admitted to the ICU for the first time during their initial hospitalization. After applying the inclusion and exclusion criteria, 3811 patients were included in the study (Fig. 1). Table 1 presents the baseline characteristics of patients with acute myocardial infarction (AMI) categorized according to the quartiles of the GPR index, which are defined as follows: Quartile Q1 (0.20–1.40), Q2 (1.40–1.70), Q3 (1.70–2.25), and Q4 (2.25–40.57). The mean age of the participants was 77 years, with a male representation of 70.38%. It was observed that patients with a higher GPR index tended to be older, exhibited a lower percentage of males, and had a reduced proportion of Caucasians, alongside an increased representation of Black individuals and other racial groups. Furthermore, these patients demonstrated a higher prevalence of HF, stroke, ARF, CKD, diabetes andcardiogenic shock. The proportion of revascularization is relatively low. They also presented with lower levels of potassium, chloride, PT, and INR, while exhibiting elevated levels of GLU, anion gap, WBC, RBC, PLT, Hb, RDW, HCT, APTT, UA, and Cr, with all comparisons yielding p-values less than 0.05. The prevalence of hypertension was notably higher in the Q2 and Q3 groups, whereas it was lower in the Q1 and Q4 groups. Additionally, an increase in the GPR index was associated with a rise in the 1-year all-cause mortality rate, a trend that was similarly reflected in the 180-day all-cause mortality rate (all p < 0.05).
Primary outcomes
The Kaplan–Meier survival analysis curves depicting the primary outcomes across the quartile groups of the GPR index are illustrated in Fig. 2. Patients exhibiting elevated GPR index values demonstrated a markedly increased 1-year mortality rate in comparison to those with lower GPR index values (log-rank P < 0.001). A comparable pattern was noted during the 180-day follow-up period (log-rank P < 0.001).
Table 2 delineates three multivariate Cox proportional hazards models employed to evaluate the relationship between the GPR index and primary outcomes. For the purpose of sensitivity analysis, three distinct Cox proportional hazards models were developed. In the fully adjusted Model 3, a 10-unit increment in the GPR index was correlated with a 70% heightened risk of all-cause mortality at 1 year (HR: 1.70; 95% CI 1.34–2.15; P < 0.001) and an 71% increased risk of all-cause mortality at 180 days (HR: 1.71; 95% CI 1.36–2.17; P < 0.001). To further clarify the association between the GPR index and primary outcomes, the study stratified the GPR index into quartiles. In the unadjusted model [HR: 2.34; 95% CI 1.97–2.78; P < 0.001], partially adjusted model [HR: 1.52; 95% CI 1.27–1.82; P < 0.001], and fully adjusted model [HR: 1.70; 95% CI 1.40–2.07; P < 0.001], higher GPR index values (Q4 vs. Q1) were significantly associated with increased 1-year mortality, demonstrating a clear upward trend in mortality with increasing GPR index.Analysis of 180-day all-cause mortality using quartile stratification of the GPR index yielded similar results, where higher GPR index values (Q4 vs. Q1) were significantly associated with increased 180-day mortality: unadjusted model [HR: 2.50; 95% CI 2.07–3.02; P < 0.001], partially adjusted model [HR: 1.63; 95% CI 1.34–1.98; P < 0.001], and fully adjusted model [HR: 1.87; 95% CI 1.51–2.31; P < 0.001].Furthermore, restricted cubic spline regression modeling indicated a “J”-shaped nonlinear relationship between the GPR index and the risks of both 1-year all-cause mortality (all P for nonlinearity < 0.001) (Fig. 3). The ROC curve analysis suggested that the GPR index possesses moderate predictive capability for 1-year all-cause mortality (AUC = 0.77; 95% CI 0.75–0.79), with an optimal cut-off point of 1.891, specificity of 74%, and sensitivity of 69% (Fig. 4, Table 3).
(A) Results of one-year all-cause mortality from RCS analysis, (B) Results of 180-day all-cause mortality from RCS analysis. Results of 180-day all-cause mortality from restricted cubic spline (RCS) analysis. The horizontal dashed line represents a hazard ratio of 1.0. HR hazard ratio; CI confidence interval.
