Abstract
Enhancement in cell killing by proton beams in the presence of boron (natural mixture natB: 80% 11B, 20% 10B) was reported, selectively in the Bragg peak region, putatively due to the proton-11B capture reaction. However, as some groups observed no such enhancement or assigned it to secondary neutron-10B capture, proton-boron capture therapy (PBCT) remains controversial. We previously validated this concept for U-87 MG glioblastoma cells. To test its generality and potential applicability for these tumours, we assessed PBCT using three further cell lines widely used in glioblastoma research. In U251 cells, natB enhanced cell killing by protons in Bragg peak but also in plateau regions, effects of 10B were even higher, and were found also for 18MV but not 6 MV photon beams (above and below photo-neutron production thresholds, respectively), suggesting a key role of secondary neutrons. For A172 and T98G cells, no enhancement was found at all. This variability among cell lines may stem from differences in boron uptake and/or in intercellular signalling likely needed to amplify the initial events in a few hit cells to population-level effects. Together with recent negative studies, the results suggest that potential clinical applications of PBCT are less promising than originally thought.
Similar content being viewed by others
Introduction
Radiation therapy is among the key modalities for combating cancer; about a half of cancer patients receive radiotherapy as a part of their treatment1. In comparison with conventionally used photon beams, proton beams offer more conformal dose distributions, so that tumour doses can be increased and normal tissue burden reduced, which likely translates to improved treatment outcomes and drives a growing use of proton radiotherapy worldwide2,3. The underlying principle is the difference in energy deposition between photons, which possess exponentially decreasing depth-dose profiles, and protons, which lose energy roughly inversely proportionally to their velocity squared, leading to a sharp peak in energy deposition just before the particles stop. Behind this Bragg peak, virtually no energy (dose) is deposited. In the plateau region in front of the peak, the dose is relatively low. The depth of this Bragg peak can be adjusted to the tumour position by varying the beam energy, and the target volume covered by active scanning with a pencil beam or by a passively spread-out beam4.
The presence of boron was suggested to increase the effectiveness of proton irradiation via a proton-boron capture nuclear reaction, p + 11B → 3α. The cross-section of this reaction possesses a sharp peak at proton energies below 1 MeV, i.e. at the very end of the proton track, just about 20 μm before its stopping. Thus, the reaction is effectively confined to the targeted tumour region, without the need for boron to be selectively accumulated there. Pioneering experiments on so-called proton-boron capture therapy (PBCT) showed significant increases in proton-induced cell killing upon the presence of boron6. In DU145 human prostate cancer cells, the presence of 40–80 ppm 11B led to an increase in proton-induced cell killing as if the dose increased by as much as 75%. Boron was added in the form of sodium mercaptododecaborate (BSH), as is widely used in boron-neutron capture therapy7; the applied boron levels were within clinically relevant ones, i.e. up to 100 ppm with available boron carriers8. The boron-mediated enhancement of cell killing was not observed in the plateau but only in the Bragg peak region, effectively increasing the degree of conformity in dose deposition by proton beams, with likely clinical benefits. Furthermore, an enhanced induction of complex chromosomal aberrations was reported6, which is characteristic of densely ionizing radiation such as alpha particles, supporting the proposed mechanism based on proton-boron capture. As tumours resistant to conventional, sparsely ionizing radiation may respond favourably to densely ionizing radiation9, this suggests an additional benefit of PBCT.
Given the potential clinical implications of these findings, several groups have investigated the PBCT phenomenon. However, their reports have been inconclusive or negative10,11,12, casting doubts on PBCT. Other groups have questioned the suggested underpinning mechanism13,14,15. Further groups have ascribed the boron-mediated enhancements to secondary neutrons, and proposed to call this potential treatment modality as neutron capture-enhanced particle therapy (NCEPT)16,17. Indeed, though avoiding neutrons generated from proton reactions with scattering elements needed in passive beam modulation techniques, even actively scanned proton beams inevitably include neutrons from nuclear interactions of protons with hydrogen in water and other molecules in the patient body, both in plateau and Bragg peak regions. Especially when slowed down to thermal energies, neutrons may be captured by 10B contained in the natural boron isotope mixture natB (80% 11B + 20% 10B) used in the majority of PBCT experiments. The PBCT concept and its mechanism thus remain rather controversial.
We have recently provided an independent validation of the PBCT concept18. First, we have done so for the same DU145 prostate cancer cell line as used in the pioneering experiments6,19,20. Second, we have extended the PBCT validity to U-87 MG glioblastoma cells. Dedicated experiments with natBSH i.e. BSH containing natB as in the pioneering studies or 10BSH containing virtually purely 10B have shown that secondary neutrons including thermal ones present in the proton fields do not play a major role. This conclusion was corroborated by additional experiments with therapeutic 18MV and 6 MV photon beams. While 18 MV photon fields contain considerable amounts of secondary neutrons generated by nuclear reactions of high-energy photons with heavy metal elements in the linac head components, 6 MV beams are free of this contamination as their energies do not reach corresponding reaction thresholds (> 6 MeV)21. If the presence of boron enhanced the effectiveness of 18MV but not 6 MV photons, this would point to a key role of (thermal) secondary neutrons captured by 10B. However, the experiments demonstrated that boron (added either as natBSH or 10BSH) affected the killing of U-87 MG cells by neither 18MV nor 6 MV photon beams. The observed boron-mediated enhancements were selective to protons, the Bragg peak region, and the presence of 11B (not 10B or other components of the BSH molecule) in U-87 MG cells18. These results strongly supported the concept of PBCT based on the p + 11B → 3α capture reaction.
In this work, we further address the generality of the PBCT concept and its potential applicability to glioblastoma by examining the effect of boron on cell killing by proton and photon beams for three additional human cell lines widely used in glioblastoma research, namely U251, A172 and T98G cells. We show that there are large variations among boron effects in these cell lines: The boron-mediated enhancement is selective to protons in the Bragg peak region and 11B in U-87 MG cells ‚18, with little or no contribution of secondary neutrons and 10B. In U251 cells, the selectivity to the Bragg peak region is poorer, and effects from 10B are more pronounced than those from natB. In A172 and T98G cells, the studied boron levels do not lead to any clear enhancement in proton-induced cell killing. Experiments with 6MV and 18 MV photon beams agree with this interpretation. Unfortunately, the results suggest that potential clinical applications of PBCT may not be as promising as originally thought.
Materials and methods
The present work extends our previous study on PBCT in U-87 MG glioblastoma cells18 to three further glioblastoma cell lines: U251, A172 and T98G. The applied methodology follows the previous one, and is briefly outlined below. As the observed data suggested a key contribution of secondary neutrons captured by 10B for U251 cells, both in proton and photon irradiations, quantitative estimates on their fluence were performed by Monte Carlo simulations; their methodology is described below in detail.
