Abstract
While the perihematomal region of a spontaneous intracerebral hemorrhage (ICH) provides a potential therapeutic target, the pathophysiological processes in that region remain unclear. To evaluate the nature, extent and localization of perihematomal changes, we investigated glucose uptake, water diffusion, and cell counts in the acute phase of collagenase-induced ICH. Sprague-Dawley rats were intrastriatally injected with collagenase or saline (control group). 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) PET, T2-weighted and diffusion-weighted MRI were performed at baseline (n = 30) and day 1 (n = 28), 3 (n = 14) and 7 (n = 14) after injection. Astrocytes, microglia and neurons in the piriform cortex were quantified using immunohistochemistry on brain slices obtained at day 2 (n = 14) or 8 (n = 14) after injection. Decreased [18F]FDG uptake and increased ADC values were seen in the perihematomal region, and were most pronounced on day 1 and 3 in the piriform cortex and basal ganglia. Significantly less neurons and more glia were found ipsilateral compared to contralateral at day 2 and 8 (p < 0.05), with no significant difference between timepoints. The unchanged glial and neuronal numbers are in contrast to the [18F]FDG uptake that partially recovered over time, suggesting that the absolute numbers of cells do not correlate directly to the perihematomal changes in glucose metabolism following ICH.
Similar content being viewed by others
Introduction
Spontaneous intracerebral hemorrhage (ICH) accounts for 15% of all strokes with an estimated incidence of 26.47 per 100 000 person-years1 and is associated with high mortality2,3. Currently, effective therapies for ICH are lacking and therapeutic strategies are limited to blood pressure lowering interventions and optimal medical management, besides neurosurgical interventions in selected patients. Unraveling the pathophysiological mechanisms underlying ICH may advance the development of new treatment options.
Primary brain damage develops within minutes to hours after ICH and is attributable to the mechanical pressure of accumulating blood in the brain parenchyma. Secondary injury occurs due to pathophysiological processes associated with the presence of intraparenchymal blood, such as edema, inflammation, oxidative stress, and metabolic dysfunction. Furthermore, hemoglobin-induced vasospasm can cause secondary ischemia, however, based on findings of decreased oxygen consumption, altered metabolite concentrations and disturbed glucose uptake in the perihematomal region (PHR), the existence of metabolically disturbed tissue rather than a pure ischemic region surrounding an ICH has been hypothesized4,5,6. Previous research using 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) PET in ICH has revealed varying results with regard to the presence of hyper- or hypometabolism in the PHR at different timepoints post ICH4,5,7,8.
A link between changes in glucose metabolism and edema following ICH has been hypothesized but has not been proven5. Presence of edema can be indicated by changes in the apparent diffusion coefficient (ADC), which is a quantitative MRI measure for the magnitude of diffusion within a tissue and is acquired through diffusion-weighted imaging (DWI). Increased ADC values typically indicate increased extracellular diffusion of water due to the presence of vasogenic edema, while decreased ADC values are indicative of cytotoxic edema1. Whether changes in [18F]FDG uptake and ADC changes following ICH occur simultaneously and/or in the same regions has not yet been determined.
The precise mechanisms and nature of altered glucose uptake or metabolism following ICH remain unclear. The association between post-ICH glucose uptake and diffusion changes is unclear, and it is not known whether neuronal cell death, decreased neuronal activity or the effects of inflammation play a role in [18F]FDG PET signal changes1,11. Immunohistochemical stainings for neuronal and glial cells in the regions of altered glucose uptake can be useful to investigate the cellular changes associated with glucose uptake changes7. Additionally, stainings for c-Fos may provide insight into neuronal cell activity14.
Animal models for ICH are useful in this line of research since they make it possible to compare imaging characteristics between the pre- and post-ICH brain and to histologically study the cellular layout of the post-mortem brain15. In this study, the rat collagenase model for ICH was used, in which an intraparenchymal injection of collagenase induces the breakdown of blood vessel walls and subsequent leakage of blood into the brain tissue17.
The aim of this study was to evaluate the nature, extent and localization of perihematomal glucose metabolism and diffusion changes in the acute phase of ICH in the rat collagenase model and to investigate underlying cellular changes using histology. We hypothesized that the PHR is heterogenous in nature, characterized predominantly by hypometabolism and increased diffusion due to edema.