Subgroup analysis
In order to further elucidate the association between the GPR index and subsequent mortality, a series of subgroup analyses were performed, as depicted in Fig. 5. These analyses encompassed variables such as age, gender, BMI, diabetes status and cardiogenic shock status. The findings indicated that the relationship between the GPR index and the risk of 1-year all-cause mortality remained stable across most subgroups, with no significant interactions detected (P for interaction > 0.05). However, it is noteworthy that among patients with a BMI of 24 or greater, the predictive capacity of the GPR index was significantly enhanced [BMI < 24, HR (95% CI): 1.41 (0.90–2.20) vs. BMI ≥ 24, HR (95% CI): 2.56 (1.74–3.75), interaction P value = 0.022]. Comparable patterns were observed in the subgroup analysis concerning 180-day all-cause mortality.
(A) Subgroup analysis of the association between the GPR and 1-year all-cause mortality, (B) Subgroup analysis of the association between the GPR and 180-day all-cause mortality. Adjusted for age, sex, BMI, race, tobacco use, hypertension, HF, stroke, ARF, CKD, diabetes, cardiogenic shock, revascularization status, potassium, chloride, GLU, AG, WBC, red blood cell count, PLT, Hb, RDW, HCT, PT, APTT, BUN, and Cr, excluding the subgroup factors themselves.
Discussion
This research represents the inaugural investigation establishing a significant correlation between the GPR index and the 1-year mortality risk among critically ill patients with AMI. Notably, this association persists even after controlling for potential confounding variables. Consequently, the GPR index may function as a valuable indicator for risk stratification within this patient population and could be utilized as a tool for clinical decision-making.
Cardiovascular diseases have become a significant global health concern, especially in low-income nations1. AMI is marked by its abrupt onset, critical nature, and considerable associated morbidity and mortality. Notwithstanding the progress made in percutaneous coronary intervention (PCI), the mortality rate associated with AMI has been increasing at a concerning pace7. Timely identification of patients at high risk for adverse outcomes is essential for the implementation of personalized management strategies aimed at enhancing patient prognosis.
Blood glucose and blood potassium serve as readily accessible biological markers in clinical settings8. The heart predominantly utilizes glucose as its energy source, making stable glucose metabolism crucial for optimal cardiac function. Research indicates that fluctuations in blood glucose levels may enhance the expression of myocardial fibrosis markers, including type III collagen and α-smooth muscle actin21. A substantial body of literature consistently identifies blood glucose as a significant prognostic indicator in patients with cardiovascular disease22,23. For instance, the study conducted by Chen et al. demonstrates a correlation between blood glucose variability and the prognosis of critically ill patients suffering from cardiovascular conditions24. Elevated blood glucose levels can exacerbate cellular dysfunction, induce oxidative stress, activate intracellular inflammatory pathways, and trigger apoptosis25,26. In patients with ST-segment elevation myocardial infarction complicated by cardiogenic shock, hyperglycemia at admission is an independent predictor of in-hospital mortality, highlighting its role in the stratification of high-risk populations27. Similarly, in a group of acute coronary syndrome patients requiring intra-aortic balloon pump support, poor glycemic control is associated with prolonged ICU stays and an increased incidence of arrhythmias28. The Intermountain Risk Score validated by Mert İlker, H., et al. uses blood glucose as a key parameter for predicting the short- and long-term mortality of patients with cardiogenic shock29. Notably, even in the absence of diabetes, transient hyperglycemia during acute stress episodes in critically ill cardiovascular patients is associated with an increased risk of short-term mortality30. Following an AMI, the activation of the sympathetic nervous system results in heightened levels of cortisol and catecholamines, which contribute to stress-induced hyperglycemia20. This condition subsequently elevates free fatty acid concentrations, potentially exerting toxic effects on the infarcted myocardium31,32. Furthermore, blood glucose levels have been linked to postoperative wound infection rates following cardiac surgery, which may influence patient prognosis33.