Cell culture and boron treatment
Exponentially growing non-synchronized populations of adherent U251, A172 and T98G human glioblastoma cells (Sigma-Aldrich, Germany) were used. These are standard cell lines widely used in glioblastoma research. The U251 (ECACC 9063001) human glioblastoma astrocytoma cell line was originally isolated from a 75-year-old male with an astrocytoma tumour, the A172 line (ECACC 88062428) was derived from a glioblastoma removed from a 53-year-old male, and the T98G line (ECACC 92090213) from a glioblastoma multiforme tumour from a 61-year-old male.
Cells were cultured in Minimum Essential Medium Eagle (MEME, Sigma-Aldrich, Germany) supplemented with 10% Fetal Bovine Serum (FBS, Biosera, France), 2 mM glutamine, 1% non-essential amino acids solution, 1 mM sodium pyruvate, 100 U/ml penicillin and 0.1 mg/ml streptomycin (all: Sigma-Aldrich, Germany) in an incubator maintained at 37 °C in 5% CO2 atmosphere. Two days prior to the planned irradiation with proton or photon beams, the cells were seeded in T25 culture flasks (25 cm2), with 4 ml media, at cell density of 1200–8000 cm−2. One day before irradiation, 1 ml of media was added, with no boron supplement in control samples and with boron in test ones. Boron was added in the form of sodium mercaptododecaborate (BSH, also known as mercapto-undecahydro-closo-dodecaborate or sodium borocaptate, Na2B12H11SH, Katchem, Czech Republic). To assess the potential roles of proton-11B vs. neutron-10B capture reactions, two forms of BSH were used alternatively: (i) natBSH with the natural mixture of boron isotopes (natB, i.e. 80% 11B + 20% 10B), at a final concentration of 0.04 mg/ml natB (i.e. 40 ppm natB, corresponding to 0.083 mg/ml natBSH), or (ii) 10BSH containing virtually 100% 10B, at a final concentration of 0.04 mg/ml 10B (i.e. 40 ppm 10B, 0.070 mg/ml 10BSH).
Irradiations
Proton irradiations were performed at the Proton Therapy Center Czech in Prague, Czech Republic using monoenergetic irradiation maps of 190.6 MeV (range in water of 23.9 cm) with the pencil beam scanning mode (spot spacing of 4 mm, spot full-width at half maximum 7.4 mm), irradiation area of 20 cm × 20 cm at the isocentre, with the gantry positioned at 180°, i.e. irradiating the sample from below, through the patient couch. The cells were irradiated at two positions: in the plateau area at total water-equivalent thickness (WET) of 2.1 cm, and in the Bragg peak region at 23.6 cm WET, using solid water (RW3) and polymethyl methacrylate (PMMA) slabs (Bragg peak position: 17 cm RW3, 4 cm PMMA and 3 mm RW3 plates placed between the treatment table and the samples; plateau position: 8 mm RW3). The applied doses were calculated by the treatment planning system XiO (ELEKTA, Sweden) and benchmarked by a plane parallel ionization chamber (PPC05, IBA Dosimetry, Belgium). The beam range was verified using a multi-layer ionization chamber ZEBRA (IBA Dosimetry, Belgium). Dose uncertainties due to range or positioning errors were estimated not to exceed 2%. All irradiations were repeated 3 times using independent cell cultures.
Photon irradiations were performed at the Bulovka Hospital in Prague, Czech Republic using Varian Clinac 2100 C/D photon beams of 6MV or 18 MV in SSD geometry of 100 cm and field size of 20 cm × 20 cm at the isocentre. Doses were determined by the treatment planning system Monaco 5.11 (ELEKTA); based on previous assessments, the dose delivery uncertainty was estimated as 0.5%. Samples were positioned horizontally on the treatment couch and 5 cm RW3 slabs that assured a dose rate of 2 Gy/min; additional RW3 slabs were placed above the samples to include backscattered radiation. The samples were irradiated from below, through the patient couch. All irradiations were repeated 3 times (for T98G cells: 4 times) using independent cell cultures.
For both proton and photon irradiations, doses up to 8 Gy were applied to U251 and T98G samples; for the more radiosensitive A172 cells, the doses were limited by 4 Gy.
Cell incubation and clonogenic cell survival assay
After returning from the irradiation facilities, the cells were washed twice by 1X Phosphate Buffered Saline (Sigma-Aldrich, Germany) and trypsinized using 1X Trypsin-EDTA (Biosera, France) for 3 min in the incubator at 37 °C in 5% CO2 atmosphere. Then 4 ml of boron-free medium were added, and cell concentrations were counted by a MUSE cell analyzer (EMD Millipore) using the Muse Count & Viability Assay Kit (Merck Millipore/Luminex, MCH100102). Cells were then re-seeded to 6-well plates with fresh, boron-free medium at appropriate densities estimated to yield about 30 colonies per well, and incubated for 12 days at 37 °C in 5% CO2 atmosphere. The formed colonies were fixed and stained using Crystal violet dye (Sigma-Aldrich, Germany) solution. Colonies containing at least 50 cells were counted manually, and dose-dependent survival fractions were calculated.
Statistical analysis of survival data
Cell survival fraction (SF) in dependence on applied dose (D) was fitted by the linear-quadratic (LQ) model, SF = exp(-(αD + βD2)), with parameters α and β, using the maximum likelihood fitting for Poisson-distributed data implemented in CFAssay tool22. ANOVA-based tests for one-way designs in this tool were applied to assess statistical significance of the observed differences of survival curves, e.g. natBSH-treated vs. control ones, rather than analysing differences in measured survival values at single dose levels separately. The number of repeats per datum (cell line, dose, radiation type, and boron presence) was reflected by CFAssay, and affected the datum’s uncertainty, the uncertainty of α and β parameters, as well as the resulting significance (p-values) of differences between curves. The LQ parameters α and β were further used to estimate doses, D10%, leading to 10% cell survival, from which two further quantities were derived: First, for each radiation type and presence or absence of boron, its relative biological effectiveness at 10% cell survival, RBE10%, was calculated as the ratio of D10% value for 6 MV photons, used as the reference radiation type here, to D10% of the test radiation, with the same boron presence or absence. Second, for each radiation type, boron-related dose-modifying factor at 10% cell survival, DMF10%, was calculated as the ratio of D10% values without vs. with the presence of boron. Uncertainties in RBE10% and DMF10% were estimated from those of α and β by error propagation techniques.
Monte Carlo simulations on the potential role of secondary neutrons
To help quantitatively estimate the potential role of neutrons in the observed boron effects for the employed irradiation setups, a series of dedicated Monte Carlo (MC) simulations were performed, using the general-purpose MC code MCNP version 6.223. The simulated geometries corresponded to the actual irradiation setups.
Simulations for photon irradiations at the Bulovka University Hospital were based on a previously developed MC model of Varian Clinac 2100 C/D, used in the experiments, without explicitly modelling the treatment room24. RW3 phantoms of 10 cm thickness in front of and 4 cm behind the sample were included. Photons were generated from monoenergetic 6MeV or 18 MeV electrons.
