Abstract
This study evaluated the acute cardiovascular responses to resistance exercise (RE) sessions with different set configurations in normotensive and hypertensive postmenopausal females. 50 physically active postmenopausal females performed a control (CON) and three RE sessions matched for total volume (144 repetitions), load (12-repetition maximum load), and total rest time (360s) but differing in set configuration: 4 sets of 9 repetitions with 120 s rest (9 S); 6 sets of 6 repetitions with 72 s rest (6 S); and 9 sets of 4 repetitions with 45 s rest (4 S). Heart rate (HR) was recorded during exercise, while HR variability, baroreflex sensitivity, arterial stiffness, and blood pressure were assessed before and after each session. Peak and mean HR values were higher during exercise in 9 S (p ≤ 0.026). All RE protocols induced cardiac parasympathetic withdrawal, reduction in baroreflex sensitivity, and increased post-exercise arterial stiffness compared to CON. However, in 4 S, cardiac parasympathetic withdrawal and baroreflex impairment were attenuated without a significant increase in arterial stiffness. Additionally, a post-exercise hypotensive response was observed only after 9 S in hypertensive participants (p = 0.004). Shorter set configurations attenuate chronotropic response during RE and mitigate impairments in cardiac autonomic and baroreflex control following RE sessions, without affecting arterial stiffness or blood pressure.
Similar content being viewed by others
Introduction
Hormonal changes during menopause negatively impact women’s health, increasing cardiometabolic risk stemming from increases in body weight and central fat accumulation, worsening cardiac health, decreased insulin sensitivity, and impaired vascular function. This period is also characterized by accelerated bone loss, reduced muscle mass, decreased physical activity, and neuropsychological symptoms, which together also contribute to compromising balance, mobility, and overall quality of life1,2,3,4,5. In this context, physical exercise, particularly resistance training, is an effective strategy to mitigate or slow these changes in postmenopausal women6,7,8,9,10,11,12.
Robust evidence supports resistance training’s multifactorial benefits on metabolic, neuromuscular, cardiovascular, and functional aspects, and highlights its association with reduced risk and mortality from major non-communicable diseases13,14,15,16. However, concerns persist about its safety in certain populations (i.e., populations at cardiovascular risk), due to the abrupt hemodynamic response during resistance exercise (RE)17 and cardiac parasympathetic withdrawal post-exercise, as noted in recent review articles18,19. These responses depend on load parameters, including muscle mass involved, contraction intensity, exercise duration, recovery periods, time under tension, and the Valsalva maneuver19,20,21,22,23,24.
Set configuration, defined as the percentage of performed repetitions relative to the maximum possible with a given load, modulates cardiovascular responses to RE25. Studies in young, physically active individuals show that shorter set configurations (< 40% of maximum repetitions) attenuate hemodynamic responses and post-exercise reductions in cardiac autonomic and baroreflex control26,27,28,29,30,31,32,33,34,35. Conversely, longer set configurations may enhance post-exercise hypotension, particularly in hypertensive individuals29,36. However, most studies have focused primarily on young, active males, with women often underrepresented. This underrepresentation contributes to the sex data gap37and limits the applicability of findings to female populations, particularly in the context of cardiovascular health2. Given that menopause is associated with deterioration in autonomic and cardiovascular function2,4it is plausible that set configuration may exert differential effects in postmenopausal women. Exploring these effects in this population is therefore critical to inform tailored exercise prescriptions aimed at mitigating cardiovascular risk.
Only one study has examined set configuration effects in normotensive and hypertensive postmenopausal females38 but it did not assess key physiological mechanisms such as cardiac autonomic control and baroreflex function. Since menopause-related changes overlap with aging and sedentary lifestyle effects, analyzing acute cardiovascular responses in physically active postmenopausal women in early menopause is crucial to better characterize the specific physiological responses associated with this period.
This study aimed to comprehensively characterize the acute cardiovascular responses of normotensive and hypertensive postmenopausal females to RE sessions with different set configurations. Based on prior research, we hypothesized that shorter set configurations would minimize reductions in cardiac autonomic and baroreflex control, whereas longer configurations would enhance post-exercise hypotension, particularly in hypertensive postmenopausal women.
Methods
Participants
Fifty physically active postmenopausal women (37 normotensive, 13 hypertensive; Supplementary Table S1) were recruited from sports facilities. A medical consultation confirmed eligibility based on: (i) age 50–65 years; (ii) ≥ 12 months since last menstruation; (iii) physically active (150–300 min/week moderate or ≥ 75 min/week vigorous activity); (iv) ≤ 3 cardiovascular risk factors; (v) no cardiovascular (except hypertension), metabolic, or renal diseases. Hypertensive participants had well-controlled grade 1 hypertension on a single medication. Exclusion criteria: (i) grade 2–3 hypertension; (ii) hypertension managed with ≥ 2 medications or drugs affecting cardiovascular responses (e.g., beta-blockers); (iii) current/past hormone replacement therapy; (iv) hypertensive response to exercise. Participants provided written informed consent. The study was approved by the regional ethics committee (Galicia-Spain; ref. number: 2022/313) and adhered to the Declaration of Helsinki.
A priori power analysis was conducted using G*Power v3 (Universität Kiel, Düsseldorf, Germany). Considering the research design, the sample size calculation was carried out for a statistical power of 80% (1– β) and assumed Type Error I of 0.05 in order to detect a small effect size (f = 0.12) for the interaction between the within-subject factor (i.e., set configuration 1–3 and control session) and between-subjects factor (i.e., normotensive and hypertensive groups) in an Analysis of Variance test (ANOVA), and assuming a correlation of 0.75 between repeated measures. As a result of this analysis, a total sample size of 25 women per group (i.e., a total sample of 50 participants) would be sufficient to detect small set configuration × group interaction effects.
Study design
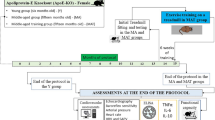
A randomized crossover design examined the acute effects of set configuration on cardiovascular responses during a resistance exercise (RE) session with multiple exercises. This study is part of a clinical trial registered on December 7, 2022, at ClinicalTrials.gov under the registration number NCT05544357 (https://clinicaltrials.gov/study/NCT05544357). The study was conducted from February 3, 2023, to December 22, at the facilities of the Faculty of Sports Sciences and Physical Education of the University of A Coruna, in Spain. Participants attended the lab on eight days, with ≥ 72-hour intervals. The protocol included medical evaluation, familiarization, two 12-repetition maximum (12RM) test sessions, three experimental RE sessions with different set configurations, and a control session (CON). A schematic representation is shown in Fig. 1A. Further details on procedures are available in the full protocol39.
RE sessions consisted of four exercises performed in the following order: (a) horizontal leg press (N956, SportsArt, Leggia, Switzerland), (b) bench press (Multipower Smith Machine 4SHO004/0, Telju Fitness, Toledo, Spain), (c) prone leg curl (BT101, Biotech Fitness Solutions, Brazil), and (d) lat pull-down (BT6215, Biotech Fitness Solutions, Brazil).
Medical evaluation
After initial screening, a treadmill ergometry test following the Bruce protocol40 was conducted. BP was measured at each stage’s end with a sphygmomanometer (Minimus® II, Rudolf Riester GmbH, Jungingen, Germany) to detect hypertensive responses. Maximal oxygen consumption was assessed using a portable gas analyzer (MetaMax3B; Cortex Biophysik, Leipzig, Germany)41.
