Abstract
This study presents the development of a high-performance resistive humidity sensor based on a cetyltrimethylammonium bromide (CTAB)-assisted tin oxide (SnO₂) nanostructured thin film integrated with a Poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) (PEDOT: PSS)/SnO₂ heterojunction. The sensor design incorporates CTAB at varying weight percentages (0%, 6%, 11%, 16%, and 20%) during the hydrothermal synthesis of SnO₂ to regulate crystal growth, morphology, and surface area. The sample with 20 wt% CTAB (SnO-5) exhibited a flower-like stacked nanostructure, confirmed via field emission scanning electron microscopy (FESEM), which significantly enhanced water molecule adsorption and charge transport pathways. X-ray diffraction (XRD) analysis confirmed the tetragonal rutile phase of SnO₂ with decreasing crystallite size from 12.2 nm (nm) to 4.8 nm as CTAB concentration increased. The incorporation of PEDOT: PSS, a p-type conducting polymer, onto the SnO₂ layer via spin coating formed a p–n heterojunction, which improved charge separation and reduced recombination, thereby enhancing electrical conductivity and sensor performance. Electrochemical impedance spectroscopy (EIS) and current-voltage (J-V) measurements demonstrated that SnO-5 exhibited a low internal resistance (1.1 kilo ohms (kΩ)), a minimal cut-in voltage (0.071 Volts (V)), and a high current response (2.645 micro Amps.(µA)), indicating efficient carrier transport. The optimized SnO-5 sensor achieved a high sensitivity of 85.7%, a rapid response time of 14 s (s), and a quick recovery time of 7 s, with low hysteresis (1.60%) across a broad humidity range (5–97% Relative Humidity (RH)), outperforming several existing humidity sensing platforms. The synergistic effects of CTAB-induced nanostructuring and heterojunction engineering played a pivotal role in improving moisture interaction, charge mobility, and structural stability. Furthermore, to validate real-time application feasibility, machine learning (ML) algorithms were implemented to model and predict sensor behavior. Among the tested models, Random Forest (RF) Regression achieved the highest predictive accuracy (R² = 0.99), confirming the sensor’s robustness and reproducibility in dynamic environments. The proposed sensor’s outstanding performance, in combination with ML-enhanced evaluation, positions it as a promising candidate for next-generation humidity monitoring systems in industrial, environmental, and biomedical applications, including respiratory diagnostics and non-invasive health monitoring.
Similar content being viewed by others
Introduction
Humidity sensing is essential in diverse fields, including environmental monitoring, healthcare, industrial processes, and agriculture. Accurate humidity measurement is crucial for maintaining optimal conditions in critical environments such as clean rooms, incubators, and respiratory monitoring systems, ensuring efficiency, safety, and reliability1,2,3,4. These sensors function by detecting variations in physical, chemical, or optical properties when exposed to moisture in the environment. Regarding this, a variety of materials have been investigated, including carbon-based materials like graphene, graphene oxide, and carbon nanotubes; polymers like polyvinyl alcohol and polyaniline; and semiconductor metal oxides (SMOs) like ZnO, SnO₂, TiO₂, CuO, and NiO. Through variations in electrical and optical properties such as resistance, capacitance, impedance, refractive index, surface acoustic waves, and quartz crystal microbalance (QCM) responses, these materials make it easier to detect humidity5,6,7,8,9. Among the various sensing materials, SnO₂ stands out as a preferred choice for humidity detection due to its excellent semiconducting properties, high surface reactivity, and stability. As an n-type metal oxide semiconductor (MOS), SnO₂ exhibits significant variations in electrical resistance upon exposure to moisture, making it highly suitable for resistive-based humidity sensors10. However, achieving precise control over SnO₂ nanoparticles remains challenging, as synthesis variations often lead to size inconsistencies, surface defects, and agglomeration. These structural irregularities reduce the material’s surface area and active sites, thereby affecting its sensing efficiency. To address these limitations, optimized synthesis techniques and surface modifications, including the incorporation of dopants, are essential for enhancing dispersion, stability, and overall sensor performance in humidity and energy applications11,12. To further enhance the structural and functional properties of SnO₂, CTAB is introduced as a surfactant to regulate nanoparticle growth. By preventing aggregation, CTAB ensures uniform particle distribution, controlled size, and improved crystallinity, all of which contribute to enhanced sensing performance and stability. Additionally, CTAB micelles play a crucial role in shaping SnO₂ nanostructures, effectively controlling morphology and porosity. This increased surface area provides more active sites, making CTAB-assisted SnO₂ highly suitable for applications in catalysis, sensing, and energy storage13. The precise effect of CTAB on the structural and functional characteristics of SnO₂ has not been thoroughly examined, even though numerous studies have examined its involvement in the synthesis of different metal oxides. Variations in CTAB concentration can significantly alter nanoparticle size, crystallinity, and surface characteristics, yet a comprehensive analysis of these effects on SnO₂ is still lacking. Understanding how CTAB influences SnO₂ properties is crucial for optimizing its performance in sensing and catalytic applications. A systematic investigation in this area could lead to advancements in synthesis techniques, enhancing material efficiency and functionality. Beyond SnO₂, other materials such as carbon-based compounds have also been employed in sensing applications. Additionally, polymers like polyvinyl alcohol and polyaniline have demonstrated effectiveness in detection-based technologies. Bhuvaneswari et al. reported that CTAB incorporation in SnO₂ synthesis plays a vital role in preventing agglomeration, increasing surface area, and modifying morphology. Their study revealed, through scanning electron microscopy (SEM) analysis, the formation of nanorods, nanosheets, and plate-like structures, which improve the surface-to-volume ratio, enhancing sensing capabilities14. Furthermore, EIS and J-V measurements demonstrated that CTAB-assisted SnO₂ exhibits lower internal resistance, improved conductivity, and efficient charge transport, contributing to stable sensor performance. Ultraviolet-Visible (UV-Vis) spectroscopy further confirmed that CTAB modification can fine-tune the bandgap of SnO₂, directly influencing its sensing response and overall efficiency. As demonstrated by15, the hydrothermal method has been extensively used among the many synthesis procedures for the production of SnO₂, allowing for the formation of distinct structures such flower-like, nanosheet, and plate-like morphologies. Building upon its role in modifying SnO₂ properties, CTAB-assisted synthesis has also been shown to enhance the crystallinity and uniform dispersion of NiO nanoparticles, resulting in superior humidity and gas sensing performance. Numerous studies have been conducted on the application of CTAB-modified SnO₂ nanostructures in humidity sensing, which provide distinct advantages, including more active sites for water adsorption, which improve sensitivity. Additionally, the efficient charge transfer mechanisms facilitated by CTAB improve response and recovery times, ensuring