Simulations for proton irradiations at PTC were performed with a dedicated MC model of the irradiation room prepared according to the provided technical drawing. The beamline structure was not modelled. Monoenergetic 190.6 MeV protons were started in air and emitted vertically towards ceiling in 51 × 51 spot beamlets of a Gaussian shape with 7.4 mm full width at half maximum, with spot spacing of 4 mm, as in the experiments. The sample was irradiated from below, through the treatment table, which was modelled by 8.3 mm Kevlar corresponding to its measured WET of 12 mm. RW3 and PMMA slabs defined in the MC model matched the geometry used in the experiments. The cell culture flask placed on top of the slabs was also included.
Protons, neutrons and photons were tracked in the simulations. Default parameters of particle physics (PHYS card) were kept, except as follows: ion recoils were set on (parameters recl = 1 and coilf = 1), and stopping power energy spacing (efac) for protons was increased to 0.977 to enhance the resolution of sampled multiple-scattering tables resulting in smoother proton depth-dose curves. The Coulomb diffusion (FermiLab angular deflection model with Vavilov straggling), elastic and inelastic scatterings, and photonuclear interactions were considered. Cut-off energies were set to 1 MeV for protons and photons. Delayed particles from non-fission nuclear interactions were also considered (ACT card). The simulations were run for 2500 min of CPU time generating about 5 × 107 primary protons.
Photon interactions were simulated using the electron-photon relaxation library EPRDATA1425 and the photonuclear reaction library ENDF/B-VII.026‚27. For neutrons, the library ENDF/B-VIII.0 at 293.6 K was used, with the table-based physics cut-off set to mix and match (parameter tabl = −1 on the PHYS: N card for neutrons). Proton interactions were simulated using evaluations of ENDF/B-VII data28. Outside the energy ranges of the libraries, the Bertini INC with Dresner evaporation model was used (parameter tabl = −1 on the PHYS: H card for protons).
Spectral fluence distributions, integral thermal neutrons flux, and absorbed dose from protons or photons were calculated using F4-type or F6-type tally in appropriate scoring volumes or surfaces.
Results
Boron-mediated alterations in glioblastoma cell killing by proton or photon beams
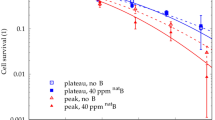
To facilitate comparisons with the other glioblastoma cell lines, we first reprint our previously published data18 for U-87 MG glioblastoma cells (Fig. 1). Adding 40 ppm natB (i.e., 32 ppm 11B and 8 ppm 10B) in the form of natBSH led to a significantly enhanced cell killing by 190 MeV protons selectively in their Bragg peak region (p-value < 0.001). Only an indication (p > 0.05) for a slight enhancement was seen for 10B, in the same chemical form (10BSH) and concentration (40 ppm 10B). Virtually no effects were observed in the plateau region. Likewise, the presence of neither natBSH nor 10BSH affected the killing of U-87 MG glioblastoma cells by 6 or 18 MV photon beams. These results provided an independent validation of the PBCT phenomenon 18 , and demonstrated that it was selective to protons in their Bragg peak and the presence of 11B, with no large contribution from secondary neutrons or 10B18.
Survival of U-87 MG glioblastoma cells irradiated by (a) 190.6 MeV proton beams in their Bragg peak position, (b) in plateau region, (c) by 6 MV or (d) 18 MV photons, in the absence (black) or presence of natBSH (red) or 10BSH (green) at a concentration corresponding to 40 ppm natB or 10B, respectively. Symbols depict mean survival values from at least 3 independent experiments, error bars show standard errors of the mean, and lines are linear-quadratic fits to the data. Data replotted from18. Stars highlight significant differences between survival curves without and with boron (*** p < 0.001; not marked: not significant, p > 0.05).
Also for U251 glioblastoma cells, the presence of 40 ppm natB (i.e., 32 ppm 11B and 8 ppm 10B) in the form of natBSH led to a notable increase of cell killing by 190 MeV protons in their Bragg peak region, particularly at doses of 4–8 Gy (Fig. 2a, red: with natB vs. black: without B, p = 2.4 × 10−6). This finding is consistent with the pioneering experiments on PBCT in DU145 prostate and PANC-1 pancreatic cancer cells6,19,20 and our previous results for DU145 prostate cancer and U-87 MG glioblastoma cells18. However, unlike in U-87 MG cells, an even greater enhancement was observed for 40 ppm 10B added as 10BSH (green: with 10B vs. black: without B, p = 8.1 × 10−9). This suggests that 10B capturing secondary neutrons (especially thermal ones) inevitably present in the proton field is capable of significantly reducing U251 cell survival. Furthermore, even in the plateau region, an indication was found for enhanced cell killing by the proton beam upon the presence of 40 ppm natB (Fig. 2b, red: with natB vs. black: without B, p = 0.12), and for 40 ppm 10B, the enhancement was statistically significant (green: with 10B vs. black: without B, p = 2.4 × 10−5) although its degree was smaller than in the Bragg peak. For 6 MV photon beams (Fig. 2c), the enhancement of cell killing by boron was very small; the indication did not reach statistical significance (red: with natB vs. black: without B, p = 0.053; green: with 10B vs. black: without B, p = 0.16). Larger enhancements in cell killing were observed for 18 MV photon beams (Fig. 2d) which, contrary to 6 MV ones, do contain neutrons (red: with natB vs. black: without B, p = 0.012; green: with 10B vs. black: without B, p = 0.0043).
Survival of U251 glioblastoma cells irradiated by (a) 190.6 MeV proton beams in their Bragg peak position, (b) in plateau region, (c) by 6 MV or (d) 18 MV photons, in the absence (black) or presence of natBSH (red) or 10BSH (green) at a concentration corresponding to 40 ppm natB or 10B, respectively. Symbols depict mean survival values from 3 independent experiments, error bars show standard errors of the mean, and lines are linear-quadratic fits to the data. Stars highlight significant differences between survival curves without and with boron (* p < 0.05; **p < 0.01; *** p < 0.001; not marked: not significant, p > 0.05).
For A172 cells, the measured data (Fig. 3) did not reveal any statistically significant differences between cell survival in the absence of boron or presence of either 40 ppm natB or 40 ppm 10B, for either Brag peak or plateau regions of 190 MeV proton beams or for 18MV or 6 MV photons (p > 0.05 for all pairs of curves). To investigate whether the absence of boron-mediated enhancement might be due to insufficient boron concentrations, additional experiments were conducted using 80 ppm of natB (n = 2 independent experiments) or 10B (n = 1), leading however to no clear effects observed, either (data not shown).
Survival of A172 glioblastoma cells irradiated by (a) 190.6 MeV proton beams in their Bragg peak position, (b) in plateau region, (c) by 6 MV or (d) 18 MV photons, in the absence (black) or presence of natBSH (red) or 10BSH (green) at a concentration corresponding to 40 ppm natB or 10B, respectively. Symbols depict mean survival values from 3 independent experiments, error bars show standard errors of the mean, and lines are linear-quadratic fits to the data. None of the differences between survival curves without and with boron was found statistically significant (all p > 0.05).
