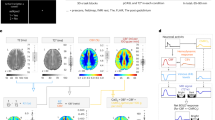
Abstract
Magnetic resonance images of normal brains were analyzed in order to clarify the relationship of resting state BOLD signals in white matter to cortical neural activity. We quantified the degree to which spontaneous activities in the cortex, which are reflected in low frequency fluctuations in BOLD signals from gray matter, modulate corresponding resting state BOLD signals in white matter. The similarity between the resting state BOLD signals from selected cortical regions and white matter voxels, measured using the inner product of their time series, was found to be directly proportional to the BOLD signal power in each cortical volume. From measurements of resting state correlations we find cortical networks supporting more basic level functions tend to contribute more to correlated fluctuations in white matter than those of higher level functions. In addition, each cortical network exhibits a distinct spatial pattern of modulating effects on white matter BOLD signals, and their magnitudes are strongly correlated with the myelination level of the cortical network. Our findings confirm that resting state BOLD signals in white matter encode the spontaneous activity of specific cortical networks and are affected by the cytomyeloarchitecture of the cortex.
Similar content being viewed by others
Introduction
Magnetic resonance imaging (MRI) based on blood-oxygenation-level-dependent (BOLD) signals has become a primary functional neuroimaging technique that is widely used by the neuroscience community. BOLD functional MRI (fMRI) has been used for localizing cortical activities1 and mapping their inter-regional correlations2 but the significance of BOLD effects in white matter has not been recognized to a similar extent3,4. However, there is evidence that metrics of neural activity are encoded in white matter BOLD signals5,6,7,8 which is further supported by white matter structural organization9 glucose metabolism10 energetics11 electrophysiology12 and transcriptomic profiling13. However, concerns persist that the BOLD signal changes in white matter could be driven by physiological or experimental confounds that are not of neural origin3.
In this note, we address some of these concerns by establishing relationships between neural activities within the cortex at rest and corresponding BOLD signals in white matter. Previous reports have demonstrated that the cortex exhibits regionally-varying levels of neural activity with different temporal profiles14 and that neural signals from cortical regions traverse the white matter in patterns that reflect the network architecture of the human brain15,16. Given these findings, we hypothesized that cortical networks showing higher baseline neural activity might modulate BOLD signals in white matter more strongly, and that each network would manifest via distinct patterns of their effects on white matter signals. Here we evaluate these hypotheses and also quantify the influence of cortical myelin content in order to clarify the relationships between resting state cortical activities and white matter BOLD signals.
Results
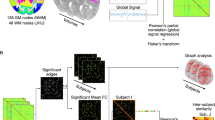
We analyzed functional images of 120 healthy young adults sourced from publicly available data. For each subject, the cortical gray matter was parcellated into 75 regions of interest (ROIs), and the fractional amplitude of low-frequency fluctuations (fALFF) of BOLD signals was computed for each ROI (see Methods for details). Figure 1a shows the relationship between the fALFF of cortical BOLD signals and their mean white matter projections (i.e. the mean value of the inner products of the BOLD time series from each gray ROI and those from white matter voxels). For the 75 ROIs studied, the mean white matter projection averaged over the 120 subjects studied was strongly correlated (r = 0.8110, p < 0.0001) with the subject-averaged fALFF (see Supplementary Fig. S1 in Supplementary Information for correlation results from each individual subject). To examine whether the observed correlations were spurious, we randomly perturbed the time order of BOLD signals in white matter and repeating the above analysis with 60 subjects, which yielded a mean r=-0.1967 ± 0.2665 (see Supplementary Fig. S2 in Supplementary Information for detailed results).
(a) Relationship between subject-averaged fALFF of cortical BOLD signals and their subject-averaged mean white matter projection. Each data point represents subject-averaged measures for an ROI in the cortex. (b) Mean white matter projection of BOLD signals in the cortical functional networks analyzed. The vertical line at the top of each bar represents standard error across the 120 subjects studied. Abbreviations: prim = primary, DMN = default mode network. LECN = left executive control network. RECN = right executive control network.
In a control experiment using signal from skull bone marrow, we excluded datasets with visible image distortion or insufficient thickness of bone marrow in the skull, and thus ended up with 24 subjects with robustly defined skull bone marrow (see Supplementary Fig. S3a–e for a typical example of bone marrow voxel locations). Applying the same procedure as for white matter (without correction for time delay) the correlation coefficient between the fALFF of cortical BOLD signals and their mean bone marrow projection was r = 0 (see Supplementary Fig. S3f). On the individual subject level (see Supplementary Fig. S4 for definitions of skull bone marrow for individual subjects), the mean correlation coefficient was r = 0.1310 ± 0.2445.