Materials and methods
Ethical approval
This study followed all applicable institutional and/or national guidelines for the care and use of animals. All procedures were in accordance with the European guidelines (directive 2010/63/EU) and the protocol was approved by the local Animal Ethical Committee of Ghent University (ECD 19/80).
Animals
Thirty male Sprague-Dawley rats (Envigo, The Netherlands) of 10 weeks old and with a weight of 317 ± 26 g (mean ± SD) were included in this study. An overview of the experimental procedures can be found in Fig. 1. Animals were randomly allocated to the collagenase or saline group within batches of three using the randombetween() function in Microsoft Excel. The study is reported in accordance with the ARRIVE Essential 10 guidelines.
Timeline of experimental procedures. Animals were injected with collagenase (ncol=17) or saline (nsal=11) in the left striatum (D0). [18F]FDG PET, T2 and DWI were performed one day before (baseline, n = 30) and one day after (D1) injection (ncol=17, nsal=11). 14/28 (ncol=6, nsal=8) animals were perfused on day 2 (D2) following an odor stimulus. 14/28 (ncol=11, nsal=3) animals were additionally scanned at day 3 (D3) and 7 (D7) post injection and perfused at day 8 (D8). After perfusion, histological analysis was performed. Created in BioRender. Labo, 4. (2025) https://BioRender.com/8yw860j.
Animals were housed under controlled conditions (12 h/12 h light/dark cycle, 20–24 °C and 40–60% relative humidity. They were group-housed until the start of the experiments (baseline scans) where they were separated and housed individually until the end of the experiment. Animals received food and water ad libitum and were food deprived during the nights before [18F]FDG PET scanning to lower their blood glucose levels.
ICH induction
Animals were anesthetized with 2% isoflurane in medical O2 and stereotactically injected with 0.6 U collagenase (type VII-S, C2399, Sigma-Aldrich) dissolved in 0.7 µl saline (collagenase group, ncol=19), or 0.7 µl saline (control group, nsal=11) in the left striatum (0.5 AP, 3.5 ML relative to bregma, 6.0 DV relative to brain surface) at a flowrate of 0.14 µl/min using a Neuros syringe (model 7001, point style 4, Hamilton) and a Quintessential Stereotaxic Injector (Stoelting). After injection, the needle was left in place for 5 min to prevent backflow.
Scanning procedures
PET, and T2 and DWI MRI scans were acquired one day before (baseline; n = 30) and on day 1 (ncol=17, nsal=11), 3 (ncol=11, nsal=3) and 7 (ncol=11, nsal=3) after injection. Two of the animals included in the baseline scanning did not survive to day 1.
For PET imaging, 32.5 ± 7.8 MBq (mean ± SD) [18F]FDG was administered intravenously into a tail vein under short anesthesia (2% isoflurane in O2). After injection, animals were placed in a heated cage in a dark room to reduce tracer uptake in brown fat and Harderian glands. Sixty minutes after tracer administration, a 15-min static PET scan was performed (β-CUBE, MOLECUBES NV) under general anesthesia (2% isoflurane in O2) while body temperature was maintained at 37 °C. PET data were recorded in list-mode and iteratively reconstructed by an Ordered Subset Expectation Maximization (OSEM) algorithm18 using 30 iterations and 1 subset into a 196 × 196 × 384 matrix with 400 μm voxel size, with an energy window of 30% centered on the 511 keV photopeak.
TurboRARE T2-weighted and DWI MRI were performed 3 h after PET imaging under 2% isoflurane anesthesia on a 7 T system (PharmaScan 70/16, Bruker) using a transmit/receive volume coil with 40 mm inner diameter (Bruker) while maintaining body temperature and monitoring respiratory rate. After optimizing the magnetic field homogeneity, the following acquisition parameters were used for T2-weighted imaging: repetition time 3700 ms, echo time 37 ms, inplane slice resolution 109 × 109 μm², 30 contiguous slices of 600 μm thickness, matrix size 320 × 320, 4 averages, resulting in a total acquisition time of 9 min. DWI acquisitions were recorded using an encoding scheme of 32 gradient directions with a bvalue of 800 s/mm² and with 5 b0 images. Other DWI parameters were: repetition time 6250 ms, echo time 24 ms, inplane slice resolution 333 × 333 μm², 25 slices of 500 μm thickness, 600 μm interslice distance, 30 × 30 mm² field of view, 4 segments, 15 min total acquisition time.
