Abstract
The rising prevalence of severe skin infections, particularly those linked to multidrug-resistant (MDR) bacteria, poses an increasing public health challenge. Although the systemic administration of broad-spectrum antibiotics such as colistin sulfate (CS) represents a last-resort treatment for these infections, its use is associated with serious, life-threatening damage to vital organs. The current investigation aimed to develop and assess the efficacy of a CS-loaded nanoemulgel (NEG) as an anti-microbial therapy. Initially, a nanoemulsion (NE) was prepared using high speed homogenization, employing Labrafil as oil, Tween 80 as the surfactant and Transcutol as the co-surfactant. The formulation was then characterized for its pH, thermodynamic stability, particle size, drug content and entrapment efficiency. CS-NE formulation was then converted into CS-NEG using Carbopol 940 as a thickening agent. The resulting formulation was assessed for spreadability, viscosity, in-vitro drug release, ex-vivo skin permeation and antimicrobial activity. The particle size of the optimized CS-NE and CS-NEG formulations ranged from 193.2 ± 9.52 nm to 169.3 ± 10.22 nm and 274.9 ± 11.59 nm to 303.2 ± 20.36 nm, respectively, while the zeta potential of the developed CS-NE and CS-NEG formulations ranged from − 11.7 ± 0.11 mV to -42.3 ± 0.08 mV and − 11.8 ± 0.02 mV to -20.6 ± 0.51 mV, respectively. FTIR and thermal analysis (TGA/DSC) confirmed the absence of interactions between the drug and excipients. Both the CS-NE and CS-NEG formulations exhibited favorable physico-chemical properties suitable for transdermal applications. The drug content was within the official limits of ± 10%. pH of all formulations ranged from 5.6 ± 0.23 to 6.6 ± 0.56. The developed CS-NEG formulation exhibited relatively higher viscosity values ranged from 34,510 ± 99.85 cP to 38,810 ± 103.77 cP with excellent spreadability. The CS-NEG formulations achieved a cumulative drug release ranging from 73.6 ± 2.01% to 84.31 ± 5.28% over 24 h. Furthermore, these formulations demonstrated enhanced skin permeability following topical application and significantly improved antimicrobial activity (zone of inhibition = 21.23 mm) compared to CS solution. Overall, the findings suggest that the NE-based approach offers a promising therapeutic strategy for combating complex bacterial infections, including those caused by MDR pathogens.
Similar content being viewed by others
Introduction
The skin, the body’s largest organ, serves as the primary barrier against external pathogens. After an injury, the natural healing process can be hindered by opportunistic bacteria, potentially prolonging recovery and increasing the risk of severe infections1. Chronic wound infections often involve complexities such as antibiotic resistance, biofilm formation and hypoxia, which collectively limit the effectiveness of antibiotics and allow bacteria to persist2. Skin and soft tissue infections (SSTIs) are frequently caused by pathogens like Escherichia coli, Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus. The emergence of multidrug-resistant (MDR) bacteria poses a significant global health challenge, affecting individuals across all age groups and signaling an escalating threat of antibiotic resistance3. Complicated SSTIs triggered by MDR bacteria require potent antibiotics, often used alone or in combination. Colistin, also referred to as polymyxin E, is part of the polymyxin family, which includes cationic polypeptides. These are positively charged and cyclic decapeptides attached to a fatty acid and derived from various Bacillus polymyxa species4. It is employed as a last-resort therapy for infections initiated by MDR Gram negative bacteria. Its primary mode of action involves disrupting the cell membrane by attachment to the anionic component of lipopolysaccharide (LPS). This interaction results in a detergent-like effect, altering membrane permeability, leading to leakage of cellular contents, and ultimately causing cell death5. Systemic administration of colistin remains the standard approach for treating deep skin infections caused by MDR bacteria. However, systemic administration of such type of antibiotics often results in serious side effects, including nephrotoxicity and hepatotoxicity, which can hinder patient recovery and elevate morbidity and mortality rates. Plasma concentrations of colistin required for anti-microbial action also heighten the risk of nephrotoxicity, emphasizing the need for safer and more effective treatment methods6.
Nanotechnology has emerged as a promising approach to augment the therapeutic effects of drugs while reducing their adverse effects. Topical transdermal formulations offer numerous advantages, such as targeted drug delivery, fewer systemic side effects, greater patient compliance and improved therapeutic efficacy. These formulations are effective when the drug penetrates the skin layers and forms a localized depot7. However, the therapeutic efficacy of certain drugs may be limited by their physicochemical properties and the constraints of conventional formulations like ointments, gels and creams, which often have larger drug particle sizes. The drug’s effectiveness at its action site depends on the size of its particles8. Nanoemulsions (NEs) are advanced drug delivery systems characterized by their small droplet size (100–1000 nm) and kinetic stability9. Comprising two immiscible liquids stabilized by surfactants or co-surfactants, NEs improve drug solubility, dissolution and bioavailability10. They allow higher drug loading compared to traditional topical formulations like creams or gels and can accommodate both hydrophilic and hydrophobic drugs while enabling sustained drug release11. Despite these benefits, they often suffer from low viscosity, which poses challenges for transdermal applications. Incorporating gelling agents like polymers can address this issue by enhancing viscosity and improving therapeutic performance12.
Gels are widely preferred for dermatological use due to their numerous beneficial characteristics, such as non-staining, easy to remove, offering excellent spreadability, being non-greasy, highly compatible with various excipients, water solubility and displaying pseudo-plastic behavior13,14. Gels can be combined with NEs to produce nanoemulgels (NEGs). In these systems, the polymers within the gels form a three-dimensional hydrogel network that encapsulates the NE globules. NEGs are typically composed of either oil-in-water (O/W) or water-in-oil (W/O) NEs, which are gelled by incorporating appropriate gelling agents. These formulations combine the advantages of both NEs and gels, resulting in enhanced patient acceptability15. Upon extensive literature review, it was scarce to find Colistin based nanoemulsion or nanoemulgel drug delivery systems. However, recent studies underscore the antimicrobial potential of colistin when used in innovative drug delivery systems. For instance, colistin combined with amikacin in nanostructured lipid carriers demonstrated effectiveness against wound and lung infections16. Another research showed enhanced anti-microbial efficacy of colistin in combination with glabrol by the development of micelle based delivery system17. These available few studies utilized a combination approach and did not explore colistin as a single entity. Additionally, compared to micellar dispersions, NEs offer superior solubilization capacity and thermodynamic stability. Building on this, the current study focuses on developing, characterizing and evaluating the anti-microbial activity of colistin sulfate (CS) loaded NEG for transdermal application, contributing to ongoing efforts to improve existing anti-microbial therapies.