A detailed regional analysis reveals that BOLD signals in 13 derived cortical functional networks had varying levels of white matter projection, and overall, those of more basic level functions (primary/higher visual, auditory and sensorimotor) had greater signal projection onto white matter than those of higher level functions (see Fig. 1b).
We also computed spatial distributions of BOLD signal projections in white matter for the cortical functional networks. Each network produced a distinct spatial pattern in white matter, with high projection regions generally close to the network cortices. Remote white matter projections were also commonly seen, indicating BOLD effects transferred through white matter to remote cortices within the network or to other related networks. To illustrate the distinctness of the spatial distribution patterns of individual networks, the distribution maps of white matter projections of the primary visual, sensorimotor and precuneus networks are compared in Figs. 2, 3 and 4 (see Supplementary Fig. S5 in Supplementary Information for distribution maps of all the 13 cortical networks studied). It can be appreciated that these cortical networks tended to have greater signal projection respectively along the optic radiations (primary visual), along the projection pathways (sensorimotor), and in the parietal lobe (precuneus).
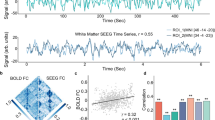
Spatial distributions of white matter projections of BOLD signals from the primary visual network. Data are averaged across the 120 subjects, and dark gray color denotes cortical regions of the primary visual network. The red and blue curves in the bottom row are 0.01–0.15 Hz bandpass filtered BOLD time courses of one subject at the gray and white matter locations pointed by the red and blue arrows respectively. Note that the value of white matter projections across the brain has been normalized into zero mean and unit variance, which is then capped to the range [-3 3].
Spatial distributions of white matter projections of BOLD signals from the sensorimotor network. Data are averaged across the 120 subjects, and dark gray color denotes cortical regions of the sensorimotor network. The red and blue curves in the bottom row are 0.01–0.15 Hz bandpass filtered BOLD time courses of one subject at the gray and white matter locations pointed by the red and blue arrows respectively. Note that the value of white matter projections across the brain has been normalized into zero mean and unit variance, which is then capped to the range [-3 3].
Spatial distributions of white matter projections of BOLD signals from the precuneus network. Data are averaged across the 120 subjects, and dark gray color denotes cortical regions of the precuneus network. The red and blue curves in the bottom row are 0.01–0.15 Hz bandpass filtered BOLD time courses of one subject at the gray and white matter locations pointed by the red and blue arrows respectively. Note that the value of white matter projections across the brain has been normalized into zero mean and unit variance, which is then capped to the range [-3 3].
We further compared the white matter projection maps of the three cortical networks with their corresponding structural connectivity maps (shown in Figs. 5, 6 and 7) generated by diffusion-based fiber tracking (see Methods for technical details). Upon close inspection it can be seen that the structural connectivity maps appear grossly similar to the spatial distributions of medium to high white matter projections (with color value of ~ 0–3) for these cortical networks. When the normalized projection value is thresholded at 0 and the structural connectivity value is thresholded at 20, the Dice coefficient between the two maps is 0.8133, 0.8394 and 0.8244 for the primary visual, sensorimotor and precuneus networks respectively.
The myelin content across the mid-thickness cortical surface is graphically rendered in Fig. 8a. In keeping with several other reports17,18 the cortical regions of heavier myelination tended to concentrate in the occipital, temporal and parietal lobe, where the primary/higher visual, auditory and sensorimotor networks reside. Figure 8b shows the mean myelin content for the 13 cortical networks analyzed. Networks engaged in more basic functions tended to have heavier myelination than those of higher level functions. The cortical myelin content is correlated against the mean white matter projection of cortical BOLD signals in Fig. 8c. There was a strong positive correlation between the two measures (r = 0.7199, p < 0.0001), suggesting that the cortical networks of heavier myelination tended to impart greater signals to white matter.
(a) Mid-thickness cortical surface map of mean myelin content across the 120 subjects studied. Left: lateral surface of the right hemisphere. Right: medial surface of the right hemisphere. (b) Mean myelin content in the cortical functional networks analyzed. The vertical line at the top of each bar represents standard error across the 120 subjects studied. Abbreviations: prim = primary, DMN = default mode network. LECN = left executive control network. RECN = right executive control network. (c) Relationship between myelin content and mean white matter projection of BOLD signals in the cortical functional networks analyzed. Each data point represents the subject-averaged measures for a cortical functional network.
