Abstract
Large-extent movements (LEMs) are vital for daily activities, and their recovery remains a challenge in neurorehabilitation due to inevitable loading on muscles. Since inhibitory cTBS may facilitate recovery but also disrupt task control, we ask whether a previously identified linear relationship between load and cortical activation would retain, after cTBS over the contralateral supplementary motor area (SMA). It will set a benchmark for choosing resistance during brain stimulation. Hemodynamic activities of SMA and M1 during LEMs were monitored by functional Near-Infrared Spectroscopy (fNIRS) in this study. Twelve healthy participants received 40s of cTBS over SMA, before and after which the participants were instructed to complete LEMs with varying loads (0, 3, and 15 pounds) repetitively. When lifting 0-pound and 3-pound weights, the β of the left SMA and M1 increased significantly after cTBS (p < 0.05). Also, the random intercepts of individual subjects increased significantly in the left SMA, left M1, and right SMA, but the slopes were not significantly affected by cTBS. Data also showed a significant positive correlation between the intercept of left M1, right SMA&M1 areas and age (all p < 0.05). Despite cTBS-induced inhibition, the supplementary motor area (SMA) exhibited enhanced fNIRS responses to increased loads, and the positive correlation between load inertia and motor-cortical activation remained unaltered.
Similar content being viewed by others
Introduction
Activities of daily living (ADL) typically involve rapid, long-range limb movements against effort-demanding loads1i.e. large-extent movements (LEMs)2. Impaired LEMs present a major challenge in neurorehabilitation3as they generally require substantial participant effort during therapeutic exercises4. Consequently, approximately 50–60% of stroke survivors still experience some degree of gross motor impairment after standard rehabilitation5. Substantial evidence indicates that improving motor dysfunction requires patients to exert significant effort, which can be achieved through increased load/resistance6, speed7 and accuracy8. Moreover, perceived effort may be modulated by continuous theta-burst transcranial magnetic stimulation (cTBS) over the supplementary motor area (SMA)9suggesting potential effects of cTBS on the progress of motor recovery. Although indirect evidence from transcranial magnetic stimulation has demonstrated efficacy in enhancing motor function10evidence from cTBS remains limited whether/how cTBS modulates motor-related cortices during large-extent movements that are meaningful in daily exercises. Incorporating external loading during arm-lifting movements proportionally enhances activity in motor-related regions11. However, it remains unclear whether combining loaded exercise with inhibitory brain stimulation retains12 or disrupts13. Clinically, combining exercise and neuromodulation shows potential for neurological recovery14. Therefore, understanding the cortical responses to loading provides a unique opportunity for optimized neurorehabilitation.
One factor affecting LEMs performance is resistance, which increases movement difficulty and trial-to-trial variability15. Higher resistance enhances motor cortex activation11,16potentially aiding post-stroke recovery17. LEMs performance is also sensitive to temporal kinematic requirements (e.g., overall speed18,19peak velocity20tempo21. A common pattern is stronger hemodynamic responses during faster task completion22. Slow movements tend to evoke greater frontal activation related to cognitive control, while faster movements trigger stronger responses in motor cortices23,24. Thus, controlling elements affecting LEMs is crucial for understanding how brain stimulation influences cortical responses.
Non-invasive brain stimulation benefits post-stroke recovery, potentially via motor cortex inhibition. For example, repetitive 1 Hz TMS and cTBS can decrease cortical excitability25enhancing motor-evoked potentials (MEP)26 and upper limb function27. cTBS over SMA affects effort perception (i.e., participants’ subjective rating of exertion during tasks)9 but not behavior12. Most TMS studies focus on M128due to its role in fine movements29; however, cortical responses following SMA stimulation are rarely reported. For LEMs, SMA hemodynamic response may be more relevant30,31. Direct evidence of cTBS effects over SMA is limited, but inhibitory TMS protocols can produce enhanced32null33or suppressed34 hemodynamic responses. Notably, neuroimaging studies increasingly report paradoxical hemodynamic enhancement following inhibitory stimulation35. This phenomenon may reflect increased cerebral blood flow (CBF)32,36supported by fNIRS measurements showing elevated oxy-hemoglobin (oxy-Hb) concentrations. Given this debate, we sought to investigate whether the addition of cTBS to LEMs would further modulate cortical blood flow responses.
This study focuses on upper-limb LEMs to explore how cTBS inhibition of motor regions (SMA and M1) affects large-movement control. Both cTBS and fNIRS were applied to the SMA guided by a Transcranial Brain Atlas (TBA)-based navigation system37. We hypothesized that cortical inhibition would enhance neural responses to task loading, despite that the effect of cTBS was commonly found to be inhibitory. The clinical relevance of this study was to inform parameter settings for resistance training combined with cTBS to recover meaningful daily movements.
Methods
Participants
Twelve healthy subjects participated in this study (5 males, 7 females; all right-handed, according to the Edinburgh handedness inventory38. None of the participants had any history of neurological or musculoskeletal disorders. Before the beginning of the experiment, the subjects were explained the study content and signed an informed consent form. The study was approved by the Ethics Committee of Shanghai Jiao Tong University Ruijin Hospital (NO.209 of 2020), all methods were performed in accordance with the Declaration of Helsinki.
The inclusion criteria for participants were as follows:
-
1.
No history and chief complaint of any neurological disorders and dyskinesia;
-
2.
Right-handed, as assessed by the Edinburgh Handedness Inventory (EHI);
-
3.
Above 18 years old;
-
4.
No abnormalities detected in head MRI scans;
-
5.