Material & Methods
Materials
Colistin sulfate (CS) was generously gifted by Zafa Pharma Pvt. Ltd. (Karachi 75950, Sindh, Pakistan). Transcutol and Labrafil M1944 CS were obtained from Gattefossé Co. (Chemin De Genas 69800 Saint-Priest, France). Tween 80, phosphate buffer (pH 7.4) and triethanolamine were purchased from Sigma Aldrich (St. Louis, MO 63103, USA). Carbopol 940P was procured from Lubrizol (29400 Lakeland Boulevard Wickliffe, Ohio 44092, USA). Distilled water was used in all experiments. Analytical grade chemicals and reagents were utilized.
Methodology
Standard calibration curve
The standard calibration curve is mandatory and signifies in finding out any unknown concentration under various conditions. Firstly, the standard stock solution of CS was prepared by solubilizing its 100 mg in 100 ml PBS, pH 7.4 to yield solution of strength 1 mg/ml. From this stock solution, various dilutions, i.e. 150 µg/ml to 9.375 µg/ml, were prepared utilizing the same solvent. The drug absorbance was recorded at 288 nm, and a calibration curve was constructedvia UV/Vis Spectrophotometer (UV-1800 Shimadzu Japan) using the linearity data18.
Pre screening of formulation ingredients
Pre formulation studies are mandatory in case of nano-based systems to get a clue about remarkable drug dissolution and permeation profiles. To select suitable oil phase, surfactant and co surfactant, saturation solubility experiments was employed as previously reported with minor modifications. This experiment utilized dissolving excess amount of Colistin sulphate in about 2 ml of various oils (olive oil, almond oil, Labrafil, oleic acid), surfactants (Tween 80, Span 80, Tween 60, Span 60) and Co surfactants (Glycerol, PEG400, Transcutol, Iso propyl alcohol). The required quantities of drug and oils, surfactants and co surfactants were separately sealed in the vials and subjected to stirring at 37 ± 0.5 °C for 48 h followed by centrifugation at 5000 rpm for 10 min to separate any insoluble drug residues. The supernatant was further filtered through 0.45µ filter paper and quantification was done by utilizing UV/Vis Spectrophotometry at 288 nm, using phosphate buffer solution pH 7.4 as blank. Determinations were conducted in triplicate.
Preparation of blank and drug loaded NE and NEG formulation
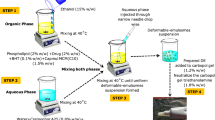
A high shear homogenization method was used to fabricate CS loaded NE formulation, based on a previously published study with slight modifications19. The formulation included Labrafil as oily phase, while Tween 80 and Transcutol served as surfactant and co-surfactant, respectively. To prepare the oil phase, Labrafil and Transcutol were mixed under magnetic stirring at 700 rpm and 70 °C for 1 h. Meanwhile, the aqueous phase was formulated by the dissolution of CS and Tween 80 in distilled water, which was stirred continuously for 1 h at the same temperature. The oil phase was then slowly added to the aqueous phase, and the mixture was stirred continuously to form the final NE formulation. Trial and error method was used for formulation optimization by adjusting the concentrations of oily phase, surfactant, and co-surfactant to achieve the desired clarity and homogeneity. To assess phase separation, the produced NE was sonicated for 15 min and then left at room temperature. A similar procedure was followed to produce blank NE formulation (Table 1).
To enhance skin contact time and improve patient usability, the optimized NE was converted into NEG formulation. Various polymeric plain gels were fabricated to determine the gel base deemed fit for our optimized formulation. These included HPMC (1%, 2%, 3%), Sodium alginate (2%, 2.5%, 3%), Chitosan (2%,3%, 4%) and Carbopol 940 (1%,1.5%,2%). Out of all these fabricated plain gel bases Carbopol 940 presented ideal viscosity and homogeneity. Moreover, the stability of the formulation was also superb, thus we selected Carbopol 940 2% gel as a carrier for loading our optimization formulation. Carbopol 940 (2%) gel base was prepared in distilled water using a hot plate magnetic stirrer at 500 rpm for 1 h. Finally, the NE and gel base were combined in a 1:1 ratio with constant stirring at 1,000 rpm for 10 min, and the pH was adjusted with triethanolamine. The schematic development process of colistin loaded nanemulsion gel explained in Fig. 1.
Physico-chemical assessment of optimized NE formulations
Thermodynamic stability studies
The formulated CS-NE systems were tested under various stress conditions, including heating cooling (4 °C and 40 °C) and freeze thaw cycles (−21 °C and 25 °C), with the system being stored at each specified temperature for 48 h. In centrifugation test, 1 ml of the CS-NE system was diluted with distilled water to 100 ml and subjected to centrifugation at 5,000 rpm for half an h. These experiments were repeated for up to three cycles, and any signs of phase separation were visually monitored20.
PS, PDI and ZP measurement
The globule size and polydispersity index (PDI) of various CS-NE formulations were measured using dynamic light scattering (DLS) at 25 °C with a Zetasizer (Nano ZS90; Malvern Instruments, UK). Additionally, the zeta potential of CS-NE formulations was assessed using identical device21.
Entrapment efficiency (EE)
The entrapment efficiency (EE) of the optimized CS-NE formulations was assessed to estimate the amount of drug encapsulated in the formulated NE system. The formulations were placed in a centrifuge tube, followed by their centrifugation at 15,000 rpm for half an h. The supernatant was then separated and further diluted to measure the unencapsulated drug in the NE, with absorbance readings taken at 288 nm using a UV spectrophotometer. The absorbance values were converted into drug concentration using a mathematical calculation. The percentage of EE was calculated from the equation as follows22:
Drug content
The drug content of optimized CS-NE formulations was measured using a slightly modified version of a previously reported method23. In short, the samples were diluted twice with methanol, then centrifuged at 3,500 rpm for 15 min before being analyzed using the UV method at 288 nm.
pH determination
The pH of the developed CS-NE formulations was determined at 25 °C by a calibrated pH meter (Mettler Toledo, Langacher, Switzerland). The experiment was repeated three times, and the results were averaged24.
FTIR analysis
The FTIR spectra of CS, Labrafil, Tween 80, Transcutol and the optimized CS-NE formulations were obtained using FTIR spectroscopy (Perkin Elmer, USA). This analysis was conducted to assess the compatibility of the drug as well as polymer with the formulation constituents and to identify the functional groups as well as wavenumbers in the formulations. A diamond crystal was used to hold the components and samples, which were then pressed using a knob. The spectra for each sample were noted within the wavenumber range of 400–4000 cm−1, and measurements were taken in triplicate25.
Thermal analysis
The thermal stability of CS-NE formulations was assessed using both thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) simultaneously with a TGA–DSC thermal analyzer (HT 3 Mettler Toledo). The thermal properties of both the optimized and blank NE formulations were evaluated with a heating rate of 10 °C per min. Each 10 mg sample was subjected to heating from 25 °C to 600 °C under a nitrogen gas flow of 10 ml per min26.