reliable and stable sensor operation. A key benefit of these sensors is their low hysteresis, which enhances repeatability and long-term stability in practical applications16. Beyond structural enhancements, the addition of CTAB to SnO₂ synthesis is essential for modifying the material’s electrical characteristics. By enhancing surface area, dispersion, and uniformity, CTAB-assisted SnO₂ promotes the formation of well-defined heterojunctions when combined with other semiconductors. These hetero-junctions significantly improve charge transfer dynamics, leading to optimized humidity sensing performance. The fabrication of extremely sensitive and stable humidity sensors with exceptional response and recovery qualities is made possible by the combination of heterojunction engineering and surfactant-assisted synthesis. Heterojunctions are essential in advancing nanostructured humidity sensors by improving charge separation and transport efficiency. At the interface of two semiconductor materials, a potential barrier is created, which enhances sensitivity, response time, and stability. For instance, a p-type Co₃O₄/p-type AgO heterojunction demonstrated high sensitivity across a wide humidity range due to enhanced charge carrier dynamics17. Similarly, a g-C₃N₄/MoS₂ heterojunction sensor exhibited low hysteresis and rapid response/recovery times, attributed to optimized electronic interactions18. Another study on ZnO/porous GaN hetero-junction sensors highlighted the advantages of structural and electronic modifications at the interface, leading to enhanced performance19. Furthermore, hetero-junction structures are highly effective in improving charge transport efficiency and minimizing recombination losses, making them valuable for sensor performance enhancement. The controlled engineering of nanostructures, as demonstrated in TiO₂/SnO₂ systems, plays a key role in optimizing both electrical and surface properties, further contributing to sensor efficiency and stability20. Hetero-junction structures play a crucial role in enhancing charge transport and overall sensor performance by effectively minimizing recombination losses. Among various hetero-junction configurations, the SnO₂/NiO heterojunction has demonstrated significant improvements in electronic properties and sensitivity, emphasizing the importance of interface engineering in optimizing device efficiency21. In order to increase humidity sensing capability, the current study investigates the development of a PEDOT: PSS/SnO₂ heterojunction, which improves charge separation and transport. A p-type conductive polymer that has been extensively researched, PEDOT: PSS, is well-known for its ability to create a p-n hetero-junction and improve the performance of SnO₂-based sensors. By adjusting the depletion region at the interface, this hetero-junction increases effective charge transfer, which has a direct impact on sensor stability and response. Furthermore, studies on organic–inorganic hetero-junctions have shown notable improvements in rectifying behavior and sensitivity in photo-detectors and gas sensors.
SnO₂ is widely explored in optoelectronics due to its wide bandgap and strong response to UV light. Ozel et al.22 investigated n-SnO₂/p-Si heterojunction photodetectors and found that the photoresponsivity (PR) was highest at low light intensities. In this range, PR remained almost constant and was mainly affected by the device’s dark current. The study reported a maximum PR of 46.3 A/W. Such insights are valuable for SnO₂-based sensor design, where similar charge transport mechanisms are involved. Tin oxide (SnO₂) is a promising semiconductor known for its temperature-dependent electrical properties, making it ideal for sensing devices. Its conduction behavior strongly depends on fabrication parameters and operational temperature. According to Serin et al.,23 thermionic emission dominates above 100 K, while tunneling is significant below 100 K. This variation in conduction mechanisms underscores the need for precise control over material processing. Such understanding is essential for improving the performance and reliability of SnO₂-based sensors. SnO₂-based p–n heterojunctions are widely studied for their simple fabrication and effective electronic properties. Ozel et al.24 reported a hydrothermally grown n-SnO₂/p-Si diode with strong rectifying behavior. The device showed a rectification ratio of 4754 and a turn-on voltage of ~ 3.89 V. Key energy band parameters were extracted to explain carrier transport. These findings support SnO₂’s suitability for sensing and optoelectronic devices.
These findings suggest that the integration of PEDOT: PSS with SnO₂ could significantly improve humidity sensing capabilities by optimizing charge transport mechanisms and amplifying signal response25,26. ML is increasingly used to interpret and analyze complicated sensor data to improve accuracy, dependability, and efficiency. ML models can detect patterns and correlations in sensor signals using both supervised and unsupervised algorithms, which enhances their performance in a variety of applications, including industrial processes, healthcare, and environmental monitoring. V. V. Kornienko et al. improved the precision and dependability of humidity readings by integrating machine learning algorithms to examine the sensor’s optical responses27. A deep learning method was used by Rodić, L.D. et al. to suggest a system that can accurately identify soil moisture levels based only on measurements of signal strength28. As a whole, by facilitating precise data analysis, pattern recognition, and prediction across a range of applications, machine learning greatly improves sensor performance. In this study, different weight percentages of CTAB (0%, 6%, 11%, 16%, and 20%) were incorporated to modify the structural and surface properties of SnO₂ for humidity sensing applications. The controlled dispersion of nanoparticles enhanced the active surface area, improving sensor efficiency. Furthermore, a p-n hetero-junction was established by integrating PEDOT: PSS as a p-type material onto the SnO₂ layer via spin coating, facilitating efficient charge transfer and transport. The optimized sensor showed outstanding humidity detection capability with a high sensitivity of 85.7%, a quick reaction time of 14 s, and a recovery time of 7 s. Notably, the sensor with 20 wt % CTAB concentration exhibited a hysteresis of 1.60%, ensuring stable and repeatable operation. Additionally, the fabricated sensor achieved an extended humidity detection range from 5 to 97% RH, highlighting its effectiveness for wide-range humidity monitoring. The synergistic effect of CTAB-assisted SnO₂ nanostructures and the PEDOT: PSS hetero-junction significantly enhanced sensor performance by improving charge transport and surface interactions. The optimized PEDOT: PSS/SnO₂ hetero-junction, when combined with CTAB-mediated nanostructuring, has high potential for biomedical applications such as real-time bio sensing, non-invasive breath analysis for disease detection, and respiratory monitoring because of its superior electrical and optical characteristics. The results of this study offer significant novel data for developing heterojunction sensors based on SnO₂ modified by CTAB for use in biomedical and industrial applications.
Experimental section
Materials and methods
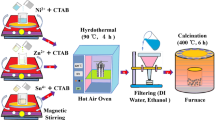
A stacked flower-like SnO₂ nanostructure was fabricated by altering the molar ratio of CTAB and keeping the temperature of tin chloride (Sn⁴⁺) constant. The organic components were then eliminated by calcination. The hydrothermal method was used for synthesis, as shown in Fig. 129.