Well cognitive and able to fully complete LEMs.
The exclusion criteria for this study were:
-
1.
Presence of severe neurological diseases, mental disorders, severe somatic diseases, or history of craniocerebral trauma;
-
2.
Difficulties in communication;
-
3.
Women who were pregnant or lactating;
-
4.
Inability to complete the experimental protocol due to other reasons.
Experimental design
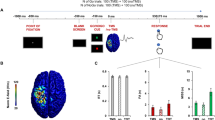
The experiment was divided into 4 steps. First, the subjects completed a session of fNIRS measurement during weight-adjusted LEMs (Fig. 1A). Second, the scalp localization of the left SMA was performed on each subject and marked with a black pen (Fig. 1B). Third, cTBS was applied to the marked target position (Fig. 1C). Fourth, another session of fNIRS measurement (identical to the 1 st step) was conducted 40 min (10 min of SMA brain localization, 15 min of rest and 15 min of cTBS and transfer) after cTBS to measure the altered effect of cTBS on brain excitability (Fig. 1D). Prior to the formal experiment, we recorded MEPs in with a volunteer to verify the inhibitory effect of cTBS on cortical excitability. Specific details are provided in the Supplementary Materials.
Apparatus
In this study, the cTBS procedures were performed via a CCY-I magnetic stimulator (Wuhan Yiruide Medical Equipment New Technology Co., Ltd., Wuhan, China). Brain-hemodynamic signals were acquired using a multichannel fNIRS device (model ETG-4100, HITACHI Inc., Tokyo, Japan). A TBA-based navigation system (DPen) with a 3D magnetic digitizer (Polhemus Patriot, Polhemus Inc., Colchester, VT, USA) was used for guidance of fNIRS probe placement, acquisition of MNI coordinates for each fNIRS channel, and localization of the stimulating site for cTBS37.
Movement task and hemodynamic signal acquisition using fNIRS
Participants sat in chairs without armrests to ensure their arm movement was unrestricted. They lifted and lowered their right hand at 1 Hz, guided by a metronome, lifting from a resting position to maximum height along the midaxillary line (Fig. 2A). All practiced the task beforehand. The exercise was conducted under three load conditions: 0-pound (bare hand), 3-pounds (1.36 kg), and 15-pounds (6.80 kg). The 3-pound condition was chosen because it was the entry-level weight for strength training39; the 15-pound condition was chosen in reference to previous studies40and also because this was the maximum weight that allowed pilot subjects to finish the protocol without excessive fatigue.
Brain-hemodynamic signals were acquired using ETG-4100 at a 10-hertz sampling rate. The device evaluated the absorption of near-infrared light at two wavelengths (695 nm and 830 nm) and computed the corresponding oxy-Hb and deoxy-hemoglobin (deoxy-Hb) density following the modified Beer–Lambert Law41. We used a high-density 3 × 10 probe with 16 sources and 14 detectors, a total of 44 channels to place on the head. We selected the Supp_Motor_Area_L&R Precentral_L&R labeled by anatomical automatic labeling (AAL) as the imaging ROI. The TBA-based navigation system (DPen) was used to guide the placement of the fNIRS probe array37. The configuration of the probe is shown in Fig. 2B.
A block design containing large-extent movements was used during fNIRS data collection. The session of data collection was divided into 12 blocks, and the order of blocks was randomized. Subjects were requested to perform corresponding tasks (0-pound, 3-pounds, and 15-pounds) in each block and repeat them 12–14 times for about 25 s. A 30-second rest was given after the end of each block (Fig. 2C). The entire experimental process involved LEMs before and after cTBS. fNIRS detected changes in the functional status of the motor brain areas of subjects during the LEMs paradigm (a flowchart of the experimental procedure is provided in the Supplementary Materials).
Experimental design of fNIRS test. (A) Illustration of LEMs including three different tasks. (B) The high-density fNIRS probe. (C) Block design of three tasks under fNIRS, 0/3/15 represents grasping 0/3/15 pounds of three different loads for LEMs, with 30 s rest after each task (0 lb: green; 3 lbs: pink; 15 lbs: blue).
Localization of stimulating site and procedure of cTBS
According to the TBA of left supplementary motor area labeled by AAL, the scalp point with the highest probability of being labeled SMA would be (0.44, 0.48) in CPC scalp space (Fig. 3). The TBA-based system (DPen) guided the application of cTBS on this point, with a figure-eight-shaped coil (70 mm) tightly placed tangentially to the scalp, figure-eight coil was placed tangentially over the left SMA with the handle oriented posteriorly at a 45° angle to the scalp42. The participants were instructed to relax and remain silent during stimulation. The cTBS consisted of 600 pulses in total, which included 3 bursts of three pulses at 50 Hz that being repeated every 0.2s (i.e., 5 Hz)43. Stimulation intensity was set at 70% of resting motor threshold (RMT), and the RMT was the minimum stimulator output recording the motor evoked potentials higher than 50 µV at the resting first dorsal interbone muscle of the right hand for at least 5 out of 10 trials44.
The probability heat map of left SMA localization according to TBA. The CPC point with the highest SMA probability is marked. (A) CPC space for planar visualization; Figure (B) CPC space on an example real scalp displays the orientation of the skull. (Nz: nasion; Iz: inion; AL: left preauricular point; AR: right preauricular point.).
Post-hoc cortical localization
To verify the positioning of ROI, we used the TBA-based system (DPen) to obtain the MNI coordinates of each channel, as well as the corresponding AAL label with probability. Data from a representative subject are listed in Table 1.
