Abstract
In modern Boron neutron capture therapy (BNCT) treatment planning, 18F-BPA (18F-boronophenylalanine) PET (positron emission tomography) imaging is used to assess boron uptake and guide accurate dose delivery. This study evaluates the geometric and dosimetric differences between target volumes defined by MRI (magnetic resonance imaging) and PET images in accelerator-based BNCT using the NeuPex system. The GTV (gross tumor volume) was defined based on MRI (GTVMRI) and PET images with SUV thresholds of 2.0 (GTVPET2.0) and 2.5 (GTVPET2.5) in patients with head-and-neck cancer (HNC) and malignant glioma (MG). GTVPET2.5 was approximately 50% smaller, while GTVPET2.0 showed closer volumetric agreement, with ∆V% of 23.52 ± 17.44 (HNC) and 28.71 ± 12.57 (MG), and corresponding Dice similarity coefficients of 0.71 ± 0.14 and 0.86 ± 0.17. Dosimetric analysis revealed that GTVPET2.0 achieved higher dose coverage than GTVMRI, with mean doses of 24.07 ± 6.42 Gy-Eq (HNC) and 26.36 ± 9.77 Gy-Eq (MG), and D80 values of 19.01 ± 6.90 Gy-Eq and 21.00 ± 7.51 Gy-Eq, respectively. Tumor-to-blood ratio (TBR) and tumor-to-normal ratio (TNR) analyses further indicated substantial heterogeneity in boron uptake, with mean TBR and TNR of 2.71 ± 0.53 and 2.97 ± 0.79 for GTVPET2.0. These results suggest that GTVPET2.0 provides a balanced and biologically relevant target for BNCT planning. A TBR threshold of ≥ 2.0 is proposed as a practical criterion for patient selection. While this study focuses on geometric and dosimetric endpoints, future work correlating these findings with clinical outcomes is warranted. Overall, this study supports the integration of functional PET imaging with anatomical MRI to improve the precision and efficacy of BNCT treatment planning.
Similar content being viewed by others
Introduction
Boron neutron capture therapy (BNCT) is a biologically targeted radiotherapy that exploits the nuclear reaction between thermal neutrons and boron-10 (10B) to produce high-linear energy transfer (LET) particles, specifically alpha particles and lithium nuclei, within tumor cells. These particles have a short range (5–10 μm), comparable to the diameter of a single cell, enabling highly localized tumor cell destruction while sparing surrounding normal tissues1,2. The therapeutic efficacy of BNCT is contingent upon the selective accumulation of 10B in tumor cells relative to normal tissues, a phenomenon that is primarily governed by the pharmacokinetics of boron delivery agents, such as L-para-boronophenylalanine (BPA)3.
In recent years, BNCT has gained recognition as a promising treatment modality for recurrent or locally advanced cancers, particularly head-and-neck cancer (HNC) and malignant glioma (MG)4,5. The success of BNCT hinges on the precise delineation of target volumes, which traditionally relies on anatomical imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI). However, the unique mechanism of BNCT, which depends on the spatial distribution of 10B within tumors, necessitates the integration of functional imaging techniques, such as positron emission tomography (PET), to accurately assess boron uptake and optimize treatment planning6.
Despite the growing adoption of BNCT, there remains a lack of standardized guidelines for target volume delineation, particularly with respect to the integration of 18F-BPA PET imaging. Previous studies have proposed using tumor-to-normal tissue ratio (TNR) and tumor-to-blood ratio (TBR) as key parameters for patient selection and dose calculation7,8. However, the variability in boron uptake between tumors and normal tissues, as well as the inherent limitations of PET imaging, pose challenges for accurate dose estimation and treatment planning9.
This study aims to address these challenges by evaluating the geometric and dosimetric differences between target volumes defined by MRI and 18F-BPA PET imaging in patients undergoing accelerator-based BNCT. By analyzing the volume, shape, and dose distribution of gross tumor volumes (GTVs) delineated using different imaging modalities, we seek to establish a standardized framework for target delineation in BNCT. Furthermore, we explore the implications of these differences for treatment planning and patient selection, with the goal of optimizing therapeutic outcomes and minimizing radiation-induced toxicity.