Blood potassium is essential for sustaining the automaticity and excitability of myocardial cells. Abnormal blood potassium levels are widely acknowledged in the cardiovascular domain as factors that elevate the risk of sudden cardiac death34. Both hyperkalemia and hypokalemia can disrupt the resting membrane potential of myocardial cells, leading to an increased likelihood of sudden cardiac death. Stress-induced elevations in catecholamines can interfere with potassium transport via the sodium–potassium adenosine triphosphatase (Na + /K + -ATPase), resulting in hypokalemia. Research by Shiyovich et al. has established a relationship between blood potassium levels and in-hospital mortality among AMI patients35. Additionally, in individuals undergoing maintenance hemodialysis, greater variability in blood potassium levels is associated with an increased risk of cardiovascular mortality, even within normal physiological ranges36.
The relationship between potassium and glucose is intricate and multifaceted. ATP-sensitive potassium channels are integral to the regulation of glucose homeostasis, facilitating glucose uptake in skeletal muscle and enhancing counter-regulatory mechanisms against hypoglycemia through the increased secretion of hormones such as glucagon. However, the specific mechanisms by which the GPR index influences prognosis remain ambiguous13. An elevation in the GPR index may signify heightened sympathetic nerve activation, which could correlate with disease severity, thereby serving as a potential prognostic indicator for mortality risk. Recent studies have corroborated these findings. The GPR index has been the subject of extensive investigation within the field of neurology, where it has been established as an early prognostic marker for various conditions, including aneurysmal subarachnoid hemorrhage, acute intracerebral hemorrhage, severe traumatic brain injury, and ischemic stroke14,15,16,17. In cardiovascular research, a study conducted by Chen et al. demonstrated that a high GPR index is a significant predictor of in-hospital mortality following A-type aortic dissection surgery, thereby highlighting the association between the GPR index and postoperative outcomes in cardiovascular interventions18. Furthermore, a national multicenter study from Turkey confirmed the GPR index as a reliable predictor of in-hospital mortality risk within coronary care units, aligning with prior research. Additionally, a cohort study in the United States focusing on heart failure with preserved ejection fraction indicated that the GPR index possesses substantial predictive value, particularly among non-diabetic patients and those exhibiting milder heart failure symptoms37. The GPR index has also demonstrated prognostic utility in other medical conditions, including abdominal blunt trauma and carbon monoxide poisoning38,39.
The GPR index demonstrates a “J”-shaped relationship with the mortality risk among critically ill patients suffering from AMI. Both low and high values of the GPR index are associated with an elevated risk of mortality. A low GPR index may correlate with elevated serum potassium levels and diminished glucose levels. Elevated potassium levels can result in arrhythmias, reduced myocardial contractility, muscular weakness, cognitive impairment, acid–base disturbances, and renal injury40. Conversely, low glucose levels can adversely affect cerebral function, leading to neuronal damage and the potential for seizures. Additionally, hypoglycemia may provoke sympathetic nervous system activation, thereby increasing myocardial oxygen demand41. Further investigation is warranted to delineate the optimal range of the GPR index and its prognostic implications, with the aim of enhancing clinical outcomes.
In the subgroup analysis, a significant interaction was observed between BMI and the GPR index. Specifically, in the analysis of 1-year mortality risk, the prognostic impact of the GPR index was not significant for individuals with a BMI of less than 24. Conversely, for individuals with a BMI of 24 or greater, the GPR index had a significant prognostic impact, indicating a higher risk. Similar findings were noted in the 180-day subgroup analysis. These results suggest that elevated BMI amplifies the influence of changes in the GPR index on the prognosis of patients with AMI, indicating a potential synergistic effect. Increased BMI is frequently associated with metabolic disturbances, such as insulin resistance, which can disrupt glucose and lipid metabolism and impair vascular endothelial function42,43. In this context, metabolic signaling pathways related to glucose and potassium may be more prone to inducing cellular damage and dysfunction, thereby heightening the risk of adverse outcomes44,45. Furthermore, obesity is characterized by a chronic inflammatory state, with adipose tissue secreting various inflammatory mediators, including interleukin-646. This chronic inflammation can compromise the integrity of the vascular endothelium, and subsequent elevations in glucose and potassium levels may activate inflammatory pathways, exacerbating myocardial injury47. Additionally, obesity contributes to increased cardiac workload and myocardial hypertrophy48. An elevation in the GPR index can disrupt normal cardiovascular regulatory mechanisms, thereby increasing the likelihood of arrhythmias in the presence of myocardial hypertrophy. Therefore, in the treatment of obese AMI patients, weight management should be emphasized to improve prognosis risk.