Figure 1 depicts how many animals were scanned at the different timepoints. The numbers of included PET scans for analysis were: 28 at baseline, 23 (14 collagenase, 9 control) at day 1 and 10 (collagenase) at day 3 and 7. Included DWI scans: 29 at baseline, 26 (16 collagenase, 10 control) at day 1 and 10 (collagenase) at both day 3 and 7. Most control animals were included only for baseline and the first post-surgery scans before being euthanized on the second day after saline injection. These timepoints were deemed sufficient to confirm that the injection surgery itself does not affect imaging or histological outcomes. Details on the number of animals used for PET, MRI and histology and exclusion criteria are listed in Suppl. Table 1 (see supplementary material).
Perfusion and histology
Animals were euthanized on day 2 or day 8 post injection with an overdose of sodium pentobarbital (200 mg/kg i.p.) followed by transcardial perfusion with PBS and 4% paraformaldehyde (PFA). Afterwards, the brains were post-fixated in PFA for 24 h and cryoprotected in 30% sucrose solution. The brains were then snap-frozen in isopentane and stored at −80 °C. A cryostat (Leica) was used to make 40 μm axial sections.
In the region where PET images showed consistent decreased [18F]FDG uptake, slices were selected for immunofluorescence staining: GFAP + Iba-1 (day 2 and day 8 tissue), and NeuN + c-Fos (day 2 tissue) or NeuN only (day 8 tissue). One region where histological analysis was performed was chosen because it showed hypometabolism in all animals. This region, located in the piriform-insular region, was termed region of interest 1 (ROI1; see Fig. 2). Additionally to the already mentioned stainings, c-Fos protein expression, indicative of neuronal activation, was investigated in the piriform cortex (PC). This region was targeted because not only did it display pronounced hypometabolism in our study, but a previous study had linked lesion extension into and hemosiderin deposits inside the PC to post-ICH epileptic seizures1, highlighting its relevance for further investigation. The subregion of the PC that showed the most prominent c-Fos activation was termed ROI2 (see Fig. 2) and included in stereological quantification. It was located in the second layer of the PC.
Odor stimulus
To investigate neuronal activation in the PC, a region that is involved in the perception of smell, histological analysis of c-Fos protein expression in PC neurons following exposure to an odor stimulus was performed in the subset of animals euthanized on the second day after collagenase/saline injection (ncol=6, nsal=8). Those animals were exposed to an odor stimulus for 60 min and euthanized immediately after. The stimulus was delivered in a 30 × 30 × 50 cm transparent cage with a paper-covered wire-grid floor under which a cotton pad with 60 µl with Isoamyl Acetate (W205508, Sigma-Aldrich) had been placed 60 min before animal entry20,21.
Immunofluorescence
Immunofluorescence was performed as described in Larsen et al.22. For a blocking buffer, PBS with 0.4% Fish Skin Gelatin and 0.2% Triton X was used. As primary antibodies mouse anti-GFAP (1:1000, Dako, Z033401-2), rabbit anti-Iba-1 (1:500, Abcam, ab178846), mouse anti-NeuN (1:1000, Sigma-Aldrich, MAB377) and rabbit anti-c-Fos (1:1000, Abcam, ab192089) were used. As secondary antibodies goat anti-mouse Alexa Fluor 594 (1:1000, Abcam, ab15088) or goat anti-rabbit Alexa Fluor 488 (1:1000, Abcam, ab150113) were used. After nuclear DAPI staining, sections were mounted on a glass slide and coverslipped with Fluoroshield (Sigma-Aldrich).
Stereological quantification
Stereological quantification was performed by researchers blinded for the animal group (collagenase vs. saline). In tissue of day 2 (ncol=6, nsal=8) and 8 (ncol=10) after injection, GFAP, Iba-1 and NeuN positive cells were counted on five sections per animal in ROI1. c-Fos + NeuN double positive cells, as well as NeuN, GFAP and Iba-1 positive cells were additionally counted in ROI2 in the tissue of day 2 post injection. The fraction of neurons expressing c-Fos was calculated as a measure for odor-induced neuronal activation.
The numbers of cells in the ROIs were estimated by a random sampling stereological counting procedure, the optical fractionator method23using a computerized system (Stereologer, SRC Biosciences) and a Leica DM6 B upright fluorescent microscope (40x magnification/0.8 NA dry).