Methods and materials
Patients and tumor characteristics
Between September 2022 and April 2023, a total of 14 patients were treated BNCT at Xiamen Humanity Hospital, which used Neuboron Medical Group’s NeuPex system10 and the boron-containing drug NBB-001, also known as BPA. Nine patients, comprising six with HNC and three with MG, were included in this study. Two patients received BNCT twice. All patients underwent 18F-BPA PET imaging, except for five who were excluded due to COVID-19-related complications. Patient demographics, tumor characteristics, and prior treatments are summarized in Table 1.
The investigator-initiated trial (IIT) was conducted in accordance with the principles of the Declaration of Helsinki and the Guidelines for Good Clinical Practice. Ethical approval was obtained from the Medical Ethics Committee of Xiamen Humanity Hospital (Approval No. HAXM-MEC-20220901-005-02). Written informed consent was obtained from all participating patients prior to their inclusion in the study. The trial was registered in the Chinese Clinical Trial Registration Center (Registration No. ChiCTR2200066473, 06/12/2022) and the Medical Research Registration and Archival Information System of the National Safety and Health Security Information Platform.
Imaging acquisition and target volume delineation
All patients were immobilized using thermoplastic masks and underwent CT, MRI, and 18F-BPA PET imaging within a two-week period. CT scans were performed using a Philips Brilliance CT Big Bore with slice thicknesses of 3.0 mm for HNC and 2.5 mm for MG. MRI scans were conducted using a GE Discovery MR 750 3.0 T system. 18F-BPA PET imaging was performed approximately one hour after intravenous injection of 0.1–0.2 mCi/kg 18F-BPA, using a GE Discovery PET/CT 600 scanner. MRI and PET images were co-registered with CT images using the treatment planning system (TPS), which employs the Normalized Gradient Field (NGF) algorithm. This algorithm optimizes the transformation parameters between images by minimizing differences in gradient fields, ensuring accurate multimodal image fusion.
"Following image registration and fusion, the GTV was delineated by an experienced oncologist based on MRI, referred to as GTVMRI. Additionally, target volumes were automatically defined on PET images (GTVPET) using standardized uptake value (SUV) thresholds of 2.0 and 2.5, referred to as GTVPET2.0 and GTVPET2.5, respectively. The SUV values were normalized to the patient’s body weight and the injected dosage of 18F-BPA11. These PET-based volumes were defined with distinct clinical rationales:
-
GTVPET2.0 (SUV ≥ 2.0): Represents a biologically relevant target volume corresponding to the clinically validated tumor-to-blood ratio for effective BNCT12.
-
GTVPET2.5 (SUV ≥ 2.5): A more conservative volume targeting subregions with the highest tracer uptake, historically used in clinical trials to ensure robust treatment efficacy13.
Treatment planning and dose calculation
Treatment planning and dose calculations were performed using the NeuMANTA (Multifunctional Arithmetic for Neutron Transportation Analysis) system13, which incorporates the Monte Carlo-based dose calculation engine COMPASS (COMpact Particle Simulation System)14. The system utilizes DICOM image data to simulate homogeneous or heterogeneous materials and boron distribution, enabling the creation of a detailed voxel-based Monte Carlo calculation model15,16.
The total bioequivalent dose in BNCT is calculated by considering three primary components:
-
1.
Boron dose: this represents the high LET dose resulting from the 10B(n, α)7Li reaction, which occurs when thermal neutrons interact with 10B within tumor cells.
-
2.
Neutron dose: this component includes the dose generated by the interaction of fast neutrons with hydrogen in tissues (1H(n, n′)p) and the interaction of thermal neutrons with nitrogen (14N(n, p)14C), both of which release protons.
-
3.