First, in a beaker filled with 100 ml of distilled water, hydrated tin chloride (Sn⁴⁺), CTAB (at an SnO: CTAB wt% ratio of 100:20), and urea (CH₄N₂O) were dissolved while being continuously stirred with a magnetic stirrer. The resulting mixture was then heated in a hot air oven for 24 h at 120 °C and placed in an autoclave lined with Teflon. Impurities were then eliminated by centrifugation using ethanol and deionised water. The final product was dried overnight at 100 °C in an oven. Using a mortar and pestle, the dried materials were crushed into a fine powder before being calcined for 6 h at 400 °C as displayed in Fig. 1a. The same procedure was applied to the following Table 1.
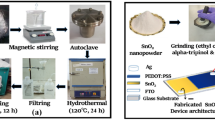
Device fabrication
Figure 1b shows the SnO₂ thin-film device fabrication process. Ethyl cellulose and a mortar and pestle were used to grind the synthesized SnO₂ nanopowder into a paste. The doctor blade method was then used to apply this paste to an FTO substrate, and it was annealed for an hour at 350 °C. After annealing, a thin layer of PEDOT: PSS, which acts as the hole-collecting layer was coated by spin-coating for 30 s at 3000 revolutions per minute (rpm) and then letting it dry for 10 min. The electrical connections were manually applied to the sensor using silver paste, as seen in Fig. 1b.
Results and discussion
Structural properties (XRD)
Typical XRD patterns of the materials produced under various hydrothermal conditions are displayed in Fig. 2. Peak positions were in good agreement with the standard data for SnO2 (JCPDS card no. 41-1445). The pattern showed no extra crystalline residues, suggesting that the as-prepared materials had a more pure, useful structure. This result indicated that the temperature and duration of hydrothermal treatment are crucial for obtaining the pure phase SnO2 with suitable crystallinity. The rutile SnO₂ crystal’s (110), (101), (211), and (112) planes were found to correlate to peaks at 2θ values of 26.8°, 33.1°, 51.7°, and 64.1°, respectively30.
The crystallite sizes of SnO-1, SnO-2, SnO-3, SnO-4, and SnO-5 are 12.2 nm, 9.5 nm, 7.3 nm, 6.1 nm, and 4.8 nm, respectively, as shown in Table-2. When the CTAB molar ratio is increased during SnO₂ synthesis, the average crystallite size decreases from 12.2 nm to 4.8 nm, as Table 2 illustrates. The exceptional purity of the SnO₂ samples is confirmed by the absence of extra peaks from impurities. Additionally, the size of the crystallite was determined using the Scherrer Eq. (1)31.
Where, D is the typical crystal size in nm. \(\lambda\) is the wavelength of X-rays (CuKα radiation) is 0.15406 nm. \(\beta\) is the peak’s full width at half-maximum (FWHM). and \(\theta\)represents the corresponding Bragg diffraction angle.
Micro-strain (ε)
Micro-strain represents the internal lattice distortions within a crystal structure, typically resulting from imperfections, grain boundary stresses, or nanoscale effects. It has significant effects on the physical and chemical characteristics of the material. The Williamson-Hall Eq. (2) can be used to estimate the micro-strain (ε)32.
Where θ is the Bragg angle in radians and β is the FWHM in radians. Micro-strain can positively impact device performance by increasing the density of reactive surface sites. This lattice distortion enhances sensitivity by facilitating adsorption and desorption processes during sensing applications.
Dislocation density (δ)
The amount of dislocations in a crystal per unit area is known as the dislocation density. By serving as active sites, these defects improve sensor performance by enhancing the adsorption of moisture. The dislocation density (δ) is calculated using Eq. (3)33.
Where D is the crystallite size, as calculated by the Debye-Scherrer equation, expressed in nm. In sensing applications, an optimal level of dislocation density is beneficial, as it enhances surface reactivity, facilitates charge carrier transport via defect-assisted pathways, and provides additional active sites for molecular interactions. This leads to improved sensitivity, faster response and recovery times, and enhanced overall sensor performance.
Morphological characterization (FESEM)
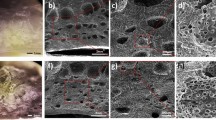
FESEM was used to further characterize the structural properties of the SnO2 powders. Figure 3 displays FESEM pictures of the synthesized nanoparticles. The uneven spherical morphology of the SnO2 nanocomposite in device SnO-1 is depicted in Fig. 3a. In Fig. 3b, the particles of device SnO-2 appear Rock-like structures in shape. This exhibits a dense and compact morphology, resulting in a reduced surface area and restricted surface exposure limits how the material interacts with humidity and environmental elements that could have an impact on its overall performance.
FESEM images and elemental analysis of SnO₂ samples synthesized with different CTAB concentrations: (a) SnO-1, (b) SnO-2, (c) SnO-3, (d) SnO-4, and (e) SnO-5. Each image includes a scale bar representing 200 nm. (f) Elemental mapping of SnO-3 showing the spatial distribution of elements C, Au, O, and Sn. (g–j) Individual elemental maps for (g) C, (h) Sn, (i) Au, and (j) O. (k) EDX spectrum of SnO-3 showing the presence of Sn, O, Cl, and Au. The X-axis denotes energy in keV, and the Y-axis indicates signal counts.
The gradual growth of a distinct flower-like stacked structure as the molar ratio of CTAB rises is obviously shown in Fig. 3c–e. The main cause of this morphological change is the adsorption of CTA⁺ cations on particular SnO₂ nanoparticle facets, which directs anisotropic development and promotes the creation of thin nanosheets that self-assemble into a layered, petal-like structure34. The surface area is greatly increased by such a structure, which also creates mesoporous channels that enable effective interaction with moisture molecules. These features are advantageous for sensing applications, as they contribute to faster response and recovery times. Moreover, the uniform stacking observed across the sample surface indicates consistent nucleation and controlled growth. This highly porous, flower-like structure also provides improved pathways for charge transport, reducing interfacial resistance and enhancing sensor performance, particularly when interfaced with a conductive polymer like PEDOT: PSS. The elemental mapping in Fig. 3f-j shows that Sn, O, Zn, and C are distributed uniformly in area, indicating that the material is consistently composed and that each component is successfully integrated throughout the sample. Detailed mapping data are summarized in Table 3.