Also for T98G cells (Fig. 4), none of the setups showed a statistically significant effect of either natB or 10B at a concentration of 40 ppm (p > 0.05 for all pairs of curves). The observed variations among the cell survival curves thus likely represented just biological variability. Even when using 80 ppm of natB (n = 2) or 10B (n = 1), no clear effects were observed (data not shown).
Survival of T98G glioblastoma cells irradiated by (a) 190.6 MeV proton beams in their Bragg peak position, (b) in plateau region, (c) by 6 MV or (d) 18 MV photons, in the absence (black) or presence of natBSH (red) or 10BSH (green) at a concentration corresponding to 40 ppm natB or 10B, respectively. Symbols depict mean survival values from 3 (for protons) or 4 (for photons) independent experiments, error bars show standard errors of the mean, and lines are linear-quadratic fits to the data. None of the differences between survival curves without and with boron was found statistically significant (all p > 0.05).
Parameters of the linear-quadratic (LQ) fits to the data for all studied glioblastoma cell lines are listed in Table 1. Also included in Table 1 are the values of relative biological effectiveness (RBE10%) at 10% cell survival, using as the reference radiation the case of 6 MV photons with the same presence or absence of boron. For protons, no significant deviation of their RBE from unity is observed in the plateau region, while in the Bragg peak, the RBEs are clearly higher than unity (apart from T98G cells for which they suffer from large uncertainties). This agrees with the known slightly enhanced RBE of protons compared to photons, which actually increases in the Bragg peak region and especially in its distal part, but is commonly approximated 29 by an overall value of 1.1. RBEs for 18 MV photons relative to 6 MV ones are not significantly different from unity. Further, Table 1 also includes boron-elicited dose-modifying factors DMF10%, again obtained from the LQ fits as the ratios of doses leading to 10% survival level, without vs. with boron. The values agree with the above-discussed significant effects of natBSH for protons in their Bragg peak and 10BSH in both peak and plateau regions for U251 cells and the lack of effects for A172 and T98G cells. For U251 cells irradiated by 18 MV photons, the statistical significance demonstrated by comparing the whole survival curves would not be revealed by the DMF10% values; this highlights the superiority of the employed whole-curve analysis.
Taken together, these results suggest that for U251 cells the observed enhancements could be predominantly attributed to secondary neutrons captured by 10B, with just a little if any contribution from protons captured by 11B. This is an opposite result to U-87 MG cells, where the proton-11B capture clearly dominated over the neutron-10B one. No boron-mediated effects were observed for A172 and T98G cells.
Potential roles of the proton-11B versus neutron-10B capture reactions
The proton-11B capture produces a spatially and temporarily correlated triplet of alpha particles that share the reaction energy of 8.7 MeV. The energies of the generated alpha particles span a continuous spectrum; typically, two alpha particles possess an energy around 4 MeV and the third one about 1 MeV, corresponding to linear energy transfer (LET) values around 150 keV/µm and 175 keV/µm and ranges around 27 μm and 6 μm, respectively3031. The proton-11B reaction is a rather rare event that occurs in a very few cells only: At 40 ppm natB (32 ppm 11B, corresponding to the number density of 11B atoms 1.1 × 1024 m−3), this reaction occurs in the Bragg peak region with the frequency of 1.1 × 1024 m−3 × 10−28 m2 × 3 × 108 cm−2 Gy−1 = 0.33 mm−3 Gy−1; here, to provide an upper limit, the maximal interaction cross section of 1 b = 10−28 m2 was taken, and the fluence of 190 MeV protons (i.e., energy used in18 and herein) was approximated as 3 × 108 cm−2 Gy−1 in the Bragg peak32. This spatial frequency translates to relative dose enhancement of the order of 8.7 MeV × 0.33−3 Gy−1 = 8.7 × 106 eV × 1.6 × 10−19 J eV−1 × 0.33 mm−3 Gy−1 × 103 kg m−3 = 4.6 × 10−7, i.e., in the Bragg peak region, the alpha particle mediated dose enhancement at 32 ppm 11B is 6–7 orders of magnitude smaller than the proton dose. Very similar estimates were reported in13. With cell masses of human cells 33 spanning the broad range of 10−9 – 10−5 g, corresponding to volumes of the order of 10−6 – 10−2 mm3, the proton-11B reaction occurs in roughly one out of a million to one out of a hundred cells in the Bragg peak region at 40 ppm natB (32 ppm 11B) at doses of 2–3 Gy typically used per fraction in radiotherapy. In fact, these figures represent an upper estimate, as even in the Bragg peak region only a minority of protons possess energies in the range where the proton-11B cross section peaks19; accounting for this would reduce the given estimate by roughly an order of magnitude13. The estimate cannot be much increased by individual alpha particles hitting multiple cells, due to their very short ranges (< 30 μm). In the plateau region, the proton-11B capture rates are even smaller13, as protons possess high energies there, at which the reaction cross sections are much smaller.
The potential role of the neutron-10B capture reaction is governed by the fluences of thermal neutrons, as its cross section is inversely proportional to neutron velocity. In this work, the fluences of secondary neutrons and their energy spectra for the four applied irradiation setups were assessed by a series of dedicated Monte Carlo simulations. As expected, 6 MV photon beams were predicted to be virtually free of secondary neutrons (not shown). As shown in Fig. 5, proton beams in plateau and peak regions as well as 18 MV photon beams include thermal (< 0.5 eV considered herein), intermediate and high-energy neutrons. The high-energy components of neutron spectra from the three beams differ in shapes, total yields, and highest energies. However, the thermal components, which are of importance for boron-neutron capture, are remarkably similar, and differ only in thermal neutron fluence, simulated as 1.3 × 107 cm−2 Gy−1 for 18 MV photons, 3.0 × 106 cm−2 Gy−1 for Bragg peak and 3.8 × 105 cm−2 Gy−1 for plateau region of 190.6 MeV protons, respectively (these values correspond to summing up the fluence values in energy bins plotted in Fig. 5 over energies up to 0.5 eV). Here, to facilitate comparisons between different irradiation scenarios, the fluence was normalized per unit dose, considering the simulated dose delivered by primary protons or photons. With the effective 34 for thermal neutron capture by 10B of 3840 b34, at the 40 ppm 10B concentration applied, these values result in 68, 16, and 1.9 neutron-10B capture reactions per mm3 per Gy; for 8 ppm 10B present in 40 ppm natB, these figures are 5 times smaller, 14, 3.2, and 0.4 neutron-10B capture reactions per mm3 per Gy for 18 MV photons, Bragg peak or plateau regions of the 190.6 MeV proton beam, respectively. Very similar reaction yields result (not shown) if the simulated neutron fluence spectra are folded with detailed energy-dependent boron-neutron capture cross-sections, as provided by the EXFOR nuclear reaction database35,36. The neutron-10B capture reaction releases the energy of 2.8 MeV; in 94% of events, the reaction produces 1.47 MeV alpha particles, 0.84 MeV 7Li and 0.48 MeV photons, while the remaining 6% lead to 1.77 MeV alpha particles and 1.02 MeV 7Li nuclei37. The formed alpha particles and 7Li nuclei are short-ranged (2–10 μm), densely ionizing particles (LET approximately 180 keV/µm for α and 400 keV/µm for 7Li)31,38.