Analysis
Image analysis
Voxel-based analysis
PET images were normalized using a data-driven approach that reduces intra- and inter-subject variability as described in more detail in our previous work24. In summary, after co-registration to a normal [18F]FDG PET rat brain atlas, each image was voxel-wise divided by the normal rat brain template24. Histograms of the resulting ratio images were generated and the location of the peak of the histogram (i.e. the most prevalent ratio) was used as normalization factor for each individual image25.
Acquired DWI images were denoised and corrected for Gibbs ringing artefact in MRtrix326, and then corrected for eddy current, EPI and motion distortions in ExploreDTI27. Using the corrected DWIs, ADC maps were generated using MRtrix3.
Subsequent analyses were performed using MRTrix3 and are described in our previous work24. A normal [18F]FDG PET rat brain atlas and a normal ADC rat brain atlas were generated using the baseline [18F]FDG PET scans and baseline ADC maps, respectively. [18F]FDG PET images and ADC maps acquired post ICH were analyzed by calculating voxel-wise Z-scores: \(\:Z=\frac{{x}_{i}-{\mu\:}_{i}}{{\sigma\:}_{i}}\), where \(\:{x}_{i}\) represents the voxel value of a post-ICH image in voxel \(\:i\), and \(\:{\mu\:}_{i}\) and \(\:{\sigma\:}_{i}\) are the mean and standard deviation in the corresponding normal rat brain atlas in voxel \(\:i\), respectively. Voxels with a Z-score smaller than − 3 or larger than + 3 were considered as significantly altered [18F]FDG uptake or significantly altered ADC values compared to baseline.
Lesion extent and severity
Using PMOD (PMOD Technologies 3.405), Z-maps were overlayed on T2-weighted images. On the T2-weighted images, the ICH-lesion was delineated and its volume calculated. On the Z-maps, regions of significantly altered [18F]FDG uptake and regions of significantly altered ADC values that were situated around the ICH-lesion were delineated separately. Next, the volumes and mean Z-scores of these perihematomal regions were calculated, representing perihematomal lesion extent and severity respectively. Additionally, the mean Z-scores within ROI1 and ROI2 (see Fig. 2) were calculated.
Region-based analysis
The [18F]FDG uptake and ADC values were analyzed in ROI1, ROI2 and the following brain structures: basal ganglia (BG), corpus callosum (CC), PC, other olfactory areas (i.e. the olfactory tubercle and lateral olfactory tract), amygdala, insular area, somatosensory cortex, and motor cortex. These structures were identified on T2-weighted MR images using the multidimensional rat brain atlas developed by Johnson et al.28which was based on the Paxinos-Watson stereotaxic rat brain atlas29. The previously delineated ICH-lesion was excluded from these structures. The relative volume of each brain structure that showed significantly altered [18F]FDG uptake or significantly altered ADC values on the Z-maps was calculated by dividing the significantly altered volume by the total volume of each brain structure.
Statistical analysis
Volumes of the PHR and ICH-lesions were compared between timepoints by paired t-tests (including only subjects where data was available at both timepoints) and the p-values were adjusted for multiple comparisons using Bonferroni correction. Linear mixed models using brain structure, group and time as fixed factors were performed to analyze the relative volumes of significant [18F]FDG uptake or ADC alterations for each of the eight selected brain structures. Additionally, the volumes and mean Z-scores of the perihematomal regions that showed significantly altered [18F]FDG uptake and significantly altered ADC values (extent and severity) were correlated with each other using Pearson correlation.
Stereological cell counts in ROI1 and ROI2 were analyzed by linear mixed model analysis with group and hemisphere as fixed factors and Bonferroni corrected. The mean [18F]FDG uptake Z-scores in ROI1 and ROI2 were correlated with the neuronal number in these regions using Pearson correlation.
Results
[18F]FDG PET and ADC analysis
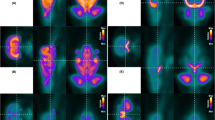
Example preprocessed PET and ADC images of a representative ICH animal are depicted in Fig. 3. PET data are displayed in standard uptake values.
Voxel-based analysis
The volume of the hematoma in the collagenase group did not differ between day 1 (21.26 ± 3.06 mm³, mean ± SE) and day 3 (21.56 ± 3.02 mm³). There was a significant decrease in ICH-volume at day 7 (14.50 ± 2.99 mm³) compared to day 3 post ICH (p = 0.003) (Suppl. Figure 1).