Familiarization session
The familiarization session included anthropometric and body composition assessments. Body mass and body composition (fat mass, fat-free mass, and visceral fat) were measured using a digital multi-frequency bioelectrical impedance scale (Omron BF-508, Omron Healthcare Co., Kyoto, Japan). Height was measured with a stadiometer (Seca 202, Seca Ltd., Hamburg, Germany), and body mass index was calculated as kg/m². Bone mineral density was assessed via ultrasound densitometer (Sonost 3000, Osteosys Corp., Seoul, South Korea). Participants were then familiarized with the exercises, with individual machine adjustments to standardize range of motion. After a warm-up (5 min cycling at 60–80 rpm on a cycle ergometer [Monark 828E; Monark Exercise AB, Vansbro, Sweden] and joint mobilization), they performed three sets of each exercise with progressively increasing loads and 90 s rest between sets42.
12RM test
During the third and fourth sessions, the 12-repetition maximum (12RM) load (i.e., the maximum load a participant can lift no more than 12 times) was determined following Iglesias-Soler et al.42. After a general warm-up, participants performed two sets of 12 repetitions at 50% of the estimated 12RM load, with a 2-minute rest between sets. The 12RM load was then determined within one to four attempts, following the previously described exercise sequence, with 6-minute rest periods between attempts. The highest load lifted in each exercise during the two sessions was recorded as the 12RM load. This procedure has shown high reliability in this population42.
Experimental and CON sessions
All participants completed three resistance exercise (RE) sessions and a control session (CON) in a randomized order. The randomization was performed using a randomized block design in a spreadsheet software. They were instructed to: (i) fast for at least 2 h, (ii) avoid high-intensity exercise for 24 h before each session, and (iii) refrain from consuming caffeinated beverages, drugs, or alcohol for at least 8 h prior. Participants were also advised to maintain stable hydration and dietary habits throughout the study. Cardiovascular assessments were performed before and after each protocol.
In the experimental sessions, participants began the RE protocol with a general warm-up, followed by 5 min of core stability and strength exercises. As a specific warm-up, they performed one set of 10 repetitions of each exercise at 70% of their 12RM load. All experimental sessions maintained the same total volume for each exercise (repetitions × kg) and an equivalent work-to-rest ratio, but with different set configurations: 9 sets of 4 repetitions per exercise (33% intensity of effort; 4 S) with 45 s of inter-set recovery; 6 sets of 6 repetitions per exercise (50% intensity of effort; 6 S) with 72 s of inter-set recovery; and 4 sets of 9 repetitions (75% intensity of effort; 9 S) with 120 s of inter-set recovery. A 4-minute rest period between exercises was established. In the CON session, participants remained seated at rest for 45 min (Fig. 1B). All sessions were separated by at least 72 h and performed at the same time of day (± 1 h) to minimize circadian rhythm effects on cardiovascular outcomes.
Physiological data recording
Both the experimental and control (CON) sessions began with a cardiovascular assessment at rest. Participants lay on a stretcher in a supine position with a 45° trunk inclination in a quiet room, remaining silent and avoiding voluntary movement. Cardiovascular variables were recorded for 15 min using the Task Force® Monitor (CNSystems, Graz, Austria)43. Heart rate (HR) was measured with a three-lead electrocardiogram at 1000 Hz, while beat-by-beat blood pressure (BP), including systolic (sBP) and diastolic BP (dBP), was recorded via photoplethysmography. Finger cuffs were placed on the proximal phalanx of the index and middle fingers of the right hand, positioned at the level of the fourth intercostal space. Following this, the stretcher was adjusted to a fully horizontal position for arterial stiffness (AS) assessment. AS was measured using the pOpmètre device (Axelife SAS, Saint-Nicolas-de-Redon, France), which employs photodiode sensors on the finger and toe. This procedure requires a prior BP measurement for device calibration44obtained using an oscillometric device (Omron MIT Elite Plus, Kyoto, Japan) with the cuff on the left arm. At least two AS measurements were performed, ensuring a maximum difference in finger-to-toe pulse wave velocity (ft-PWV) of 0.5 m·s−1. If this criterion was not met, a third measurement was taken. In a previous study, this device demonstrated acceptable agreement between ft-PWV values obtained in postmenopausal women and carotid-femoral pulse wave velocity values recorded using the SphygmoCor system, which is considered the non-invasive gold standard45. In contrast to SphygmoCor, the simplicity of the ft-PWV technique allows for immediate post-exercise assessment of AS, making it more appropriate for protocols evaluating acute effects.
During RE sessions, HR was continuously monitored using a chest-strap HR monitor (Polar H10; Polar Electro Oy, Kempele, Finland) and recorded via the mobile application Elite HRV44. After completing the RE protocol and CON session, cardiovascular assessments were conducted for 50 min. AS evaluation was performed immediately after the session and again 60 min post-exercise (Fig. 1C).
Data analysis
During the RE protocols, peak heart rate (HRpeak) and mean heart rate (HRmean), including both exercise and recovery periods, were calculated. Cardiac autonomic modulation was assessed through heart rate variability (HRV) analysis46 using linear (time- and frequency-domain) and non-linear methods. Time-domain variables included the standard deviation of R-R intervals (SDNN) and the square root of the mean squared differences between adjacent R-R intervals (RMSSD). In the frequency domain, high-frequency power (HF; 0.15–0.4 Hz) in absolute units (ms²) was obtained using the Fast Fourier Transform method. Additionally, sample entropy (SampEn) was calculated as an indicator of HR signal complexity.
All variables were derived using Kubios HRV software v3.4.2 (The Biomedical Signal and Medical Imaging Analysis Group, Department of Applied Physics, University of Kuopio, Finland) after applying automatic artifact correction (medium correction threshold) and a detrending process using the smooth priors method (Lambda: 500). The mean artifact correction was 0.36 ± 1.22%. All HRV variables were analyzed over 5-minute intervals, as recommended for short-term recordings46. The last 10 min of baseline recording were analyzed before the sessions (two intervals: Pre5-10 and Pre10-15). Post-exercise data were analyzed for 45 min across nine intervals: Post5-10, Post10-15, Post15-20, Post20-25, Post25-30, Post30-35, Post35-40, Post40-45, and Post45-50.
Baroreflex sensitivity (BRS) was quantified using the sequence method47,48. The following BRS variables were analyzed: (i) the mean slope of detected sequences (BRSslope), (ii) the total number of sequences (BRSevents), and (iii) the baroreflex effectiveness index (BEI), which represents the percentage of times the baroreflex is actively regulating HR in response to sBP oscillations49. A reduced BEI is associated with baroreflex dysfunction50. Additionally, low-frequency activity (0.04–0.15 Hz) of sBP (LFsBP) in absolute units (ms²), calculated using the autoregressive spectral method, as well as standard deviation of both sBP (SDsBP) and mean arterial BP (SDMAP) were obtained to estimate sympathetic vasomotor tone51,52. Data analysis of BRS variables, LFsBP, SDsBP and SDMAP was performed using TFM software v2.3 (CNSystems, Graz, Austria) over a 10-minute interval53. The analysis included one pre-exercise interval (Pre5-15) and four post-exercise intervals: Post5-15, Post15-25, Post30-40, and Post40-50.
Arterial stiffness (AS) was assessed through foot-toe pulse wave velocity (ft-PWV) and the ankle-brachial index (ABI) using pOplog® software v3.1.67 (Axelife SAS, Saint-Nicolas-de-Redon, France). ft-PWV serves as an index of AS, with higher values indicating increased arterial stiffness54while ABI is an indicator of lower-limb perfusion55. The mean of the two closest measurements was considered for three time points: Pre, Immediately post-exercise, and Post.
BP was obtained from beat-to-beat recordings before and after the sessions and averaged over 10-minute intervals. The percentage change in systolic (sBP) and diastolic BP (dBP) was calculated for Post5-15 and Post40-50 time points. This analysis was complemented by central BP estimations (cSBP and cDBP), which were obtained using pOplog® software v3.1.67 (Axelife SAS, Saint-Nicolas-de-Redon, France)56.