Physico-chemical assessment of CS-NEG formulations
pH determination
The pH of CS-NEG formulations was measured using a digital pH meter (Mettler Toledo, Langacher, Switzerland) at 25 ± 0.5 °C. In this case, 1 g of NEG was dispersed in double distilled water (100 ml) and allowed to equilibrate for 2 h. A pH electrode was then immersed in the formulation, and the determination of pH was done at 25 °C. Three separate readings were taken, and the results were presented as the mean ± SD27.
Zeta analysis
The particle size, PDI and zeta potential of CS-NEG formulations were analyzed using a Zetasizer (Nano ZS90; Malvern Instruments, UK). The analysis was carried out at 90º scattering angle and 25 °C temperature28.
Viscosity
A Brookfield viscometer (DV-II + Pro, USA) was used to measure the viscosity of the prepared CS-NEGs, utilizing spindle No. 6 at a rotation speed of 10 rpm, maintained at a temperature of 37 ± 0.5 °C29.
Spreadability
The spreadability of each CS-NEG formulation was evaluated by a glass plate, based on a previously described method with minor adjustments30,31. A precise amount of drug loaded NEG was placed on a glass plate that had a 2 cm circle marked on it. Another glass plate was then placed on top, followed by a 0.5 kg weight, which was left for ten min. Triplicate readings were noted.
In-vitro drug dissolution & kinetic modeling
An in-vitro drug release test was conducted based on a previously published study with minor modifications32. The experiment used a Franz diffusion cell having two compartments: a donor (3 ml capacity) and a receptor (6 ml capacity). Prior to adding the CS-NEG formulations, the temperature was maintained at 32 ± 0.5 °C, and the stirring speed was set to 300 rpm. To study the in-vitro drug release, an artificial membrane was used, specifically a Tuffryn membrane (Sartorious, Germany), which was placed between the donor and receptor chambers. The donor chamber was filled with 1 g of the formulation, while the receptor chamber contained sodium acetate buffer, pH 5.5. At predetermined intervals of 0, 1, 2, 4, 8, 12, 18 and 24 h, 2 ml aliquots were taken out from the receptor chamber using a spiral syringe and replaced with fresh buffer to maintain sink conditions. The samples were then analyzed using UV spectrophotometry at 288 nm to assess the drug release profile. The drug release mechanism was investigated by the fitting of drug dissolution data into the Korsmeyer-Peppas model. This model gave “n” value that served as predictor of mechanism of drug release, whether it is Fickian diffusion or Non Fickian diffusion.
Ex-vivo skin permeation
To Before conducting the ex-vivo permeation analysis, formal approval (NOC) was obtained from the Pharmacy Ethical Committee of the Faculty of Pharmacy at Bahauddin Zakariya University, Multan (Protocol approval No. 272/PEC/2024, dated 22/11/2024). All procedures involving experimental animals followed international guidelines set by the OECD for Environment, Health, and Safety. Male Albino rats, weighing between 200 and 300 g, were supplied by the in-vivo Research Laboratory at BZU, Multan. The rats were housed under a 12-hour light/dark cycle for seven days to allow acclimatization. They had free access to food (standard diet) and water (ad libitum). The environmental conditions were carefully maintained at a temperature of 25 ± 2 °C with a relative humidity of 50 ± 10%. For the procedure, all animals were anesthetized using intravenous ketamine (87 mg/kg body weight) and xylazine (13 mg/kg body weight) before being euthanized via cervical dislocation. The abdominal area was shaved with a razor blade, and the skin was carefully shaved. The excised skin was then washed with a normal saline solution (0.9% NaCl), and any adhering fat was removed using a scalpel. Afterward, the skin was wrapped in aluminum foil and stored at −20 °C in a deep freezer until further use. Prior to use, thawing of the skin was performed at room temperature and immersed in phosphate buffer for at least 1 h to ensure proper hydration of the membrane. The permeation study was carried out by applying a measured number of CS-NEG formulations over abdominal rat skin (pre-treated), which was placed in the donor chamber of Franz diffusion assembly (diffusional area 1.13 cm²). The receptor chamber contained 10 mL of solution. Samples (0.5 mL) were collected at 0, 2, 4, 6, 8, 10 and 12 h. Subsequent filtration of the samples was done via 0.45 μm filter and analyzed for drug content at a wavelength of 288 nm33.
Anti-microbial activity
The minimum inhibitory concentration (MIC) of CS-NEG formulations was assessed using the broth dilution method on the Escherichia coli strain, which is commonly found in damaged skin. Different concentrations (20 to 200 mg/ml) of the formulations were prepared and placed in 96 well microtiter plates containing Mueller–Hinton broth (MHB). This media is widely used because it supports the growth of most bacteria, making it ideal for determining the MIC of antibiotics. Each well was then inoculated with culture (100 µl) having approximately 5 × 10⁵ colony forming units (CFU)/ml of test microbe. The plates were subjected to incubation at 35 ± 1 °C for 24 h which were then examined spectrophotometrically, and the extent of turbidity of the medium in each well was recorded. MIC was identified as the lowest concentration that inhibited visible bacterial growth. Triplicate readings were noted.
The outcomes from the agar well diffusion technique was used to compare the anti-bacterial activity of CS solution (positive control) and the CS-NEG formulations, with E. coli as the test organism. CS was dissolved in distilled water (1 ml), and CS-NEG formulation (0.5 g) was dissolved in 1 ml of phosphate buffer solution (pH 7.4) which were then added to different wells. Regarding the anti-microbial activity, a comparison was made between positive control and test samples, with the blank formulation serving as negative control. All tests were performed thrice, and the mean ± SD of diameters of the inhibition zones were determined34,35.
Statistical analysis
All experiments were conducted in triplicate, and the results were presented as the mean ± SD. Statistical comparisons were made using analysis of variance (ANOVA) and Student’s t-test, with GraphPad Prism (version 8.0.2) software (San Diego, CA, USA). A p-value of less than 0.05 was considered statistically significant.
Results & discussion
Standard calibration curve
The calibration curve demonstrated a linear relationship between drug concentrations and absorbance, with a regression coefficient of 0.9818, a slope of 0.0001696, and a Y-intercept of 0.007542, as shown in Fig. 2. The analytical method developed proved to be precise, specific, and robust.