Neutron spectral fluence at sample for irradiation by 18 MV photons (blue circles) or 190.6 MeV protons in their Bragg peak region (water-equivalent thickness 23.6 cm; orange triangles) or plateau region (water-equivalent thickness 2.1 cm; grey squares), as predicted by Monte Carlo simulations, normalized to unit dose deposited to the sample.
According to the above-performed estimates, the capture of secondary thermal neutrons by 10B is therefore more frequent than the capture of primary protons by 11B, in both plateau and Bragg peak positions in the presence of natB, consistent with the findings in Refs16,17. Yet, e.g. at 40 ppm natB and Bragg peak dose of 2–3 Gy, even the neutron-10B reaction occurs in only about one out of 105 cells to one per ten cells, depending on the cell size, assuming that intracellular boron levels match the extracellular ones. The produced pairs or triplets of densely ionizing particles are confined to a very close vicinity (< 30 μm) of the interaction point. As pointed out above and as resulted from previous PBCT simulations13,14,15, at boron levels < 100 ppm such as used in this work, the dose enhancement resulting from energy deposited by the produced alpha particles is several (six to seven) orders of magnitude smaller than the dose by the primary protons and hence negligible. Even though the neutron-10B capture is 10 times more frequent, the related dose enhancement is still negligible. Taken together, purely physical mechanisms of boron-mediated cell killing enhancements are hardly feasible, either via proton-11B or neutron-10B capture reactions.
Discussion
In pioneering experiments on PBCT6,19,20, natBSH at 40–80 ppm 11B was shown to significantly enhance cell killing by proton beams, as if the applied dose increased, selectively in the Bragg peak region, by up to 75%. The observed enhancements were proposed to originate from proton-11B capture reactions producing triplets of alpha particles. A pre clinical study on the proposed proton-boron capture therapy in mice with subcutaneously injected U-87 MG glioblastoma cells as a xenograft model of this tumour provided promising results39. However, several other groups have reported inconclusive or negative results in vitro, failing to observe such an enhancement in cell killing in the presence of boron10,11,12. Our previous study with DU145 prostate cancer cells and with U-87 MG human glioblastoma cells provided the first independent validation of the boron-mediated enhancements18.
Glioblastoma are aggressive brain tumours with a very poor prognosis. Any improvement to existing therapies, such as expected from PBCT, would be highly beneficial. Motivated by such considerations, the present study aimed at extending the previous results for U-87 MG glioblastoma cells to further human glioblastoma cell lines, namely U251, A172 and T98G cells. Obviously, the in vitro nature of the present work may be seen as a limitation compared to the recent preclinical study with U-87 MG cells grafted into mice39. Its strength, on the other hand, lies in studying three diverse glioblastoma cell lines, together with the previous data on U-87 MG cells representing the same tumour type18. Contrary to the previous findings that showed boron-mediated enhanced killing of cancer cells by protons selectively in their Bragg peak, for U251 glioblastoma cells the enhancements were observed in both Bragg peak and plateau regions for protons, and also for 18 MV but not 6 MV photons. In A172 and T98G glioblastoma cells, no statistically significant effects of boron were observed for any of the beams investigated. This suggests that the enhancement is predominantly due to primary protons captured by 11B for U-87 MG cells, secondary neutrons captured by 10B for U251 cells, and is not present or does not reach statistical significance for A172 and T98G cells.
Potential mechanisms underpinning PBCT remain controversial. According to first PBCT simulations, the presence of boron and the production of triplets of densely ionizing, short-ranged alpha particles from the proton-11B capture reaction could largely increase dose deposition to the tumour and hence also the killing of cancer cells5,40. Dose enhancements as high as 80% were reported, assuming the target region to contain purely boron5, 100% 11B, with a density of 2.08 g cm−3. However, subsequent simulations13,14,15 as well as the estimates reported in this work indicated that at clinically relevant boron levels, i.e. roughly up to 100 ppm 11B with presently available boron carriers, the alpha-mediated dose enhancement is negligible, several orders of magnitude smaller than the dose deposited by protons. Due to the very low boron levels, similarly negligible are also the effects of dose increase from increased density and interaction cross sections. Although the 10B-neutron capture may be more frequent than the proton-11B one, the dose enhancement from 10B-neutron capture is negligible as well, given the relatively low secondary neutron fluences in actively scanned proton fields or therapeutic photon beams, contrary to boron neutron capture therapy (BNCT) applications with neutron beams. Purely physical considerations may not explain the observed increase in biological effects, interpreted as either PBCT or NCEPT.
BSH was previously suggested to act as a general radiosensitizer, increasing cell killing by both protons and photons10. However, the present data do not support this concept; clearly, there was no effect of boron (either natB or 10B) universally enhancing cell killing by photon and proton beams for any cell line studied. On the contrary, one might be tempted to interpret the data for T98G as suggesting a trend towards a general slightly radioprotective effect of BSH, compensated by PBCT in the Bragg peak region (Fig. 4). This would be in line with the previously reported radioprotective effect of BSH on plasmid DNA, potentially related to the scavenging of •OH radicals by the thiol (SH) group in BSH41. However, this interpretation remains largely speculative, as the observed variations among survival curves may well represent just a common biological variability.
The reported quantitative estimates highlight that at clinically relevant boron concentrations only a small fraction of cells are hit by the produced triplets of alpha particles from proton-11B or alpha + 7Li pairs from neutron-10B capture reactions. With glioblastoma cell volumes 42 of the order of 10−6 – 10−5 mm3, much less than a single triplet of alpha particles or a single alpha + 7Li pair would be produced per cell volume, even at the highest studied dose of 8 Gy. The produced alpha particles and 7Li have short ranges (< 30 μm), so they cannot traverse many additional (neighbouring) cells. Assuming that boron does not accumulate massively in the cells but is roughly homogeneously distributed in the irradiated sample (i.e., assuming that intracellular boron levels are not orders of magnitude higher than the prescribed concentrations in the culture medium), only a few cells in the studied population are thus hit by the triplets of alpha particles or pairs of an alpha particle + 7Li ion. For effects such as reduced cell survival to be observed, the initial effects in these few hit cells need to be amplified to a population level.
The hit cells, their nuclei or subcellular organelles such as mitochondria are damaged heavily43. In cell populations where only a few cells are hit by a few alpha particles, leaving other cells unirradiated, bystander effects are known: population-level responses in terms of DNA damage induction or cell killing were observed that largely exceed the fractions of hit cells44,45,46. These bystander effects involve intercellular signalling from the initially hit cells to their neighbours, either through direct gap-junctional communication or via medium-borne signals44. In analogy, we suggested a crucial role of intercellular communication in amplifying the initial events of the few alpha particles on top of a sea of protons in PBCT and triggering an additional population-level response on top of the proton-induced one47. We suggest that a similar amplification through intercellular signalling is needed for the initial events triggered by alpha + 7Li pairs in the few hit cells where the neutron-10B capture occurred in order to manifest on top of a proton or photon wash, as shown by the present data for U251 cells.
