Abstract
Although hyperlipidemia is a key factor in cardiovascular disease, there are limited safe and effective therapies for disorders of lipid metabolism. This study investigated the therapeutic potential of Ginkgolide B (GB), a bioactive component of Ginkgo biloba leaves, in ameliorating hyperlipidemia and explored its underlying mechanisms. Utilizing a high-fat diet-induced hyperlipidemic rat model and lipidomics analysis, the study showed that GB significantly decreased serum total cholesterol, triglyceride, and low-density lipoprotein levels. Lipidomics revealed that GB reversed dysregulated sphingolipid metabolism, notably decreasing ceramides levels and increasing sphingomyelins, which are implicated in metabolic inflammation and oxidative stress. Mechanistically, GB activated PPARα, thereby enhancing fatty acid oxidation and upregulating the Nrf2 pathway to mitigate oxidative damage. These dual effects were validated in vitro using HepG2 cells, where GB reduced lipid accumulation and improved antioxidant defenses. Overall, these findings highlight GB as a promising therapeutic agent for hyperlipidemia by restoring sphingolipid homeostasis and targeting the interplay between lipid metabolism and oxidative stress.
Similar content being viewed by others
Introduction
Hyperlipidemia, characterized by elevated circulating lipid levels, contributes to over 50% of global cardiovascular mortality1. Current pharmacotherapies (e.g., statins) often face challenges such as drug resistance and myopathy, necessitating safer alternatives targeting lipid metabolic dysregulation2. Natural products, particularly those exhibiting both lipid-modulating and antioxidant activities, have gained increasing attention for their potential to address limitations associated with current medications for cardiovascular diseases3,4,5.
Extracted from the leaves of Ginkgo biloba, Ginkgolide B (GB) is a powerful antagonist of the platelet-activating factor (PAF) receptor6,7. GB exhibits a range of biological activities, including neuroprotection8, enhancement of vascular endothelial function9, anti-inflammatory properties, and antioxidant effects10. Furthermore, GB protects mitochondrial function, attenuates oxidative stress, and inhibits inflammation11,12, all of which positively impact lipid metabolism and reduce liver damage.
Accumulating evidence supports the role of Ginkgo biloba extracts, particularly ginkgolides, in lipid homeostasis13,14. GB alleviated hepatic lipid accumulation in high-fat diet fed mice by suppressing ferroptosis via Nrf2 activation15. Ginkgolide C activated Sirt1/AMPK in oleic acid-induced hepatocytes, downregulating PPARγ/SREBP-1c to inhibit lipogenesis while upregulating PPARα and CPT-1 to enhance fatty acid β-oxidation16. Further validating this, ginkgolide C attenuated Western diet-induced Non-Alcoholic Steatohepatitis (NASH) via AMPK signaling17. These findings indicate that GB is a promising candidate for treating metabolic dysregulation.
By integrating lipidomics with molecular pharmacology, this study examined the mechanisms through which GB alters lipid metabolism in hyperlipidemia. We hypothesized that GB could alleviate dyslipidemia by restoring sphingolipid balance and synergistically activating lipid catabolism and antioxidant pathways, representing a novel therapeutic strategy. This study is the first to integrate lipidomics with dual-pathway validation (involving PPARα and Nrf2) to elucidate GB’s role in sphingolipid metabolism. Distinct from previous work that focused on GB’s antioxidant properties, this study identifies ceramides/sphingomyelins (Cers/SMs) homeostasis as an innovative treatment target.
Materials and methods
Materials
Total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), glutathione peroxidase (GPx), superoxide dismutase (SOD), and malondialdehyde (MDA) kits were obtained from Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Nrf2 rabbit monoclonal antibody was purchased from Abotek Biotechnology Co., Ltd., Wuhan, China. CPT-1 monoclonal antibody was purchased from Wuhan Sanying Biotechnology Co., Ltd., Wuhan, China. Nuclear and cytoplasmic protein extraction kits, DAPI and FITC fluorescent dyes, and RIPA cell lysis buffer were sourced from Shanghai Biyun Tian Biotechnology Co., Ltd., Shanghai, China. Oleic acid (OA) and palmitic acid (PA) were sourced from Selleck Chemicals LLC, Houston, TX, USA. GB (purity ≥ 98%) was obtained from Hubei Jusheng Technology Co., Ltd., Hubei, China. Simvastatin (purity ≥ 98%) was purchased from Shanghai McLean Biotechnology Co., Ltd., Shanghai, China. DMEM high-glucose medium and fetal bovine serum (FBS) were sourced from Life Technologies Corporation, Carlsbad, CA, USA. Penicillin/streptomycin solution was sourced from Dalian Meilun Biotechnology Co., Ltd., Dalian, China. Isopropyl alcohol, acetonitrile, and methanol were sourced from Thermo Fisher Scientific Inc., Waltham, MA, USA. The CCK-8 kit was purchased from Bimake Inc., USA. Sprague-Dawley (SD) rats were provided by the Animal Center of Jinzhou Medical University. HepG2 human liver cancer cells were obtained from Shenzhen Haodi Huatuo Biotechnology Co., Ltd., Shenzhen, China.
Animal model and administration regimen
The SD rats, with weights between 200 and 240 g, were maintained under controlled conditions at 25 ± 2 °C, 40–70% relative humidity, and a 12-hour cycle of light and darkness. The institution’s guidelines for the care and use of laboratory animals were followed in all animal procedures.
Following a week-long period of acclimatization, 30 healthy male SD rats were allocated randomly into control, model, and GB groups (n = 10). The control group was administered a standard diet, while the model and GB groups were fed a high-fat diet designed to induce mixed hyperlipidemia, characterized by elevated levels of TG, TC, and LDL-C. This diet was composed of 20% sucrose, 15% lard, 10% casein, 1.2% cholesterol, 0.2% sodium cholate, and 52.2% standard diet18. The modeling process spanned a duration of four weeks, during which the rats’ weight was recorded. The blood lipid levels were regularly assessed to ensure the model’s successful establishment.
Following the successful model establishment, the GB group received 4 mg/kg of GB raw material via intragastric administration19,20. The GB raw material was prepared as a 1 mg/mL suspension in distilled water with 0.5% PVP K30 and administered intragastrically at a volume of 4 mL/kg. The control and model groups received 4 mL/kg of distilled water via intragastric gavage once daily for four consecutive weeks21,22. During the administration period, all three groups of rats were fed a standard diet, and their body weight and daily food intake were monitored. Pre-weighed food pellets were provided to each cage, with residual food collected and weighed after 24 h.