Data processing
We collected data on oxy-Hb and deoxy-Hb. In the context of neurovascular coupling, the directions of changes in oxy-Hb always align with changes in CBF, whereas the direction of changes in deoxy-Hb is determined by alterations in venous blood oxygenation and volume. Although oxy-Hb is the most sensitive indicator of CBF changes in fNIRS measurements45 we still compared oxy-Hb and deoxy-Hb before conducting further statistical analysis. The purpose of this comparison was to control for these confounding factors and ensure that the observed changes in oxy-Hb reflect cortical activity rather than systemic effects. Subsequently, we focused on the oxy-Hb data processed by the imaging system and analyzed the data46.
So The NIRS-KIT toolbox (version 2.0)47 in MATLAB R2013b (The MathWorks Inc., Natick, MA, USA) was used for brain hemodynamic signal processing. The pre-processing of oxy-Hb and deoxy-Hb consisted of 3 steps: (1) the raw oxy-Hb and deoxy-Hb signals were detrended using the linear-detrending method; (2) motion artifacts were removed using temporal derivative distribution repair (TDDR); (3) the oxy-Hb and deoxy-Hb signals were band-pass filtered with cut-off frequencies at 0.01 Hz and 0.08 Hz (third-order Butterworth) to remove physiological artifacts (such as heartbeats, breath, and Mayer wave) and high-frequency noise.
The general linear model (GLM) approach48 was used to characterize the hemodynamic response of oxy-Hb and deoxy-Hb in all 44 channels under 3 conditions.
Beta coefficient (β), of which the sign and magnitude indicate the direction (positive/negative) and intensity of change in oxy-Hb or deoxy-Hb, was generated for each subject, channel, and condition. To distinguish the β corresponding to oxy-Hb and deoxy-Hb, they were referred to as β-oxy and β-dxy, respectively. β was referred to interchangeably with cortical hemodynamic response in this manuscript unless otherwise specified. The functional connectivity processing included a total of 12 functional connections including LM1-LSMA, LM1-RM1, LM1-RSMA, LSMA-RSMA, LSMA-RM1, RSMA-RM1 before and after cTBS.
Statistical analysis
Correlation and difference statistical analyses for β-oxy and β-dxy were first conducted using SPSS 28.0. β values for each region of interest (ROI) underwent Shapiro-Wilk tests to confirm normality prior to analyzing differences. Normally distributed data were reported as mean ± standard deviation (mean ± SD) and analyzed using Pearson’s correlation test and T-test. Non-normally distributed measurements were represented by the interquartile range (IQR) as [M (Q1, Q3)] and analyzed using Spearman’s correlation test and Mann-Whitney U tests. The β-oxy and β-dxy before and after cTBS under LEMs was tested by paired T test. Cortical changes in hemodynamic response in left and right SMA and M1 brain areas before and after cTBS were mainly observed. To account for the risks of Type I and II errors due to multiple comparisons, we controlled the False Discovery Rate (FDR) using the Benjamini-Hochberg procedure49 and q < 0.05 was considered statistically significant.
Effects of weight, time (before and after cTBS) and interaction of weight and time on β (Primarily β-oxy) was analyzed using mixed-effect linear models, as follows:
β ~ weight * time + (1 | subject).
where weight represents the mass of the dumbbell and the time denotes the β at which a large degree of locomotion is stimulated before and after cTBS. The significance level in the analyses was set at 0.05. These statistical analyses were performed using R (version 4.2.1). The models were fitted using the “lmer” package, the significances were calculated using the “lmerTest” package and the conditional r-squares were calculated using the “MuMIn” package50. Conditional r-square is the proportion of total variance explained through both fixed and random effects.
At the individual level, based on the beta of brain areas in LEMs with varying resistances for each subject, the intercept and slope for each subject are calculated using the least squares method. This study compared the intercept and slope before and after cTBS and analyzed the correlation between them and basic indexes including age, height, weight, BMI, RMT threshold of baseline MEP, their recent daily average step count and functional connectivity between the ROI.
Results
Changes in the hemodynamic response between before and after cTBS
Before conducting a more detailed analysis of brain area hemodynamic response, we randomly selected CH23 channel from one of the subjects and plotted the corresponding oxy-Hb and deoxy-Hb changes in CH23 channel based on the subject’s process of performing LEMs paradigm (Fig. 4). The results showed that the change in oxyhemoglobin increased after cTBS stimulation, indicating an increase in the height of the blood flow response curve. Furthermore, we can observe that the curve changes of deoxy-Hb, represented by blue, mostly trend in the opposite direction compared to those of oxy-Hb.
Correlation and differential results between oxy-Hb and deoxy-Hb
Within each ROI, no significant correlation was found between β-oxy and β-dxy under any condition; also oxy-Hb exceeded deoxy-Hb both before and after cTBS (Table 2, significant differences in bilateral M1 under 15-lbs condition before cTBS; in left M1 under all conditions, right SMA and M1 under 15-lbs condition). These analyses were performed to rule out abnormal neurovascular uncoupling during LEMs before and after cTBS51.