The involvement of intercellular signalling and its non-linear nature could explain why the observed enhancement in U251 cell killing upon the presence of 40 ppm 10B added as 10BSH was somewhat but not 5-fold higher than with 8 ppm 10B contained in natBSH, for both protons and 18 MV photons (Fig. 2). Indeed, intercellular signalling often exhibits non-linear features in the release of signals upon exposure to stressors and/or in the response to these signals. Positive and negative feedbacks often lead to threshold-type (sigmoidal) rather than linear or exponential behaviour48. Signalling systems may include a multitude of players such as cytokines or reactive oxygen species, and cascades of biochemical reactions49, with complex spatial and temporal consequences50,51. Specifically, in the context of radiation-induced bystander effects, experiments with diluting the irradiated cell-conditioned medium52 demonstrated that the response to signals is highly non-linear; reducing the signal level by 50% led to completely diminishing bystander effects, contrary to what would be expected in systems with linear characteristics53. The release of bystander signals likely requires an above-threshold energy deposition by ionizing radiation54,55, which may also contribute to non-linear features56. Thus, if the observed boron enhancements involve non-linear intercellular signalling, 40 ppm 10B may differ from being 5-times more effective than 8 ppm 10B, in line with the data in Fig. 2.
This intercellular signalling hypothesis could also explain the observed quantitative and qualitative differences among cell lines. The capabilities to release signals upon irradiation and to respond to these signals are known to vary significantly among cell lines44,49. One cell line may be more sensitive to triplets of alpha particles than to alpha + 7Li pairs, even if the latter occur 10 times more frequently. For another cell line, the opposite may be true. The decisive factors may include the number of and/or spatial arrangement of mitochondria, their oxidative stress levels, etc. For yet another cell line, both damage patterns may be insufficient to trigger the release of signals, or the signalling insufficient to trigger a response in terms of measurable cell inactivation. Exosomes, signal-carrying vesicles, have been identified as key players in intercellular signalling57,58. In a dedicated glioblastoma cell line study, exosomes were found to be regulated by antisense transcript of hypoxia‑inducible factor‑1α (AHIF), and increased levels of AHIF expression were observed in T98G and A712 cells compared with U-87 MG and U251 cells59. This suggests that the observed differences in behaviour between U-87 MG and U251 cells on one hand, and A172 and T98G cells on the other hand, may reflect their more general biological differences, even though they were all derived from glioblastoma.
Aiming to directly demonstrate the involvement of intercellular signalling in the observed PBCT/NCEPT effects, we have investigated how it would be modulated by dimethyl sulfoxide (DMSO), ketotifen, or both present during irradiation. DMSO is a known scavenger of reactive oxygen species, while ketotifen inhibits exosome release, thus targeting two putative levels of radiation-induced signalling. Unfortunately, the performed experiments have not provided results supporting or rejecting the suggested signalling-based mechanism of boron effects (not shown). Directly demonstrating the involvement of intercellular signalling is thus left to future work.
Obviously, differences in cellular uptake of boron (including boron in the cytoplasm, mitochondria, cell nucleus, and attached to the cell membrane) may play a key role in the observed variations among the studied cell lines. The presented frequency estimates of both capture reactions have assumed a homogenized boron presence throughout the sample. Active uptake of boron by cancer cells e.g. due to their increased metabolic rates would effectively increase the cellular frequencies of both proton-11B and neutron-10B capture reactions. Variations among cell lines or in their uptake of 11B vs. 10B (known as isotopic fractionation) would shift the reported estimates, too. Likewise, deviations from homogeneous boron concentrations, even very small in absolute terms due to the ppm levels used, could largely affect the outcomes; e.g. a small BSH grain in the vicinity of a cell, resulting from a limited BSH solubility, would dramatically increase the probability of the cell being hit by the products of both boron capture reactions. We have aimed at addressing the subcellular localization of boron experimentally; however, unfortunately, neither electron energy-loss spectroscopy nor inductively coupled plasma – optical emission spectroscopy provided clear and reproductive results for BSH (with either natB or 10B) on cell membranes or inside U-87 MG and U251 cells (results not shown).
The conditions used in the present study were identical to those used in our previous one18, to the extent to which they could have been controlled practically. Proton beam energies, field sizes, spot sizes, thicknesses of RW3 and PMMA plates used etc. were identical. However, the culture media, sera and other chemicals used in the present study originated from different batches than those used previously. While their specified composition was identical, differences might have been present in non-specified items, e.g. in the levels of serotonin which may affect radiation-induced signalling60,61,62. Potential differences in experimental setup, such as timing or cell densities, media or sera composition may also explain the inconclusive or negative PBCT data reported recently10,11,12, given the sensitivity of intercellular signalling to these factors44,49.
Finally, the present results together with the mentioned inconclusive or negative studies suggest that, unfortunately, the potential clinical applications of proton-boron capture therapy may not be as promising as originally hoped. PBCT was not found unequivocally effective by independent groups10,11,12 using the same DU145 prostate cancer cell line as in the pioneering experiments6,19,20. Among diverse model cell lines representing glioblastoma multiforme, a single diagnosis, it was found clearly effective and beneficial for just a single one, U-87 MG18, but not for others (this study); for U251, the presence of boron did enhance cell killing but not selectively for protons in the Bragg peak. Thus, in vitro studies conducted so far suggest that PBCT in its present form may not provide a sufficiently robust and clinically reliable approach. Yet, we hope that future research will help identify details of the underpinning mechanisms and enable controlling their key players such as potentially serotonin affecting intercellular communication. Although this may be very challenging especially in clinical applications, the potential benefits of PBCT are worth such efforts.
Data availability
Data will be available from the corresponding author upon request.
References
Delaney, G., Jacob, S., Featherstone, C. & Barton, M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104, 1129–1137. https://doi.org/10.1002/cncr.21324 (2005).
Mohan, R. A review of proton therapy – Current status and future directions. Prec Radiat. Oncol. 6, 164–176. https://doi.org/10.1002/pro6.1149 (2022).
Chen, Z., Dominello, M. M., Joiner, M. C. & Burmeister, J. W. Proton versus photon radiation therapy: A clinical review. Front. Oncol. 13, 1133909. https://doi.org/10.3389/fonc.2023.1133909 (2023).
Newhauser, W. D. & Zhang, R. The physics of proton therapy. Phys. Med. Biol. 60 https://doi.org/10.1088/0031-9155/60/8/R155 (2015).
Yoon, D. K., Jung, J. Y. & Suh, T. S. Application of proton boron fusion reaction to radiation therapy: a Monte Carlo simulation study. Appl. Phys. Lett. 105, 223507. https://doi.org/10.1063/1.4903345 (2014).
Cirrone, G. A. P. et al. First experimental proof of proton boron capture therapy (PBCT) to enhance protontherapy effectiveness. Sci. Rep. 8 https://doi.org/10.1038/s41598-018-19258-5 (2018).