Following the final treatment administration, all experimental groups were fasted overnight for 12 h before body weight measurement. After inducing anesthesia via intraperitoneal injection of 1% sodium pentobarbital, the anesthetized animals were positioned in dorsal recumbency on surgical platforms. Aortic blood samples were collected into heparinized vacuum tubes (5 mL capacity) and gently inverted to ensure anticoagulant mixing. At 4 °C, samples underwent centrifugation at 3000 rpm for 15 min, followed by careful separation of the plasma with sterile pipettes. Plasma aliquots were immediately cryopreserved by rapid dipping into liquid nitrogen and stored at −80 °C. After blood collection, euthanasia was performed by an intraperitoneal injection of an overdose of sodium pentobarbital (300 mg/kg, dissolved in physiological saline), administered at a rate of 0.2 mL/s. Animals were monitored until complete absence of corneal reflex, respiratory movement, and heartbeat was confirmed. To ensure definitive death, cervical dislocation was subsequently performed. Liver tissue was rapidly excised for gravimetric analysis, with representative sections preserved through immersion in 4% phosphate-buffered paraformaldehyde for subsequent histological processing. The animal experiments were approved by the Animal Experiment Ethics Committee of Jinzhou Medical University (Ethics batch number: 2023034). All experimental procedures adhered to nationally established guidelines for laboratory animal welfare and ethical research practices, and the study was reported in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (PLoS Bio 8(6), e1000412, 2010).
Biochemical markers and histological examination
Serum lipid profiles, such as TG, TC, and LDL-C, were analyzed using manufacturer-recommended protocols with commercially available assay kits.
Hepatic tissues were precisely weighed, standardized to uniform size, and fixed in neutral-buffered 4% formaldehyde for structural preservation. Following fixation, tissues underwent dehydration through a graded ethanol series, clearing with xylene, and paraffin embedding. Using a microtome, 4-μm-thick sections were cut, mounted on glass slides, and deparaffinized in xylene followed by rehydration via a descending ethanol gradient. For H&E staining, sections were incubated with Mayer’s hematoxylin for 5 min, rinsed to blue nuclei, differentiated in 1% hydrochloric acid in 70% ethanol for 10 s, counterstained with 0.5% eosin Y for 3 min, dehydrated through an ascending ethanol series, cleared in xylene, and coverslipped with a xylene-based medium23.
Tissue staining was imaged using a bright-field microscope (Olympus, Tokyo, Japan) at 400× magnification. Five non-overlapping fields of view were randomly captured per slide. Images were quantitatively analyzed using ImageJ software (National Institutes of Health, Bethesda, Maryland, USA)24. First, images were converted to grayscale, and thresholding was applied to segment stained regions (nuclei and cytoplasm) from white areas (lipid vacuoles). Original and thresholded images were compared to ensure no omission or over-inclusion of lipid vacuoles. The percentage of white area relative to the total tissue area (encompassing both stained and white regions) was calculated25. Thus, a lower percentage of white area (lipid vacuoles) indicates less hepatic lipid accumulation.
Plasma lipidomics
Plasma sample processing
Aliquots of plasma (50 μL) were subjected to biphasic extraction, beginning with the addition of 200 μL of deionized H₂O and vortexing for 30 s. Lipid partitioning was achieved by sequential addition of solvents: 800 μL of methyl tert-butyl ether (vortexed for 3 min), followed by 240 μL of ice-chilled methanol (vortexed for 3 min). The mixture was then sonicated in a 4 °C water bath for 20 min, allowed to equilibrate at 25 °C for 30 min. Then, the mixture was centrifuged at 10 °C for 15 min at 14,000 g to achieve phase separation. Under nitrogen, the organic supernatant was evaporated, reconstituted in 200 μL of chilled isopropanol/acetonitrile (9:1 v/v) with 30 s of vortexing, and clarified by repeated centrifugation (14,000 g, 10 °C, 15 min).
Chromatographic conditions
Lipidomic profiling was performed using a Shimadzu Nexera LC-30 A UHPLC system coupled to a Waters BEH Amide C18 column (2.1 × 100 mm, 1.7 μm). The separation in chromatography was achieved by maintaining the column at 45 °C, using a flow rate of 300 μL/min, and employing a mobile phase of (A) acetonitrile: water (6:4, v/v) with 0.1 mM ammonium formate and 0.1% formic acid, and (B) acetonitrile: isopropyl alcohol (1:9, v/v) with 0.1 mM ammonium formate and 0.1% formic acid. The gradient elution program was as follows: 30% B for the first 2 min, followed by a linear gradient to 100% B over 23 min, and then 100% B maintained from 25 to 35 min. Samples were analyzed in randomized order without interruption (autosampler maintained at 10 °C) to minimize instrumental drift.
Parameters for mass spectrometry
Dual-polarity ionization analysis (positive and negative ESI modes) was conducted using a QExactive HF-X hybrid quadrupole-Orbitrap mass spectrometer coupled with ultra-high-performance liquid chromatography (UHPLC). The optimized ionization source parameters were as follows: vaporizer temperature, 300 °C; sheath gas flow, 45 arbitrary units (arb); auxiliary gas flow, 15 arb; purge gas flow, 1.0 arb; capillary voltage, 3.0 kV; and ion transfer capillary temperature, 350 °C. The mass analyzer was operated with the S-Lens RF at 50% intensity, acquiring spectral data across the 200–1800 m/z range in MS¹ mode. Instrumental configurations included: MS¹ scans at 70,000 resolution (FWHM at m/z 200); data-dependent MS² acquisition (top 10 precursors per cycle) with 17,500 resolution.
Data processing
LipidSearch 4.2 was used to perform relative quantitative analysis of lipids in samples26,27. The raw files were analyzed with these parameters: retention time alignment (± 0.2 min window), spectral noise filtering (minimum signal-to-noise ratio of 3:1), and mass accuracy thresholds (parent ion mass accuracy: 5 ppm; fragment ion mass error: 5 ppm; minimum diagnostic ion intensity: 5%). Processed datasets were subjected to initial quality control before further analysis. System suitability was monitored by injecting pooled quality control (QC) samples intermittently (every 10th injection), with data retained only when the relative standard deviation across QC batches remained below 15%.