Preparation of CS-NE formulation based on screening of ingredients
A pre-formulation study conducted for adequate screening of formulation ingredients made it obviously clear to find out suitable excipients for the final development of formulation. Solubility experiment paved the way in the selection of requisite oil phase, surfactant and co- surfactant. The results of solubility experiment suggested that among all the tested solvents (Data not shown), Labrafil, Tween 80 and Transcutol were found more compatible with the drug and could be tailored in the nanoemulsion formulation as oil phase, surfactant and co-surfactant respectively. A total of eight CS-NE formulations were successfully prepared by high pressure homogenization technique using Labrafil, Tween 80 and Transcutol as oil phase, surfactant and co-surfactant, respectively. It is widely recognized that the composition of NE formulation can influence its various characteristics. Therefore, different concentrations of oil and surfactant were chosen for the study. According to previous research, higher oil concentrations can lead to larger droplet sizes in a NE, so lower oil concentrations were preferred36. It is also suggested that ionic surfactants cause irritation, thus non-ionic surfactants in low concentrations were selected. Therefore, for each oil concentration, the minimal necessary amount of Smix was used37.
Thermodynamic stability studies
NEs are thermodynamically unstable, as they form under specific ratios of oil, Smix and water without exhibiting phase separation, creaming or cracking. Thermodynamic stress testing was conducted to identify any metastable NEs within the screened formulations. This process evaluates the flow of free energy between the system and its environment. Table 2 presents the findings of the thermodynamic stability analysis for various CS-NE formulations. Some formulations showed turbidity during the physical stability test, while others underwent phase separation. The instability of certain NEs was attributed to Ostwald ripening, where smaller droplets merge through a diffusion mechanism, resulting in larger droplets driven by surface free energy10,38.
Three selected NE formulations (CS NE3, CS NE4 and CS NE5) demonstrated stability during heating cooling, freeze thaw cycles and centrifugation studies. These formulations showed no signs of phase separation, creaming, or cracking and were deemed suitable for further investigations. Their stability is likely due to the high zeta potential, which ranged between − 11.8 ± 0.02 mV to −20.6 ± 0.51 mV (Table 3). The magnitude of surface charges directly influences the stability of the NEs, as strong repulsive forces between droplets minimize the likelihood of coalescence as well as physical instability. NEs offer specific benefits, including thermodynamic and physical stability39.
Characterization of optimized CS-NE formulations
Zeta analysis
The particle size of the selected CS-NE formulations was assessed and is detailed in Table 3. It was observed that particle sizes ranged from 121.7 ± 8.61 nm (CS-NE4) to 193.2 ± 9.52 nm (CS-NE3). A visual representation of various characteristics of CS-NE3 is provided in Fig. 3. Particle size plays a significant role in drug permeation through the skin and enhances drug retention in the epidermis. According to published studies, nanocarrier sizes between 80 and 250 nm is ideal for achieving effective transfollicular and transcellular penetration in the skin40. Furthermore, some studies suggest that NEs with particle sizes below 500 nm are also acceptable41. Therefore, all formulations in this study fall within the acceptable nanosize range. Our findings indicated that CS-NE3 exhibited the largest droplet size, followed by CS-NE5 and CS-NE4. The increase in particle size corresponds to higher lipid concentrations, likely due to the insufficient amount of surfactant to solubilize the drug and lower interfacial tension. When surfactant concentration is low, particle aggregation occurs, leading to increased particle size42. Conversely, increasing surfactant concentration decreases particle size, as surfactants reduce the available space for the drug to aggregate43. In this study, Tween 80, an ester of long-chain fatty acids, was used as the surfactant, effectively reducing particle size. The proper surfactant concentration significantly impacts particle size44.
The polydispersity index (PDI), a measure of globule size uniformity, is crucial for stabilizing NEs. PDI values typically range from 0 to 1. In the current investigation, all formulations demonstrated PDI values below 0.5 (Table 3), indicating a narrow and consistent size distribution. Smaller particle sizes and lower PDI values are considered beneficial for formulation stability and improved drug penetration into the skin45.
Similarly, the zeta potential of the developed CS-NE formulations ranged from − 11.7 ± 0.11 mV to −42.3 ± 0.08 mV. These values represent desirable surface charges for topical applications, as they enhance skin permeability and promote deeper penetration. The negative charge of the formulations is attributed to the anionic groups of fatty acids and glycols present in the oil, surfactant, and co-surfactant. For small molecules, higher zeta potential values correlate with greater stability, as the repulsion between droplets minimizes aggregation46.
Drug content
The drug content in the optimized CS-NE formulations ranged from 89.3 ± 7.11% to 94.6 ± 2.35% (Table 3). This parameter reflects the drug loading capacity and plays a critical role in determining the amount of the drug that reaches the blood. It also affects the drug release profile from the NE formulation. Drug content close to 100% is typically anticipated in NE formulations. The high drug content values indicate the uniform distribution of drug throughout the formulations, minimizing drug loss during various stages of formulation development. This further validates the effectiveness of the preparation method47.
Entrapment efficiency
The drug EE of the optimized CS-NE formulations was analyzed using a UV spectrophotometer at a wavelength of 288 nm. All formulations demonstrated good drug entrapment efficiency, with values ranging from 74.2 ± 6.55% to 84.2 ± 5.89% (Table 3). These findings are consistent with previously reported values in the literature48. The amount of surfactant was found to significantly influence drug entrapment efficiency. Specifically, an increase in surfactant concentration, as observed from CS-NE5 to CS-NE3, corresponded to a rise in drug entrapment.
pH determination
The pH values of the optimized CS-NE formulations ranged from 5.6 ± 0.23 to 6.6 ± 0.56 (Table 3), which are considered suitable and unlikely to cause skin irritation upon application, indicating the stability of the formulations. Typically, the pH of adult human skin is ranged from 4 to 6.5, but values within the range of 5 to 8 are generally acceptable for skin application49.
FTIR analysis
The potential molecular interactions between CS, excipients and CS-NE formulations were examined using FTIR (Fig. 4). The pure drug (CS) showed distinct FTIR peaks at 1696.19 cm−1 corresponding to C = O stretching of amide I, 1587.66 cm−1 and 1485.52 cm−1 that indicated N-H bending of amide II and 1023.48 cm−1 that was linked to C-N stretching vibrations50. The FTIR spectrum of Labrafil showed peaks at 2925.90 cm−1, 2850.88 cm−1, 1737.68 cm−1 and 1459.18 cm−1, corresponding to N-H bending of primary amines and O-H bending of carboxylic groups, respectively51. Tween 80 displayed numerous sharp absorption bands due to the various functional groups within the molecule. The hydroxyl group showed an absorption peak at 2924.30 cm−1, while the band at 2856.47 cm−1 was linked to –CH2 stretching. Another peak at 1743.27 cm−1 corresponded to C = O stretching, and 1098.49 cm−1 was due to C–O–C stretching52. The FTIR peaks of the optimized CS-NE formulations showed a similar pattern to those of the individual drug and excipients, with only minor differences and no new bands appeared, indicating absence of chemical interactions between the drug and excipients53.