Discussion
We calculated the projection of BOLD signals in defined cortical ROIs onto resting state BOLD signals in white matter, and averaged their values across ROIs engaged in specific networks to quantify the influence of cortical activity on white matter BOLD signals. Detailed regional analysis shows that different cortical networks modulate white matter BOLD signals differently, with each network exhibiting distinct, structurally interpretable, spatial distribution patterns of signal contributions. Quantitatively, the cortical networks of more basic functions tend to exert stronger influence on BOLD signals in white matter than those of higher level functions at rest. This spectrum of resting state activity agrees well with cortical myelin distributions observed in this study, and with previous reports on cortical structural connectivity profiles19 functional connectivity gradients20,21 cortical microstructure gradients22 and evolutionary expansion patterns23.
While our findings on the relationships between resting state cortical activities and white matter signals are consistent with other lines of independent evidence, further exploratory analysis into subcortical structures might provide additional insights. To this end, we analyzed resting state BOLD signals in the basal ganglia using the same imaging datasets as for the cortical networks, and found that their mean white matter projection is smaller than that of all the 13 cortical networks studied (see Supplementary Fig. S6 in Supplementary Information). This suggests that fluctuations in white matter BOLD signals are primarily driven by functionally more specialized cortical activities rather than by subcortical activities.
Low frequency fluctuations in cortical BOLD signals are closely related to spontaneous neural activity2 and our findings from this study confirm that fluctuations in white matter BOLD signals encode neural activity similarly to gray matter. The observation that components of white matter BOLD signals are closely associated with spontaneous activities of individual cortical networks, which exhibit network-specific spatial distribution patterns, can be most reasonably explained as being driven by neural activities rather than other mechanisms. However, there are potential concerns of signal contamination from nearby gray matter, which could arise from limited imaging resolution, imperfect image registration, spatial smoothing and other technical issues. This study performed a voxel-wise analysis with conservative white matter masking which should largely ameliorate these concerns. In addition, effects of progressively tighter white matter masking were also examined, and these showed that the relationship between fALFF and mean white matter projection of cortical BOLD signals continued to hold well. In particular, when white matter was masked to contain only a small deep region with a total of 57 voxels, where signal contaminations from gray matter could be certainly ignored, the correlation as we observed in Fig. 1a was still as high as r = 0.7108 (see Supplementary Fig. S7 in Supplementary Information for detail), although the signal-to-noise ratio in this small deep white matter region was lower.
Another potential concern is that the relationship we observed between BOLD signals in the cortex and white matter might be mediated by global signal changes across the brain rather than by network-specific neural signals. The issue of global signal has been quite perplexing within the fMRI field as inferences regarding brain connectivity profiles are sensitive to whether or not the global signal is regressed out24,25. Recognizing that global signal computed from brain parenchyma may contain neural information26 we chose to regress out the mean cerebrospinal fluid signal (CSF) in this work, with the expectation that the effect of global signal is reduced with a minimal loss of neural information8,27. Notwithstanding the regression of CSF signal (along with head movements, cardiac and respiratory signals and linear temporal trends), it is still possible that residual global signal, from low-frequency drifts and imaging system instabilities or changes in brain physiology26 might have created artificial correlations between the cortex and white matter. To examine this possibility, we used skull bone marrow, which contains no neural activity, as a control tissue for white matter, and correlated signal fluctuations in the bone marrow against cortical fALFF, which yielded zero correlation coefficient (r = 0, p = 0.7282). In addition, we correlated the projection of cortical BOLD signals in white matter against cortical fALFF without timing correction and found that, on average, the correlation coefficient was reduced ~ 6%. Mechanistically, the effect of global signal would have been more pronounced without any timing corrections to a particular tissue type. Therefore, this finding, along with that from our bone marrow experiment, have basically ruled out the global signal as a major contributor to the correlation patterns observed in this work.
Finally, there is a possibility that the fluctuations in white matter BOLD signals might be driven by effects of vessels draining from upstream gray matter. This is however unlikely because gray matter and white matter have two distinct venous systems28 and arterial supplies29 that have no mutual spatial overlap. Moreover, an earlier finding that the brain parenchyma has quite uniform oxygen extraction fraction is indeed a counter-argument against the vessel draining speculation30. Nonetheless, to fully eliminate the possibility of vessel draining effects, additional in vivo human experiments are needed but clearly justified.