This study is subject to several limitations. The data were sourced from a single database, which restricts the generalizability of the findings. Additionally, the retrospective design of the study complicates the establishment of causal relationships, thereby diminishing the robustness of the conclusions drawn. Furthermore, potential confounding variables that were not measured, such as serum hormone levels of catecholamines and cortisol, as well as echocardiographic data, cannot be excluded. It is advisable to undertake further multi-center, prospective studies to validate these findings. Moreover, this study concentrated on the GPR index recorded at the initial admission, neglecting the dynamic fluctuations of the GPR index over time.
Conclusion
In conclusion, the GPR index demonstrates a significant association with unfavorable outcomes in critically ill patients suffering from AMI, exhibiting a “J”-shaped nonlinear relationship. Consequently, the GPR index holds promise as a swift predictive marker for this patient population.
Data availability
Access to the data needs to be applied for through the official channels of the MIMIC-IV database. Once the access permission is granted, the relevant data subset used in this study can be obtained from the corresponding author. The corresponding author will ensure that data sharing complies with all ethical and legal requirements.
References
Virani, S. S. et al. Heart disease and stroke statistics-2020 update: A report from the American heart association. Circulation 141(9), e139–e596 (2020).
Mercado-Lubo, R., Yarzebski, J., Lessard, D., Gore, J. & Goldberg, R. J. Changing trends in the landscape of patients hospitalized with acute myocardial infarction (2001 to 2011) (from the Worcester Heart Attack Study). Am. J. Cardiol. 125(5), 673–677 (2020).
Aissaoui, N. et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur. J. Heart Fail. 22(4), 664–672 (2020).
Hunziker, L. et al. Twenty-year trends in the incidence and outcome of cardiogenic shock in AMIS plus registry. Circ. Cardiovasc. Interv. 12(4), e007293 (2019).
Janse, M. J. & Wit, A. L. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol. Rev. 69(4), 1049–1169 (1989).
Ibanez, B. et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 39(2), 119–177 (2018).
Moroni, F., Gurm, H. S., Gertz, Z., Abbate, A. & Azzalini, L. In-hospital death among patients undergoing percutaneous Coronary intervention: A root-cause analysis. Cardiovasc. Revasc. Med. Incl. Mol. Interv. 40S, 8–13 (2022).
Lopaschuk, G. D., Karwi, Q. G., Tian, R., Wende, A. R. & Abel, E. D. Cardiac energy metabolism in heart failure. Circ. Res. 128(10), 1487–1513 (2021).
Delanaye, P. et al. A simple modification of dialysate potassium: Its impact on plasma potassium concentrations and the electrocardiogram. Clin. Kidney J. 14(1), 390–397 (2019).
van Melle, J. P. et al. Diabetes, glycemic control, and new-onset heart failure in patients with stable coronary artery disease: Data from the heart and soul study. Diabetes Care 33(9), 2084–2089 (2010).
Keskin, M. et al. The effect of serum potassium level on in-hospital and long-term mortality in ST elevation myocardial infarction. Int. J. Cardiol. 221, 505–510 (2016).
Tekkesin, A. I. et al. The use of monocyte to HDL ratio to predict postoperative atrial fibrillation after aortocoronary bypass graft surgery. North. Clin. Istanb. 4(2), 145–150 (2017).
McTaggart, J. S., Clark, R. H. & Ashcroft, F. M. The role of the KATP channel in glucose homeostasis in health and disease: More than meets the islet. J. Physiol. 588(Pt 17), 3201–3209 (2010).