JV-studies
The electrical characteristics of the proposed SnO2 nanomaterials were evaluated through photoconductivity analysis. The surfactant-modified, temperature-dependent flower-like SnO₂ nanoparticles function as an n-type sensing layer. The energy band diagram of PEDOT: PSS and SnO₂ is shown in Fig. 4a, which shows that the barriers for hole injection from Ag to the Highest Occupied Molecular Orbital (HOMO) level of PEDOT: PSS are 0.78 eV and 2.3 eV, respectively, from the HOMO level of PEDOT: PSS to the valence band (Ev) of SnO₂35.
Its electron injection barrier between the Fermi level of FTO and the conduction band (Ec) of SnO₂ is 0.32 eV, while the barrier between the conduction band of SnO₂ and the Lowest Unoccupied Molecular Orbital (LUMO) of PEDOT: PSS is 0.41 eV respectively. As seen in Fig. 4a, this results in a substantial build-up of electrons and holes at the PEDOT: PSS/SnO₂ interface under a positive bias. Figure 4b shows the p-n junction properties of the fabricated Sno-1, SnO-4, and SnO-5 devices, showing that forward current increases as bias voltage rises. The turn-on (TON) voltages for SnO-1, SnO-2, SnO-3, SnO-4, and SnO-5 devices, as described in Table 4, are 0.425 V, 0.321 V, 0.218 V, 0.162 V, and 0.071 V, respectively, confirming the presence of a p-n junction. The turn-on voltage refers to the voltage at which a p-n junction begins to conduct current in the forward direction.
EIS studies
Figure 5 presents the Nyquist plot of the proposed devices obtained through frequency response analysis using spectroscopy. In the case of the proposed sensing device, the resonance frequency (‘) is inversely proportional to the RC time constant (TRC) and it is shown in Eq. (4)36.
Where f’ is the Resonance frequency (Hz). As shown in Eq. (4), the calculated time constant (TRC) and the resonant frequency, where Z” reaches its peak for the proposed samples, are summarized in Table-5. The internal resistance can be computed from the diameter of the curve along the axis indicating the real part of the impedance (Z’) because the RC impedance is primarily resistive at lower frequencies. The internal capacitance (Cint) of the proposed samples are determined using Eq. (4). An important barrier to electron-hole recombination is shown by higher internal resistance for the suggested CTAB-modified samples. Consequently, the complex impedance spectrum depicted in Fig. 5 tends to exhibit a semicircular shape, and there is an impedance shift between each device. These impedance-shifting properties make the material highly suitable for applications involving humidity adsorption. More chemical reaction sites are made available by the increased specific surface area, as seen in Table-5. In fields such as heterogeneous catalysis, this can lead to improved catalytic activity37, accelerated reaction rates, and greater efficiency. The larger surface area also facilitates increased adsorption and absorption of moisture. Significant advancements have been made as a result of the addition of surfactant CTAB and heterojunction. These include decreased Rct for improved charge transport across grains, increased Cit for more active sites for sensing interactions, improved TRC for increased sensitivity by extending analyte interaction with the sensing material, and decreased f’, which indicates improved performance at low frequencies necessary for real-time sensing.
HR-TEM analysis
The HR-TEM pictures of the synthesised SnO-5 sample are shown in Fig. 6a, emphasising the ultra-fine crystallite character of the material. A significant degree of crystallinity is confirmed by the pronounced lattice fringes seen in the pictures. In accordance with the (110) plane of SnO₂, these fringes have an interplanar spacing of about 0.33 nm. The Selected Area Electron Diffraction (SAED) pattern in Fig. 6b shows five expanded concentric rings that correspond to the (110), (101), (211), (301), and (321) planes of cassiterite-phase SnO₂38. The tin (Sn) and oxygen (O) elements are confirmed to be present in the sample by the TEM-EDX spectrum shown in Fig. 6c. The modest carbon (C) peak is thought to be the result of minor surface contamination during sample preparation, while the copper (Cu) signals are ascribed to the supporting TEM grid. The XRD analysis and these HR-TEM observations agree, as shown by the d-spacing values in Table 6.
(a) HR-TEM image of SnO-5 nanoparticles showing crystallographic planes with interplanar spacings of 0.17 nm (211), 0.26 nm (101), 0.14 nm (321), and 0.33 nm (110); the central image indicates selected regions. (b) SAED pattern with rings indexed to (110), (101), (211), (301), and (321) planes of cassiterite SnO₂. (c) EDX spectrum confirming Sn and O presence.
A comparison between the crystallite sizes (r) found in the TEM-EDX pictures and those obtained from the XRD patterns using the Scherrer equation is shown in Table 2. The results from both methods show a significant correlation, which supports the structural analysis from XRD and shows that the synthesised SnO₂ samples are made up of equally sized nanocrystals.
Sensor characterization
Experimetal set-up
The fabricated humidity sensor’s ability to detect humidity was carefully tested in a glass chamber under control. To determine the sensor’s sensing response, resistance variations were recorded throughout a range of RH from 5 to 97%. Humidity regulation within the chamber was achieved by precisely controlling the introduction of dry and humidified air. To adjust the humidity levels, an ultrasonic humidifier was used to create a stream of water vapor-saturated air that was injected into the chamber via an inlet valve. Throughout the experimental process, a commercial thermo-hygrometer continuously monitored and recorded the temperature and RH to ensure accurate environmental conditions. A thorough evaluation of the sensor’s sensitivity, response time, recovery time, and overall performance was made possible by the real-time recording of its electrical response to changing humidity levels.
A high-precision digital multimeter (PICOTEST M3510A) was utilized to accurately measure resistance fluctuations corresponding to different RH levels, ensuring the reliability of the sensor’s performance assessment. A schematic representation of the humidity measuring setup is shown in Fig. 7. RH levels were gradually raised to 97% in order to assess the sensor’s reaction, and resistance readings were taken once every second. To decrease the humidity within the chamber, high-purity dry nitrogen gas was gradually introduced, ensuring a controlled reduction in moisture levels. This process was repeated until the sensor exhibited a complete adsorption and desorption cycle across the full RH range. The uniform distribution of humidity inside the chamber was facilitated through an inlet positioned at the bottom left. The variations in resistance corresponding to different RH levels were precisely measured using a PICOTEST M3510A Digital Multimeter. The proposed sensor’s sensitivity-humidity relationship is shown in Fig. 8. The effectiveness of the surfactant-assisted PEDOT: PSS/SnO2 humidity sensor was evaluated by analyzing its performance over a range of RH from 5 to 97%. Sensitivity calculations were performed using Eq. (5)39.