Methods
Image data and study subjects
This study used publicly available data (the Human Connectome Project (HCP) database31, from which images of 120 young adults (female = 60, age range = 26–35 years) were randomly selected. The resting-state fMRI data used in this study were acquired with multiband gradient-echo echo‐planar imaging sequences with the following parameters: repetition time (TR) = 720 ms, echo time = 33.1 ms, voxel size = 2 × 2 × 2 mm3 number of dynamics (Nd) = 1200. The fMRI data were minimally preprocessed32 and were further processed in this study to regress out nuisance variables from head movements, cardiac and respiratory signals33 and the mean CSF signal, followed by removal of linear temporal trends.
Image data analysis
Power spectra of cortical BOLD signals
The gray matter of each study subject was parcellated into 85 functional ROIs using an atlas defined previously34. These ROIs were confined to a common gray matter mask created using the MNI152 template. Note that the original atlas contains 90 Gy matter ROIs, but five of them that reside in the basal ganglia network were excluded from our analysis. Also, ten cortical ROIs had insufficient numbers of voxels in the region (< 7) and thus were further excluded so that only 75 cortical ROIs entered our subsequent analysis. Second, the power spectra of BOLD signals were computed for each of the cortical ROIs using Welch’s method35. Briefly, the BOLD time series from each voxel was segmented into eight consecutive and overlapping blocks, from which a periodogram was derived for each block and these were then averaged across the blocks. From the averaged periodogram, the fALFF was calculated as the ratio of average power across the frequency range of 0.01–0.08 Hz to the average power across all frequencies above 0.01 Hz. Finally, the resulting fALFF values were averaged across each of the cortical ROIs.
Projections of cortical BOLD signals onto white matter
A common white matter mask was created using the MNI152 template with a tight threshold of 0.8. To avoid signal contaminations from nearby gray matter, the most superficial white matter was eroded by using a structuring element of six directly adjacent neighbors. BOLD signals in the eroded white matter mask were then slightly smoothed with an FWHM = 2 mm Gaussian kernel, bandpass filtered to retain signals in the frequency range from 0.01 to 0.15 Hz, and normalized into unit variance. Meanwhile, cortical BOLD signals were similarly bandpass filtered and normalized.
For each voxel in the cortical ROIs, the inner product of its BOLD time series and that in a white matter voxel was computed to represent the projection of the cortical BOLD time series onto the white matter voxel. Mathematically, if gmTS = (gmTS(1), gmTS(2), … gmTS(Nd)) and wmTS = (wmTS(1), wmTS(2), … wmTS(Nd)) denote the normalized time series in a cortical and white matter voxel respectively, the inner product between them (Pgm_wm) is defined as:
Geometrically, Pgm_wm measures the amount that the vector gmTS projects onto wmTS in an Nd-dimensional space. This projection, or equivalently inner product, is the same as the cross-correlation at zero lag. To take into account possible time delays in white matter BOLD signals8 progressive delays of 1–10 TR were added in moving from superficial to deep white matter prior to computations of the signal projections. This process yielded a (time delay corrected) white matter projection map for each voxel in the cortical ROIs. The average of the white matter projection maps for all voxels in a cortical ROI was defined to be the white matter projection map of the ROI.
Projections of cortical BOLD signals onto skull bone marrow
As a control experiment for white matter, a portion of bone marrow in the skull was analyzed using the same procedure as above. Specifically, a cuboid region that contained 5 × 16 × 5 voxels was first defined in each side of the head in temporally averaged BOLD data. These voxels were thresholded such that each cuboid retained 60 voxels of high intensity value, which were used as bone marrow control voxels. BOLD time series from the cortical ROIs were then projected onto these control voxels.
Network-based analysis
The 75 cortical ROIs were grouped into 13 functional networks34 which contained basic functional networks (such as auditory and visual circuits) as well as cognitive networks (such as language and default mode network). A projection map was derived for each of these networks by averaging the white matter projection maps of all the cortical ROIs within the network, which was rescaled by voxel-wise normalization of the summed projection across the 13 networks into unity.