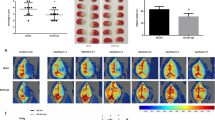
Statistical voxel-based analysis of [18F]FDG PET images revealed a region of significantly decreased [18F]FDG uptake around the ICH-lesion in all ICH animals. An example of such a PHR can be found in Fig. 4a. The volume of this region was larger at day 1 and 3 (49.87 ± 4.15 mm³ and 51.38 ± 9.32 mm³ resp.) compared to day 7 post ICH (10.99 ± 2.59 mm³) (D1 vs. D7: p = 0.003, D3 vs. D7: p = 0.003) (Fig. 4b). The mean (negative) Z-score in this region increased over time (D1 vs. D3: p = 0.010; D1 vs. D7: p = 0.003; D3 vs. D7: p = 0.002) (Suppl. Figure 2a), indicating decreased lesion severity. In control animals, only a very small number of voxels, scattered across the brain, had a Z-score lower than − 3.
(a) Example of a delineated hypometabolic PHR acquired from [18F]FDG PET voxel-based analysis (upper panel) and example of an increased diffusion region acquired from ADC map voxel-based analysis (lower panel) at day 1 post ICH, overlayed on T2-weighted images. The ICH is delineated in white. (b-c) Volumes of the (b) hypometabolic regions and (c) increased diffusion regions in ICH animals over time. Paired t-tests were performed between the different timepoints, excluding subjects for which data was not available at both timepoints. Error bars represent the standard error of the mean. *p < 0.05,**p < 0.01,***p < 0.001.
The same statistical voxel-based analysis of ADC maps revealed PHRs of significantly increased ADC value in all ICH animals (example in Fig. 4a). The volumes of these regions were significantly larger at day 1 (20.93 ± 2.66 mm³) compared to day 3 and 7 post ICH (7.75 ± 2.37 mm³ and 1.67 ± 0.58 mm³, resp.) (D1 vs. D3: p = 0.036, D1 vs. D7: p < 0.001) (Fig. 4c). The mean (positive) Z-score in this region was significantly lower at day 3 compared to day 1 (p = 0.001), indicating improvement in lesion severity (Suppl. Figure 2b). In control animals, no regions of increased diffusion were seen.
There were no significant correlations between the volumes of hypometabolism and increased diffusion at any of the timepoints, nor was there a significant correlation between the mean PET and mean ADC Z-score within the delineated hypometabolic region (p > 0.05).
Region-based analysis
Figure 5a shows the eight selected brain regions on a T2-weighted MRI of a normal rat brain. The relative volumes of each brain structure showing significantly decreased glucose metabolism or significantly increased diffusion at the ipsilateral side of ICH are plotted in Fig. 5b-e. In all brain regions, there was a significant increase in the number of voxels with significantly decreased glucose metabolism and increased diffusion between control and ICH animals at day 1 (Fig. 5b-c). These volumes were largest at day 1 or 3 post ICH in all regions except for the motor cortex (Fig. 5d-e).
(a) Eight brain structures delineated on a T2-weighted image of a normal rat brain used in subsequent analysis. (b-c) Volume percentages of each of the eight brain structures showing (b) significant hypometabolism and (c) significantly increased diffusion in ICH and control animals at day 1. (d-e) Volume percentages of each of the eight brain structures showing (d) significant hypometabolism and (e) significantly increased diffusion at the different timepoints. Error bars represent the standard error of the mean. *p < 0.05,**p < 0.01,***p < 0.001.
The PC and the BG contained the highest numbers of voxels with significantly lower glucose metabolism relative to their total voxel count (volume percentage of hypometabolism), with linear mixed models revealing that their volume percentages were significantly higher compared to all remaining regions at day 1 post ICH (p < 0.05). Also the amygdala, insula, and other olfactory areas contained large relative volumes of hypometabolism, although the differences to smaller regions were not statistically significant.
Similarly, the highest numbers of voxels with significantly increased diffusion relative to their total voxel count (volume percentage of increased diffusion) were found in the PC, BG, and CC. Linear mixed model analysis at day 1 revealed that the BG had a significantly higher volume percentage of increased diffusion than all the remaining regions (p < 0.01). The CC showed a significantly higher volume percentage compared to all the other structures (p < 0.05) apart from the other olfactory areas, while the PC showed significant differences only compared to the somatosensory and motor cortices (p < 0.001).