Photon dose: this primarily arises from background photons generated by the neutron beam, with additional contributions from neutron interactions with hydrogen (1H(n, γ)2H) and other tissue elements.
The total bioequivalent dose, expressed in Gy-Eq, accounts for the relative biological effectiveness (RBE) of each dose component. The RBE for the neutron dose is set to 3.2, while the compound biological effectiveness (CBE) for the boron dose is 3.8 for tumors, 2.5 for mucosal and skin tissues, and 1.3 for other tissues17. Dose constraints were applied based on tumor type: HNC patients, the maximum mucosal dose was limited to 11.5–15.0 Gy-Eq, while for MG patients, the mean brain dose was constrained to 2.5–3.0 Gy-Eq.
Geometric and dosimetric evaluation
To quantitatively evaluate differences between GTVMRI and GTVPET, this study conducted the following four analyses:
-
1.
Volume Difference: The volumes of GTVMRI, GTVPET2.0, and GTVPET2.5 were recorded. In traditional radiotherapy, GTVMRI is considered the reference benchmark, as MRI-based target delineation is the gold standard for photon, proton, and heavy ion therapies. The percentage volume difference (∆V%) between GTVPET and GTVMRI was calculated using the following Eq. 1.
$$\Delta {\text{V}}\% = \frac{{GTV_{MRI} - GTV_{PET} }}{{GTV_{MRT} }}\%$$(1) -
2.
Shape Consistency: The Dice Similarity Coefficient (DSC) was used to evaluate the spatial overlap between GTVMRI and GTVPET. The DSC ranges from 0 to 1, with values greater than 0.7 indicating high overlap18. The DSC was calculated as Eq. 2, where \({V}_{{GTV}_{MRI}}\) and \({V}_{{GTV}_{MRI}}\) represent the volumes of GTVMRIand GTVPET respectively19.
$${\text{DSC}} = \frac{{2\left| {V_{{GTV_{MRI} }} \cap V_{{GTV_{PET} }} } \right|}}{{\left| {V_{{GTV_{MRI} }} } \right| + \left| {V_{{GTV_{PET} }} } \right|}}$$(2) -
3.
SUV, TNR, and TBR Comparison: The minimum, maximum, and mean values of SUV, tumor-to-normal tissue ratio (TNR), and tumor-to-blood ratio (TBR) were compared between GTVMRI and GTVPET. TNR was calculated as the ratio of SUV in the tumor (SUVGTV) to SUV in normal tissue (SUVN), as shown in Eq. 3. TBR was calculated as the ratio of SUVGTV) to SUV in the left ventricular blood pool (SUVB), as shown in Eq. 4
$${\text{TNR}} = \frac{{SUV_{GTV} }}{{SUV_{N} }}$$(3)$${\text{TBR}} = \frac{{SUV_{GTV} }}{{SUV_{B} }}$$(4) -
4.
Dosimetric Comparison: The dose coverage of GTVs was evaluated using dose-volume histograms (DVH), two-dimensional dose maps, and key dosimetric parameters, including the minimum dose (Dmin), maximum dose (Dmax), mean dose (Dmean), and the dose received by 80% of the target volume (D80).
Results
Volume and shape comparison
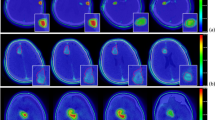
A total of 12 18F-BPA PET scans were performed on 9 patients, with one patient having a dual target structure, resulting in 13 sets of GTVs for analysis. Figure 1 illustrates representative MRI, PET, and CT images for a MGpatient and a HNC patient. The volumes of GTVMRI, GTVPET2.0, and GTVPET2.5 are shown in Fig. 2. For HNC cases, the average volumes of were GTVMRI, GTVPET2.0, and GTVPET2.5 are 110.2 ± 130.9 cc, 82.9 ± 107.9 cc, and 52.3 ± 95.1 cc respectively, while for MG cases, they are 66.8 ± 62.0 cc, 51.4 ± 47.1 cc, and 26.1 ± 25.5 cc respectively.