Statistical analysis
Data are presented as mean ± standard deviation or mean ± standard error. Linear mixed models included hypertension status, condition (4 S, 6 S, 9 S, CON), and time (except for HR during exercise) as fixed factors. Set configuration and time were repeated measures. Models were selected via maximum likelihood estimation, comparing nested models with the likelihood ratio test57,58,59 and non-nested models via the Akaike Information Criterion. Variance-covariance structures (first-order autoregressive or compound symmetry) and random intercepts nested within subjects were considered.
As no main effect of hypertension or Hypertension×Condition×Time interaction emerged, hypertension remained a fixed factor only for BP variables. Residuals normality was assessed visually (histograms, Q-Q plots) and via kurtosis/skewness statistics. Variables with improved normality after transformation were log-transformed (LnSDNN, LnRMSSD, LnHF, LnBRSslope, LnLFsBP, Lnft-PWV). Significant interactions underwent Bonferroni-adjusted post-hoc tests, reporting only within-group comparisons against baseline. Hedge’s g (G) and confidence intervals assessed effect sizes.
Statistical analyses used IBM SPSS v27.0 (IBM Corp, Armonk, NY, USA), GraphPad Prism v5.01 (GraphPad Software, San Diego, CA, USA), and R v3.6.1 (R Foundation, Vienna, Austria). Effect sizes were computed with the “esc” R package60. Statistical significance was set at p < 0.05.
Results
HR
A significant effect of condition was detected for both HRpeak (p < 0.001) and HRmean (p = 0.004) during exercise. Post-hoc analysis showed lower HRpeak in 4 S (127 ± 15 bpm) than in 6 S (133 ± 15 bpm; p < 0.001; G = 0.564; 95%CI: 0.144,0.987) and 9 S (139 ± 17 bpm; p < 0.001; G = 1.116; 95%CI: 0.676, 1.556). Additionally, HRpeak in 6 S was lower than in 9 S (p < 0.001; G = 0.540; 95%CI:0.115, 0.966). For HRmean, 4 S (93 ± 11 bpm; p = 0.026; G = 0.443; 95%CI: 0.029, 0.857) and 6 S (94 ± 11 bpm; p = 0.019; G = 0.352; 95%CI: 0.069, 0.773) were lower than 9 S (97 ± 11 bpm).
Resting HR changes throughout sessions are shown in Fig. 2. All experimental sessions resulted in higher HR than CON (p < 0.001). HR was higher in 9 S than in 4 S (p = 0.002), while 6 S showed only a trend (p = 0.056). A significant Condition×Time interaction was observed (p < 0.001). Paired post-hoc contrasts showed HR in 4 S was higher than baseline during Post5-10 (p < 0.001 vs. Pre5-10 and Pre10-15; G = 0.849; 95%CI:0.440, 1.259 and G = 0.853; 95%CI:0.443, 1.263, respectively) and Post10-15 (p < 0.001 vs. Pre5-10 and Pre10-15; G = 0.859; 95%CI:0.449, 1.269 and G = 0.855; 95%CI:0.445, 1.265, respectively). In 6 S, HR remained elevated post-session at Post5-10 (p < 0.001 vs. Pre5-10 and Pre10-15; G = 0.963; 95%CI:0.535, 1.390, and G = 0.936; 95%CI:0.509, 1.362), Post10-15 (p < 0.001 vs. Pre5-10 and Pre10-15; G = 0.586; 95%CI:0.173, 0.999 and G = 0.576; 95%CI:0.164, 0.989), and Post15-20 (p = 0.029 and p < 0.001 vs. Pre5-10 and Pre10-15; G = 0.370; 95%CI:0.038, 0.778 and G = 0.372; 95%CI:−0.036, 0.780).
Heart rate (HR) response before and after each condition (4 S: green circles; 6 S: orange triangles; 9 S: blue squares; CON: grey diamonds). # Significantly lower values in CON compared to all experimental conditions at the specific time point (p < 0.001 in all cases); * Significant difference between 4 S and 9 S at the specific time point (p < 0.05); ** Significant difference between 4 S and 9 S at the specific time point (p ≤ 0.001); @ Significant difference between 6 S and 9 S at the specific time point (p < 0.05).
In 9 S, HR remained elevated compared to baseline during Post5-10 (p < 0.001 vs. Pre5-10 and Pre10-15; G = 1.240; 95%CI:0.803, 1.677 and G = 1.310; 95%CI:0.873, 1.747), Post10-15 (p < 0.001 vs. Pre5-10 and Pre10-15; G = 1.101; 95%CI:0.671, 1.530 and G = 1.162; 95%CI:0.734, 1.591), Post15-20 (p < 0.001 vs. Pre5-10 and Pre10-15; G = 0.800; 95%CI:0.384, 1.216 and G = 0.838; 95%CI:0.425, 1.251), Post20-25 (p = 0.001 and p < 0.001 vs. Pre5-10 and Pre10-15; G = 0.733; 95%CI:0.320, 1.147 and G = 0.734; 95%CI:0.325, 1.144), and Post25-30 (p = 0.016 and p = 0.004 vs. Pre5-10 and Pre10-15; G = 0.584; 95%CI:0.176, 0.993 and G = 0.556; 95%CI:0.152, 0.959). Conversely, HR after CON was significantly lower than baseline (p < 0.001).
HRV
The evolution of SDNN (logarithmic scale) is in Fig. 3A. Post-hoc analysis showed a main effect of condition, with higher values in CON vs. all RE sessions (p < 0.001) and in 4 S vs. 6 S (p < 0.001) and 9 S (p = 0.008). A Condition × Time interaction was found (p < 0.001). Compared to baseline, 4 S had lower values at Post5-10 (p = 0.007, p < 0.001 vs. Pre5-10, Pre10-15; G = 0.488; 95%CI: 0.091, 0.886 and G = 0.505; 95%CI: 0.106, 0.903). In 6 S, reductions occurred at Post5-10 (p < 0.001 vs. Pre5-10, Pre10-15; G = 0.825; 95%CI: 0.408, 1.242 and G = 0.791; 95%CI: 0.375, 1.207) and Post10-15 (p = 0.013, p < 0.001; G = 0.680; 95%CI: 0.264, 1.096 and G = 0.686; 95%CI: 0.269, 1.102). In 9 S, reductions persisted at Post5-10 (p < 0.001; G = 0.674; 95%CI: 0.259, 1.090 and G = 0.723; 95%CI: 0.310, 1.137), Post10-15 (p < 0.001; G = 0.733; 95%CI: 0.315, 1.151 and G = 0.793; 95%CI: 0.377, 1.209), and Post15-20 (p = 0.035, p < 0.001; G = 0.447; 95%CI: 0.038, 0.857 and G = 0.506; 95%CI: 0.100, 0.913). After CON, LnSDNN increased from Post10-15 (p < 0.001).
Cardiac autonomic control before and after each condition (4 S: green circles; 6 S: orange triangles; 9 S: blue squares; CON: grey diamonds). (A) LnSDNN: logarithm of the standard deviation of the R-R interval; (B) LnRMSSD: Logarithm of the root mean square of successive differences between adjacent R-R intervals; (C) LnHF: logarithm of the high-frequency power of the R-R interval in absolute values; (D) SampEn: sample entropy. # Significantly higher values in the control session at that time point compared to all experimental conditions (p < 0.001 in all cases); * Significant difference at the specific time point between 4 S and 6 S (p < 0.05); @ Significant difference at the specific time point between 4 S and 9 S (p < 0.05).