Network Pharmacology
Candidate therapeutic targets of GB were screened through cross-referencing predictions from the Comparative Toxicogenomics Database (CTD)28 and PharmMapper29. Hyperlipidemia-associated genes were compiled from three bioinformatics platforms: CTD, DisGeNET v7.030, and GeneCards31 using standardized disease ontology filters. Human protein ortholog normalization and gene symbol standardization were performed using UniProt32. Overlapping targets between GB and hyperlipidemia were visualized using Venn diagrams generated by Venny 2.133.
Identified target proteins were analyzed for protein-protein interactions (PPI) using the STRING database34. Network topology analysis was conducted with Cytoscape 3.7.2 to identify key targets exhibiting high degrees of connectivity. Using DAVID v6.8, Gene Ontology (GO)34 and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses35,36,37 were carried out to characterize the identified targets. Data visualization, particularly for target identification and KEGG pathway mapping, was facilitated using results from a bioinformatics cloud platform. Ultimately, a network combining compounds, targets, and pathways was built using Cytoscape 3.7.2, with nodes symbolizing drugs, targets, and signaling pathways, and edges depicting the interactions among these nodes.
Molecular Docking
GB’s molecular structure was obtained from PubChem, and the corresponding target protein configurations were obtained from the RCSB Protein Data Bank. Molecular docking simulations were performed using AutoDock Vina 1.2 after preparing both molecules38. GB was treated as the flexible ligand, and the target protein as the rigid receptor. Protein preparation involved removing co-crystallized ligands, solvent molecules, and extraneous organic moieties using PyMOL 4.3.0. Electrostatic parameterization was performed using AutoDockTools, including the addition of hydrogen atoms and assignment of Gasteiger-Marsili partial charges, along with the specification of AD4 atom types. A grid box centered on the binding site was constructed. Ligand flexibility was defined by selecting torsional bonds and assigning a hierarchical root. Both macromolecular (.pdb) and ligand files were converted to.pdbqt format. Following docking, binding affinity was assessed using Vina’s scoring function, and intermolecular interactions were analyzed using Discovery Studio. PyMOL was used to generate three-dimensional visualizations of the binding poses, which were combined with two-dimensional interaction schematics generated by Discovery Studio to comprehensively characterize the ligand-receptor binding.
HepG2 cell experiments
Cell viability
HepG2 cells were incubated at 37 °C in a humidified 5% CO2 atmosphere using high-glucose DMEM containing 10% FBS and 1% penicillin-streptomycin39. When the cell monolayers reached 90% confluence, they were washed with PBS and detached using 0.25% trypsin-EDTA.
For the cell viability assay, the detached HepG2 cells were resuspended and seeded into 96-well plates at a suitable density. After 24 h of incubation to allow cell attachment and recovery, the culture medium was replaced with fresh medium containing different concentrations of GB solution (ranging from 1 to 80 μM). A negative control group was set up, where cells were treated with blank DMEM without any test compound. The effect of GB on HepG2 cell viability was quantitatively evaluated using the CCK-8 assay after a 2-hour exposure period.
Establishment of the cell model
Logarithmically growing cells were seeded into 24-well plates at a density of 1 × 105 cells/well and randomly assigned to one of six experimental groups: untreated control, OA/PA-induced model, GB-L (4 μg/mL), GB-M (8 μg/mL), GB-H (16 μg/mL), and simvastatin (20 μmol/L)40. Following cell attachment, the designated groups received media containing the corresponding treatments. Lipid overload was induced by a 24-hour exposure to a 2:1 (v/v) mixture of OA and PA at a total concentration of 1 mM15. Control cells were maintained in a standard medium without lipid or pharmacological treatment.
Biochemical markers
After 24 h of intervention, cellular monolayers were washed with phosphate-buffered saline (PBS, 4 °C) three times to halt reactions. Ice-cold RIPA lysis buffer was used to extract cellular components for 30 min, and then centrifuged at 12,000 g for 10 min to get clear lysates. Quantification of TC, TG, SOD, MDA, and GPx levels was performed using commercial assay kits according to the manufacturers’ protocols.
Immunofluorescence assay
Following induction, cells were processed sequentially as follows: rinsing with cold (4 °C) PBS; fixing with PFA (4%, 15 min); permeabilizing with Triton X-100 (0.5%, 10 min); and blocking with BSA (5%, 1 h). Primary antibodies against CPT-1 and Nrf2 (diluted in 1% BSA) were then used and cells were incubated at 4 °C overnight. Cells were incubated in the dark at 25 °C for 2 h with FITC-conjugated anti-rabbit IgG secondary antibodies after being washed three times with PBS. Nuclear counterstaining was performed using DAPI (10 min, ambient light) following removal of secondary antibody and PBS rinses. Fluorescence signals were subsequently captured using an inverted epifluorescence microscope.
Results
Assessment of body mass and biochemical markers in rats
Figure 1A illustrates that the initial body weights were similar across the groups (p > 0.05). Following modeling, all groups exhibited progressive weight gain; however, the model and GB groups displayed accelerated growth rates (11.9% and 15.0% greater than the control group, respectively, at week 2; p < 0.05). The GB intervention, which began in week 4, decreased the rate of weight gain, resulting in a 5.0% lower final body mass in the GB group than in the model group by week 8 (p < 0.05).
The absolute daily food intake (g/day) was slightly reduced in the GB group compared to the model group throughout the 4-week administration period, but this difference did not reach statistical significance (p = 0.1017; Figure S1). When normalized to body weight, the food intake/weight ratio remained comparable across groups (p = 0.4373; Figure S2), suggesting that GB-induced weight loss may drive reduced absolute intake through metabolic adaptation rather than appetite suppression.
As shown in Figs. 1B-D, diet-induced hyperlipidemia was successfully established, with model rats exhibiting marked elevations compared to controls in TG (2.9-fold), TC (2.2-fold), and LDL-C (4.8-fold) (p < 0.0001). Four-week treatment with GB significantly attenuated these dysregulations, resulting in a 37.3% decrease in TG, a 26.1% reduction in TC, and a 22.5% decline in LDL-C relative to the model group (p < 0.05), demonstrating a trend toward normalization of lipid levels.
Histological examination of rat liver
As shown in Fig. 1E, hepatocytes from the control group rats exhibited uniform size, orderly arrangement, and no lipid vacuole accumulation. In contrast, the model group exhibited significant lipid vacuole accumulation within hepatocytes, accompanied by noticeable nuclear displacement. The lipid vacuole proportion in this group was 34.94% ± 3.08% (n = 5), indicating diet-induced hepatic lipid accumulation. Following treatment with GB, a significant reduction (p < 0.0001) in hepatic lipid vacuoles was observed, with the proportion decreasing to 17.86% ± 3.34% (n = 5), indicating the effective amelioration of liver lipid accumulation by GB.