The ∆V% between GTVMR and GTVPET2.5 was significant, with GTVPET2.5 being approximately 50% smaller than GTVMRI . In contrast, GTVPET2.0 demonstrated better volumetric agreement with GTVMRI , showing a ∆V% of 23.52 ± 17.44 for HNC cases and 28.71 ± 12.57 for MG cases. The DSC between GTVMRI and GTVPET2.0 was 0.71 ± 0.14 for HNC and 0.86 ± 0.17 for MG, indicating good spatial concordance. In comparison, the DSC for GTVPET2.5 was lower—0.49 ± 0.16 for HNC and 0.65 ± 0.10 for MG—suggesting reduced spatial overlap (Table 2).
SUV, TNR, and TBR Analysis
The SUV, TNR, and TBR values for GTVMRI, GTVPET2.0, and GTVPET2.5 are summarized in Table 3. The mean SUVB was 1.15 ± 0.17, and the mean SUVN was 0.91 ± 0.13, consistent with the assumption that boron uptake in blood and normal tissues is similar. The mean TNR for GTVPET2.0 was 2.97 ± 0.79, compared to 2.60 ± 0.59 for GTVMRI, representing a 12.5% difference. However, the minimum TNR for GTVPET2.0 (1.96 ± 0.46) was significantly higher than that for GTVMRI (0.77 ± 0.33), with a 60.7% difference. Similarly, the mean TBR for GTVPET2.0 was 2.71 ± 0.53, compared to 2.28 ± 0.63 for GTVMRI. Figure 3 illustrates the distribution of SUV, TNR, and TBR values across the GTVs, highlighting the variability in boron uptake and its impact on dose calculation.
Dosimetric evaluation
The dosimetric parameters for GTVMRI, GTVPET2.0, and GTVPET2.5 are presented in Table 4. For HNC cases, GTVPET2.5 showed significantly higher Dmin (15.77 ± 7.79 Gy-Eq), Dmean (28.08 ± 6.00 Gy-Eq), and D80 (23.95 ± 6.65 Gy-Eq) compared to GTVMRI (6.81 ± 2.98, 21.18 ± 4.60, and 14.63 ± 4.29 Gy-Eq, respectively), with all differences reaching statistical significance (p < 0.01). GTVPET2.0 exhibited intermediate values Dmin: 10.52 ± 6.67 Gy-Eq; Dmean: 24.07 ± 6.42 Gy-Eq; D80: 19.01 ± 6.90 Gy-Eq), also significantly higher than those of GTVMRI. Similarly, in MG cases, GTVPET2.5 resulted in the highest dose parameters, with Dmin, Dmean, and D80 of 20.41 ± 7.95, 30.46 ± 11.72, and 26.89 ± 10.13 Gy-Eq, respectively. These values were significantly greater than those for GTVMRI (9.57 ± 4.45, 24.02 ± 9.45, and 17.44 ± 6.65 Gy-Eq), with p < 0.01. GTVPET2.0 again showed intermediate results. No significant differences in Dmax were observed across the three contours for either cohort.
Figure 4 shows the dose maps and DVHs for an MG patient and an HNC patient. The dose distribution within the tumor was not uniform, with lower doses observed in regions of GTVMRI that lacked significant boron uptake (i.e., regions excluded from GTVPET). The DVH for GTVMRI exhibited a flatter curve due to its larger volume and wider dose distribution compared to GTVPET.
Discussion
Integration of anatomical and functional imaging
The observed discrepancies between GTVMRI and GTVPET highlight the limitations of relying solely on anatomical imaging for target delineation in BNCT. While MRI provides excellent soft tissue contrast and is widely regarded as the gold standard for defining tumor extent in conventional radiotherapy, it does not account for the heterogeneity of boron uptake within tumors. In contrast, 18F-BPA PET imaging offers a functional assessment of boron distribution, enabling the identification of biologically active tumor regions that are most likely to benefit from BNCT.