Acute RMSSD changes (logarithmic scale) are in Fig. 3B. Higher global values were found in CON vs. all RE sessions (p < 0.001) and in 4 S vs. 6 S (p = 0.025). In 4 S, Pre5-10 values were higher than Post5-10 and Post10-15 (p < 0.001, p = 0.047; G = 0.317; 95%CI: 0.077, 0.712 and G = 0.171; 95%CI: 0.216, 0.564), while Pre10-15 differed only from Post5-10 (p < 0.001; G = 0.350; 95%CI: 0.045, 0.745). In 6 S, Pre5-10 exceeded Post5-10 and Post10-15 (p < 0.001; G = 0.947; 95%CI: 0.524, 1.369 and G = 0.881; 95%CI: 0.462, 1.301), with reductions vs. Pre10-15 extending until Post20-25 (p < 0.001, p < 0.001, p = 0.017, p = 0.045; G = 0.910; 95%CI: 0.489, 1.330; G = 0.885; 95%CI: 0.465, 1.304; G = 0.475; 95%CI: 0.070, 0.881; G = 0.531; 95%CI: 0.123, 0.938). In 9 S, Post5-10 (p < 0.001; G = 0.523; 95%CI: 0.120, 0.926 and G = 0.711; 95%CI: 0.302, 1.119), Post10-15 (p < 0.001; G = 0.595; 95%CI: 0.190, 1.000 and G = 0.697; 95%CI: 0.287, 1.107), and Post15-20 (p = 0.003, p < 0.001; G = 0.413; 95%CI: 0.013, 0.814 and G = 0.489; 95%CI: 0.085, 0.893) were lower than Pre5-10 and Pre10-15. After CON, values remained elevated vs. baseline (p ≤ 0.045).
Figure 3C shows HF evolution (logarithmic scale). Global 4 S values were higher than 6 S (p = 0.021) and 9 S (p = 0.032), while all experimental sessions had lower HF power than CON (p < 0.001). In 4 S, pre-session values were higher than the first post-exercise recordings (p = 0.029, p = 0.002; G = 0.425; 95%CI: 0.029, 0.821 and G = 0.413; 95%CI: 0.017, 0.809). In 6 S, differences from baseline lasted until Post15-20, with lower LnHF at Post5-10 (p < 0.001 vs. Pre5-10, Pre10-15; G = 0.829; 95%CI: 0.412, 1.246 and G = 0.843; 95%CI: 0.425, 1.260), Post10-15 (p < 0.001; G = 0.769; 95%CI: 0.349, 1.188 and G = 0.666; 95%CI: 0.250, 1.081), and Post15-20 (p = 0.008, p < 0.001; G = 0.549; 95%CI: 0.141, 0.957 and G = 0.570; 95%CI: 0.162, 0.978). Similarly, in 9 S, baseline values exceeded Post5-10 (p < 0.001; G = 0.690; 95%CI: 0.278, 1.102 and G = 0.639; 95%CI: 0.232, 1.045), Post10-15 (p < 0.001; G = 0.707; 95%CI: 0.294, 1.119 and G = 0.645; 95%CI: 0.239, 1.052), and Post15-20 (p = 0.002, p < 0.001; G = 0.482; 95%CI: 0.076, 0.888 and G = 0.556; 95%CI: 0.044, 0.968). Conversely, LnHF remained elevated for 40 min after CON vs. baseline.
Figure 3D represents SampEn evolution. Post-hoc analysis detected a global decrease in 4 S and 9 S vs. CON (p < 0.001), with only a trend in 6 S (p = 0.076).
Cardiovagal BRS and peripheral sympathetic tone
Changes in LnBRSslope are shown in Fig. 4A. The condition factor revealed higher values in CON than in 4 S, 6 S, and 9 S (p = 0.001 in all). In 4 S, LnBRSslope decreased at Post5-15 vs. baseline (p = 0.017; G = 0.469; 95%CI: 0.067, 0.870). In 6 S, reductions were observed at Post5-15 and Post15-25 (p < 0.001; G = 0.602; 95%CI: 0.179, 1.024 and G = 0.613; 95%CI: 0.194, 1.031). Similar decreases occurred in 9 S (p < 0.001 and p = 0.003 for Post5-15 and Post15-25; G = 0.432; 95%CI: 0.018, 0.846 and G = 0.478; 95%CI: 0.072, 0.884). In CON, baseline values were lower than Post5-15 (p = 0.001; G = 0.382; 95%CI: 0.014, 0.778).
Cardiovagal baroreflex and peripheral sympathetic tone responses before and after each condition (4 S: green circles; 6 S: orange triangles; 9 S: blue squares; CON: grey diamonds). (A) LnBRSslope: logarithm of baroreflex sensitivity magnitude; (B) BRSevents: number of baroreceptor sequences detected; (C) BEI: baroreflex effectiveness index; (D) LnLFsBP: logarithm of low-frequency power of systolic blood pressure; (E) SDsBP: standard deviation of systolic blood pressure; (F) SDMAP: standard deviation of mean arterial pressure. # Significantly higher values in the control session at that time point compared to all experimental conditions (p < 0.001 in all cases); $ Significantly higher values in the control session at that time point compared to 6 S and 9 S; * Significant difference at the specific time point between 4 S and 6 S (p < 0.05); @ Significant difference at the specific time point between 4 S and 9 S (p < 0.05); & Significantly higher values in the control session at that time point compared to 4 S and 6 S.
For BRSevents (Fig. 4B), the condition factor significantly affected event count, with lower values in CON than in 4 S (p < 0.001), 6 S (p = 0.001), and 9 S (p = 0.006). Figure 4C shows BEI changes. CON had higher values than 6 S (p = 0.014) and 9 S (p < 0.001), with a trend vs. 4 S (p = 0.069). No significant changes from baseline occurred in 4 S, while a trend toward reduction was seen post-6 S (Post5-15; p = 0.058; G = 0.402; 95%CI: 0.007, 0.810), and a significant decrease in 9 S (p < 0.001; G = 0.603; 95%CI: 0.181, 1.026).
For LnLFsBP and SDsBP, no condition or Condition × Time interaction effects were found (Fig. 4D and E). Regarding SDMAP (Fig. 4F), a significant Condition × Time interaction was observed (p = 0.026), although no significant changes from baseline in 4 S and 9 S conditions. In contrast, pretest values were significantly lower than Post30-40 (p = 0.047; G = 0.325; 95%CI: −0.070, 0.728) and Post15-25 (p = 0.025; G = 0.465; 95%CI: 0.067, 0.862) in 6 S and CON conditions, respectively.
Vascular stiffness
Figure 5A displays ft-PWW results on a logarithmic scale. CON showed lower global values than 4 S (p < 0.001) and 6 S (p = 0.037), with a tendency versus 9 S (p = 0.067). Lnft-PWV increases post-session were significant only in 6 S (p = 0.025; G = 0.335; 95% CI: 0.072, 0.743) and 9 S (p < 0.001; G = 0.632; 95% CI: 0.226, 1.038). Figure 5B summarizes ABI results. CON global values were significantly lower than 4 S (p = 0.009), 6 S (p = 0.006), and 9 S (p < 0.001). The Condition × Time interaction revealed significant baseline reductions only in CON (p = 0.001 and p = 0.003 for the first and second measurements, respectively; G = 0.619; 95% CI: 0.217, 1.020 and G = 0.437; 95% CI: 0.040, 0.834).
Arterial stiffness response before, immediately after, and 60 min after each condition (4 S: green circles; 6 S: orange triangles; 9 S: blue squares; CON: grey diamonds). (A) Lnft-PWV: logarithm of finger-to-toe pulse wave velocity; (B) ABI: ankle-brachial index. # Significantly lower values in the control session at that time point compared to all experimental conditions (p < 0.001 in all cases); $ Significantly lower values in the control session at that time point compared to 9 S (p < 0.001).