Lipidomics
Multidimensional statistical analysis
Principal Component Analysis (PCA) is a method for reducing dimensions that uses linear transformations to change original lipidomic variables into orthogonal principal components, maximizing the retention of variance. This unsupervised multivariate approach facilitates the visualization of inter- and intra-group variation through coordinate system reconstruction41. Comparative PCA modeling generated scatter plots (Fig. 2A and D) with PC1 (t[1]) and PC2 (t[2]) axes, enclosed by 95% confidence ellipses. Colocalized data points represent biological replicates, with spatial distribution patterns illustrating group-specific clustering and within-group heterogeneity. Significant segregation between control and model cohorts, as well as between GB and model pairs, confirmed treatment-specific lipidomic remodeling. Cross-validated PCA models demonstrated stability, exhibiting R²X values of 0.523 for the control-model comparison and 0.6 for the three-group comparison.
Orthogonal partial least squares discriminant analysis (OPLS-DA), a supervised discriminant analysis method, enhances class separation by filtering out orthogonal noise. Score plots (Fig. 2B and E) revealed distinct metabolic trajectories across the experimental conditions. Model validation metrics (control model: R²X = 0.582, R²Y = 0.996, Q² = 0.945; three-group model: R²X = 0.604, R²Y = 0.987, Q² = 0.817) confirmed robust predictive capacity. Random permutation testing (Fig. 2C and F) validated model robustness by demonstrating a progressive decline in R² and Q² values in randomized datasets, effectively precluding overfitting.
Changes in lipid subclasses
Comprehensive lipidomic profiling, utilizing both positive and negative ionization modes, identified 43 distinct lipid subclasses encompassing 1,700 molecular species. Comparative analysis (Fig. 3A) revealed significant perturbations (p < 0.05) in 21 lipid subclasses between model and control groups. The model group exhibited marked increases in TG, cholesterol ester (ChE), phosphatidylglycerol (PG), lysophosphatidylserine (LPS), phosphatidylserine (PS), and ceramide (Cer) levels. Conversely, substantial decreases were observed in acyl-Coenzyme A (AcCA), coenzyme (Co), ganglioside GM3 (GM3), phosphatidylinositol (PI), and sphingomyelin (SM) levels, indicative of systemic lipid dysregulation. GB treatment induced significant metabolic adjustment, attenuating TG, ChE, PG, PS, and Cer concentrations in treated animals in comparison to the model group (p < 0.05). Simultaneously, GB administration restored AcCA, Co, GM3, PI, and SM levels toward physiological baselines (p < 0.05), demonstrating a multi-target regulatory capacity.
Differential lipid molecular analysis
Univariate statistical approaches, including fold-change (FC) analysis and parametric/non-parametric tests, were used for intergroup lipidomic comparisons. Applying dual thresholds (FC > 2 or < 0.5, p < 0.05) revealed 640 dysregulated lipids in model rats compared to controls (238 upregulated, 402 downregulated), confirming diet-induced lipid metabolic disruption. GB administration demonstrated marked bidirectional regulation, with 31 lipid species showing increased abundance and 31 exhibiting decreased levels relative to the model group.
OPLS-DA-derived variable importance in projection (VIP) scores were utilized to assess the contribution of lipidomic features to class discrimination, identifying 320 candidate lipids (VIP > 1, p < 0.05). Stringent FC filtering further isolated 55 signature lipids, of which 54 exhibited normalization with GB treatment (Table S1). Figure 3B-D illustrates the dynamics of Cer subclasses: model rats displayed elevated levels of Cer (d34:1), Cer (d42:1), Cer (d42:2), Cer (m19:0/21:2), Cer (m19:0/23:2), and Cer (m41:1 + O) compared to controls (p < 0.05), with subsequent attenuation observed following GB administration. Conversely, SM (SM (d35:2), SM (d37:1), SM (d39:2), SM (d40:7), SM (d41:1)) and cardiolipin (CL (79:11), CL (80:8), CL (83:11), CL (84:8)) were depleted in the model group (p < 0.05), with levels restored by GB intervention.
Volcano plots (Fig. 3E) were generated to visualize lipidomic perturbations by plotting log2 FC on the x-axis against -log10 p-value on the y-axis. Lipids exhibiting significant up- or downregulation (FC > 2 or < 0.5, p < 0.05) are indicated in crimson and azure, respectively. Lipids in the top decile of FC underwent hierarchical clustering (Fig. 3F), revealing group-specific lipid expression patterns. In the generated heatmap, rows denote individual lipids, columns denote samples, and high and low expression levels are shown in red and blue. The expression profiles of samples treated with GB were intermediate between those of the control and model groups, validating the selected biomarkers.
Network Pharmacological analysis
Bioinformatic analysis identified 175 putative targets of GB using CTD and PharmMapper, while 347 hyperlipidemia-associated targets were compiled from CTD, DisGeNET, and GeneCards. Intersection analysis using Venny 2.1 revealed 38 overlapping therapeutic targets. A PPI network (Fig. 4A) pinpointed crucial hub targets (AKT1, TNF, TP53, CASP3, PPARα, HMOX1, MMP9, MAPK3, EGFR, APOE, NFE2L2, MAPK8, CYBB) based on topological centrality metrics. KEGG pathway enrichment analysis identified 132 pathways, with the top 20 visualized in Fig. 4B, including atherosclerosis dynamics, lipid metabolism, AGE-RAGE/TNF/IL-17 signaling cascades, and hypoxia adaptation (HIF-1). A Cytoscape-generated network (55 nodes, 252 edges; Fig. 4C) was generated to illustrate the multi-target therapeutic mechanisms of GB.
GB attenuates OA/PA-Induced metabolic dysregulation
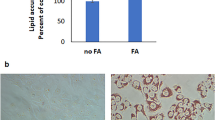
The results of the cell viability study demonstrated that GB did not exhibit significant cytotoxic effects on HepG2 cells within the concentration range of 1–80 μM in vitro (Figure S3). OA/PA co-induction significantly increased lipid accumulation (TG: 5.3-fold increase, TC: 7.7-fold increase compared to control, p < 0.0001) and induced redox imbalance (SOD: 58% decrease, GSH-Px: 61% decrease; MDA: 3.0-fold increase, p < 0.0001). GB dose-dependently reversed these effects, with the highest dose achieving maximal efficacy (TG: 46% decrease, TC: 47% decrease; SOD: 2.0-fold increase, GSH-Px: 1.9-fold increase; MDA: 46% decrease compared to the model group, p < 0.0001; Figs. 5A-E).