Thermal analysis
The thermograms shown in Fig. 5 present the results from our combined TGA/DSC analysis of both optimized drug loaded and blank NE formulations. Literature suggests that CS melts within the range of 200–220 °C54. The DSC thermograms of the CS-NE formulations display endothermic peaks at 118.31 °C (CS-NE3), 120.25 °C (CS-NE4), 123.21 °C (CS-NE5), and 124.12 °C (blank NE), which are likely associated with the evaporation of water. However, the endothermic peak corresponding to CS was absent in the DSC thermograms of the formulations (Fig. 5), suggesting that the drug has been fully dispersed at the molecular level within the oil phase. This type of heat analysis determines the heat flux during phase transitions and chemical reactions55.
TGA is commonly used in the development of various materials (both solid and liquid) to assess their thermal stability as well as composition. TGA was employed to examine the degradation temperatures of the developed CS-NEs under a nitrogen atmosphere, ranging from 20 °C to 500 °C. The TGA thermograms of the optimized and blank NE formulations show a single primary degradation step, with weight losses of 88.55% (CS-NE3), 89.76% (CS-NE4), 88.51% (CS-NE5) and 91.04% (blank NE), which is attributed to the loss of surface water. As a result, the preparation of NE formulations containing Labrafil, Tween 80 and Transcutol was confirmed through TGA/DSC analysis56.
Physico-chemical characterization of developed CS-NEG formulations
The particle size, PDI and zeta potential of the CS-NEGs were determined, with the results shown in the Table 4; Fig. 6. The particle sizes of the CS-NEGs exhibited a well-distributed range and were found to be within a narrow size spectrum. These findings align with those of Khalifa et al., who reported a particle size of 227 nm for an azithromycin and tea tree oil-based NEG formulation45. The pH of CS-NEG formulations ranged from 7.36 ± 0.23 to 7.60 ± 0.36 (Table 4). The pH of the prepared formulations was within the typical pH of human skin (4.5–6), suggesting the safety of the NEG for topical application, which could otherwise be compromised by an inappropriate pH. These results are consistent with Soliman et al., who verified the uniformity of a myrrh oil-based NEG and noted its pH range was between 5.58 and 6.730.
Viscosity is a critical property for topical formulations. Therefore, the viscosity of the developed CS-NEG formulations was evaluated, with the results depicted in the Table 4. It was found that the CS-NEGs had relatively high viscosity values (34,510 ± 99.85 cP to 38,810 ± 103.77 cP), which is within an acceptable range. These findings align with those of Upadhyay et al., who noted that the developed gel had sufficient viscosity and followed a Newtonian flow pattern23. Moreover, CS-NEG3 displayed the highest viscosity among the formulations, likely due to the greater concentration of Tween 80 used in the CS-NEG3 formulation. The viscosity needs to be optimized for improved skin adherence and spreadability57,58.
Spreadability is a key factor for homogenous application of topical treatments and ensuring patient compliance59. All formulations demonstrated excellent spreadability with minimal shear force, making them easy for patients to apply (Table 4). These findings align with those of Razzaq et al., who emphasized the importance of spreadability for even distribution and patient adherence in glimepiride-loaded NEG formulations41.
In-vitro drug release & kinetic modeling
The viscosity and drug release from the NEG formulation are influenced by the polymer and surfactants. As their concentration increases, the formulation becomes thicker and more rigid, slowing down the drug’s release from the dosage form. Accordingly, CS-NEG3, CS-NEG4 and CS-NEG5 demonstrated cumulative drug release of 73.6 ± 2.01%, 79.42 ± 2.25% and 84.31 ± 5.28% over 24 h, respectively (Fig. 7). There is statistically significant difference between drug release of CS-NEG3 & CS-NEG5 (p < 0.05, one-way Anova). These results suggest a delayed drug release from the formulations, likely due to the gel matrix’s ability to hinder drug release, coupled with a reduced water content. All formulations initially showed a burst release, where unentrapped drug rapidly diffused into the release medium, followed by a sustained release. These release patterns align with previous research findings22. Such type of drug release profile is beneficial for topical formulations, as the burst release delivers the requisite dose to the target site, achieving therapeutic concentrations, while the sustained release maintains those levels. When the drug dissolution data were fitted to the Korsmeyer-Peppas model, the n value was determined to be 0.48 (< 0.5), suggesting that the drug release follows Fickian diffusion60.
Ex-vivo skin permeability
The drug skin permeability was analyzed using CS-NEG formulations with the Franz diffusion cell and the results are shown in the Fig. 8. It is evident that the cumulative drug amount that permeated through the skin was 189.81 ± 10.23 µg/cm² for CS-NEG3 and 120.7 ± 12.69 µg/cm² for the plain CS gel producing statistically significant difference (Student t-test, p < 0.05). Also, a statistically significant difference existed between permeation profiles of CS-NEG3 & CS-NEG5 (p < 0.05, one-way Anova). The increased skin permeability of CS in the CS-NEG formulation could be attributed to its encapsulation within the NE system. Encapsulating the drug within nano oil droplets helps improve its ability to permeate the skin61. The enhanced drug permeation from NEG formulations is mainly due to their nanometric size and the inclusion of Tween 80 in the Smix formulation. Tween 80 acts as a surfactant as well as permeation enhancer, promoting dermal drug delivery. By interacting with the skin’s stratum corneum and water molecules within the cells, Tween 80 modifies the lipid and protein structures of the skin, thus increasing the its permeability to drugs20.
Anti-microbial activity
The MIC assay results demonstrated strong anti-microbial activity of the tested CS-NEG formulations, with MIC values ranged from 1.25 to 1.50 µg/ml against the E. coli strain. A comparison of anti-bacterial susceptibility between the CS solution and the CS-NEG formulation indicated that, once the drug was incorporated into the nano formulation, it exhibited superior antibacterial activity (CS-NEG3) over a 24-h period (ANOVA, p < 0.01), compared to both the drug alone and the control (Fig. 9). The improved effectiveness of CS-NEG3 can likely be ascribed due to the oil in the form of nano-sized globules within the formulation62. These findings align with earlier studies, which also showed that nano formulations had enhanced anti-bacterial effects63.
Conclusion
Anti-microbial resistance is alarmingly posing serious threats and needs urgent addressal. Colistin is popular last available weapon that can eradicate Gram negative organisms responsible for inducing sepsis. However, its toxicity limits its use and requires a novel captivating strategy to minimize its toxicity and maximize its efficacy. A successful stable topical nanoemulsion gel system was crafted with uniform dispersion of colistin using the high-pressure homogenization technique and investigated for various physico-chemical attributes with main focus on ex-vivo skin permeability and anti-microbial potential of the formulation. Among these formulations, CS-NEG3 showed promise in enhancing drug permeability and antibacterial effectiveness compared to the pure drug solution for topical use. The colistin encapsulation in nanoemulsion system yielded a droplet size around 108.10 nm using optimal surfactant and co-surfactant concentrations. This easy method of high shear homogenization is of significance for industrial scalability of nanoemulsion based drug delivery systems loaded with therapeutic moieties having poor biopharmaceutical attributes. However, further in-depth in-vivo studies and an exploration of the underlying mechanisms of anti-microbial activity are recommended to advance this formulation from the lab to clinical application.