Blood pressure
Analysis of sBP percentual changes detected main effects of time (p = 0.031), condition (p = 0.013), and a Hypertension × Condition interaction (p = 0.010). Post-hoc contrasts showed lower global values in 9 S versus CON (p = 0.004) and a tendency versus 6 S (p = 0.051) in hypertensive participants. Differences between hypertensive and normotensive participants were observed only in 9 S (p = 0.018) (Fig. 6A). For cSBP, main effects of Hypertension (higher in hypertensive participants), Time, and Condition were significant (p < 0.001). Post-hoc contrasts indicated higher global cSBP in 4 S versus CON (p = 0.014) and 9 S (p = 0.017). The Hypertension × Condition interaction approached significance (p = 0.051). Post-hoc contrasts revealed lower values in normotensive participants across all conditions (p < 0.001), while hypertensive participants showed differences between CON and 4 S (p = 0.009) and 6 S (p = 0.026) (Fig. 6B). No main effects or interactions were found for peripheral dBP changes. For cDBP, main effects of Hypertension (p < 0.001; higher in hypertensive participants), Condition (p < 0.001), and Time (p = 0.003) were detected. Post-hoc contrasts showed higher cDBP in 4 S versus CON (p = 0.008) and 9 S (p = 0.015).
Blood pressure response before and after each condition (4 S: green circles; 6 S: orange triangles; 9 S: blue squares; CON: grey diamonds). (A) ΔsBP: relative changes in peripheral systolic blood pressure measured via photoplethysmography; (B) cSBP: central systolic blood pressure. In both cases, values represent estimated marginal means ± standard error of the mean and correspond to the interaction between hypertensive status and condition. # Significant differences compared to the control session in the hypertensive group; $ Differences with 6 S within the hypertensive group (trend: p = 0.051); * Differences between hypertensive and normotensive groups for a specific condition.
Discussion
The main findings of this study were: (i) Regardless of set configuration, a resistance exercise (RE) session involving major muscle groups acutely reduces parasympathetic tone and modulation and impairs cardiovagal baroreflex sensitivity compared to a control session in physically active postmenopausal women; (ii) a short set configuration (4 S) mitigates this acute impairment in cardiovascular control; (iii) For some variables, the interaction between a moderate effort level and relatively short rest intervals (6 S) is associated with greater cardiovascular stress; and (iv) long set configurations (9 S) appear to produce an acute hypotensive effect in hypertensive postmenopausal women, though at the cost of greater cardiac parasympathetic loss compared to short sets.
Regardless of set configuration, all RE sessions caused an acute decrease in parasympathetic tone (i.e., resting HR) and modulation (i.e., HRV) compared to the control session. The acute withdrawal of cardiac parasympathetic activity following an RE session has been previously observed in various populations18,19. However, the magnitude and duration of these changes depended on set configuration. Our data suggest that, as observed in young participants27,28,29,31,34,61 a short set configuration (4 S) mitigates RE-induced impairment of cardiac vagal control in physically active postmenopausal women. Similar to previous studies in young populations26,30,32,33,34,35ower chronotropic responses (i.e., HRpeak and/or HRmean) were recorded during 4 S sessions, suggesting reduced parasympathetic withdrawal and/or sympathetic activation compared to 6 S and 9S62. This response was followed by decreased vagal tone, measured by resting HR63⁹, after all training sessions, with a more pronounced and prolonged reduction (i.e., higher HR) in 9 S and 6 S than in 4 S. These changes appear to follow a dose-response pattern, with stronger effects as set length increases.
Similarly, HRV analysis showed that the reduction in cardiac parasympathetic modulation was attenuated in 4 S compared to 6 S and 9 S, which exhibited similar responses. It can be speculated that proximity to muscular failure in 9 S and the interaction between effort level and short rest periods in 6 S had comparable effects on cardiac autonomic modulation. Based on these findings, set configurations not exceeding 30% of the maximum number of repetitions may help attenuate vagal modulation loss in postmenopausal women after an RE session. Since vagal withdrawal during and after exercise increases cardiovascular risk in both healthy individuals64 and particularly in susceptible ones65,66this recommendation is especially relevant for postmenopausal women, who face menopause-related cardiovascular changes4that elevate their risk2. These results align with studies in young active participants27,28,29,31,34,61but to our knowledge, this is the first study confirming the effect of set configuration on cardiac autonomic modulation in postmenopausal women. Conversely, although SampEn was slightly lower in RE sessions than in CON, differences were minor, with no significant time-condition interaction. Previous studies in judo athletes26 and sport science students28 also reported a limited effect of set configuration. SampEn, reflecting RR interval irregularity, is considered an independent parasympathetic marker67; thus, our findings and prior research26,28 suggest it is less sensitive than HRV variables for detecting RE-induced changes in cardiac parasympathetic modulation across different set configurations in both young and postmenopausal women.
Similar to cardiac vagal modulation, BRS and BEI were affected by the RE session, with changes in 4 S being shorter in magnitude and duration than in 6 S and 9 S compared to baseline. Notably, 4 S had no effect on BEI, as indicated by the absence of differences from CON and baseline. Given that total event count was higher in RE than in CON, these findings suggest a greater number of ineffective BP ramps in 6 S and 9 S, but not in 4 S. A limited impact of short sets on cardiac baroreflex sensitivity and effectiveness has also been reported in previous studies for single28 or multiple exercise61 RE sessions, suggesting an attenuated shift in the arterial baroreflex operating point compared to longer sets68. Additionally, within-session comparisons showed that AS (i.e., ft-PWV) increased immediately after 6 S and 9 S but not after 4 S. This suggests that RE sessions exceeding 30% effort increase AS, potentially reducing vessel wall deformation and baroreceptor sensitivity69. It has been suggested that abrupt and intermittent BP surges during high-intensity resistance training may alter arterial structure70partly explaining the increased AS observed post-exercise. A recent meta-analysis identified an acute increase in AS following RE sessions, suggesting that both cardiovascular (abrupt changes in BP, HR, and left ventricular ejection time) and non-cardiovascular (elevated acute inflammatory markers) mechanisms contribute to acute changes in pulse wave velocity71. Among these factors, set configuration plays a key role, as it can modulate BP, HR, and rate-pressure product during exercise, with shorter sets eliciting a lower cardiovascular response compared to longer set structures26,30,32,33,34,35,72,73. The cardiovascular nature of changes in pulse wave velocity is partially attributed to the greater chronotropic response observed in 6 S and 9 S compared to 4 S. Further studies should clarify how set configuration influences AS-regulating mechanisms, potentially involving vasomotor tone. In this study, LFsBP, was used as an estimate, but no significant condition effect or time-condition interaction were observed, aligning with findings ed for young61 and healthy middle-aged individuals74. This raises doubts about LFsBP’s sensitivity in detecting post-exercise vasomotor changes75,76. Complementarily, blood pressure variability was assessed using SDsBP and SDMAP, given the sensitivity of these indices to changes in sympathetic transduction52. Similar to LFsBP, neither main effects nor interaction effects were observed for SDsBP, whereas some specific changes were detected for SDMAP, although their magnitude was limited. Overall, our findings are not conclusive regarding the involvement of vasomotor tone in the changes in pulse wave velocity observed after resistance training sessions.
An interesting finding of this study was that RE counteracted the decrease in ABI observed after the sedentary period performed in CON, regardless of set configuration. An interesting finding of this study was that RE counteracted the decrease in ABI observed after the sedentary period in CON, regardless of set configuration. ABI is a noninvasive measure used to diagnose lower-extremity peripheral artery disease when values are low (i.e., ≤ 0.90) and arterial wall calcification when abnormally high values are detected (i.e., > 1.40)77. In all conditions, participants exhibited normal ABI values before and after the sessions. However, while the sedentary period led to a decrease in ABI, this reduction was not observed following exercise, which may be attributed to the detrimental effects of prolonged sitting on endothelial function78.