ROI hemodynamic response analysis between before and after cTBS
According to the registration records, the channels and 3D locations in the ROI were marked in Fig. 5A, The 3D hemodynamic response (oxy) of the motor brain area before and after cTBS under different conditions is illustrated in Fig. 5B. The spatial registrations of all the channels for a representative subject are listed in Table 1. Based on the probability of channel registration, the channels with the highest probability of being located in the left and right SMA and M1 were included in the predetermined ROI range, and the β values were calculated based on a weighted average within the ROI. Therefore, the weight-adjusted hemodynamic signal for each ROI was:
where βoxy(dxy)ROI represented the β-oxy and β-dxy of each ROI, n represented the number of matching channels for different subjects on ROI, \(\:{\upbeta\:}oxy\left(\text{d}\text{x}\text{y}\right)i\) and \(\:\text{P}i\) represented the β-oxy and β-dxy and probability of channel i out of n.
(A) Three-dimensional location of each channel. We used NFRI fNIRS tools incorporated in NIRS-SPM to calculate the spatial registration of each channel from real space to MNI (Montreal Neurological Institute templates) coordinates and plotted them on the standard brain. We considered channels 19, 20, 21, 28, and 29 (red box) as the area of right M1, channels 24, 25, 26, 34, and 35 (red box) as the area of left M1 and channels 31,40 (blue box) as the area of right SMA, 32,41 (blue box) as the area of left SMA based on the probabilistic registration. (B) The 3D hemodynamic response maps of the motor brain areas before and after cTBS under different conditions are presented. From left to right, the conditions include the 0-pound, 3-pounds, and 15-pounds tasks. The upper row represents the pre-cTBS state, while the lower row represents the post-cTBS state.
The results regarding the β-oxy are as follows, in the 0-pound condition, the β-oxy of left SMA and M1 after cTBS was significantly higher than that before cTBS (t=−3.015, p = 0.012, q = 0.048; t=−3.464, p = 0.005, q = 0.036) (Fig. 6A and D), their effect size reached 0.870 and 0.999, respectively. In the 3-pounds condition, the β-oxy of left M1 after cTBS was significantly higher than that before cTBS (t=−3.439, p = 0.006, q = 0.036) (Fig. 6E), effect size reached 0.993. β-oxy showed no significant differences before versus after cTBS in other conditions (all q > 0.05). While for the β-dxy, the results showed no significant difference in the β-dxy for all conditions (all q > 0.05) before and after cTBS (Fig. 6). The detailed results are listed in the Supplementary Materials.
Comparison of ROI’ β-oxy and β-dxy before and after cTBS under different LEMs, the part shown in the picture shows the difference in the T-test of paired samples on the left SMA and M1. (A) - (C) Comparison of β-oxy in the left SMA before and after cTBS under the LEMs paradigm of 0 pound, 3 pounds, and 15 pounds, respectively. (D) - (F) Comparison of β-oxy in the left M1 before and after cTBS under the LEM paradigms of 0 pound, 3 pounds, and 15 pounds, respectively. In the 0-pound condition, the β-oxy of left SMA and M1 had a significant difference before and after cTBS. In the 3-pounds condition, the β-oxy of left SMA and M1 had a significant difference between before and after cTBS. (A1) - (F1) Comparison of β-dxy in the left SMA and M1 before and after cTBS under the LEMs paradigm of 0 pound, 3 pounds, and 15 pounds, respectively. *q-values < 0.05.
Correlation analysis of the impact on βs
We ran a linear model in each ROI to examine the impact of load magnitudes, time and interaction of weight and time on the cortical hemodynamic response. As shown in Fig. 7, the regression lines of all subjects were displayed to show whole trends. First, we discovered positive correlations between the load magnitudes and the β in four ROI areas. Specifically, before cTBS, the β increased by 0.003689/pounds (conditional r2 = 0.5283, p < 0.001) in left SMA, 0.003181/pounds (conditional r2 = 0.7530, p < 0.001) in left M1, 0.002682/pounds (conditional r2 = 0.6177, p < 0.001) in right SMA and 0.0034608/pounds (conditional r2 = 0.6372, p < 0.001) in right M1. Second, time effected the β of the left SMA, left M1 and right SMA, i.e., cTBS effected the β (p < 0.05). However, we did not discover correlations between the time and the β in the right M1. Third, the β was not affected by interaction of weight and time.
Correlation analysis of the impact on individual intercept and slope
Differences in intercepts and slopes before and after cTBS were computed and the results showed that in the left SMA, left M1 brain areas (t = 2.847, p = 0.016, q = 0.032; t = 3.991, p = 0.002, q = 0.008), cTBS significantly altered the intercepts and increased them significantly after cTBS. However, under other conditions, the intercepts and slopes were not significantly affected by cTBS (all q > 0.05) (Fig. 8). Relationship between individual slopes and loads are plotted in Supplementary Materials.
Comparison of random intercepts and slopes before and after cTBS. (A) - (D) The intercept values of four brain areas, including the left SMA, left M1, right SMA, and right M1 before and after cTBS. (E) - (I) The slope values of four brain areas, including the left SMA, left M1, right SMA, and right M1 before and after cTBS. (A) and (B) show a significant increase in the intercepts of the left SMA and left M1 after cTBS. * q-values < 0.05.
We explored the effects of age, height, weight, BMI, baseline RMT threshold of MEP, and recent daily average step count on the intercept and slope of left SMA and M1. The results showed that age was positively correlated with the intercept, and age significantly affected the intercept in the left M1, right SMA and right M1 area. Other indicators did not significantly affect the intercept and slope (Fig. 9).
Linear mixed effect model of intercept and basic indicators of subjects. (A) Linear mixed effect model of intercept and age in the left SMA brain area. (B) Linear mixed effect model of intercept and age in the left M1 brain area. (C) Linear mixed effect model of intercept and age in the right SMA brain area. (D) Linear mixed effect model of intercept and age in the right M1 brain area.