Computation of network-specific structural connectivity maps
To assist anatomically interpreting the white matter projection maps, we tracked fibers from a group-average template that was constructed from 1065 diffusion scans in the HCP database. The diffusion scans used a multi-shell diffusion scheme with b-values of 990, 1985 and 2980 s/mm2 along each of 90 diffusion sampling directions. The voxel size was 1.25 mm isotropic, which was resampled to 2.0 mm isotropic in the MNI space using q-space diffeomorphic reconstruction36 to obtain the spin distribution function37 with a restricted diffusion model38.
Three sets of seed ROIs, namely Brodmann Area 17, Brodmann Areas 1–4 and Brodmann Area 7, were defined for the primary visual, sensorimotor and precuneus networks respectively. For each network, a total of 1,000,000 random samples were drawn in the seed ROI, from which deterministic fiber tracking39 was performed with augmented tracking strategies40. The tracking step size was one voxel, the anisotropy threshold was randomly selected between 0.5 and 0.7 otsu threshold41 and angular threshold was randomly chosen in 45–90°. Tracts with length < 30 mm or > 200 mm were discarded. Finally, structural connectivity maps were computed for each network as voxel-wise distributions of the number of fiber tracts tracked from the seed ROI.
The similarity between the structural connectivity and white matter projection maps was measured using Dice coefficients, which are defined as 2(|A∩B|/(|A|+|B|)), where |X| denotes the number of voxels in X, and ∩ denotes the intersection operation. Note that, in this study, all image slices containing the brainstem were excluded from the Dice coefficient computation.
Quantification of cortical Myelin content
Cortical myelin maps of the selected subjects were sourced from the same datasets as the fMRI images, and the mean myelin content for each of the 13 functional networks in each subject was calculated by averaging across the network.
Data availability
Processed data are available through request to the corresponding author Zhaohua Ding. Data in the supplementary information will be available online after acceptance for publication.
References
Ogawa, S., Lee, T. M., Kay, A. R. & Tank, D. W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. 87, 9868–9872 (1990).
Fox, M. D. & Raichle, M. E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711 (2007).
Gawryluk, J. R., Mazerolle, E. L. & D’Arcy, R. C. Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Front. NeuroSci. 8, 239 (2014).
Gore, J. C. et al. Functional MRI and resting state connectivity in white matter-A mini-review. Magn. Reson. Imaging 63, 1–11 (2019).
Peer, M., Nitzan, M., Bick, A. S., Levin, N. & Arzy, S. Evidence for functional networks within the human brain’s white matter. J. Neurosci. 37, 6394–6407 (2017).
Ji, G. J., Liao, W., Chen, F. F., Zhang, L. & Wang, K. Low-frequency blood oxygen level-dependent fluctuations in the brain white matter: More than just noise. Sci. Bull. 62, 656–657 (2017).
Ding, Z. et al. Detection of synchronous brain activity in white matter tracts at rest and under functional loading. Proc. Natl. Acad. Sci. 115, 595–600 (2018).
Li, M., Newton, A. T., Anderson, A. W., Ding, Z. & Gore, J. C. Characterization of the hemodynamic response function in white matter tracts for event-related fMRI. Nat. Commun. 10, 1140 (2019).
Wang, P. et al. The organization of the human corpus callosum estimated by intrinsic functional connectivity with white-matter functional networks. Cereb. Cortex. 30, 3313–3324 (2020).
Guo, B., Zhou, F., Li, M., Gore, J. C. & Ding, Z. Correlated functional connectivity and glucose metabolism in brain white matter revealed by simultaneous mri/positron emission tomography. Magn. Reson. Med. 87, 1507–1514 (2022).
Harris, J. J. & Attwell, D. The energetics of CNS white matter. J. Neurosci. 32, 356–371 (2012).
Huang, Y. et al. Intracranial electrophysiological and structural basis of BOLD functional connectivity in human brain white matter. Nat. Commun. 14, 3414 (2023).
Li, J. et al. Transcriptomic and macroscopic architectures of intersubject functional variability in human brain white-matter. Commun. Biol. 4, 1417 (2021).
Biswal, B. B. et al. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. 107, 4734–4739 (2010).
Gonzalez-Castillo, J. et al. Whole-brain, time-locked activation with simple tasks revealed using massive averaging and model-free analysis. Proc. Natl. Acad. Sci. 109, 5487–5492 (2012).
Schilling, K. G. et al. Whole-brain, gray, and white matter time-locked functional signal changes with simple tasks and model-free analysis. Proc. Natl. Acad. Sci. 120, e2219666120. https://doi.org/10.1073/pnas.2219666120 (2023).