The effect of RE sessions on sBP may primarily affect hypertensive postmenopausal females, as indicated by the detected Hypertension×Condition interaction. A significant percentage decrease in peripheral sBP was observed in 9 S, but only in hypertensive participants, with no changes in cSBP compared to CON. In contrast, 4 S and 6 S exhibited higher sBP values than CON. These findings suggest that proximity to failure may contribute to a post-exercise hypotensive effect or attenuate the acute systolic pressor response following the training session in hypertensive postmenopausal females, which is consistent with findings in other populations29,79. Several mechanisms have been proposed to explain the hypotensive effect of RE, along with individual factors that may influence this response80,81,82,83. Thus, it can be speculated that these factors interact or are particularly pronounced in hypertensive postmenopausal females following RE sessions with sets performed close to muscular failure. However, it is important to consider that this hypotensive effect, which may be beneficial in principle, is observed under conditions in which cardiovascular regulatory mechanisms are more impaired compared to shorter set structures. Consequently, in hypertensive participants, 9 S may induce an acute hypotensive response at the expense of a more pronounced impairment in cardiac regulatory mechanisms. Given that acute reductions in BP following RE do not appear to be directly linked to autonomic control84 and that the hypotensive effect may be attenuated after a period of training85,, performing RE training near failure may not be ideal for physically active postmenopausal females, particularly hypertensive ones, as the short-term costs may not outweigh the potential long-term benefits. Future studies should examine the chronic effects of resistance training with different set configurations in both normotensive and hypertensive postmenopausal females.
There are some limitations in this study that should be considered. Firstly, the sample size of hypertensive participants was relatively small, which may have limited the sensitivity to detect the interaction between hypertension and other variables, aside from BP. The limited number of hypertensive participants resulted from the fact that the experimental design focused on physically active postmenopausal females to avoid the potential confounding effects of sedentary behaviour on the specific responses of this population. As a result, many hypertensive females interested in participating did not meet the inclusion criteria. Additionally, potential confounders such as menopausal status, aging, and sex may have influenced the observed responses. Future studies should consider these factors by including control groups (e.g., pre- vs. postmenopausal women, men vs. women, younger vs. older individuals) to explain their influence on acute cardiovascular responses. Secondly, although we evaluated several key cardiovascular and autonomic outcomes, the inclusion of additional non-cardiovascular (e.g., pro-inflammatory markers) and cardiovascular variables (e.g., flow-mediated dilation) would have been useful in interpreting the underlying mechanisms of the observed responses. Furthermore, the exploratory nature of the study may limit the generalizability of the findings. Nonetheless, our study provides a detailed analysis of the acute cardiovascular responses of postmenopausal females to RE sessions with varying set configurations and offers preliminary insights that may guide future research involving larger samples and broader physiological profiling. Thirdly, chronotropic state differences between conditions (i.e., HR differences) could have influenced the HRV results, considering the potential mathematical effect of HR on HRV previously described86,87. However, it must be noted that: (i) the analyses performed with HRV data normalized to mean RR interval preserved the main findings obtained from the raw data; and (ii) although the 4 S and 6 S sessions induced similar HR responses, differences in HRV outcomes were still observed. This reinforces the notion that HRV responses were not merely a mathematical artifact resulting from differences in HR, as previously discussed in the literature88.
Despite the aforementioned limitations, the ecological validity of our study design enhances the translational relevance of the findings. The exercises employed were multi-joint movements commonly performed in clinical and fitness settings, targeting major muscle groups. Furthermore, the set configurations were based on a simple and practical method—calculating a percentage of the maximum number of repetitions performed—which increases their feasibility in both research and applied contexts. This approach is accessible to practitioners and individuals without requiring advanced equipment, close supervision, or extensive training experience. Compared to more complex strategies such as velocity-based training or RIR-based prescriptions, it better reflects real-world practices. Additionally, the experimental setting closely resembled conventional training conditions, further supporting the applicability of the results to the daily routines of postmenopausal women and their direct implementation in health and fitness programs.
Conclusion
Set configuration can modulate the cardiovascular response during and after exercise in physically active postmenopausal females. Shorter set configurations (around 30% of the effort intensity) elicit a lower chronotropic response during exercise, thereby attenuating parasympathetic withdrawal, reducing baroreceptor sensitivity loss, and limiting increases in arterial stiffness, without triggering a post-exercise hypotensive effect. Therefore, shorter set configurations are recommended for physically active postmenopausal females to optimize the benefits of resistance exercise while minimizing the cardiovascular risks associated with the session.
Data availability
The data that support the findings of this study are not yet publicly available but can be obtained from the corresponding author upon reasonable request.
References
Maltais, M. L., Desroches, J. & Dionne, I. J. Changes in muscle mass and strength after menopause. J. Musculoskelet. Neuronal Interact. 9, 186–197 (2009).
Vogel, B. et al. The lancet women and cardiovascular disease commission: reducing the global burden by 2030. Lancet 397, 2385–2438 (2021).
Messier, V. et al. Menopause and sarcopenia: A potential role for sex hormones. Maturitas 68, 331–336 (2011).
Hulteen, R. M., Marlatt, K. L., Allerton, T. D. & Lovre, D. Detrimental changes in health during menopause: the role of physical activity. Int. J. Sports Med. 44, 389–396 (2023).
de Villiers, T. J. Bone health and menopause: osteoporosis prevention and treatment. Best Pract. Res. Clin. Endocrinol. Metab. 38, 101782 (2024).
Shojaa, M., von Stengel, S., Kohl, M., Schoene, D. & Kemmler, W. Effects of dynamic resistance exercise on bone mineral density in postmenopausal women: a systematic review and meta-analysis with special emphasis on exercise parameters. Osteoporos. Int. 31, 1427–1444 (2020).
Lin, Y. Y. & Lee, S. Da. Cardiovascular benefits of exercise training in postmenopausal hypertension. Int J. Mol. Sci 19 (2018).
Leite, R. D., Prestes, J., Pereira, G. B., Shiguemoto, G. E. & Perez, S. E. A. Menopause: highlighting the effects of resistance training. Int. J. Sports Med. 31, 761–767 (2010).
Khalafi, M. et al. Influence of exercise type and duration on cardiorespiratory fitness and muscular strength in post-menopausal women: a systematic review and meta-analysis. Front Cardiovasc. Med 10 (2023).
He, M. et al. Effect of resistance training on lipid profile in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 288, 18–28 (2023).
Choudhry, D. N., Saleem, S., Hatim, S. & Irfan, R. The effect of resistance training in reducing hot flushes in post-menopausal women: A meta-analysis. J. Bodyw. Mov. Ther. 39, 335–342 (2024).
Capel-Alcaraz, A. M., García-López, H., Castro-Sánchez, A. M., Fernández-Sánchez, M. & Lara-Palomo, I. C. The efficacy of strength exercises for reducing the symptoms of menopause: A systematic review. J Clin. Med 12 (2023).
Maestroni, L. et al. The benefits of strength training on musculoskeletal system health: practical applications for interdisciplinary care. Sports Med. 50, 1431–1450 (2020).
El-Kotob, R. et al. Resistance training and health in adults: an overview of systematic reviews. Appl. Physiol. Nutr. Metab. 45, S165–S179 (2020).
Momma, H., Kawakami, R., Honda, T. & Sawada, S. S. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: a systematic review and meta-analysis of cohort studies. Br. J. Sports Med. 56, 755–763 (2022).
Mcleod, J. C., Stokes, T. & Phillips, S. M. Resistance exercise training as a primary countermeasure to Age-Related chronic disease. Front Physiol 10 (2019).
MacDougall, J. D., Tuxen, D., Sale, D. G., Moroz, J. R. & Sutton, J. R. Arterial blood pressure response to heavy resistance exercise. J. Appl. Physiol. 58, 785–790 (1985).
Kingsley, J. D. & Figueroa, A. Acute and training effects of resistance exercise on heart rate variability. Clin. Physiol. Funct. Imaging. 36, 179–187 (2014).