Here, S stands for sensitivity, and (Rm) and () indicate the resistance of the device at the lowest humidity level (5% RH) and the resistance variation that corresponds to variations in RH, respectively. As the percentage RH increases, the resistivity of the as-prepared samples shows a linear reduction, as seen in Fig. 8a. The resistivity variation with respect to % RH is categorized into three distinct regions: (I) from 10 to 30% RH, (II) from 40 to 70% RH, and (III) from 80 to 97% RH. This classification provides a structured approach to identifying specific application domains based on humidity levels, allowing for targeted utilization of the sensor in various environmental and industrial settings.
Figure 8b shows the sensitivity graph. A systematic analysis of the fabricated humidity sensor’s resistance at various moisture levels was conducted. The resistance measurements were carried out at an ambient temperature of approximately 25° C while gradually increasing the RH from 5 to 97% in incremental steps. During each step, careful measures were taken to stabilize the RH at a constant level to ensure reliable and consistent resistance readings. The sensor’s electrical conductivity increases at higher RH levels, because the adsorption of water molecules on its surface enhances charge carrier movement within the sensing material. Resistance changes in response to changes in humidity become more noticeable as a result of this phenomenon. Surprisingly, sensitivity increased significantly when the RH was changed from 80 to 97%, resulting in a sensitivity rating of 85.7%.
Humidity sensing mechanism
Adsorption of water molecules onto the detecting surface, which affects its electrical conductivity, is the basis for the humidity sensor’s operation. Water molecules are adsorbed by physisorption and chemisorption when moisture from a humidifier reaches the sensor, as shown in Fig. 9.
At first, van der Waals forces cause water molecules to cling shakily to the surface.Stronger interactions develop as water molecules split into hydrogen ions (H⁺) and hydroxyl ions (OH⁻) when humidity rises, creating chemical connections with the surface’s active sites40,41. As a result of this adsorption, electrical resistance changes. A conductive and hydrophilic polymer called PEDOT: PSS is added, which greatly improves charge carrier transport over the sensor film42,43. Water molecules build up in greater quantities when the humidity level rises, creating proton-conductive routes and enhancing ionic mobility. The overall resistance of the sensor is lowered as a result. Depending on the humidity range, the sensor responds differently. At low relative humidity (10–30% RH), resistance changes somewhat due to negligible water adsorption. Because of improved proton hopping, water absorption rises and considerably reduces resistance in the intermediate range (40–70% RH). High humidity (80–97% RH) creates a continuous layer of water, which stabilises the resistance by creating a highly conductive surface. The electrical signal that represents the relative humidity of the surrounding air is then produced from this change in resistance.
Response and recovery time study
The proposed humidity sensors’ sensing capabilities are depicted in Fig. 10. The SnO-1 to SnO-5 samples’ response and recovery characteristics are shown in Fig. 10a, c, e, g, and i. These characteristics, which include a response time and a recovery time, emphasize the sensor’s effectiveness in detecting changes in humidity. Specifically, Fig. 10b, d, f, h, and j present the adsorption and desorption characteristics of the proposed sensors when subjected to a RH cycle ranging from 5 to 97% and back to 5%. Recovery time is the amount of time needed for the sensor to return to its initial resistance state when the humidity drops, whereas response time is the amount of time needed for the sensor to reach 90% of its overall resistance change after being subjected to a specific humidity level. Due to the increased charge carrier mobility made possible by the interaction of water molecules, the resistance of the sensing material falls as the relative humidity rises during the adsorption phase. On the other hand, during the desorption phase, the humidity sensor’s resistance increases as RH falls. It can be seen from the response and recovery characteristics of SnO-2, SnO-3, SnO-4, and SnO-5 that the developed sensors’ response and recovery times gradually decrease as the CTAB molar concentration rises. By increasing the amount of oxygen vacancies on ZnO’s surface, CTAB modifies its surface characteristics and improves the material’s ability to adsorb water molecules.
Response and recovery characteristics of humidity sensors SnO-1 to SnO-5. Subfigures (a), (c), (e), (g), and (i) show resistance variation under humidity cycling (5–97% RH), while (b), (d), (f), (h), and (j) depict the corresponding response and recovery times. Improved performance with higher CTAB content is observed, with SnO-5 exhibiting the fastest response due to its flower-like morphology and PEDOT: PSS-assisted charge transport.
The repeatability of the sensor was confirmed through testing over five consecutive cycles. The PEDOT: PSS/SnO₂ heterojunction and CTAB integration improve surface structure, charge transport, and adsorption-desorption efficiency, all of which are critical for improving the sensor’s response and recovery times. CTAB assists in forming flower-like SnO₂ nanostructures (SnO-5), increasing surface area and enabling faster water molecule adsorption and desorption, which accelerates the sensing process. Meanwhile, the PEDOT: PSS/SnO₂ hetero-junction enhances charge transfer, where PEDOT: PSS facilitates hole conduction and SnO₂ supports electron movement, creating an internal electric field that speeds up sensor response. Additionally, the oxygen vacancies in SnO₂ improve charge mobility, ensuring stable operation and repeatability. The response/recovery time details for each sensor are listed in Table 7. The combined effect of CTAB-assisted structural modifications and hetero-junction-driven charge transport leads to a rapid response time of 14 s and a recovery time of 7 s, making the SnO-5 sensor highly effective for real-time humidity monitoring.
Hysteresis/long term stability analysis
In the presence of moisture, adsorption and chemical interactions take place on the sensor layer’s surface, leading to changes in its conductance (or conductivity). This variation in resistance, which directly correlates with the amount of moisture in the surroundings, serves as an indicator of these alterations. The hysteresis error can be calculated using Eq. (6)47.
Here, FS stands for the full-scale output value, and is the largest impedance difference that was seen between the adsorption and desorption operations. The measured hysteresis errors as shown in Fig. 11a-c for the fabricated sensors were 3.4%, 2.34%, and 1.6%, with the highest hysteresis observed for the SnO-1 sensor (3.4%). There are other important aspects that contribute to the decrease in hysteresis when CTAB concentration rises. Hysteresis was exacerbated in the SnO-1 sensor due to ineffective water molecule adsorption and retention caused by the lack of CTAB, which also resulted in limited surface area and irregular porosity. Additionally, the hydroxyl (OH-) functional groups on the surface were not well-controlled, causing moisture to remain trapped, delaying desorption, and thereby increasing the hysteresis error. However, with the incorporation of CTAB as a surfactant, the sensor exhibited improved surface morphology, enhancing adsorption-desorption balance and reducing moisture retention. Previous studies have demonstrated that CTAB-assisted synthesis improves the surface area and porosity of SnO₂ nanomaterials, facilitating better interaction with humidity and thereby enhancing sensing properties48. Furthermore, the PEDOT: PSS heterojunction contributed to better charge transport, preventing charge accumulation and ensuring a more stable electrical response. Research has shown that PEDOT: PSS functionalization in humidity sensors significantly enhances charge mobility, leading to improved sensor performance49. As a result, the optimized SnO-5 sensor configuration achieved a significant hysteresis reduction, reaching 1.6%, demonstrating improved accuracy and reliability in humidity sensing applications. The Table 8 lists the hysteresis error performance of the different sensors.