Limitation of the study
While the developed CS-loaded nanoemulgel demonstrated excellent physicochemical properties and antimicrobial potential, two key limitations remain. First, the long-term stability of the formulation under varying environmental conditions was not comprehensively assessed, which is essential for ensuring product consistency and shelf-life. Second, although enhanced antimicrobial activity was observed, the specific mechanism by which the nanoemulsion system improves colistin’s antibacterial action was not fully elucidated. Understanding how the formulation interacts at the microbial and cellular levels would provide critical insights for further optimization. Addressing these limitations will be vital to support future clinical translation and regulatory acceptance.
Data availability
All data generated or analysed during this study are included in this published article and rest of data available as a supplementary file S1.
References
Refai, H. et al. Enhanced wound healing potential of spirulina platensis nanophytosomes: metabolomic profiling, molecular networking, and modulation of HMGB-1 in an excisional wound rat model. Mar. Drugs. 21 (3), 149. https://doi.org/10.3390/MD21030149/S1 (2023).
Uberoi, A., McCready-Vangi, A. & Grice, E. A. The wound microbiota: microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 22 (8), 507–521. https://doi.org/10.1038/s41579-024-01035-z (2024).
Salam, M. A. et al. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 11 (13), 1946. https://doi.org/10.3390/HEALTHCARE11131946 (2023).
Moubareck, C. A. Polymyxins and bacterial membranes: A review of antibacterial activity and mechanisms of resistance. Membranes 10 (8), 181. https://doi.org/10.3390/MEMBRANES10080181 (2020).
Trimble, M. J., Mlynárčik, P., Kolář, M. & Hancock, R. E. W. Polymyxin: alternative mechanisms of action and resistance. Cold Spring Harbor Perspect. Med. 6 (10), a025288. https://doi.org/10.1101/CSHPERSPECT.A025288 (2016).
Pulakat, L. et al. Transdermal delivery of high molecular weight antibiotics to deep tissue infections via droplette micromist technology device (DMTD). Pharmaceutics 14 (5), 976. https://doi.org/10.3390/PHARMACEUTICS14050976 (2022).
Benson, H. A. E., Grice, J. E., Mohammed, Y., Namjoshi, S. & Roberts, M. S. Topical and transdermal drug delivery: from simple potions to smart technologies. Curr. Drug Deliv. 16 (5), 444–460. https://doi.org/10.2174/1567201816666190201143457 (2019).
Choudhury, H. et al. Recent update on nanoemulgel as topical drug delivery system. J. Pharm. Sci. 106 (7), 1736–1751. https://doi.org/10.1016/J.XPHS.2017.03.042 (2017).
Jindal, A., Kumar Sharma, P. & Kumar, A. Self-nanoemulsifying drug delivery system (SNEDDS) as nano-carrier framework for permeability modulating approaches of BCS class III drug. J. Drug Target. 1–21. https://doi.org/10.1080/1061186X.2025.2469751 (2025).
Kumar, A., Singh Arya, P. K. & Jindal, A. Modulation of intestinal permeability of 5-fluorouracil via phospholipid interaction based lipophilic complex designing and Pharmacokinetic assessment. J. Dispers. Sci. Technol. 46 (8), 1315–1327. https://doi.org/10.1080/01932691.2024.2325398 (2025).
Pavoni, L., Perinelli, D. R., Bonacucina, G., Cespi, M. & Palmieri, G. F. An overview of Micro- and nanoemulsions as vehicles for essential oils: formulation, Preparation and stability. Nanomaterials 10 (1), 135. https://doi.org/10.3390/NANO10010135 (2020).
Kassem, M. A., Ghalwash, M. M. & Abdou, E. M. Development of nanoemulsion gel drug delivery systems of cetirizine; factorial optimisation of composition, in vitro evaluation and clinical study. J. Microencapsul. 37 (6), 413–430. https://doi.org/10.1080/02652048.2020.1771446 (2020).
Albash, R. et al. Sonophoresis mediated diffusion of caffeine loaded Transcutol® enriched cerosomes for topical management of cellulite. Eur. J. Pharm. Sci. 201, 106875. https://doi.org/10.1016/J.EJPS.2024.106875 (2024).
Saraogi, G. K. et al. Formulation development and evaluation of Pravastatin-Loaded nanogel for hyperlipidemia management. Gels 8 (2), 81. https://doi.org/10.3390/GELS8020081 (2022).
Alyoussef, A. et al. The beneficial activity of Curcumin and Resveratrol loaded in nanoemulgel for healing of burn-induced wounds. J. Drug Deliv. Sci. Technol. 62, 102360. https://doi.org/10.1016/J.JDDST.2021.102360 (2021).
Vairo, C., Villar Vidal, M., Hernandez, M., Igartua, R., Villullas, S. & M., & Colistin- and amikacin-loaded lipid-based drug delivery systems for resistant gram-negative lung and wound bacterial infections. Int. J. Pharm. 635, 122739. https://doi.org/10.1016/J.IJPHARM.2023.122739 (2023).
Liu, Y. et al. Synergistic antimicrobial efficacy of glabrol and colistin through micelle-based co-delivery against multidrug-resistant bacterial pathogens. Phytomedicine 137, 156371. https://doi.org/10.1016/J.PHYMED.2025.156371 (2025).
Noreen, M. et al. Formulation and in vitro characterization of sustained release tablets of lornoxicam. Lat. Am. J. Pharm. 38 (4), 701–711 (2019).
Rashid, S. A. et al. Development, characterization and optimization of methotrexate-olive oil nano-emulsion for topical application. Pak. J. Pharm. Sci. 34 (1), 205–215. https://doi.org/10.36721/PJPS.2021.34.1.SUP.205-215.1 (2021).
Algahtani, M. S. et al. Preparation and characterization of Curcumin nanoemulgel utilizing ultrasonication technique for wound healing: in vitro, ex vivo, and in vivo evaluation. Gels 7 (4), 213. https://doi.org/10.3390/GELS7040213 (2021).
Mahmood, A. et al. Enhanced intestinal permeability of cefixime by Self-Emulsifying drug delivery system: In-Vitro and Ex-Vivo characterization. Molecules 28 (6), 2827. https://doi.org/10.3390/MOLECULES28062827 (2023).