Marasingha-Arachchige, S. U., Rubio-Arias, J. Á., Alcaraz, P. E. & Chung, L. H. Factors that affect heart rate variability following acute resistance exercise: A systematic review and meta-analysis. J. Sport Health Sci. https://doi.org/10.1016/j.jshs.2020.11.008 (2020).
de Sousa, N. M. F. et al. Continuous blood pressure response at different intensities in leg press exercise. Eur. J. Prev. Cardiol. 21, 1324–1331 (2014).
Lamotte, M., Niset, G. & van de Borne, P. The effect of different intensity modalities of resistance training on beat-to-beat blood pressure in cardiac patients. Eur. J. Cardiovasc. Prev. Rehabil. 12, 12–17 (2005).
Matos-Santos, L., Farinatti, P., Borges, P. J., Massaferri, R. & Monteiro, W. Cardiovascular responses to resistance exercise performed with large and small muscle mass. Int. J. Sports Med. 38, 883–889 (2017).
Gjøvaag, T. F. et al. Hemodynamic responses to resistance exercise in patients with coronary artery disease. Med. Sci. Sports Exerc. 48, 581–588 (2016).
Hackett, D. A. & Chow, C. M. The Valsalva maneuver: its effect on intra-abdominal pressure and safety issues during resistance exercise. J. Strength. Cond Res. 27, 2338–2345 (2013).
Iglesias-Soler, E., Carballeira, E., Sánchez-Otero, T. & Mayo, X. Fernández-del-Olmo, M. Performance of maximum number of repetitions with cluster-set configuration. Int. J. Sports Physiol. Perform. 9, 637–642 (2014).
Iglesias-Soler, E. et al. Effect of set configuration on hemodynamics and cardiac autonomic modulation after high-intensity squat exercise. Clin. Physiol. Funct. Imaging. 35, 250–257 (2015).
Kassiano, W. et al. Parasympathetic nervous activity responses to different resistance training systems. Int. J. Sports Med. 42, 82–89 (2021).
Mayo, X., Iglesias-Soler, E. & Carballeira-Fernández, E. Fernández-Del-Olmo, M. A shorter set reduces the loss of cardiac autonomic and baroreflex control after resistance exercise. Eur. J. Sport Sci. 16, 996–1004 (2016).
Mayo, X., Iglesias-Soler, E., Fariñas-Rodríguez, J., Fernández-Del-Olmo, M. & Kingsley, J. D. Exercise type affects cardiac vagal autonomic recovery after a resistance training session. J. Strength. Cond Res. 30, 2565–2573 (2016).
Mayo, X., Iglesias-Soler, E., Kingsley, J. & Dopico, X. Interrepetition rest set lacks the V-Shape systolic pressure response advantage during resistance exercise. Sports 5, 90 (2017).
Nunes, J. H. C. et al. Cardiac autonomic control following resistance exercise with different set configurations in apparently healthy young men: A crossover study. Physiol. Behav. 230, 113292 (2021).
Paulo, A. C., Tricoli, V., Queiroz, A. C. C., Laurentino, G. & Forjaz, C. L. M. Blood pressure response during resistance training of different Work-to-Rest ratio. J. Strength. Cond Res. 33, 399–407 (2019).
Rial-Vázquez, J. et al. Heart rate responses and cardiovascular adaptations to resistance training programs differing in set configuration: A randomized controlled trial. Res. Q. Exerc. Sport. 94, 466–475 (2023).
Río-Rodríguez, D. & Iglesias-Soler, E. Fernández Del olmo, M. Set configuration in resistance exercise: muscle fatigue and cardiovascular effects. PLoS One. 11, e0151163 (2016).
Rúa-Alonso, M. et al. Hemodynamic response during different set configurations of a Moderate-load resistance exercise. Int. J. Sports Med. 43, 1118–1128 (2022).
Casonatto, J., Goessler, K. F., Cornelissen, V. A., Cardoso, J. R. & Polito, M. D. The blood pressure-lowering effect of a single bout of resistance exercise: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Prev. Cardiol. 23, 1700–1714 (2016).
Cowley, E. S., Olenick, A. A., McNulty, K. L. & Ross, E. Z. Invisible sportswomen: the sex data gap in sport and exercise science research. Women Sport Phys. Act. J. 29, 146–151 (2021).
Dias, R. K. N., Noronha, Á. S. N. & Coswig, V. S. Cluster-set promotes hemodynamic responses similar to traditional resistance training in menopaused and elderly women. Brazilian J. Exerc. Prescription Physiol. 16, 645–654 (2023).
Nine, I. et al. Acute cardiovascular responses of postmenopausal women to resistance training sessions differing in set configuration: A study protocol for a crossover trial. PLoS One. 19, e0311524 (2024).
Bruce, R. A., Blackmon, J. R., Jones, J. W. & Strait, G. Exercising testing in adult normal subjects and cardiac patients. Ann. Noninvasive Electrocardiol. 9, 291–303 (2004).
Macfarlane, D. J. & Wong, P. Validity, reliability and stability of the portable cortex metamax 3B gas analysis system. Eur. J. Appl. Physiol. 112, 2539–2547 (2012).
Iglesias-Soler, E. et al. Variability in the relationship between velocity loss and percentage of completed repetitions during horizontal leg press and bench press in postmenopausal women. J. Strength. Cond Res. 38, 1576–1583 (2024).
Fortin, J. et al. Validation and verification of the task Force® monitor. FDA Paper. 510k, 1–7 (2001).
Perrotta, A. S., Jeklin, A. T., Hives, B. A., Meanwell, L. E. & Warburton, D. E. R. Validity of the elite HRV smartphone application for examining heart rate variability in a Field-Based setting. J. Strength. Cond Res. 31, 2296–2302 (2017).
Bohn, L. et al. Arterial stiffness assessment by pulse wave velocity in postmenopausal women: comparison between noninvasive devices. Menopause 31, 709–715 (2024).
Malik, M. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European society of cardiology and the North American society of pacing and electrophysiology. Circulation 93, 1043–1065 (1996).
Parati, G. et al. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension 12, 214–222 (1988).
Blaber, A. P., Yamamoto, Y. & Hughson, R. L. Methodology of spontaneous baroreflex relationship assessed by surrogate data analysis. Am. J. Physiol. Heart Circ. Physiol. 268, H1682–H1687 (1995).
Rienzo, M. et al. Baroreflex effectiveness index: an additional measure of baroreflex control of heart rate in daily life. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, 744–751 (2001).
Johansson, M. et al. Baroreflex effectiveness index and baroreflex sensitivity predict all-cause mortality and sudden death in hypertensive patients with chronic renal failure. J. Hypertens. 25, 163–168 (2007).
Pagani, M., Montano, N., Porta, A. & Birkett, C. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation 95, 1441–1448 (1997).
Guerrero, R. V. D. et al. Resting beat-to-beat blood pressure variability in humans: role of alpha-1 adrenergic receptors. Clin. Auton. Res. 35, 277–284 (2025).
Niemelä, T. H. T. et al. Recovery pattern of baroreflex sensitivity after exercise. Med. Sci. Sports Exerc. 40, 864–870 (2008).
Alivon, M. et al. A novel device for measuring arterial stiffness using finger-toe pulse wave velocity: validation study of the pOpmètre®. Arch. Cardiovasc. Dis. 108, 227–234 (2015).
Amrani, S. et al. Assessment of the systolic rise time by photoplethysmography in peripheral arterial diseases: a comparative study with ultrasound doppler. Eur. Heart J. Open 2 (2022).
Obeid, H., Boutouyrie, P., Hallab, M., Khettab, H. & Laurent, S. Calculation of central blood pressure by analyzing the contour of the photoplethysmographic pulse measured at the finger with the pOpmètre® device. Archives Cardiovasc. Dis. Supplements. 10, 104 (2018).