Our findings demonstrate that GTVPET aligns closely with GTVMRI in both volume and shape. This suggests that GTVPET2.0 may serve as a reliable target volume for BNCT, balancing the need for comprehensive tumor coverage with the precision of functional imaging. In contrast, GTVPET2.5 was significantly smaller than GTVMRI, with a DSC of only 0.49 ± 0.16 for HNC cases and 0.65 ± 0.10 for MG cases, indicating that higher SUV thresholds may exclude regions of the tumor with lower but still clinically relevant boron uptake.
Implications for dose calculation and tumor control
The dosimetric analysis revealed that GTVPET2.0 receive Dmean of 24.07 ± 6.42 Gy-Eq for HNC and 26.36 ± 9.77 for MG. The dose covering 80% of the volume was 19.01 ± 6.90 Gy-Eq for HNC and 21.00 ± 7.51 Gy-Eq for MG. In contrast, GTVMRI exhibited lower dose coverage, particularly in regions with minimal boron uptake, underscoring the importance of integrating functional imaging to ensure adequate dose delivery to biologically active tumor regions. The observed heterogeneity in boron uptake, as reflected by the variability in SUV, TNR, and TBR values, further emphasizes the need for personalized treatment planning in BNCT. While the TNRmean and TBRmean for GTVPET2.0 were 2.97 ± 0.79 and 2.71 ± 0.53, respectively, the TNRmin (1.96 ± 0.46) and TBRmin (1.66 ± 0.34) were significantly lower, highlighting the potential for underdosing in regions with suboptimal boron accumulation. These findings suggest that BNCT treatment plans should incorporate both anatomical and functional imaging to account for spatial variations in boron uptake and ensure comprehensive tumor coverage.
Recommendations for clinical practice
Based on our results, we recommend using GTVPET2.0 as the primary target volume for BNCT treatment planning. This approach balances the need for accurate boron distribution assessment with the anatomical information provided by MRI, ensuring optimal dose delivery to biologically active tumor regions. Additionally, we propose a TBR threshold of 2.0 as a screening criterion for BNCT eligibility, using the left ventricular blood pool as a reference region due to its consistent physiological uptake and well-defined anatomical location, which allows for reliable and reproducible SUV normalization across patients.
Limitations and future directions
This study has several limitations that warrant consideration. First, the relatively small sample size (9 patients with 13 lesions) limits the statistical power and generalizability of the findings. The cohort includes both HNC and MG, which, while demonstrating the feasibility of BNCT across tumor types, introduces heterogeneity that may confound dosimetric comparisons. Subgroup analyses stratified by tumor type could elucidate potential differences in boron uptake patterns, target volume delineation, and dose distribution. These analyses may reveal tumor-specific considerations in defining optimal SUV thresholds and predicting treatment efficacy.
Furthermore, given the known inter-patient variability in 10B uptake, the use of fixed SUV thresholds (e.g., 2.0 or 2.5) may not fully capture intra-tumoral and inter-patient heterogeneity. Adaptive or personalized segmentation strategies—such as those based on TNR or TBR—could enhance biological relevance and improve target definition accuracy. Additionally, advanced computational techniques, including radiomics and machine learning, may enable more individualized modeling of boron distribution and support data-driven threshold optimization in future BNCT planning systems. While fixed SUV thresholds offer simplicity, adaptive approaches—such as TBR-scaled isocontouring or gradient-based delineation—could personalize target definition. For example, a tumor volume could be dynamically contoured where SUV exceeds 2.0 × the blood pool SUV (measured from the left ventricle). Such methods may better account for pharmacokinetic variability but require validation in prospective BNCT trials.
Another limitation is the absence of clinical outcome data. While this study focuses on geometric and dosimetric metrics—such as Dice similarity coefficients and D80 values—there is no evaluation of local tumor control, progression-free survival, or overall survival. Although dosimetric parameters serve as useful surrogates, their clinical validity must be confirmed through prospective trials with long-term follow-up and integrated clinical endpoints.