PPARα pathway activation
Molecular docking studies revealed high-affinity binding of GB to PPARα (ΔG = −7.6 kcal/mol; Fig. 6A), suggesting potential activation of this receptor to promote fatty acid metabolism. GB targets the ligand-binding domain (LBD) anterior wall (H3 helix) and C-terminal H12 helix, with additional interactions at H8/H11. Hydrogen bonds with Glu315 and Met467 anchor GB, inducing H12 helix conformational changes, while hydrophobic interactions with Arg434/Pro389 stabilize binding to form an AF-2 hydrophobic pocket for coactivator recruitment.
Consistently, HepG2 cell experiments showed that the model group exhibited a 59% reduction in CPT-1 (a key enzyme in fatty acid β-oxidation regulated by PPARα) compared to the control group, as quantified by immunofluorescence (p < 0.0001). Notably, GB reversed this reduction in a dose-dependent manner, with the high-dose GB group (GB-H) demonstrating a 2.3-fold increase in CPT-1 expression (p < 0.0001; Figs. 6B-C). These findings indicate that GB activates PPARα to enhance CPT-1 expression, thereby mediating regulatory effects on lipid metabolism.
(A) Molecular docking of GB (Compound ID: 11973122) with PPARα (PDB ID: 1I7G). Three-dimensional structures were rendered with PyMOL 4.3.0 (https://pymol.org) (B) CPT-1 immunofluorescence. (C) Fluorescence intensity quantification.
Nrf2 antioxidant pathway regulation
Molecular docking studies demonstrated strong binding affinity of GB to the Kelch domain of Keap1 (ΔG = −9.8 kcal/mol; Fig. 7A). GB forms hydrogen bonds with Arg326, Val561, Val606, and Val608, hydrophobic interactions with Cys513 and Ile559, and a C-H hydrogen bond with Thr560, establishing a multi-residue interaction network in the Keap1 Kelch domain. This binding is predicted to disrupt the Keap1-Nrf2 interaction, thereby preventing Nrf2 ubiquitination and degradation.
HepG2 cell experiments showed that nuclear Nrf2 expression was reduced by 74% in the model group compared to controls (p < 0.0001). GB dose-dependently restored nuclear Nrf2 levels, with the high-dose group (GB-H) exhibiting a 3.3-fold increase relative to the model group (p < 0.0001; Figs. 7B-C). These results indicate that GB binding to the Keap1 Kelch domain disrupts its inhibitory interaction with Nrf2, promoting Nrf2 nuclear translocation and accumulation.
(A) Molecular docking of GB (Compound ID: 11973122) with Keap1 Kelch domain (PDB ID: 2FLU). Three-dimensional structures were rendered with PyMOL 4.3.0 (https://pymol.org) (B) Nuclear Nrf2 fluorescence. (C) Fluorescence intensity quantification.
Mechanistic integration
Integrative analysis revealed that GB effectively ameliorated hyperlipidemia through the dual modulation of sphingolipid metabolism and oxidative-lipolytic pathways. In rats subjected to a high-fat diet, GB effectively lowered serum TG, TC, and LDL-C levels (p < 0.0001), concurrently reducing hepatic lipid vacuole accumulation (p < 0.001). Lipidomics identified 54 differentially expressed lipids, including a marked decrease in pro-inflammatory Cers (e.g., Cer(d34:1)) and an increase in membrane-stabilizing SMs (e.g., SM(d35:2)). Mechanistically, GB activated PPARα to enhance fatty acid oxidation and upregulated Nrf2 to mitigate oxidative stress, as validated in HepG2 cells. These findings suggest that GB acts as a multi-target agent against lipid metabolic disorders. The putative mechanism by which GB affects lipid metabolism is illustrated in Fig. 8.
GB’s lipid-modulating mechanism. Green: differential lipid classes; red arrows: model vs. control changes; blue arrows: GB vs. model reversals. This image is sourced from the KEGG database (http://www.kegg.jp) and its use has been permitted by the Kanehisa Laboratory. Citation follows the KEGG citation guidelines (http://www.kegg.jp/kegg/kegg1.html).
Discussion
Previous studies have reported the potential of GB in lipid regulation. For instance, Yang et al. demonstrated that GB attenuates ferroptosis in high-fat diet-induced NAFLD by reducing hepatic lipid peroxidation15. Similarly, Huang et al. revealed that Ginkgolide C (structurally homologous to GB) reduces lipid accumulation in hepatocytes by activating the Sirt1/AMPK pathway to suppress PPARγ/SREBP-1c-mediated lipogenesis and enhance PPARα/CPT-1-driven β-oxidation16. While these studies highlight the lipid-modulating properties of ginkgolides, our work is the first to establish that GB specifically targets sphingolipid remodeling (Cers/SMs balance) through dual activation of PPARα and Nrf2 pathways, providing a novel mechanistic dimension. Unlike statins, which primarily inhibit cholesterol synthesis40, this dual mechanism addresses both lipid accumulation and oxidative damage, two interconnected drivers of hyperlipidemia progression42. This synergistic effect may explain GB’s superior efficacy in reducing hepatic steatosis compared to single-target agents.
Ceramides and sphingomyelins are dynamically regulated through the sphingomyelin cycle. Specifically, sphingomyelin is hydrolyzed by sphingomyelinases (SMases) to generate ceramide, and ceramide can be reconverted to sphingomyelin via sphingomyelin synthases (SMSs)43. This balance is crucial for cellular homeostasis, as its disruption underlies the pathogenesis of various diseases. For instance, studies have shown that ceramide feeding or genetic manipulations that increase ceramide levels are closely associated with cardiac dilation and systolic dysfunction in Drosophila44. In mice lacking serine palmitoyltransferase 2 (SPTLC2), the rate-limiting enzyme in de novo ceramide synthesis, de novo ceramide synthesis is reduced, and a large amount of sphingomyelin is hydrolyzed into ceramide, leading to compensatory accumulation of ceramide in the liver. This, in turn, results in chronic liver damage in mice, including inflammatory cell infiltration, apoptosis, and progressive fibrosis45. Additionally, Pulsatilla chinensis saponins affect sphingomyelin metabolism in hepatocytes, disrupt the Cers/SMs balance, cause lipid metabolism disorders and cell apoptosis, and ultimately lead to liver damage46. Furthermore, abnormal accumulation of ceramide can block insulin signaling, promote adipose tissue inflammation, and is closely related to the occurrence of diseases such as metabolic syndrome, type 2 diabetes, and NAFLD47,48,49.