We explored the effects of brain functional connectivity between ROI in LEMs on the intercept and slope. Brain functional connectivity between ROI include left SMA and left M1, left SMA and right SMA, left M1 and right M1. In terms of the correlation of the intercept, there was a correlation between the functional connectivity of LSMA and RSMA after cTBS and the intercept of LSMA (r=−0.585, p < 0.05,) (Fig. 10). In terms of the correlation of the slope, there was a significant correlation between the functional connectivity of LSMA and LM1 before cTBS (r = 0.593, p < 0.05) (Fig. 10). There was no significant correlation between other functional connectivity and intercept or slope (all p > 0.05).
Correlation of the intercept or slope in the left SMA and ROI functional connectivity. (A)-(B) Correlation of the intercept in the left SMA and functional connectivity of LSMA-RSMA/LSMA-LM1. (C)-(D) Correlation of the slope in the left SMA and functional connectivity of LSMA-RSMA/LSMA-LM1 FC: functional connectivity.
Discussion
In this study, cTBS was applied over SMA, and motor-related cortical hemodynamic response was assessed during large-extent arm-lifting movement. Assessments were conducted both pre- and post-cTBS in 12 healthy subjects. Results showed that cTBS over SMA led to enhanced fNIRS hemodynamic response (i.e. larger intercept in “Beta ~ Load”) in SMA and M1 for lighter loads (0–3 pounds). This increase may stem from enhanced functional connectivity, notably between left/right SMA and left SMA/M1. Besides, cTBS did not seem to affect the positive correlation between load inertia and cortical hemodynamic response (SMA and M1). We also observed a positive correlation between age and increased cortical hemodynamic response before and after cTBS.
In this study, oxy-Hb and deoxy-Hb were compared beforehand to eliminate external influences, despite the common practice in most fNIRS studies to only report the results of oxy-Hb. A correlation and difference analysis between β-oxy and β-dxy was conducted in this study. The results indicated no significant correlation under any of the conditions. As can be seen in Fig. 4, oxy-Hb often changes with a reversed trend as does deoxy-Hb, which was supported by our tests (Table 2) and compatible with previous findings51. On the contrary, if oxy-Hb correlates with deoxy-Hb, it would indicate other influences on fNIRS signals, e.g. activation from head muscles52 or neurological disorders53.
Several studies were relevant for the interpretation of our findings. In a TMS experiment when healthy participants received 1 Hz inhibitory rTMS over the ipsilateral M154, activity in M1 increased in the contralateral side; these results agreed with ours in that inhibitory TMS might enhance cortical hemodynamic response, but our stimulation was on the contralateral side rather than ipsilateral. Another study reported increased oxy-Hb in the ipsilateral M1 after contralateral TMS (inhibitory)55 - a finding further substantiated by recent systematic reviews with meta-analyses56which were possibly explained by the theory of interhemispheric inhibition57 but this theory is insufficient to explain our data. Overall, there is a possibility that increased hemodynamic activity did not necessarily reflect higher/lower cortical excitability58and increased cerebral blood flow59 might enhance the cortical hemodynamic response after cTBS. In addition, greater hemodynamic changes suggest increased vasomotor reactivity after cTBS60.
It is of particular interest that the slope of “Beta ~ Load”(i.e. the increase in hemodynamic response against per unit increase in load) did not significantly alter after cTBS, albeit the intercept showed a significant increase. Note that all participants accomplished the tasks since cTBS unlikely disrupted motor performance12but the same task ought to require more effort after inhibitory cTBS. If so, a logical corollary is that the slope of “Beta ~ Load” likely associated with task accomplishment, and the intercept might reflect perception of effort. This is a conjecture but not groundless, since perceived level of effort was changed after SMA inhibition9,61.
The SMA has dense, direct connections to M162, which is crucial for the execution of voluntary movements63. Previous studies have indicated that SMA is able to raise the MEP in M164. Here, we employed cTBS to inhibit the SMA, which resulted in enhanced hemodynamic responses in M1. This could be attributed to that the SMA-induced effects subsequently triggered a similar response in M1; another possibility was the reduction on inhibition of the SMA on M1, which was frequently observed during motor imagery65,66,67.
The significant post-cTBS hemodynamic response in SMA and M1 observed under 0- and 3-pound loads, but not under the 15-pound condition (Fig. 6), may reflect cortical activity saturation at higher loads, where hemodynamic responses plateau despite sustained neural demands68. Notably, reduced β values in the 15-pound condition—particularly for participants like S09 reporting fatigue—suggest that motor control strategies or metabolic constraints may diverge under heavy inertia, potentially due to intensified neuromuscular fatigue69 or compensatory adaptations to manage increased resistance70. Additionally, we observed a positive correlation between age and the intercept, suggesting increased baseline hemodynamic activity during LEMs with advancing age. Age-related hemodynamic patterns were also found in prefrontal and motor regions during motor execution71and in dorsolateral prefrontal cortex during cognitively demanding tasks72,73. Future studies should include elderly populations to inform personalized rehabilitation strategies across age groups.
Participants did not undergo the post-cTBS fNIRS test immediately (Fig. 1C and D). Instead, a 15 min break was included for resting and device switching. This interval was designed with two key considerations in mind: (1) It substantially exceeds the typical short-term memory retention window74 while falling short of the stage for long-term memory formation75. This helps to alleviate potential learning effects caused by repeated measurements. (2) It remains well within the documented persistence window of cTBS after-effects. Studies have shown that these after-effects can last up to 50 minutes76. This study introduced a comparison before vs. after cTBS, which implied that the SMA would not autonomously alter its fNIRS responses during the 40-minute data collection. Other limitations of this study would be improved by introducing sham stimulation and/or non-ROI stimulation as controls, using of load magnitude calibrated to each participant, etc.