Finally, a potential confounding factor not directly addressed is the washout of 18F-BPA between injection and imaging, which could influence SUV measurements and, consequently, PET-based tumor geometry. Although imaging was standardized to occur approximately one hour post-injection—aligned with published protocols—variations in boron pharmacokinetics between patients may affect tracer retention. The lack of dynamic or delayed PET imaging limits our ability to fully exclude washout-related effects. Future studies incorporating time-activity curve analyses or dual-time-point PET could provide deeper insights into boron stability within the tumor microenvironment.
While this study primarily focuses on geometric and dosimetric endpoints, preliminary clinical observations from our cohort (e.g., tumor response rates or symptomatic improvement in patients with higher TBR values) will be analyzed in an ongoing follow-up study. For example, early data from two HNC cases with GTVPET2.0 D80 > 20 Gy-Eq showed partial radiographic response at 3-month post-BNCT, suggesting a potential link between dosimetric parameters and short-term outcomes. Future work will systematically correlate these findings with progression-free survival and local control rates in a larger cohort.
Conclusions
This study provides preliminary evidence supporting the potential benefits of integrating anatomical MRI and functional 18F-BPA PET imaging for target delineation in BNCT. The geometric and dosimetric analyses reveal significant differences between target volumes defined by MRI (GTVMRI) and PET (GTVPET), particularly in terms of volume, shape, and boron uptake heterogeneity. The key findings include:
-
Volume and Shape Consistency: GTVPET2.0 showed high similarity to GTVMRi in both volume (∆V% = 23.52 ± 17.44 for HNC and 28.71 ± 12.57 for MG) and shape (DSC = 0.71 ± 0.14 for HNC and 0.86 ± 0.17 for MG), making it a suitable target volume for BNCT treatment planning. In contrast, GTVPET2.5 was significantly smaller and exhibited lower overlap with GTVMRI, underscoring the limitations of higher SUV thresholds.
-
Boron Uptake Heterogeneity: The variability in SUV, TNR, and TBR values highlights the heterogeneity of boron uptake within tumors, emphasizing the need for personalized treatment planning to ensure adequate dose coverage.
-
Dosimetric Outcomes: For HNC cases, GTVPET2.0 received a mean dose of 24.07 ± 6.42 Gy-Eq, with 80% of the volume receiving 19.01 ± 6.90 Gy-Eq. For MG cases, the corresponding values were 26.36 ± 9.77 Gy-Eq and 21.00 ± 7.51 Gy-Eq, respectively. These dose levels are consistent with those associated with improved tumor control probabilities.
Based on these findings, we recommend using GTVPET2.0 as the primary target volume for BNCT treatment planning, as it provides a balance between anatomical and functional imaging information. Additionally, we propose a TBR threshold of 2.0 as a screening criterion for BNCT eligibility, leveraging the clear anatomical reference provided by the left ventricular blood pool.
This study advances the clinical application of BNCT by providing evidence-based recommendations for integrating multimodal imaging into treatment planning. Future research should focus on optimizing target delineation methods and exploring adaptive strategies to account for inter-patient variability in boron uptake, further enhancing the precision and efficacy of BNCT as a targeted radiotherapy modality.
These findings lay the groundwork for future clinical validation. The proposed TBR ≥ 2.0 threshold and GTVPET2.0-based planning will be prospectively tested in our investigator-initiated trial, with tumor control and toxicity as primary endpoints. This step is critical to confirm whether the observed dosimetric advantages translate to improved patient outcomes.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Barth, R. F. et al. Current status of boron neutron capture therapy of high-grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 7, 146 (2012).
Dymova, M. A., Taskaev, S. Y., Richter, V. A. & Kuligina, E. V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. 40(9), 406–421 (2020).
Miyatake, S. I. et al. Boron neutron capture therapy of malignant gliomas. Prog. Neurol. Surg. 32, 48–56 (2018).