Glasser, M. F. & Van Essen, D. C. Mapping human cortical areas in vivo based on Myelin content as revealed by T1-and T2-weighted MRI. J. Neurosci. 31, 11597–11616 (2011).
Toschi, N. & Passamonti, L. Intra-cortical Myelin mediates personality differences. J. Pers. 87, 889–902 (2019).
Hagmann, P. et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 6, e159 (2008).
Margulies, D. et al. (ed, S.) Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. 113 12574–12579 (2016).
Huntenburg, J. M., Bazin, P. L. & Margulies, D. S. Large-scale gradients in human cortical organization. Trends Cogn. Sci. 22, 21–31 (2018).
Huntenburg, J. M. et al. A systematic relationship between functional connectivity and intracortical Myelin in the human cerebral cortex. Cereb. Cortex 27, 981–997 (2017).
Hill, J. et al. Similar patterns of cortical expansion during human development and evolution. Proc. Natl. Acad. Sci. 107, 13135–13140 (2010).
Murphy, K., Birn, R. M., Handwerker, D. A., Jones, T. B. & Bandettini, P. A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44, 893–905 (2009).
Murphy, K. & Fox, M. D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage 154, 169–173 (2017).
Liu, T. T., Nalci, A. & Falahpour, M. The global signal in fMRI: Nuisance or information?? Neuroimage 150, 213–229 (2017).
Biswal, B. et al. Transcriptomic, cellular, and functional signatures of white matter damage in alzheimer’s disease. (2024).
San Millán Ruíz, D., Yilmaz, H. & Gailloud, P. Cerebral developmental venous anomalies: Current concepts. Ann. Neurol. Official J. Am. Neurol. Assoc. Child. Neurol. Soc. 66, 271–283 (2009).
Akashi, T. et al. Ischemic white matter lesions associated with medullary arteries: classification of MRI findings based on the anatomic arterial distributions. Am. J. Roentgenol. 209, W160–W168 (2017).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl. Acad. Sci. 98, 676–682 (2001).
Van Essen, D. C. et al. The human connectome project: A data acquisition perspective. Neuroimage 62, 2222–2231 (2012).
Glasser, M. F. et al. The minimal preprocessing pipelines for the human connectome project. Neuroimage 80, 105–124 (2013).
Kasper, L. et al. The physio toolbox for modeling physiological noise in fMRI data. J. Neurosci. Methods 276, 56–72 (2017).
Shirer, W. R., Ryali, S., Rykhlevskaia, E., Menon, V. & Greicius, M. D. Decoding subject-driven cognitive States with whole-brain connectivity patterns. Cereb. Cortex 22, 158–165 (2012).
Welch, P. The use of fast fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 15, 70–73 (1967).
Yeh, F. C., Tseng, W. Y. & I. NTU-90: A high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage 58, 91–99 (2011).
Yeh, F. C., Wedeen, V. J. & Tseng, W. Y. I. Generalized ${q} $-sampling imaging. IEEE Trans. Med. Imaging 29, 1626–1635 (2010).
Yeh, F. C., Liu, L., Hitchens, T. K. & Wu, Y. L. Mapping immune cell infiltration using restricted diffusion MRI. Magn. Reson. Med. 77, 603–612 (2017).
Yeh, F. C., Verstynen, T. D., Wang, Y., Fernández-Miranda, J. C. & Tseng W.-Y. I. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PloS One 8, e80713 (2013).
Yeh, F. C. Shape analysis of the human association pathways. Neuroimage 223, 117329 (2020).
Otsu, N. A threshold selection method from gray-level histograms. Automatica 11, 23–27 (1975).
Acknowledgements
National Institutes of Health grant R01 NS129855 (ZD). National Institutes of Health grant R01 NS113832 (JCG). National Institutes of Health grant R01 MH123201 (JCG). National Institutes of Health grant R21 AG083915 (YG).
Author information
Authors and Affiliations
Contributions
Z.D. and J.C.G. conceptualized and designed the experiments. L.X., Y.T. and M.L. performed the experiments, Y.G., Y.Z. and A.W.A. contributed to data analysis. Z.D. and J.C.G. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary Material 2
Supplementary Material 4
Supplementary Material 5
Supplementary Material 7
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ding, Z., Xu, L., Gao, Y. et al. Cortical modulation of resting state BOLD signals in white matter. Sci Rep 15, 30056 (2025). https://doi.org/10.1038/s41598-025-14352-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14352-x