Data availability
Original datasets are stored off-line on an encrypted computer. The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AAL:
-
anatomical automatic labeling
- BA6:
-
Brodmann area 6
- BMI:
-
body mass index
- CBF:
-
cerebral blood flow
- cTBS:
-
continuous theta-burst stimulation
- fNIRS:
-
functional near-infrared spectroscopy
- GLM:
-
general linear model
- LEMs:
-
large-extent movements
- LF-rTMS:
-
low-frequency repetitive transcranial magnetic stimulation
- M1:
-
primary motor cortex
- SMA:
-
supplementary motor area
- TBA:
-
transcranial brain atlas
- oxy-Hb:
-
oxy-hemoglobin
- deoxy-Hb:
-
deoxy-hemoglobin
- PMC:
-
premotor cortex
- RMT:
-
resting motor threshold
- ROI:
-
region of interest
References
Edemekong, P. F., Bomgaars, D. L., Sukumaran, S. & Schoo, C. Activities of Daily Living. In StatPearls (StatPearls Publishing, 2023).
Gottlieb, G. L. A computational model of the simplest motor program. J. Mot Behav. 25, 153–161 (1993).
Hortobágyi, T. et al. Functional relevance of resistance training-induced neuroplasticity in health and disease. Neurosci. Biobehav Rev. 122, 79–91 (2021).
Goncalves, S., Le Bourvellec, M., Mandigout, S. & Duclos, N. C. Impact of active physiotherapy on physical activity level in stroke survivors: A systematic review and Meta-Analysis. Stroke 54, 3097–3106 (2023).
Gor-García-Fogeda, M. D. et al. Scales to assess gross motor function in stroke patients: a systematic review. Arch. Phys. Med. Rehabil. 95, 1174–1183 (2014).
Gómez-Carmona, C. D. et al. Lower-limb dynamics of muscle oxygen saturation during the Back-squat exercise: effects of training load and effort level. J. Strength. Cond Res. 34, 1227–1236 (2020).
Morán-Navarro, R. et al. Movement velocity as a measure of level of effort during resistance exercise. J. Strength. Cond Res. 33, 1496–1504 (2019).
de Froment, A. J., Rubenstein, D. I. & Levin, S. A. An extra dimension to decision-making in animals: the three-way trade-off between speed, effort per-unit-time and accuracy. PLoS Comput. Biol. 10, e1003937 (2014).
Zenon, A., Sidibe, M. & Olivier, E. Disrupting the supplementary motor area makes physical effort appear less effortful. J. Neurosci. 35, 8737–8744 (2015).
Savelon, E. C. J., Jordan, H. T., Stinear, C. M. & Byblow, W. D. Noninvasive brain stimulation to improve motor outcomes after stroke. Curr. Opin. Neurol. 37, 621–628 (2024).
Chen, Z. et al. Increased inertia triggers linear responses in motor cortices during Large-Extent Movements-A fNIRS study. Brain Sci. 12, 1539 (2022).
Solopchuk, O., Alamia, A., Dricot, L., Duque, J. & Zénon, A. cTBS disruption of the supplementary motor area perturbs cortical sequence representation but not behavioural performance. NeuroImage 163, 34–40 (2017).
Jung, J. & Lambon Ralph, M. A. The immediate impact of transcranial magnetic stimulation on brain structure: Short-term neuroplasticity following one session of cTBS. NeuroImage 240, 118375 (2021).
Evancho, A., Tyler, W. J. & McGregor, K. A review of combined neuromodulation and physical therapy interventions for enhanced neurorehabilitation. Front. Hum. Neurosci. 17, 1151218 (2023).
Moras, G. et al. Entropy measures detect increased movement variability in resistance training when elite rugby players use the ball. J. Sci. Med. Sport. 21, 1286–1292 (2018).
Shi, P., Li, A. & Yu, H. Response of the cerebral cortex to resistance and Non-resistance exercise under different trajectories: A functional Near-Infrared spectroscopy study. Front. Neurosci. 15, 685920 (2021).
Lee, M. J., Kilbreath, S. L., Singh, M. F., Zeman, B. & Davis, G. M. Effect of progressive resistance training on muscle performance after chronic stroke. Med. Sci. Sports Exerc. 42, 23–34 (2010).
Lamontagne, A. & Fung, J. Faster is better: implications for speed-intensive gait training after stroke. Stroke 35, 2543–2548 (2004).
DeJong, S. L., Schaefer, S. Y. & Lang, C. E. Need for speed: better movement quality during faster task performance after stroke. Neurorehabil Neural Repair. 26, 362–373 (2012).
Villepinte, C., Verma, A., Dimeglio, C., De Boissezon, X. & Gasq, D. Responsiveness of kinematic and clinical measures of upper-limb motor function after stroke: A systematic review and meta-analysis. Ann. Phys. Rehabil Med. 64, 101366 (2021).
Sethi, A., Stergiou, N., Patterson, T. S., Patten, C. & Richards, L. G. Speed and rhythm affect Temporal structure of variability in reaching poststroke: A pilot study. J. Mot Behav. 49, 35–45 (2017).
Rosenbaum, D. et al. Comparison of speed versus complexity effects on the hemodynamic response of the trail making test in block designs. Neurophotonics 5, 1 (2018).