Error and stability analysis
Resistance measurements were made over a 30-day period at five different relative humidity levels-5%, 32%, 40%, 52%, and 97%-in order to assess the sensor’s long-term dependability. Figure 11d displays the equivalent long-term stability curve with error bars. Strong long-term stability without noticeable degradation or drift was indicated by the sensor’s exceptional resistance value consistency across the observed time. Error bars shown in Fig. 11e were added to each RH level at Day 0 to confirm measurement accuracy and reproducibility. These show the standard deviation derived from three separate experiments carried out in the same circumstances. While intermediate RH values (32%, 40%, and 52%) displayed somewhat higher but still manageable variability, each with an error of ± 0.15 kΩ, the lowest and highest RH situations (5% and 97%) showed the least amount of fluctuation, with an error of ± 0.1 kΩ. The consistency and dependability of the sensing performance are highlighted by these low standard deviations. Table 9 presents a summary of the Day 0 resistance values along with the associated error estimates.
Integration of machine learning in humidity sensor analysis
Machine learning (ML) is vital for improving sensor-based systems’ precision and effectiveness. In this part, ML techniques are utilized to analyze the performance of the CTAB-mixed SnO₂-based resistive humidity sensor53. Several machine learning models are used to process the sensor’s data for both regression and classification tasks. The humidity levels are categorized into three distinct ranges-low (5–30% RH), medium (40–70% RH), and high (80–97% RH)-using models such as Logistic Regression (LR), Decision Tree (DT), and Random Forest (RF). These models are also used to predict exact humidity values based on sensor resistance. Standard metrics are used to measure each model’s efficacy, including accuracy, precision, recall, F1-score (for classification), and Mean Absolute Error (MAE), Mean Squared Error (MSE), Root Mean Squared Error (RMSE), and R-Squared (R2) score (for regression)54. By integrating ML-based classification and prediction, this study can improve sensor data analysis, enabling more accurate humidity monitoring for real-world applications. The dataset comprises sensor resistance values recorded at different RH levels. The key features considered for training include resistance (Ω), which reflects the sensor’s response to humidity variations, % RH as the target variable for regression analysis, and time (s) to observe changes in sensor response over time. These features are essential for establishing a correlation between humidity and sensor output. Three humidity levels are used to classify the data: low humidity (5–30% RH), medium humidity (40–70% RH), and high humidity (80–97% RH) in order to assess the sensor’s capacity to detect changes in humidity. This classification approach enables a detailed analysis of the sensor’s performance across different humidity conditions. For each of the five sensor configurations, a total of 1224 data points were gathered, yielding 6120 entries. To guarantee a fair model evaluation and stop data leaking, the dataset was split into 80% training and 20% testing at random. Each data subset was able to contribute to both learning and validation through the use of 5-fold cross-validation during training, which improved model dependability. In order to optimise important parameters for each method, such as n_estimators, max_depth, and min_samples_split in Random Forest, and comparable settings for Decision Tree and Logistic Regression, hyperparameter tuning was carried out using Grid Search. Figure 12 gives the details about the flow of machine learning algorithms.
The classification performance is evaluated using confusion matrix. Figure 13a, b, and c illustrates the confusion matrix for humidity (LR, DT and RF) classification respectively, showing correctly and incorrectly predicted humidity levels. The diagonal values indicate accurate classifications, while off-diagonal values represent misclassifications. This graphic helps in evaluating model performance through the use of metrics such as F1-score, recall, accuracy, and precision55.
The LR model’s poor classification performance, especially in the medium and high RH groups, indicates that it may be challenging to distinguish overlapping ranges using a linear method. In the low and medium ranges, the Decision Tree enhances categorisation; nevertheless, some medium samples are mistakenly categorised as high. This shows that, although somewhat sensitive to noise, the model can manage nonlinearity. The Random Forest model is the most effective; it accurately predicts almost all samples with very little misclassification. Its ensemble technique improves its generalisation across various humidity levels and lessens overfitting. Figure 14 presents the graphical representation of actual versus predicted humidity values using the Random Forest Regression model.
Table 10 presents the performance metrics of the regression models, providing a comparative evaluation of their accuracy. With an R2 score of 0.99, which indicates its great predictive power, the RF Regression performed the best. This highlights the effectiveness of machine learning techniques in evaluating the fabricated humidity sensor, where the RF models exhibited the highest accuracy and minimal error, demonstrating the sensor’s reliability for real-time humidity monitoring.
Conclusion
This study demonstrates the successful design, fabrication, and evaluation of a novel, high-performance resistive humidity sensor based on CTAB-assisted SnO₂ nanostructures interfaced with a PEDOT: PSS/SnO₂ heterojunction architecture. By strategically incorporating varying concentrations of cetyltrimethylammonium bromide (CTAB) during hydrothermal synthesis, the morphological and crystallographic properties of SnO₂ were precisely tailored. The optimized composition, SnO-5 with 20 wt% CTAB, exhibited a unique flower-like nanostructure with high porosity, reduced crystallite size (4.8 nm), increased dislocation density, and elevated micro-strain—all of which contributed to enhanced adsorption of water molecules and improved charge transport.
The integration of PEDOT: PSS, a highly conductive p-type polymer, with SnO₂ facilitated the formation of an efficient p–n heterojunction, which played a pivotal role in improving the electronic properties of the sensor. This heterostructure reduced the charge transfer resistance, minimized recombination losses, and resulted in a low cut-in voltage (0.071 V) and high output current (2.645 µA), as evidenced by J–V and EIS analyses. The combined effects of morphological control and heterojunction engineering led to superior sensing performance, including high sensitivity (85.7%), fast response (14 s), quick recovery (7 s), and low hysteresis (1.60%) across a broad relative humidity range of 5–97% RH. Furthermore, the sensor exhibited excellent stability and repeatability, maintaining consistent performance over multiple cycles and extended durations.
To enhance predictive capabilities and operational reliability, machine learning algorithms were deployed to analyze the relationship between sensor resistance and humidity levels. Among the evaluated models, Random Forest Regression outperformed others, achieving an R² score of 0.99, demonstrating exceptional prediction accuracy. This integration of machine learning not only validates the reliability of the sensor data but also signifies a shift towards intelligent, self-adaptive sensing systems capable of real-time monitoring and dynamic calibration.