Our research demonstrates that GB can reduce hepatic ceramide accumulation and increase sphingomyelin levels, thereby lowering serum lipids and improving hepatic steatosis in hyperlipidemic rats. In terms of the mechanism, GB activates PPARα to promote fatty acid oxidation and upregulates Nrf2-mediated antioxidant defense, addressing both abnormal lipid metabolism and oxidative stress. The regulation of Cers/SMs balance by GB represents a promising strategy, which may synergize with existing therapies (such as statins) to reduce side effects. Our work highlights the potential of Cers/SMs balance as a viable therapeutic target for metabolic disorders and positions GB as a candidate drug for the treatment of hyperlipidemia and NAFLD.
Study strengths and limitations
Strengths
This study is notable for its innovative multi-omics integration, combining lipidomics, network pharmacology, and molecular docking for the first time. This comprehensive approach systematically elucidated the dual-pathway mechanism (PPARα and Nrf2) by which GB regulates sphingolipid metabolism, providing a comprehensive understanding of GB’s multi-target actions. Beyond multi-omics integration, this study also advanced translational biomarker identification, identifying Cers and SMs as potential biomarkers for hyperlipidemia intervention. This offers a novel strategy for clinical efficacy assessment. Moreover, rigorous experimental validation was conducted, including both in vivo (high-fat diet rat model) and in vitro (HepG2 cells) experiments. These experiments validated GB’s lipid-modulating and antioxidant effects, providing robust mechanistic evidence.
Limitations
Despite its strengths, this study has limitations. Regarding the animal model, Sprague-Dawley rats were used. While this model provided valuable data, it might restrict the applicability of the findings to other species or genetic backgrounds. Future studies should incorporate diverse models (e.g., ApoE−/− mice) to enhance translational relevance.
While we controlled for food intake, the lack of direct appetite-related measurements (e.g., hormone levels, feeding behavior analysis) limits our ability to conclusively exclude central appetite modulation. In addition, energy expenditure and nutrient absorption efficiency were not assessed, which could further elucidate the relationship between weight loss and food intake. Future studies incorporating indirect calorimetry and ghrelin/leptin profiling are warranted to dissect these mechanisms.
Regarding clinical translation, although Cers/SMs dynamics were identified as potential biomarkers, further investigation is needed. Human cohort validation is required to confirm their clinical applicability. Concerning the dose range, the tested GB doses (4–16 μg/mL in vitro50; 4 mg/kg in vivo20) were selected based on previous literature. However, this range may not encompass the full therapeutic window. Dose-response studies in primates are warranted.
Finally, regarding lipidomics coverage, although 1,700 lipid molecules were analyzed, limitations remain. Low-abundance lipid species (e.g., sphingosine-1-phosphate) may be underrepresented due to technical constraints.
Conclusion
In conclusion, GB ameliorates hyperlipidemia by correcting sphingolipid imbalance (decreasing Cers and increasing SMs) and co-activating PPARα-mediated lipid oxidation and Nrf2-driven antioxidant defenses. This integrative approach, combining lipidomics with mechanistic validation, elucidates GB’s multi-target actions and proposes the Cers/SMs balance as a translational biomarker for metabolic disorders. The evidence supports the idea that GB could be used alongside statins to reduce their dosage, thereby possibly minimizing the risk of muscle issues. Future clinical trials should prioritize validation of the Cers/SMs balance as a biomarker in humans.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AcCa:
-
Acyl-CoA
- Cer:
-
Ceramide
- CerP:
-
Ceramide 1-Phosphate
- ChE:
-
Cholesterol Ester
- CL:
-
Cardiolipin
- Co:
-
Coenzyme
- CPT-1:
-
Carnitine Palmitoyltransferase-1
- DG:
-
Diglyceride
- FA:
-
Fatty Acid
- GB:
-
Ginkgolide B
- GSH-Px:
-
Glutathione Peroxidase
- HDL-C:
-
High-Density Lipoprotein Cholesterol
- LDL-C:
-
Low-Density Lipoprotein Cholesterol
- LPA:
-
Lyso-Phosphatidic Acid
- LPC:
-
Lyso-Phosphatidylcholine
- LPE:
-
Lyso-Phosphatidylethanolamine
- LPG:
-
Lyso-Phosphatidylglycerol
- LPS:
-
Lyso-Phosphatidylserine
- MDA:
-
Malondialdehyde
- MG:
-
Monoglyceride
- OAHFA:
-
(O-acyl)-1-hydroxy fatty acid
- PA:
-
Phosphatidic Acid
- PAF:
-
Platelet Activating Factor
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PG:
-
Phosphatidylglycerol
- phSM:
-
Sphingomyelin (or Phytosphingosine)
- PI/PIP/PIP2:
-
Phosphatidylinositol
- PS:
-
Phosphatidylserine
- ROS:
-
Reactive Oxygen Species
- S1P:
-
Sphingoshine 1 Phosphate
- SiE:
-
Sitosteryl Ester
- SM:
-
Sphingomyelin
- SOD:
-
Superoxide Dismutase
- SPH:
-
Sphingoshine
- ST:
-
Sterol Lipids
- StE:
-
Stigmasteryl Ester
- TC:
-
Total Cholesterol
- TG:
-
Triglycerides
- ZyE:
-
Zymosteryl
References
Alloubani, A., Nimer, R. & Samara, R. Relationship between hyperlipidemia, cardiovascular disease and stroke: A systematic review. Curr. Cardiol. Rev. 17, e051121189015. https://doi.org/10.2174/1573403X16999201210200342 (2021).
Goldberg, R. B. Dyslipidemia in diabetes: when and how to treat?? Endocrinol. Metab. Clin. North. Am. 51, 603–624. https://doi.org/10.1016/j.ecl.2022.02.011 (2022).
Chen, Y. et al. Integrated lipidomics and network Pharmacology analysis to reveal the mechanisms of Berberine in the treatment of hyperlipidemia. J. Translational Med. 20 https://doi.org/10.1186/s12967-022-03623-0 (2022).