Toma, K. et al. Movement rate effect on activation and functional coupling of motor cortical areas. J. Neurophysiol. 88, 3377–3385 (2002).
Guérin, S. M. R., Vincent, M. A. & Karageorghis, C. I. & Delevoye-Turrell, Y. N. Effects of Motor Tempo on Frontal Brain Activity: An fNIRS Study. NeuroImage 230, 117597 (2021).
Zhong, G., Yang, Z. & Jiang, T. Precise modulation strategies for transcranial magnetic stimulation: advances and future directions. Neurosci. Bull. 37, 1718–1734 (2021).
Cruciani, A. et al. Using TMS-EEG to assess the effects of neuromodulation techniques: a narrative review. Front. Hum. Neurosci. 17, 1247104 (2023).
Vink, J. J. T. et al. Continuous Theta-Burst stimulation of the contralesional primary motor cortex for promotion of upper limb recovery after stroke: A randomized controlled trial. Stroke 54, 1962–1971 (2023).
Suppa, A. et al. Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects. Brain Stimulat. 9, 323–335 (2016).
Gordon, E. M. et al. A somato-cognitive action network alternates with effector regions in motor cortex. Nature 617, 351–359 (2023).
Yang, C. L., Lim, S. B., Peters, S. & Eng, J. J. Cortical activation during shoulder and finger movements in healthy adults: A functional Near-Infrared spectroscopy (fNIRS) study. Front. Hum. Neurosci. 14, 260 (2020).
Bonnal, J. et al. Neural substrates for hand and shoulder movement in healthy adults: A functional near infrared spectroscopy study. Brain Topogr. 36, 447–458 (2023).
Mesquita, R. C. et al. Blood flow and oxygenation changes due to low-frequency repetitive transcranial magnetic stimulation of the cerebral cortex. J. Biomed. Opt. 18, 067006 (2013).
Okabe, S., Ugawa, Y., Kanazawa, I. & Effectiveness of rTMS on Parkinson’s Disease Study Group. 0.2-Hz repetitive transcranial magnetic stimulation has no add-on effects as compared to a realistic Sham stimulation in parkinson’s disease. Mov. Disord Off J. Mov. Disord Soc. 18, 382–388 (2003).
Thomson, R. H., Rogasch, N. C., Maller, J. J., Daskalakis, Z. J. & Fitzgerald, P. B. Intensity dependent repetitive transcranial magnetic stimulation modulation of blood oxygenation. J. Affect. Disord. 136, 1243–1246 (2012).
Kinney, K. R. & Hanlon, C. A. Changing cerebral blood flow, glucose metabolism, and dopamine binding through transcranial magnetic stimulation: A systematic review of transcranial magnetic Stimulation-Positron emission tomography literature. Pharmacol. Rev. 74, 918–932 (2022).
Paus, T. et al. Dose-Dependent reduction of cerebral blood flow during Rapid-Rate transcranial magnetic stimulation of the human sensorimotor cortex. J. Neurophysiol. 79, 1102–1107 (1998).
Xiao, X. et al. Transcranial brain atlas. Sci. Adv. 4, eaar6904 (2018).
Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Epstein, L. H. & Wing, R. R. Aerobic exercise and weight. Addict. Behav. 5, 371–388 (1980).
Petrofsky, J. S. & Phillips, C. A. The use of functional electrical stimulation for rehabilitation of spinal cord injured patients. Cent. Nerv. Syst. Trauma. 1, 57–74 (1984).
Delpy, D. T. et al. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 33, 1433–1442 (1988).
Or, J. K. N. & Chang, D. H. F. cTBS over ventral cortex enhances depth perception. Front. Neurosci. 18, 1499030 (2024).
Huang, Y. Z., Edwards, M. J., Rounis, E., Bhatia, K. P. & Rothwell, J. C. Theta burst stimulation of the human motor cortex. Neuron 45, 201–206 (2005).
Mittal, N. et al. Effect of neuroanatomy on corticomotor excitability during and after transcranial magnetic stimulation and intermittent theta burst stimulation. Hum. Brain Mapp. 43, 4492–4507 (2022).
Hoshi, Y. Hemodynamic signals in fNIRS. Prog. Brain Res. 225, 153–179 (2016).
Koyanagi, M. et al. The usefulness of functional Near-Infrared spectroscopy for the assessment of Post-Stroke depression. Front. Hum. Neurosci. 15, 680847 (2021).
Hou, X. et al. NIRS-KIT: a MATLAB toolbox for both resting-state and task fNIRS data analysis. Neurophotonics 8, 1 (2021).
Friston, K. J. et al. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 2, 189–210 (1994).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R Stat. Soc. Ser. B Stat. Methodol. 57, 289–300 (1995).
Chen, Z. et al. Heavier load alters upper limb muscle synergy with correlated fNIRS responses in BA4 and BA6. Cyborg Bionic Syst. Wash. DC. 4, 0033 (2023).
Kinder, K. T. et al. Systematic review of fNIRS studies reveals inconsistent chromophore data reporting practices. Neurophotonics 9, 4 (2022).
Zimeo Morais, G. A. et al. Non-neuronal evoked and spontaneous hemodynamic changes in the anterior Temporal region of the human head May lead to misinterpretations of functional near-infrared spectroscopy signals. Neurophotonics 5, 1 (2017).
Yan, C. et al. Dysfunction of the neurovascular unit in diabetes-related neurodegeneration. Biomed. Pharmacother Biomedecine Pharmacother. 131, 110656 (2020).