Barth, R. F., Zhang, Z. & Liu, T. A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Commun. 38, 36. https://doi.org/10.1186/s40880-018-0280-5 (2018).
Hideghéty, K. et al. 11Boron delivery agents for boron proton-capture enhanced proton therapy. Anticancer Res. 39, 2265–2276. https://doi.org/10.21873/anticanres.13343 (2019).
Tinganelli, W. & Durante, M. Carbon ion radiobiology. Cancers 12, 3022. https://doi.org/10.3390/cancers12103022 (2020).
Manandhar, M. et al. Effect of boron compounds on the biological effectiveness of proton therapy. Med. Phys. 49, 6098–6109. https://doi.org/10.1002/mp.15824 (2022).
Hosobuchi, M. et al. Experimental verification of efficacy of PBCT in terms of physical and biological aspects. Nucl. Instrum. Methods Phys. Res. A. 1045, 167537. https://doi.org/10.1016/j.nima.2022.167537 (2023).
Shtam, T. et al. Experimental validation of proton boron capture therapy for glioma cells. Sci. Rep. 13 https://doi.org/10.1038/s41598-023-28428-z (2023).
Mazzone, A., Finocchiaro, P., Lo Meo, S. & Colonna, N. On the (un)effectiveness of proton boron capture in proton therapy. Eur. Phys. J. Plus. 134, 361. https://doi.org/10.1140/epjp/i2019-12725-8 (2019).
Tabbakh, F. & Hosmane, N. S. Enhancement of radiation effectiveness in proton therapy: comparison between fusion and fission methods and further approaches. Sci. Rep. 10 https://doi.org/10.1038/s41598-020-62268-5 (2020).
Meyer, H. J., Titt, U. & Mohan, R. Technical note: Monte Carlo study of the mechanism of proton-boron fusion therapy. Med. Phys. 49, 579–582. https://doi.org/10.1002/mp.15381 (2022).
Safavi-Naeini, M. et al. Opportunistic dose amplification for proton and carbon ion therapy via capture of internally generated thermal neutrons. Sci. Rep. 8 https://doi.org/10.1038/s41598-018-34643-w (2018).
Jacobsen, V. L., Johansen, J. G., Fynbo, H. O. U. & Bassler, N. A Monte Carlo study of high-LET particle production in proton boron therapy. Eur. Phys. J. Plus. 138, 625. https://doi.org/10.1140/epjp/s13360-023-04235-3 (2023).
Jelínek Michaelidesová, A. et al. First independent validation of the proton-boron capture therapy concept. Sci. Rep. 14 https://doi.org/10.1038/s41598-024-69370-y (2024).
Bláha, P. et al. The proton-boron reaction increases the radiobiological effectiveness of clinical low- and high-energy proton beams: novel experimental evidence and perspectives. Front. Oncol. 11, 682647. https://doi.org/10.3389/fonc.2021.682647 (2021).
Ricciardi, V. et al. A new low-energy proton irradiation facility to unveil the mechanistic basis of the proton-boron capture therapy approach. Appl. Sci. 11, 11986. https://doi.org/10.3390/app112411986 (2021).
International Atomic Energy Agency. Handbook on Photonuclear Data for Applications Cross-sections and Spectra, IAEA-TECDOC-11m78, IAEA, Vienna. ; (2000). https://www-pub.iaea.org/MTCD/publications/PDF/te_1178_prn.pdf
Braselmann, H., Michna, A., Heß, J. & Unger, K. CFAssay: statistical analysis of the colony formation assay. Radiat. Oncol. 10, 223. https://doi.org/10.1186/s13014-015-0529-y (2015).
Werner, C. J. MCNP® User’s Manual, Code Version 6.2. Los Alamos National Laboratory LA-UR-17-29981 (2017).
Králík, M. et al. Spectral fluence of neutrons generated by radiotherapeutic Linacs. Radiat. Prot. Dosimetry. 163, 373–380. https://doi.org/10.1093/rpd/ncu192 (2015).
Werner, C. J. et al. MCNP Version 6.2 Release Notes. Los Alamos National Laboratory LA-UR-18-20808 (2018).
Chadwick, M. B. et al. ENDF/B-VII.0: next generation evaluated nuclear data library for nuclear science and technology. Nucl. Data Sheets. 107, 2931–3060. https://doi.org/10.1016/j.nds.2006.11.001 (2006).
Conlin, J. L., Haeck, W., Neudecker, D., Parsons, D. K. & White, M. C. Release of ENDF/B-VIII.0-Based ACE data files. Los Alamos National Laboratory LA-UR-18-24034 (2018).
Trellue, H., Little, R., Lee, M. B. & New, A. C. E. F. Neutron and Proton Libraries Based on ENDF/B-VII.0. Los Alamos National Laboratory LA-UR-08-1999 (2008).
Paganetti, H. Relating proton treatments to photon treatments via the relative biological effectiveness – should we revise current clinical practice? Int. J. Radiat. Oncol. Biol. Phys. 91, 892–894. https://doi.org/10.1016/j.ijrobp.2014.11.021 (2015).
Stave, S. et al. Understanding the B11(p,α)αα reaction at the 0.675 Mev resonance. Phys. Lett. B. 696, 26–29. https://doi.org/10.1016/j.physletb.2010.12.015 (2011).
ICRU Report 49. Stopping Powers and Ranges for Protons and Alpha Particles. International Commission on Radiation Units and Measurements (Bethesda, 1993).
Kundrát, P. A semi-analytical radiobiological model may assist treatment planning in light ion radiotherapy. Phys. Med. Biol. 52, 6813–6830. https://doi.org/10.1088/0031-9155/52/23/003 (2007).
Hatton, I. A. et al. The human cell count and size distribution. Proc. Natl. Acad. Sci. U S A. 120 (e2303077120). https://doi.org/10.1073/pnas.2303077120 (2023).
International Atomic Energy Agency & Vienna Advances in Boron Neutron Capture Therapy. Non-serial Publications (IAEA, 2023). https://www-pub.iaea.org/MTCD/publications/PDF/CRCP-BOR-002_web.pdfaccessed 05-06-2024.
Database, Experimental Nuclear Reaction Data (EXFOR) Database. https://www-nds.iaea.org/exfor/, accessed 02-05-2024.
Otuka, N. et al. Towards a more complete and accurate experimental nuclear reaction data library (EXFOR): international collaboration between nuclear reaction data centres (NRDC). Nucl. Data Sheets. 120, 272–276. https://doi.org/10.1016/j.nds.2014.07.065 (2014).
Sato, T., Masunaga, S. I., Kumada, H. & Hamada, N. Microdosimetric modeling of biological effectiveness for boron neutron capture therapy considering intra- and intercellular heterogeneity in 10B distribution. Sci. Rep. 8, 988. https://doi.org/10.1038/s41598-017-18871-0 (2018).