Wang, Z. Y. et al. The mechanisms of Baicalin ameliorate obesity and hyperlipidemia through a network Pharmacology approach. Eur. J. Pharmacol. 878, 173103. https://doi.org/10.1016/j.ejphar.2020.173103 (2020).
Gomez-Zorita, S. et al. Phenolic compounds apigenin, hesperidin and Kaempferol reduce in vitro lipid accumulation in human adipocytes. J. Transl Med. 15, 237. https://doi.org/10.1186/s12967-017-1343-0 (2017).
Gachowska, M., Szlasa, W., Saczko, J. & Kulbacka, J. Neuroregulatory role of ginkgolides. Mol. Biol. Rep. 48, 5689–5697. https://doi.org/10.1007/s11033-021-06535-2 (2021).
Zhang, D. et al. Efficacy of diterpene ginkgolides Meglumine injection in elderly patients with ischemic stroke: A post hoc analysis of a randomized controlled trial. Phytomedicine 106, 154391. https://doi.org/10.1016/j.phymed.2022.154391 (2022).
Maclennan, K. M., Darlington, C. L. & Smith, P. F. The CNS effects of Ginkgo Biloba extracts and Ginkgolide B. Prog Neurobiol. 67, 235–257. https://doi.org/10.1016/s0301-0082(02)00015-1 (2002).
Zhou, T. et al. Ginkgolide B protects human umbilical vein endothelial cells against xenobiotic injuries via PXR activation. Acta Pharmacol. Sin. 37, 177–186. https://doi.org/10.1038/aps.2015.124 (2016).
Lee, C. W. et al. Ginkgolide B monotherapy reverses osteoporosis by regulating oxidative stress-mediated bone homeostasis. Free Radic Biol. Med. 168, 234–246. https://doi.org/10.1016/j.freeradbiomed.2021.03.008 (2021).
Zhang, S., Chen, B., Wu, W., Bao, L. & Qi, R. Ginkgolide B reduces inflammatory protein expression in oxidized low-density lipoprotein-stimulated human vascular endothelial cells. J. Cardiovasc. Pharmacol. 57, 721–727. https://doi.org/10.1097/FJC.0b013e31821a50a8 (2011).
Feng, Z. et al. Ginkgolide B ameliorates oxidized low-density lipoprotein-induced endothelial dysfunction via modulating Lectin-like ox-LDL-receptor-1 and NADPH oxidase 4 expression and inflammatory cascades. Phytother Res. 32, 2417–2427. https://doi.org/10.1002/ptr.6177 (2018).
Kim, H., Kang, S. & Go, G. W. Exploring the multifaceted role of ginkgolides and Bilobalide from Ginkgo Biloba in mitigating metabolic disorders. Food Sci. Biotechnol. 33, 2903–2917. https://doi.org/10.1007/s10068-024-01656-3 (2024).
Wang, S. D. et al. Inhibitory effect of Ginkgo Biloba extract on fatty liver: regulation of carnitine palmitoyltransferase 1a and fatty acid metabolism. J. Dig. Dis. 13, 525–535. https://doi.org/10.1111/j.1751-2980.2012.00627.x (2012).
Yang, Y. et al. Study on the attenuated effect of Ginkgolide B on ferroptosis in high fat diet induced nonalcoholic fatty liver disease. Toxicology 445, 152599. https://doi.org/10.1016/j.tox.2020.152599 (2020).
Huang, W. C., Chen, Y. L., Liu, H. C., Wu, S. J. & Liou, C. J. Ginkgolide C reduced oleic acid-induced lipid accumulation in HepG2 cells. Saudi Pharm. J. 26, 1178–1184. https://doi.org/10.1016/j.jsps.2018.07.006 (2018).
Xie, Y. et al. Ginkgolide C attenuated Western diet-induced non-alcoholic fatty liver disease via increasing AMPK activation. Inflammation https://doi.org/10.1007/s10753-024-02086-3 (2024).
Li, F. et al. Anthelmintics Nitazoxanide protects against experimental hyperlipidemia and hepatic steatosis in hamsters and mice. Acta Pharm. Sin B. 12, 1322–1338. https://doi.org/10.1016/j.apsb.2021.09.009 (2022).
Liu, Y. et al. Enhancing both oral bioavailability and anti-ischemic stroke efficacy of Ginkgolide B by Preparing nanocrystals self-stabilized Pickering nano-emulsion. Eur. J. Pharm. Sci. 192, 106620. https://doi.org/10.1016/j.ejps.2023.106620 (2024).
Zhao, Y. et al. Polymeric Nanoparticles-Based brain delivery with improved therapeutic efficacy of Ginkgolide B in parkinson’s disease. Int. J. Nanomed. 15, 10453–10467. https://doi.org/10.2147/IJN.S272831 (2020).
Hou, Y. et al. Ginsenoside Rg1 regulates liver lipid factor metabolism in NAFLD model rats. ACS Omega. 5, 10878–10890. https://doi.org/10.1021/acsomega.0c00529 (2020).
Liu, Y. et al. Salvia-Nelumbinis naturalis improves lipid metabolism of NAFLD by regulating the SIRT1/AMPK signaling pathway. BMC Complement. Med. Ther. 22 https://doi.org/10.1186/s12906-022-03697-9 (2022).
Feldman, A. T. & Wolfe, D. Tissue processing and hematoxylin and Eosin staining. Methods Mol. Biol. 1180, 31–43. https://doi.org/10.1007/978-1-4939-1050-2_3 (2014).
Mehlem, A., Hagberg, C. E., Muhl, L., Eriksson, U. & Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 8, 1149–1154. https://doi.org/10.1038/nprot.2013.055 (2013).
Naik, R. R., Rajan, A. & Kalita, N. Automated image analysis method to detect and quantify fat cell infiltration in hematoxylin and Eosin stained human pancreas histology images. BBA Adv. 3, 100084. https://doi.org/10.1016/j.bbadva.2023.100084 (2023).
Cantor, J. R. et al. Physiologic Medium Rewires Cellular Metabolism and Reveals Uric Acid as an Endogenous Inhibitor of UMP Synthase. Cell 169, 258–272 e217 (2017). https://doi.org/10.1016/j.cell.2017.03.023
Shi, C. et al. Effect of three types of thermal processing methods on the lipidomics profile of tilapia fillets by UPLC-Q-Extractive orbitrap mass spectrometry. Food Chem. 298, 125029. https://doi.org/10.1016/j.foodchem.2019.125029 (2019).