Chiang, T. C. et al. Elevated haemoglobin levels in the motor cortex following 1 hz transcranial magnetic stimulation: a preliminary study. Exp. Brain Res. 181, 555–560 (2007).
Park, E. et al. Real-time measurement of cerebral blood flow during and after repetitive transcranial magnetic stimulation: A near-infrared spectroscopy study. Neurosci. Lett. 653, 78–83 (2017).
Chen, S. Y., Tsou, M. H., Chen, K. Y., Liu, Y. C. & Lin, M. T. Impact of repetitive transcranial magnetic stimulation on cortical activity: a systematic review and meta-analysis utilizing functional near-infrared spectroscopy evaluation. J. Neuroeng. Rehabil. 21, 108 (2024).
Pal, P. et al. Effect of Low-Frequency repetitive transcranial magnetic stimulation on interhemispheric Inhibition. J. Neurophysiol. 94, 1668–1675 (2005).
Curtin, A. et al. A systematic review of integrated functional Near-Infrared spectroscopy (fNIRS) and transcranial magnetic stimulation (TMS) studies. Front. Neurosci. 13, 84 (2019).
Tak, S. & Ye, J. C. Statistical analysis of fNIRS data: a comprehensive review. NeuroImage 85 Pt. 1, 72–91 (2014).
Gorban, C., Zhang, Z., Mensen, A. & Khatami, R. The comparison of early hemodynamic response to Single-Pulse transcranial magnetic stimulation following inhibitory or excitatory theta burst stimulation on motor cortex. Brain Sci. 13, 1609 (2023).
Chong, T. T. J. Disrupting the perception of effort with continuous theta burst stimulation. J. Neurosci. 35, 13269–13271 (2015).
Ruddy, K. L., Leemans, A. & Carson, R. G. Transcallosal connectivity of the human cortical motor network. Brain Struct. Funct. 222, 1243–1252 (2017).
Dum, R. & Strick, P. Motor areas in the frontal lobe of the primate. Physiol. Behav. 77, 677–682 (2002).
Arai, N., Lu, M. K., Ugawa, Y. & Ziemann, U. Effective connectivity between human supplementary motor area and primary motor cortex: a paired-coil TMS study. Exp. Brain Res. 220, 79–87 (2012).
Kasess, C. H. et al. The suppressive influence of SMA on M1 in motor imagery revealed by fMRI and dynamic causal modeling. NeuroImage 40, 828–837 (2008).
Tak, S., Kempny, A. M., Friston, K. J., Leff, A. P. & Penny, W. D. Dynamic causal modelling for functional near-infrared spectroscopy. NeuroImage 111, 338–349 (2015).
Bajaj, S., Butler, A. J., Drake, D. & Dhamala, M. Brain effective connectivity during motor-imagery and execution following stroke and rehabilitation. NeuroImage Clin. 8, 572–582 (2015).
Hermand, E. et al. Prefrontal cortex activation during dual task with increasing cognitive load in subacute stroke patients: A pilot study. Front. Aging Neurosci. 11, 160 (2019).
Ishii, A. et al. Decreased alpha-band oscillatory brain activity prior to movement initiated by perception of fatigue sensation. Sci. Rep. 9, 4000 (2019).
Scott, S. H. Inconvenient truths about neural processing in primary motor cortex. J. Physiol. 586, 1217–1224 (2008).
Yu, D., Wei, C., Yuan, Z. & Luo, J. fNIRS study of brain activation during multiple motor control conditions in younger and older adults. J. Integr. Neurosci. 23, 189 (2024).
de Belli, V. et al. Prefrontal cortical activity during preferred and fast walking in young and older adults: an fNIRS study. Neuroscience 473, 81–89 (2021).
Huang, W. et al. Different cortex activation and functional connectivity in executive function between young and elder people during Stroop test: an fNIRS study. Front. Aging Neurosci. 14, 864662 (2022).
Jonides, J. et al. The Mind and brain of Short-Term memory. Annu. Rev. Psychol. 59, 193–224 (2008).
Hadjiosif, A. M., Morehead, J. R. & Smith, M. A. A double dissociation between savings and long-term memory in motor learning. PLOS Biol. 21, e3001799 (2023).
Wischnewski, M. & Schutter, D. J. L. G. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimulat. 8, 685–692 (2015).
Acknowledgements
The authors thank Qiang Xu for his help with the multi-device data acquisition.
Funding
This work has been supported by grants from the Natural Science Foundation of China (82372554), the Guangci Innovative Technology Sailing Plan of Ruijin Hospital (YW20230027), the National Key R&D Program of China (2022YFC3602700).
Author information
Authors and Affiliations
Contributions
CMN, YQ, DZ and ZC carried out the data collection, statistical analyses. CMN and YQ wrote the main manuscript text. YQ, JY contributed to the recruitment, assessment of eligibility, and acquisition of consent for participants. YQ, JY and ZC prepared figures and tables. DZ carried out the cTBS intervention. XS assisted in the SMA positioning and subject transfer. CMN, QX, YZ and CZ conceived the study and participated in revising the article. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study adhered to the Declaration of Helsinki. All participants in this study have consented in written form. The study protocol, advertisement of recruitment, and statement of consent have been approved by the Ruijin Hospital Ethics Committee, Shanghai Jiao Tong University.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qiao, Y., Zhao, D., Yan, J. et al. Supplementary motor area enhanced hemodynamic responses to loading after inhibitory cTBS. Sci Rep 15, 28046 (2025). https://doi.org/10.1038/s41598-025-14103-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-14103-y