Fujiki, Y. et al. Serum glucose/potassium ratio as a clinical risk factor for aneurysmal subarachnoid hemorrhage. J. Neurosurg. 129(4), 870–875 (2018).
Wu, X. Y. et al. Serum glucose and potassium ratio as a predictive factor for prognosis of acute intracerebral hemorrhage. J. Int. Med. Res. 49(4), 3000605211009689 (2021).
Zhou, H., Tang, Y., Li, Y. & Zhu, T. Serum glucose-potassium ratio predicts prognosis of traumatic brain injury in patients undergoing emergency craniotomy: A retrospective study. Asian J. Surg. 46(7), 2958–2959 (2023).
Yan, X. et al. Relationship between serum glucose-potassium ratio and 90-day outcomes of patients with acute ischemic stroke. Heliyon 10(17), e36911 (2024).
Demir, F. A. et al. Serum glucose-potassium ratio predicts inhospital mortality in patients admitted to coronary care unit. Rev. Assoc. Med. Bras. 70(10), e20240508 (2024).
Chen, Y. et al. The blood glucose-potassium ratio at admission predicts in-hospital mortality in patients with acute type A aortic dissection. Sci. Rep. 13(1), 15707 (2023).
Dungan, K. M., Braithwaite, S. S. & Preiser, J. C. Stress hyperglycaemia. Lancet 373(9677), 1798–1807 (2009).
Liu, C. et al. Hyperglycemia aggravates atrial interstitial fibrosis, ionic remodeling and vulnerability to atrial fibrillation in diabetic rabbits. Anadolu kardiyoloji dergisi: AKD = Anatol. J. Cardiol. 12(7), 543–550 (2012).
Danaei, G. et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 378(9785), 31–40 (2011).
Hermanides, J. et al. Hypoglycemia is associated with intensive care unit mortality. Crit. Care Med. 38(6), 1430–1434 (2010).
Chen, Y. et al. Prognostic value of glycaemic variability for mortality in critically ill atrial fibrillation patients and mortality prediction model using machine learning. Cardiovasc. Diabetol. 23(1), 426 (2024).
Uijtendaal, E. V. et al. Influence of a strict glucose protocol on serum potassium and glucose concentrations and their association with mortality in intensive care patients. Crit. Care 19(1), 270 (2015).
Xie, L. et al. Mitochondrial DNA oxidative damage triggering mitochondrial dysfunction and apoptosis in high glucose-induced HRECs. Invest. Ophthalmol. Vis. Sci. 49(9), 4203–4209 (2008).
Hayıroğlu, M. İ et al. Predictors of in-hospital mortality in patients with ST-segment elevation myocardial infarction complicated with cardiogenic shock. Heart Lung Circ. 28(2), 237–244 (2019).
Hayıroğlu, M. İ et al. Clinical characteristics and outcomes of acute coronary syndrome patients with intra-aortic balloon pump inserted in intensive cardiac care unit of a tertiary clinic. Turk Kardiyoloji Dernegi arsivi: Turk Kardiyoloji Derneginin yayin organidir 46(1), 10–17 (2018).
Mert İlker, H., Faysal, S., Ahmet Çağdaş, Y., Murat, S. & Tufan, Ç. Prognostic value of intermountain risk score for short- and long-term mortality in patients with cardiogenic shock. Coron. Artery Dis. 34(2), 154–159 (2023).
Zhang, L. et al. Effects of stress hyperglycemia on short-term prognosis of patients without diabetes mellitus in coronary care unit. Front. Cardiovasc. Med. 8, 683932 (2021).
Black, C. T., Hennessey, P. J. & Andrassy, R. J. Short-term hyperglycemia depresses immunity through nonenzymatic glycosylation of circulating immunoglobulin. J. Trauma 30(7), 830–833 (1990).
Ding, X. S., Wu, S. S., Chen, H., Zhao, X. Q. & Li, H. W. High admission glucose levels predict worse short-term clinical outcome in non-diabetic patients with acute myocardial infraction: A retrospective observational study. BMC Cardiovasc. Disord. 19(1), 163 (2019).