Zhang, Y. F., Huang, Y., Ni, Y. H. & Xu, Z. M. Systematic Elucidation of the mechanism of geraniol via network Pharmacology. Drug Des. Devel Ther. 13, 1069–1075. https://doi.org/10.2147/DDDT.S189088 (2019).
Xiang, C. et al. Network Pharmacology and molecular Docking to elucidate the potential mechanism of ligusticum Chuanxiong against osteoarthritis. Front. Pharmacol. 13, 854215. https://doi.org/10.3389/fphar.2022.854215 (2022).
Xie, C., Tang, H., Liu, G. & Li, C. Molecular mechanism of epimedium in the treatment of vascular dementia based on network Pharmacology and molecular Docking. Front. Aging Neurosci. 14, 940166. https://doi.org/10.3389/fnagi.2022.940166 (2022).
Qu, M., Tao, W. & Ma, J. Network Pharmacology and molecular Docking study of Yupingfeng powder in the treatment of allergic diseases. Evid. Based Complement. Alternat Med. 2022 (1323744). https://doi.org/10.1155/2022/1323744 (2022).
Luo, L. et al. Pharmacological mechanisms of Tinglizi against chronic heart failure determined by network Pharmacology and molecular Docking. Evid. Based Complement. Alternat Med. 2022 (2152399). https://doi.org/10.1155/2022/2152399 (2022).
Xu, X. et al. Detecting potential mechanism of vitamin D in treating rheumatoid arthritis based on network Pharmacology and molecular Docking. Front. Pharmacol. 13, 1047061. https://doi.org/10.3389/fphar.2022.1047061 (2022).
Zhao, J. et al. Exploration of the molecular mechanism of polygonati rhizoma in the treatment of osteoporosis based on network Pharmacology and molecular Docking. Front. Endocrinol. (Lausanne). 12, 815891. https://doi.org/10.3389/fendo.2021.815891 (2021).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592. https://doi.org/10.1093/nar/gkac963 (2023).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Eberhardt, J., Santos-Martins, D., Tillack, A. F. & Forli, S. AutoDock Vina 1.2.0: new Docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 61, 3891–3898. https://doi.org/10.1021/acs.jcim.1c00203 (2021).
Jeong, H. S. et al. Ginkgolide A ameliorates non-alcoholic fatty liver diseases on high fat diet mice. Biomed. Pharmacother. 88, 625–634. https://doi.org/10.1016/j.biopha.2017.01.114 (2017).
Zhang, Q. et al. The effect of Simvastatin on gut microbiota and lipid metabolism in hyperlipidemic rats induced by a High-Fat diet. Front. Pharmacol. 11, 522. https://doi.org/10.3389/fphar.2020.00522 (2020).
Shi, S., Liu, Z., Xue, Z., Chen, X. & Chu, Y. A plasma metabonomics study on the therapeutic effects of the Si-miao-yong-an Decoction in hyperlipidemic rats. J. Ethnopharmacol. 256, 112780. https://doi.org/10.1016/j.jep.2020.112780 (2020).
Chen, D. et al. Cdh1 plays a protective role in nonalcoholic fatty liver disease by regulating PPAR/PGC-1α signaling pathway. Biochem. Biophys. Res. Commun. 681, 13–19. https://doi.org/10.1016/j.bbrc.2023.09.038 (2023).
Arana, L., Gangoiti, P., Ouro, A., Trueba, M. & Gomez-Munoz, A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 9, 15. https://doi.org/10.1186/1476-511X-9-15 (2010).
Walls, S. M. et al. Ceramide-Protein interactions modulate Ceramide-Associated lipotoxic cardiomyopathy. Cell. Rep. 22, 2702–2715. https://doi.org/10.1016/j.celrep.2018.02.034 (2018).
Lallement, J. et al. Hepatic deletion of Serine palmitoyl transferase 2 impairs ceramide/sphingomyelin balance, bile acids homeostasis and leads to liver damage in mice. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 1868, 159333. https://doi.org/10.1016/j.bbalip.2023.159333 (2023).
Su, D. et al. Pulsatilla chinensis saponins cause liver injury through interfering ceramide/sphingomyelin balance that promotes lipid metabolism dysregulation and apoptosis. Phytomedicine 76 https://doi.org/10.1016/j.phymed.2020.153265 (2020).
Fang, Z., Pyne, S. & Pyne, N. J. Ceramide and sphingosine 1-phosphate in adipose dysfunction. Prog Lipid Res. 74, 145–159. https://doi.org/10.1016/j.plipres.2019.04.001 (2019).
Samuel, V. T. & Shulman, G. I. Nonalcoholic fatty liver disease, insulin resistance, and ceramides. N Engl. J. Med. 381, 1866–1869. https://doi.org/10.1056/NEJMcibr1910023 (2019).
Pagadala, M., Kasumov, T., McCullough, A. J., Zein, N. N. & Kirwan, J. P. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol. Metab. 23, 365–371. https://doi.org/10.1016/j.tem.2012.04.005 (2012).
Liu, Y. et al. Highly stabilized nanocrystals delivering Ginkgolide B in protecting against the parkinson’s disease. Int. J. Pharm. 577, 119053. https://doi.org/10.1016/j.ijpharm.2020.119053 (2020).
Funding
This article was financially supported by the Liaoning Provincial Department of Education Basic Scientific Research Project, with the grant number LJ242414289010 (Yun Liu).
Author information
Authors and Affiliations
Contributions
Y. L.: Methodology, Formal analysis, Investigation, Data curation, Funding acquisition, Writing. F. W.: Methodology, Formal analysis, Writing - review & editing. H. X.: Methodology, Data curation, Resources. H. W.: Investigation, Data curation. M. L.: Investigation, Data curation. L. C.: Conceptualization, Project administration, Supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The animal experiments were approved by the Animal Experiment Ethics Committee of Jinzhou Medical University (Ethics batch number: 2023034). All experimental procedures adhered to nationally established guidelines for laboratory animal welfare and ethical research practices, and the study was reported in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (PLoS Bio 8(6), e1000412, 2010).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Wang, F., Xu, H. et al. Ginkgolide B attenuates hyperlipidemia by restoring sphingolipid homeostasis and activating PPARα and Nrf2 pathways. Sci Rep 15, 28774 (2025). https://doi.org/10.1038/s41598-025-14626-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14626-4