Hirose, K. et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan (10B) for recurrent or locally advanced head and neck cancer (JHN002): An open-label phase II trial. Radiother. Oncol. 155, 182–187 (2021).
Zhou, T. et al. The current status and novel advances of boron neutron capture therapy clinical trials. Am. J. Cancer Res. 14, 429 (2024).
Imahori, Y. et al. Positron emission tomography-based boron neutron capture therapy using boronophenylalanine for high-grade gliomas: Part I. Clin. Cancer Res. 4, 1825–1832 (1998).
Malouff, T. D. et al. Boron neutron capture therapy: A review of clinical applications. Front. Oncol. 11, 601820 (2021).
Wang, L. W. et al. Boron neutron capture therapy followed by image-guided intensity-modulated radiotherapy for locally recurrent head and neck cancer: A prospective phase I/II trial. Cancers 15(10), 2762 (2023).
Schöder, H. et al. PET/CT: a new imaging technology in nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging. 30, 1419–1437 (2003).
Advances in Boron Neutron Capture Therapy. International Atomic Energy Agency (Vienna, 2023).
Hicks, R. J. The value of the standardized uptake value (SUV) and metabolic tumor volume (MTV) in lung cancer. in Seminars in nuclear medicine 734–744 (WB Saunders, 2022).
Wittig, A. et al. Biodistribution of 10B for boron neutron capture therapy (BNCT) in a mouse model after injection of sodium mercaptoundecahydro-closo-dodecaborate and L-para-boronophenylalanine. Radiat. Res. 172(4), 493–499 (2009).
Lin, K. H. et al. Prognostic assessment of (18)F-boronophenylalanine positron emission tomography (BPA-PET) in salvage boron neutron capture therapy for malignant brain tumors. Quant. Imag. Med. Surg. 14(6), 4177–4188 (2024).
Chen, J. et al. Development of Monte Carlo based treatment planning system for BNCT. J. Phys. Conf. Ser. 2313(1), 012012 (2022).
Zhong, W. B. et al. Introduction to the Monte Carlo dose engine COMPASS for BNCT. Sci. Rep. 13(1), 11965 (2023).
Teng, Y. C. et al. Correcting for the heterogeneous boron distribution in a tumor for BNCT dose calculation. Sci. Rep. 13(1), 15741 (2023).
Teng, Y. C. et al. HU-based material conversion for BNCT accurate dose estimation. Sci. Rep. 13(1), 15701 (2023).
Hsu, F. Y. et al. Development of a tumor control probability model for boron neutron capture therapy of head and neck cancer. Front. Oncol. 13, 1249074 (2023).
Akramova, R. & Watanabe, Y. Radiomics as a measure superior to common similarity metrics for tumor segmentation performance evaluation. J. Appl. Clin. Med. Phys. 25(8), e1444 (2024).
Acknowledgements
This work was supported by the National Nature Science Foundation of China (Grant No.12261131621) and the National Key Research and Development Program of Chian (Grant No.2022YFE0107800).
Author information
Authors and Affiliations
Contributions
Conceptualization: CR Hu, YC Teng, XB Xia Data acquisition: CR Hu, YQ Lai, Y Cao Data statistics: CR Hu, YC Teng, Paper writing: CR Hu, YC Teng, Paper editing: YC Teng, Paper review: YH Liu, JJ Pan, XB Xia Funding acquisition: JJ. P
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Medical Ethics Committee of Xiamen Humanity Hospital (No. HAXM-MEC-20220901-005-02).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, CR., Lai, YQ., Cao, Y. et al. MRI and 18F-BPA PET-guided targeting in boron neutron capture therapy. Sci Rep 15, 31285 (2025). https://doi.org/10.1038/s41598-025-14642-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14642-4
Keywords
This article is cited by
-
Development and evaluation of surface-guided patient position system for boron neutron capture therapy
Scientific Reports (2026)