The convergence of surfactant-assisted nanostructuring, organic–inorganic heterojunction engineering, and machine learning analytics marks a significant advancement in the field of humidity sensing. The developed sensor is particularly promising for applications in environmental surveillance, smart agriculture, industrial process control, respiratory monitoring, and non-invasive biomedical diagnostics, where accuracy, stability, and real-time responsiveness are paramount.
Moving forward, future research can explore the scalability of the fabrication process, integration of alternative conductive polymers or metal oxides to fine-tune heterojunction characteristics, and the deployment of advanced machine learning frameworks, such as recurrent neural networks (RNNs) or federated learning, for autonomous multi-sensor networks. Additionally, long-term environmental aging tests and field-deployable prototypes will be essential to fully transition this innovation from laboratory proof-of-concept to real-world application.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Dutta, P. K. et al. IoT revolutionizes humidity measurement and management in smart cities to enhance health and wellness. Mesopotamian J. Artif. Intell. Healthc., 110–117 (2024).
Ku, C. A. & Chung, C. K. Advances in humidity nanosensors and their application. Sensors 23 (4), 2328 (2023).
Hwang, J., Shin, C. & Yoe, H. Study on an agricultural environment monitoring server system using wireless sensor networks. Sensors 10 (12), 11189–11211 (2010).
Jarmuz, P., Barrett, C. B. & Sakkas, D. Continual temperature and humidity measurement allows more efficient and accurate monitoring of incubator performance. Fertil. Steril. 102 (3), e133–e134 (2014).
Sajid, M., Khattak, Z. J., Rahman, K., Hassan, G. & Choi, K. H. Progress and future of relative humidity sensors: A review from materials perspective. Bull. Mater. Sci. 45 (4), 238 (2022).
Nunes, D. et al. Metal oxide nanostructures for sensor applications. Semicond. Sci. Technol. 34 (4), 043001 (2019).
Qian, J. et al. Humidity sensing using polymers: A critical review of current technologies and emerging trends. Chemosensors 12 (11), 230 (2024).
Gu, X. et al. A comprehensive review on Preparation and humidity sensing applications of metal-halide perovskites. Mater. Sci. Eng. B. 311, 117834 (2025).
Abdelnour, R., Bakr, M. & Ali, G. A. Z.H. and Advanced nanomaterials for humidity sensing. In Handbook of Nanosensors. Materials and Technological Applications (575–601). (Springer, 2024).
Kumar, A. et al. SnO. Nanostructured thin film as humidity sens. Its application breath Monit. Ceram. Int. 49 (15), 24911–24921 (2023).
Ponte, R., Rauwel, E. & Rauwel, P. Surface-defect tailoring in SnO2 (CNT) nanomaterials via sol-gel routes and its influence on the cycling stability. J. Mater. Sci., pp. 1–20. (2024).
Kwon, N. & Seo, J. Functionalized polymer-capped SnO2 nanoparticle electron transport layer for efficient perovskite solar cells. Korean J. Chem. Eng., pp. 1–10. (2024).
Bao, Y., Wang, T., Kang, Q., Shi, C. & Ma, J. Micelle-template synthesis of Hollow silica spheres for improving water vapor permeability of waterborne polyurethane membrane. Sci. Rep. 7 (1), 46638 (2017).
Bhuvaneswari, K., Pazhanivel, T., Palanisamy, G. & Bharathi, G. CTAB-aided surface-modified Tin oxide nanoparticles as an enhanced photocatalyst for water treatment. J. Mater. Sci. Mater. Electron. 31, 6618–6628 (2020).
Shao, T. et al. Study on the photocatalytic properties of flower-shaped SnO. Nanomaterials 12 (19), 3419 (2022).
Prayoga, A., Iqbal, M. & Saputro, A. G. CTAB-assisted hydrothermal method for tin oxide preparation as an active materials for ethylene gas detection. J. Phys. 2705 (1), 012001 (2024).
Seekaew, Y., Chin, S. X. & Wongchoosuk, C. Flexible humidity sensor by p-type Co. Nano-Struct. Nano-Objects. 38, 101157 (2024).
Yu, S., Li, P., Ding, H. & Ma, X. Construction of high-performance g-C. Heterojunction humidity sens. Invest. Its application sens. Actuators B Chem. 419, 136392 (2024).
Wang, C. et al. A zno/porous GaN heterojunction and its application as a humidity sensor. Nanoscale Adv. 1 (3), 1232–1239 (2019).
Huang, L. et al. Low-temperature growing anatase TiO multi-dimensional Heterojunctions MXene Conductive Netw high-efficient Perovskite Solar Cells. Nano-micro Lett. 12, 1–19 (2020).
Lee, J., Kim, H., Hilal, M. & Cai, Z. Core-shell SnO. J. Mater. Sci. Mater. Electron. 35 (20), 1421 (2024).
Ozel, K. & Yildiz, A. Estimation of maximum photoresponsivity of n-SnO2/p‐Si heterojunction‐based UV photodetectors. Phys. Status Solidi (RRL)–Rapid Res. Lett., 16, 2, 2100490. (2022).
Serin, T., Yildiz, A. & Serin, N. Electrical properties of polycrystalline SnO. Thin Films Appl. Phys. Express. 4 (12), 121101 (2011).
Ozel, K. & Yildiz, A. The potential barrier-dependent carrier transport mechanism in n-SnO. Sens. Actuators A Phys. 332, 113141 (2021).
Li, S. et al. Self-powered blue-sensitive photodetector based on PEDOT: pss/sno microwires organic/inorganic p-n heterojunction. Appl. Phys. A. 119, 1561–1566 (2015).
Ragab, H. M. et al. High-performance NO2 sensing with SnO2/rGO/PEDOT composite for advanced pollution control applications. Inorgan. Chem. Commun., 114133. (2025).
Kornienko, V. V. et al. Machine learning for optical gas sensing: A leaky-mode humidity sensor as example. IEEE Sens. J. 20 (13), 6954–6963 (2020).
Rodić, L. D., Županović, T., Perković, T., Šolić, P. & Rodrigues, J. J. Machine learning and soil humidity sensing: Signal strength approach. ACM Trans. Internet Technol. (TOIT). 22 (2), 1–21 (2021).
Zhao, Y. et al. Gas-sensing enhancement methods for hydrothermal synthesized SnO. Nanotechnology 28 (45), 452002 (2017).