Sullivan, L. M., Dukes, K. A. & Losina, E. Tutorial in biostatistics. An introduction to hierarchical linear modelling. Stat. Med. 18, 855–888 (1999).
Pardo, A. & Ruiz, M. A. San martín, R. How to fit and interpret multilevel models using SPSS. Psicothema 19, 308–321 (2007).
Burton, P., Gurrin, L. & Sly, P. Extending the simple linear regression model to account for correlated responses: an introduction to generalized estimating equations and multi-level mixed modelling. Stat. Med. 17, 1261–1291 (1998).
Lüdecke, D. esc: Effect Size Computation for Meta Analysis. CRAN: Contributed Packages Preprint at https://doi.org/10.32614/CRAN.package.esc (2016).
Rúa-Alonso, M., Mayo, X., Mota, J. & Kingsley, J. D. Iglesias-Soler, E. A short set configuration attenuates the cardiac parasympathetic withdrawal after a whole-body resistance training session. Eur. J. Appl. Physiol. 120, 1905–1919 (2020).
Michael, S., Graham, K. S. & Davis, G. M. Cardiac autonomic responses during exercise and Post-exercise recovery using heart rate variability and systolic time Intervals—A review. Front Physiol 8 (2017).
Peçanha, T., Silva-Júnior, N. D. & de Forjaz, C. L. Heart rate recovery: autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin. Physiol. Funct. Imaging. 34, 327–339 (2014).
Goodman, J. M., Burr, J. F., Banks, L. & Thomas, S. G. The acute risks of exercise in apparently healthy adults and relevance for prevention of cardiovascular events. Can. J. Cardiol. 32, 523–532 (2016).
Albert, C. M. et al. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl. J. Med. 343, 1355–1361 (2000).
Thompson, P. D. et al. Exercise and acute cardiovascular events. Circulation 115, 2358–2368 (2007).
Kuusela, T. A., Jartti, T. T., Tahvanainen, K. U. O. & Kaila, T. J. Nonlinear methods of biosignal analysis in assessing terbutaline-induced heart rate and blood pressure changes. Am. J. Physiol. Heart Circ. Physiol. 282, H773–H783 (2002).
Fadel, P. J. & Raven, P. B. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp. Physiol. 97, 39–50 (2012).
Heffernan, K., Collier, S., Kelly, E., Jae, S. & Fernhall, B. Arterial stiffness and baroreflex sensitivity following bouts of aerobic and resistance exercise. Int. J. Sports Med. 28, 197–203 (2007).
Burtscher, J., Strasser, B., D’Antona, G., Millet, G. P. & Burtscher, M. How much resistance exercise is beneficial for healthy aging and longevity? J. Sport Health Sci. 12, 284–286 (2023).
Pierce, D. R., Doma, K. & Leicht, A. S. Acute effects of exercise mode on arterial stiffness and wave reflection in healthy young adults: A systematic review and Meta-Analysis. Front. Physiol. 9, 73 (2018).
Paulo, A. C. et al. Blood pressure increase in hypertensive individuals during resistance training protocols with equated work to rest ratio. Front. Physiol. 11, 481 (2020).
Ribeiro-Torres, O. et al. Lower cardiovascular stress during resistance training performed with Inter-Repetition rests in elderly coronary patients. Med. (B Aires). 56, 264 (2020).
Queiroz, A. C. C. et al. Post-resistance exercise hemodynamic and autonomic responses: comparison between normotensive and hypertensive men. Scand. J. Med. Sci. Sports. 25, 486–494 (2015).
Kiviniemi, A. M. et al. α-Adrenergic effects on low-frequency oscillations in blood pressure and R-R intervals during sympathetic activation. Exp. Physiol. 96, 718–735 (2011).
Ryan, K. L., Rickards, C. A., Hinojosa-Laborde, C., Cooke, W. H. & Convertino, V. A. Arterial pressure oscillations are not associated with muscle sympathetic nerve activity in individuals exposed to central hypovolaemia. J. Physiol. 589, 5311–5322 (2011).
Aboyans, V. et al. Measurement and interpretation of the Ankle-Brachial index. Circulation 126, 2890–2909 (2012).
Thosar, S. S., Bielko, S. L., Mather, K. J., Johnston, J. D. & Wallace, J. P. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med. Sci. Sports Exerc. 47, 843–849 (2015).
De Souza, J. C. et al. Resistance exercise leading to failure versus not to failure: effects on cardiovascular control. BMC Cardiovasc. Disord. 13, 105 (2013).
Halliwill, J. R., Buck, T. M., Lacewell, A. N. & Romero, S. A. Postexercise hypotension and sustained postexercise vasodilatation: what happens after we exercise? Exp. Physiol. 98, 7–18 (2013).
Brito, L. C. et al. Postexercise hypotension as a clinical tool: a single brick in the wall. J. Am. Soc. Hypertens. 12, e59–e64 (2018).
Forjaz, C. L. M. et al. Factors affecting post-exercise hypotension in normotensive and hypertensive humans. Blood Press. Monit. 5, 255–262 (2000).
Kenney, M. J. & Seals, D. R. Postexercise hypotension. Key features, mechanisms, and clinical significance. Hypertension 22, 653–664 (1993).
Farinatti, P. et al. Postexercise hypotension due to resistance exercise is not mediated by autonomic control: A systematic review and meta-analysis. Auton. Neurosci. 234, 102825 (2021).
Moraes, M. R. et al. Effect of 12 weeks of resistance exercise on post-exercise hypotension in stage 1 hypertensive individuals. J. Hum. Hypertens. 26, 533–539 (2012).
Sacha, J. et al. How to strengthen or weaken the HRV dependence on heart rate–description of the method and its perspectives. Int. J. Cardiol. 168, 1660–1663 (2013).
Billman, G. E. The effect of heart rate on the heart rate variability response to autonomic interventions. Front. Physiol. 4, 222 (2013).
Michael, S., Jay, O., Halaki, M., Graham, K. & Davis, G. M. Submaximal exercise intensity modulates acute post-exercise heart rate variability. Eur. J. Appl. Physiol. 116, 697–706 (2016).
Acknowledgements
The authors express their gratitude to the participants and collaborators for their effort throughout the study.
Funding
This study is part of the project PID2021-124277OB-I00 funded by MCIN/AEI/10.13039/501100011033 and “ERDF/EU. M.R.A. and J.R.V. received financial support from the Spanish Ministry of Universities through the Grants for the Requalification of the Spanish University System under the Postdoctoral Margarita Salas Programme – Universidade da Coruña (RSUC.UDC.MS09 and RSUC.UDC.MS10, respectively). MRA also acknowledges the financial support received from the Xunta de Galicia (Consellería de Cultura, Educación, Formación Profesional y Universidades) through the Xunta de Galicia Postdoctoral Fellowships (ED481B-2024‐077). I.N. is supported by a predoctoral grant from Spanish Ministry of Science, Innovation and Universities (FPU23/03727).
Author information
Authors and Affiliations
Contributions
MRA participated in design of the study, analysis and interpretation, manuscript writing and manuscript revision; JRV contributed to participants’ recruitment, data collection, analysis and interpretation; IN contributed to participants’ recruitment and data collection; JF participated in participants’ recruitment and data collection; BRL contributed to participants’ recruitment and data collection; MAGG contributed to conception and design, participants’ recruitment and data collection; EIS participated in conception and design, analysis and interpretation, statistical analysis, obtaining funding, manuscript writing and manuscript revision. All authors have read and approved the final version of the manuscript and agree with the order of presentation of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rúa-Alonso, M., Rial-Vázquez, J., Nine, I. et al. Set configuration influences cardiovascular responses to resistance exercise in postmenopausal females in a randomized crossover trial from the CARE project. Sci Rep 15, 28373 (2025). https://doi.org/10.1038/s41598-025-14379-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14379-0