Mahdi, W. A. et al. Formulation and optimization of Butenafine-Loaded topical nano lipid Carrier-Based gel: characterization, irritation study, and Anti-Fungal activity. Pharmaceutics 13 (7), 1087. https://doi.org/10.3390/PHARMACEUTICS13071087 (2021).
Upadhyay, D. K. et al. Nanoemulgel for efficient topical delivery of finasteride against androgenic alopecia. J. Pharm. Innov. 16 (4), 735–746. https://doi.org/10.1007/S12247-020-09483-9/TABLES/5 (2021).
Amalyuri, A. G., Reveny, J. & Dalimunthe, A. Antibacterial potential of ethanol extract of tamarind seed bark (Tamarindus indica L.) and formulation of Anti-Acne nanogel. Int. J. Sci. Technol. Manage. 3 (3), 598–604. https://doi.org/10.46729/IJSTM.V3I3.522 (2022).
Khan, R. U. et al. Lornoxicam-Loaded Chitosan-Decorated nanoemulsion: Preparation and in vitro evaluation for enhanced transdermal delivery. Polymers 14, 1–18 (2022).
Abdullah, N. N. A. et al. Lemongrass essential oil nanoemulsion formulations based on tragacanth gum and Guar gum as durable anti-mosquito fabric finishing agents. Polym. Bull. 81 (18), 16903–16933. https://doi.org/10.1007/S00289-024-05490-9/TABLES/5 (2024).
Morsy, M. A. et al. Preparation and evaluation of Atorvastatin-Loaded nanoemulgel on Wound-Healing efficacy. Pharmaceutics 11 (11), 609. https://doi.org/10.3390/PHARMACEUTICS11110609 (2019).
Brunda, S., Kulkarni, S. V., Manjunath, K. & Pushpalatha, D. Formulation and evaluation of Oxiconazole nitrate loaded nanosponges. World J. Adv. Res. Reviews. 11 (3), 028–040. https://doi.org/10.30574/WJARR.2021.11.3.0405 (2021).
Elsewedy, H. S., Shehata, T. M. & Soliman, W. E. Tea tree oil Nanoemulsion-Based hydrogel vehicle for enhancing topical delivery of neomycin. Life 12 (7), 1011. https://doi.org/10.3390/LIFE12071011 (2022).
Soliman, W. E., Shehata, T. M., Mohamed, M. E., Younis, N. S. & Elsewedy, H. S. Enhancement of Curcumin Anti-Inflammatory effect via formulation into myrrh Oil-Based nanoemulgel. Polymers 13 (4), 577. https://doi.org/10.3390/POLYM13040577 (2021).
Anshika, Jindal, A., Saifi, A., Kumar, A. & Chaudhary, D. Quality by design (QbD) accredited rutin-loaded self-nanoemulsifying system of Krill oil for improved dissolution rate, ameliorating human neuroblastoma and hyperlipidemia. Int. J. Biol. Macromol. 320 (2), 145768. https://doi.org/10.1016/j.ijbiomac.2025.145768 (2025).
Malik, M. R. et al. Formulation and characterization of Chitosan-Decorated multiple nanoemulsion for topical delivery in vitro and ex vivo. Molecules 27 (10), 3183. https://doi.org/10.3390/MOLECULES27103183 (2022).
Firmansyah, F. et al. Development of novel Curcumin nanoemulgel: optimisation, characterisation, and ex vivo permeation. Pharm. Educ. 22 (2), 98–103. https://doi.org/10.46542/PE.2022.222.98103 (2022).
Sultan, M. H. et al. Development and optimization of Methylcellulose-Based nanoemulgel loaded with Nigella sativa oil for oral health management: quadratic model approach. Molecules 27 (6), 1796. https://doi.org/10.3390/MOLECULES27061796 (2022).
Jindal, A. & Kumar, A. Physical characterization of clove oil based self Nano-emulsifying formulations of Cefpodoxime proxetil: assessment of dissolution rate, antioxidant & antibacterial activity. OpenNano 8, 100087. https://doi.org/10.1016/j.onano.2022.100087 (2022).
Dhivya, V. et al. Formulation of sweet flag oil (Acorus calamus) nanoemulsion by spontaneous emulsification method for the management of sitophilus oryzae. Int. J. Chem. Stud. 7 (3), 2072–2076 (2019). https://www.researchgate.net/publication/333603038
González, J. N. H., de Alba, S., Velázquez, B. N., Herrera, M. M., Andrews, H. E. & G. A. C., & Optimization of clove oil nanoemulsions: evaluation of antioxidant, antimicrobial, and anticancer properties. Colloids Interfaces. 7 (4), 64. https://doi.org/10.3390/COLLOIDS7040064 (2023).
Kumar, A., Jindal, A. & Singh Arya, P. K. Cytotoxicity and bioavailability assessment from thiamin-phospholipid complexation loaded Ajwain oil based self nanoemulsifying system. J. Dispers. Sci. Technol. 45 (13), 2420–2436. https://doi.org/10.1080/01932691.2023.2266010 (2024).
Alhakamy, N. A. et al. Formulation development, statistical optimization, in vitro and in vivo evaluation of Etoricoxib-Loaded Eucalyptus Oil-Based nanoemulgel for topical delivery. Appl. Sci. 11 (16), 7294. https://doi.org/10.3390/APP11167294 (2021).
Yu, Z. et al. Recent progress in transdermal nanocarriers and their surface modifications. Molecules 26 (11), 3093. https://doi.org/10.3390/MOLECULES26113093 (2021).
Razzaq, F. A. et al. Glimepiride-Loaded nanoemulgel; development, in vitro characterization, ex vivo permeation and in vivo antidiabetic evaluation. Cells 10 (9), 2404. https://doi.org/10.3390/CELLS10092404 (2021).
Artigas, M. A., Pérez, Y. L. & Belloso, O. M. Curcumin-loaded nanoemulsions stability as affected by the nature and concentration of surfactant. Food Chem. 266, 466–474. https://doi.org/10.1016/J.FOODCHEM.2018.06.043 (2018).
Ali, A. et al. Development of nanogel loaded with Lidocaine for Wound-Healing: illustration of improved drug deposition and skin safety analysis. Gels 8 (8), 466. https://doi.org/10.3390/GELS8080466 (2022).
Sarheed, O. et al. Formation of stable nanoemulsions by ultrasound-assisted two-step emulsification process for topical drug delivery: effect of oil phase composition and surfactant concentration and Loratadine as ripening inhibitor. Int. J. Pharm. 576, 118952. https://doi.org/10.1016/J.IJPHARM.2019.118952 (2020).
Khalifa, N. E. et al. Development of tea tree oil based nanoemulgel loaded with Azithromycin for enhancing the antibacterial activity. Processes 11 (6), 1836. https://doi.org/10.3390/PR11061836 (2023).