ICRU Report 49 and 73, Stopping of Ions Heavier than Helium. International Commission on Radiation Units and Measurements & Bethesda Maryland, USA ; Errata and Addenda (2009), (2005). https://www.icru.org/wp-content/uploads/2020/12/Errata_and_Addenda_73.pdf
Cammarata, F. P. et al. Proton boron capture therapy (PBCT) induces cell death and mitophagy in a heterotopic glioblastoma model. Commun. Biol. 6, 388. https://doi.org/10.1038/s42003-023-04770-w (2023).
Jung, J. Y. et al. Comparison between proton boron fusion therapy (PBFT) and boron neutron capture therapy (BNCT): a Monte Carlo study. Oncotarget 8, 39774–39781. https://doi.org/10.18632/oncotarget.15700 (2017).
Jamborová, Z. et al. Radiation damage to DNA plasmids in the presence of borocaptates. Radiat. Prot. Dosimetry. 198, 532–536. https://doi.org/10.1093/rpd/ncac094 (2022).
Watkins, S. & Sontheimer, H. Hydrodynamic cellular volume changes enable glioma cell invasion. J. Neurosci. 31, 17250–17259. https://doi.org/10.1523/JNEUROSCI.3938-11.2011 (2011).
Friedland, W., Schmitt, E., Kundrát, P., Baiocco, G. & Ottolenghi, A. Track-structure simulations of energy deposition patterns to mitochondria and damage to their DNA. Int. J. Radiat. Biol. 95, 3–11. https://doi.org/10.1080/09553002.2018.1450532 (2019).
Prise, K. M. & O’Sullivan, J. M. Radiation-induced bystander signalling in cancer therapy. Nat. Rev. Cancer. 9, 351–360. https://doi.org/10.1038/nrc2603 (2009).
Nagasawa, H. & Little, J. B. Unexpected sensitivity to the induction of mutations by very low doses of alpha-particle radiation: evidence for a bystander effect. Radiat. Res. 152, 552–557 (1999).
Zhou, H. et al. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc. Natl. Acad. Sci. U S A. 97, 2099–2104. https://doi.org/10.1073/pnas.030420797 (2000).
Kundrát, P. et al. Boron-enhanced biological effectiveness of proton irradiation: strategy to assess the underpinning mechanism. Radiat. Prot. Dosimetry. 198, 527–531. https://doi.org/10.1093/rpd/ncac093 (2022).
Ferrell, J. E. & Xiong, W. Bistability in cell signalling: how to make continuous processes discontinuous, and reversible processes irreversible. Chaos 11 (1), 227–236. https://doi.org/10.1063/1.1349894 (2001). Woodbury.
Bauer, G. Low dose radiation and intercellular induction of apoptosis: potential implications for the control of oncogenesis. Int. J. Radiat. Biol. 83, 873–888. https://doi.org/10.1080/09553000701727523 (2007).
Kundrát, P., Bauer, G., Jacob, P. & Friedland, W. Mechanistic modelling suggests that the size of preneoplastic lesions is limited by intercellular induction of apoptosis in oncogenically transformed cells. Carcinogenesis 33, 253–259. https://doi.org/10.1093/carcin/bgr227 (2012).
Kundrát, P. & Friedland, W. Enhanced release of primary signals may render intercellular signalling ineffective due to Spatial aspects. Sci. Rep. 6, 33214. https://doi.org/10.1038/srep33214 (2016).
Ryan, L. A., Smith, R. W., Seymour, C. B. & Mothersill, C. E. Dilution of irradiated cell conditioned medium and the bystander effect. Radiat. Res. 169, 188–196. https://doi.org/10.1667/RR1141.1 (2008).
Kundrát, P. & Friedland, W. Non-linear response of cells to signals leads to revised characteristics of bystander effects inferred from their modelling. Int. J. Radiat. Biol. 88, 743–750. https://doi.org/10.3109/09553002.2012.698029 (2012).
Friedland, W., Kundrát, P. & Jacob, P. Track structure calculations on hypothetical subcellular targets for the release of cell-killing signals in bystander experiments with medium transfer. Radiat. Prot. Dosimetry. 143, 325–329. https://doi.org/10.1093/rpd/ncq401 (2011).
Kundrát, P. & Friedland, W. Track structure calculations on intracellular targets responsible for signal release in bystander experiments with transfer of irradiated cell-conditioned medium. Int. J. Radiat. Biol. 88, 98–102. https://doi.org/10.3109/09553002.2011.595874 (2012).
Kundrát, P. & Friedland, W. Mechanistic modelling of radiation-induced bystander effects. Radiat. Prot. Dosimetry. 166, 148–151. https://doi.org/10.1093/rpd/ncv170 (2015).
Hannafon, B. N. & Ding, W. Q. Intercellular communication by exosome-derived microRNAs in cancer. Int. J. Mol. Sci. 14, 14240–14269. https://doi.org/10.3390/ijms140714240 (2013).
Jella, K. K. et al. Exosomes, their biogenesis and role in inter-cellular communication, tumor microenvironment and cancer immunotherapy. Vaccines 6, 69. https://doi.org/10.3390/vaccines6040069 (2018).
Dai, X. et al. AHIF promotes glioblastoma progression and radioresistance via exosomes. Int. J. Oncol. 54, 261–270. https://doi.org/10.3892/ijo.2018.4621 (2019).
Mothersill, C., Saroya, R., Smith, R. W., Singh, H. & Seymour, C. B. Serum serotonin levels determine the magnitude and type of bystander effects in medium transfer experiments. Radiat. Res. 174, 119–123. https://doi.org/10.1667/RR2036.1 (2010).
Mothersill, C. et al. A laboratory inter-comparison of the importance of serum serotonin levels in the measurement of a range of radiation-induced bystander effects: overview of study and results presentation. Int. J. Radiat. Biol. 88, 763–769. https://doi.org/10.3109/09553002.2012.715795 (2012).
Chapman, K. L., Al-Mayah, A. H., Bowler, D. A., Irons, S. L. & Kadhim, M. A. No influence of serotonin levels in foetal bovine sera on radiation-induced bystander effects and genomic instability. Int. J. Radiat. Biol. 88, 781–785. https://doi.org/10.3109/09553002.2012.710926 (2012).
Funding
This research was funded by the Czech Science Foundation, grant number 21–06451S.
Author information
Authors and Affiliations
Contributions
Conceptualization, M. D., P. K., V. V., and A. J. M.; methodology, M. D. and A. J. M.; investigation, O. Z., P. B., I. D., A. J. M., J. V., J. Š., M. N., L. D. and M.D.; MC simulations—J. Š.; data curation, I. D., A. J. M. and O. Z.; data analysis, P. K.; writing—original draft preparation, P. K.; writing—review and editing, K. P. B., M. D., A. J. M. and P. K.; supervision and project administration, M. D. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zahradníček, O., Kundrát, P., Danilova, I. et al. Variations among glioblastoma cell lines in boron-mediated enhancement of cell killing by proton beams. Sci Rep 15, 29453 (2025). https://doi.org/10.1038/s41598-025-14658-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14658-w