Zerr, K. J. et al. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann. Thorac. Surg. 63(2), 356–361 (1997).
Aldahl, M. et al. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur. Heart J. 38(38), 2890–2896 (2017).
Shiyovich, A., Gilutz, H. & Plakht, Y. Potassium fluctuations are associated with inhospital mortality from acute myocardial infarction. Soroka acute myocardial infarction II (SAMI-II) project. Angiology 69(8), 709–717 (2018).
Men, R. et al. Associations between serum potassium variability and mortality in patients undergoing maintenance hemodialysis: A retrospective study. Sci. Rep. 14(1), 29998 (2024).
Shan, L., Zheng, K., Dai, W., Hao, P. & Wang, Y. J-shaped association between serum glucose potassium ratio and prognosis in heart failure with preserved ejection fraction with stronger predictive value in non-diabetic patients. Sci. Rep. 14(1), 29965 (2024).
Katipoğlu, B. & Demirtaş, E. Assessment of serum glucose potassium ratio as a predictor for morbidity and mortality of blunt abdominal trauma. Künt Abdominal Travma Hastalarında Serum Glikoz Potasyum Oranının Morbidite Ve Mortalite İçin Bir Öngörücü Olarak Değerlendirilmesi. Ulusal travma ve acil cerrahi dergisi = Turk. J. Trauma Emer. Surg. TJTES 28(2), 134–139 (2022).
Demirtaş, E., Korkmaz, İ, Tekin, Y. K., Demirtaş, E. & Çaltekin, İ. Assessment of serum glucose/potassium ratio as a predictor for delayed neuropsychiatric syndrome of carbon monoxide poisoning. Hum. Exp. Toxicol. 40(2), 207–213 (2021).
Gettes, L. S. Electrolyte abnormalities underlying lethal and ventricular arrhythmias. Circulation 85(1 Suppl), I70–I76 (1992).
Amiel, S. A. The consequences of hypoglycaemia. Diabetologia 64(5), 963–970 (2021).
Ahmed, B., Sultana, R. & Greene, M. W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 137, 111315 (2021).
Kelley, D. E. & Goodpaster, B. H. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 24(5), 933–941 (2001).
Sousa, L. G. O. et al. The effects of diet composition and chronic obesity on muscle growth and function. J. Appl. Physiol. 130(1), 124–138 (2021).
Zupo, R. et al. Uric acid and potassium serum levels are independent predictors of blood pressure non-dipping in overweight or obese subjects. Nutrients 11(12), 2970 (2019).
Kawai, T., Autieri, M. V. & Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 320(3), C375–C391 (2021).
Poirier, P. et al. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation 113(6), 898–918 (2006).
Bartkowiak, J. et al. The impact of obesity on left ventricular hypertrophy and diastolic dysfunction in children and adolescents. Sci. Rep. 11(1), 13022 (2021).
Acknowledgements
This study utilized the data from MIMIC-IV. Therefore, we would like to express our sincere gratitude to all the staff of the MIMIC-IV database for their hard work.
Funding
This work was supported by the Key Research and Development Program of Shaanxi Province (S2024-YF-YBSF-0667) and the National Natural Science Foundation of China (No. 82360608).
Author information
Authors and Affiliations
Contributions
W.P., T.F.J., and J.H.X. were involved in the acquisition, analysis, and interpretation of data. W.P., J.Y., and L.L. were responsible for the preparation of the manuscript. T.F.J. and B.T.H. contributed to the critical revision of the manuscript. J.H.X. provided supervision for the research. All authors have approved the final manuscript and have engaged sufficiently in the work to assume public responsibility for appropriate sections of the content. Furthermore, all authors consent to the submission and publication of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pan, W., Ji, Tf., Yang, J. et al. The ratio of serum glucose to potassium is associated with poor prognosis in patients with severe acute myocardial infarction. Sci Rep 15, 28846 (2025). https://doi.org/10.1038/s41598-025-14002-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-14002-2