Nagaraja, S., Basavarajappa, G. M., Karnati, R. K., Bakir, E. M. & Pund, S. Ion-Triggered in situ gelling nanoemulgel as a platform for Nose-to-Brain delivery of small lipophilic molecules. Pharmaceutics 13 (8), 1216. https://doi.org/10.3390/PHARMACEUTICS13081216 (2021).
Anwer, M. K. et al. Preparation of sustained release apremilast-loaded plgalga nanoparticles: in vitro characterization and in vivo Pharmacokinetic study in rats. Int. J. Nanomed. 14, 1587–1595. https://doi.org/10.2147/IJN.S195048 (2019).
Iriventi, P., Gupta, N. V., Osmani, R. A. M. & Balamuralidhara, V. Design & development of nanosponge loaded topical gel of Curcumin and caffeine mixture for augmented treatment of psoriasis. DARU J. Pharm. Sci. 28 (2), 489–506. https://doi.org/10.1007/s40199-020-00352-x (2020).
Yousef, S. A. et al. Mechanistic evaluation of enhanced Curcumin delivery through human skin in vitro from optimised nanoemulsion formulations fabricated with different penetration enhancers. Pharmaceutics 11 (12), 639. https://doi.org/10.3390/PHARMACEUTICS11120639 (2019).
Mangal, S. et al. Composite particle formulations of colistin and meropenem with improved in-vitro bacterial killing and aerosolization for inhalation. Int. J. Pharm. 548 (1), 443–453. https://doi.org/10.1016/J.IJPHARM.2018.07.010 (2018).
Sangeetha, G., Manickam, M. S. & Kumar, P. S. An investigation of ATR-FTIR compatibility studies and preformulation studies of tapentadol HCl to design and formulate transdermal proniosomal gel. Indian J. Nat. Sci. 11 (64), 29083–29094 (2021). www.tnsroindia.org.in
Rehman, A. et al. Fabrication, In Vitro, and In Vivo Assessment of Eucalyptol-Loaded Nanoemulgel as a Novel Paradigm for Wound Healing. Pharmaceutics, 14(9), 1971. https://doi.org/10.3390/PHARMACEUTICS14091971(2022).
Naseem, F. et al. Metronidazole based floating bioadhesive drug delivery system for potential eradication of H. pylori: Preparation and in vitro characterization. Polymers 14 (3), 519. https://doi.org/10.3390/POLYM14030519 (2022).
Haroun, A. A., Ayoob, F. A. & Masoud, R. A. Sol-Gel immobilization of colistin sulfate onto double walled carbon nanotubes: in vitro bone bioactivity. Nano Biomed. Eng. https://doi.org/10.26599/nbe.2024.9290088 (2024).
Sultana, N., Chauhan, D., Yadav, P. K., Chourasia, M. K. & Gayen, J. R. Enhanced oral bioavailability of Isoformononetin through nanoemulsion: development, optimization, and characterization. J. Pharm. Innov. 19 (2), 1–12. https://doi.org/10.1007/S12247-024-09821-1/FIGURES/7 (2024).
Hasssanzadeh, H., Alizadeh, M. & Bari, M. R. Formulation of Garlic oil-in-water nanoemulsion: antimicrobial and physicochemical aspects. IET Nanobiotechnol. 12 (5), 647–652. https://doi.org/10.1049/IET-NBT.2017.0104 (2018).
Miastkowska, M., Kulawik-Pióro, A. & Szczurek, M. Nanoemulsion gel formulation optimization for burn wounds: analysis of rheological and sensory properties. Processes 8 (11), 1416. https://doi.org/10.3390/PR8111416 (2020).
Zafar, A. et al. Luteolin-loaded invasomes gel for transdermal delivery: development, optimization, in-vitro, and preclinical evaluation. J. Oleo Sci. 73 (9), 1221–1240. https://doi.org/10.5650/JOS.ESS24041 (2024).
Ahmed, M. M. et al. Development of Apremilast Nanoemulsion-Loaded Chitosan gels: in vitro evaluations and Anti-Inflammatory and wound healing studies on a rat model. Gels 8 (5), 253. https://doi.org/10.3390/GELS8050253 (2022).
Algahtani, M. S., Ahmad, M. Z. & Ahmad, J. Nanoemulsion loaded polymeric hydrogel for topical delivery of Curcumin in psoriasis. J. Drug Deliv. Sci. Technol. 59, 101847. https://doi.org/10.1016/J.JDDST.2020.101847 (2020).
Elmataeeshy, M. E., Sokar, M. S., Bahey-El-Din, M. & Shaker, D. S. Enhanced transdermal permeability of terbinafine through novel nanoemulgel formulation; development, in vitro and in vivo characterization. Future J. Pharm. Sci. 4 (1), 18–28. https://doi.org/10.1016/J.FJPS.2017.07.003 (2018).
Zafar, A., Alsaidan, O. A., Mohamed, M. S., Yasir, M. & Khalid, M. Development of gentamicin bilosomes laden in situ gel for topical ocular delivery: optimization, in vitro characterization, toxicity, and Anti-microbial evaluation. Adv. Pharm. Bull. 14 (3), 646. https://doi.org/10.34172/APB.2024.057 (2024).
Bagheri, A. M. et al. Curcumin nanoemulgel: characterization, optimization, and evaluation of photoprotective efficacy, Anti-Inflammatory properties, and antibacterial activity. J. Cluster Sci. 35 (7), 2253–2272. https://doi.org/10.1007/S10876-024-02651-8/METRICS (2024).
Funding
The financial assistance from Universiti Kebangsaan Malaysia (DIP-2024-006) and Taif University, Taif, Saudi Arabia (TU-DSPP-2024-26) are kindly appreciated.
Author information
Authors and Affiliations
Contributions
Conceptualization, B. N and S. A. R.; Data curation, F. I., and B. N.; Funding acquisition, K. J. A., K. F. A. and J. L.; Investigation, F. I. and B. N.; Methodology, F. I., B. N. and A. K. A.; Resources, B. N., S. A. R.; Software, F. N., A. J., and A. R.; Supervision, B. N. and S. A. R.; Validation, A.K A., K. J. A., K. F. A. and J. L.; Visualization, F. N., A. J., A. R., A.K. A.; Writing – original draft, F. I., B. N.; Writing – review & editing, A.K A., K. J. A., K. F. A. and J. L
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics aaproval
Through reference number 272/PEC/2024, the Animal Research & Ethical Committee of the Bahauddin Zakriya University, Multan, Pakistan, certified, approved, and accepted the investigational procedures and protocols of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ijaz, F., Nasir, B., Rashid, S.A. et al. Colistin adorned topical nanoemulsion gel formulation for enhanced anti-microbial activity. Sci Rep 15, 34064 (2025). https://doi.org/10.1038/s41598-025-14440-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-14440-y