Xu, X. et al. Highly sensitive VOCs-acetone device based on Ag-decorated SnO hollow nanofibers. J. Alloys Compd. 703, 572–579 (2017).
Suvith, V. S., Devu, V. S. & Philip, D. Facile synthesis of SnO. Struct. Magn. Catalytic Prop. Ceram. Int. 46 (1), 786–794 (2020).
Jayaram, P., Pradyumnan, P. P. & Karazhanov, S. Z. Micro-strain, dislocation density and surface chemical state analysis of multication thin films. Phys. B Condens. Matter. 501, 140–145 (2016).
John, K. I. et al. Unravelling the effect of crystal dislocation density and microstrain of titanium dioxide nanoparticles on Tetracycline removal performance. Chem. Phys. Lett. 776, 138725 (2021).
Ren, H., Zhao, W., Wang, L., Ryu, S. O. & Gu, C. Preparation of porous flower-like SnO micro/nano structures their enhanced gas sens property. . J. Alloys Compd. 653, 611–618 (2015).
Wang, S., Zhu, Y., Liu, B., Wang, C. & Ma, R. Introduction of carbon nanodots into SnO electron transport layer for efficient and UV stable planar perovskite solar cells. J. Mater. Chem. A. 7 (10), 5353–5362 (2019).
Savarimuthu, K., Rajamanickam, G., Shankararajan, R., Perumal, R. & Rayarfrancis, A. Experimental study on flexible ZnO based nanogenerator using Schottky contact for energy harvesting applications. IEEE Trans. Nanotechnol. 16 (3), 469–476 (2017).
Saleh, S. M. ZnO nanospheres based simple hydrothermal route for photocatalytic degradation of Azo dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 211, 141–147 (2019).
da Silva, G. T. et al. Long-and short-range structure of SnO2 nanoparticles: Synthesis photo (electro) catalytic activity. Mater. Chem. Phys. 305, 127989 (2023).
Yoo, K. P. et al. Novel resistive-type humidity sensor based on multiwall carbon nanotube/polyimide composite films. Sens. Actuators B. 145 (1), 120–125 (2010).
Zhang, H., Zhang, H., Man, J. & Chen, C. Preparation of high performance Fe-doped SnO2 humidity sensor and its application in respiration detection. Sens. Actuators A Phys. 362, 114644 (2023).
Pan, S. et al. A sensitive humidity sensor at low pressure with SnO2 QDs. Sens. Actuators A Phys. 346, 113835 (2022).
Wang, G. et al. Fast-response humidity sensor based on laser printing for respiration monitoring. RSC Adv. 10 (15), 8910–8916 (2020).
Khasim, S. et al. PVA treated PEDOT-PSS: TiO2 nanocomposite based high-performance sensors towards detection of relative humidity and soil moisture content for agricultural applications. J. Polym. Environ. 29, 612–623 (2021).
Laera, A. M., Cassano, G., Burresi, E., Protopapa, M. L. & Penza, M. Flexible humidity sensor based on chemically reduced graphene oxide. Chemosensors 12 (12), 245 (2024).
Li, J., Wen, S., Yao, Y., Li, W. & Ling, W. Investigating the impact of crystal face on SnO2/GO-A humidity sensor via adsorption kinetics and DFT calculations. J. Alloys Compd. 1010, 177530 (2025).
Blessi, S., Manikandan, A., Anand, S., Sonia, M. M. L., Vinosel, V. M., Alosaimi, A. M., Khan, A., Hussein, M. A. & Asiri, A. M. et al. Enhanced electrochemical performance and humidity sensing properties of Al3+ substituted mesoporous SnO2 nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 133, 114820 (2021).
Bauskar, D., Kale, B. B. & Patil, P. Synthesis and humidity sensing properties of ZnSnO3 cubic crystallites. Sens. Actuators B Chem. 161 (1), 396–400 (2012).
Begum, S. & Ahmaruzzaman, M. CTAB and SDS assisted facile fabrication of SnO2 nanoparticles for effective degradation of carbamazepine from aqueous phase: A systematic and comparative study of their degradation performance. Water Res. 129, 470–485 (2018).
Yao, X. & Cui, Y. A PEDOT: PSS functionalized capacitive sensor for humidity. Measurement 160, 107782 (2020).
Xie, X. J., Si, R. J., Zheng, J., Wei, K., Zheng, X. Y., Chen, C. & Wang, C. C. et al. Synthesis of ZnO/NiO hollow spheres and their humidity sensing performance. J. Alloys Compd. 879, 160487 (2021).
Zhang, H., Zhang, H., Jia, Z., Chen, C., Yang, C., Dou, Q., Li, X., Ma, X. & Ding, P. et al. Design of humidity sensor based on poly (sodium 4-styrenesulfonate) modified SnO2 for visual monitoring of plant growth environments. Available at SSRN 5115033.
Xia, J., Wang, X., Wang, X., Majer-Baranyi, K. & Zhang, X. Hysteresis dynamic modeling and analysis of flexible nano silver–polyvinyl alcohol humidity sensor based on the microscopic process and Langmuir-Fick theory. ACS Omega. 7 (17), 14994–15004 (2022).
Potharaju, S., Tirandasu, R. K., Tambe, S. N., Jadhav, D. B., Kumar, D. A. & Amiripalli, S. S. et al. A Two-Step machine learning approach for predictive maintenance and anomaly detection in environmental sensor systems. MethodsX, 103181. (2025).
Naser, M. Z. & Alavi, A. H. Error metrics and performance fitness indicators for artificial intelligence and machine learning in engineering and sciences. Archit. Struct. Constr. 3 (4), 499–517 (2023).
Hassan, S. U., Ahamed, J. & Ahmad, K. Analytics of machine learning-based algorithms for text classification. Sustain. Oper. Comput. 3, 238–248 (2022).
Funding
This work received no funding.
Author information
Authors and Affiliations
Contributions
Poundoss Chellamuthu, Kirubaveni Savarimuthu, Gulam Nabi Alsath M: Conceptualization, Methodology, Software, Visualization, Investigation, Writing- Original draft preparation. R. Krishnamoorthy, T. Yuvaraj, Feras Alnaimat: Data curation, Validation, Supervision, Resources, Writing - Review & Editing. Mohammad Shabaz: Project administration, Supervision, Resources, Writing - Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chellamuthu, P., Savarimuthu, K., Alsath, M.G.N. et al. CTAB modified SnO₂ PEDOT PSS heterojunction humidity sensor with enhanced sensitivity stability and machine learning evaluation. Sci Rep 15, 29042 (2025). https://doi.org/10.1038/s41598-025-14184-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14184-9