As mentioned earlier, analysis was additionally focused on two ROIs: ROI1, which showed a decreased glucose metabolism in all (14/14) ICH animals at day 1, and ROI2, which was situated in layer II of the PC and showed the most prominent c-Fos activation upon odor exposure. ROI1 showed an increase in diffusion in 25% (4/16) of the animals at day 1 post ICH. ROI2 showed a decrease in glucose metabolism in 93% (13/14) of the animals and increased diffusion in 12.5% (2/16) of the animals at day 1. The relative volumes of these ROIs showing decreased glucose metabolism or increased diffusion are visualized in Suppl. Figure 3 in the supplementary data.
Histology
Representative examples of the different stainings can be found in Fig. 6.
In ROI1, the number of NeuN positive cells was lower ipsilaterally to the ICH compared to contralaterally at both timepoints (D2 and D8: p < 0.001) and compared to ipsilateral control tissue from day 2 post ICH at both timepoints (D2: p = 0.002, D8: p = 0.011) (Fig. 7c). Additionally, the numbers of GFAP positive astrocytes and Iba-1 positive microglia was higher in ROI1 at the ipsilateral side of the ICH compared to contralaterally at both timepoints (GFAP D2: p < 0.001, D8: p = 0.001; Iba-1 D2 and D8: p < 0.001) and compared to ipsilateral control tissue from day 2 post ICH (GFAP D2: p = 0.007, D8: p = 0.066; Iba-1 D2 and D8: p < 0.001) (Fig. 7a-b). No differences were found between day 2 and day 8 in cell numbers in any of the stainings.
In ROI2 there were higher numbers of astrocytes and microglia (both: p < 0.001), while neurons were lower in number (p < 0.01) ipsilaterally to the ICH compared to contralaterally as well as to the ipsilateral side in control tissue from day 2 (Suppl. Figure 4). No significant differences were found in the c-Fos/NeuN ratio in the ipsilateral ROI2 between the two groups at day 2 (p = 0.675) (Fig. 7d).
No significant correlation was found between the number of neurons and the degree of hypometabolism in both ROIs.
Discussion
In this study, we investigated perihematomal glucose metabolism as well as diffusion changes in the acute phase of collagenase-induced ICH in rats using voxel- and region-based analysis of [18F]FDG PET images and ADC maps, respectively, and associated cellular changes. We found perihematomal hypometabolism that was for the largest parts located in the PC and BG. In the hypometabolic region, we found a decreased number of neurons and an increased number of astrocytes and microglia. The volume of the hypometabolic region decreased over time along with the hematoma, while the differences in cell counts remained. Regions of increased diffusion were seen surrounding the ICH, mostly located in the BG, PC and CC, but no correlation was found with the hypometabolic region.
In our study, hypometabolism was revealed in regions close to the induced hematoma, which is in line with what we had hypothesized. Results of previous studies investigating changes in perihematomal [18F]FDG uptake are contradictory, with some reporting hypo- and others reporting hypermetabolism. Lin et al. found hypometabolism in a perihematomal rim in a cat ICH model 2–12 h following striatal ICH induction, which normalized 120 h post ICH5. An [18F]FDG PET study in mice by Han et al. revealed striatal hypometabolism at day 1 and 3, and hypermetabolism at day 7 and 14 post ICH7. In contrast, Zazulia et al. found perihematomal regions of hypermetabolism in 6/13 ICH patients 2.9 ± 0.8 days after ICH onset using [18F]FDG PET4. Hypermetabolism was also seen in the striatum on autoradiographic slices in the autologous blood rat model at 3 h post ICH, but this was no longer seen 8–72 h post ICH6. These contrasting findings may be explained by differences in methodology such as differences in timepoints and animal species/models, and the lack of control or baseline scans4,6.
A decreased number of neurons and increased numbers of astrocytes and microglia were found at day 2 and day 8 post ICH induction in the hypometabolic PHR which was expected based on previous findings13,30,31,32. However, neuronal cell loss did not correlate with the hypometabolism found in the PHR over time. Since our results show a partial recovery of hypometabolism as indicated by the decrease in hypometabolic volume by day 7 post ICH induction without associated changes in neuronal numbers, it seems likely that other processes play a role in the perihematomal hypometabolism and its recovery.
A study by Han et al. suggested that post-ICH glucose hypermetabolism after an initial hypometabolism might be caused by increased inflammatory cell activity7. However, the unchanged numbers of astrocytes and microglia between day 2 and 8 in our study do not support that idea. Oxidative stress has been linked to glucose hypometabolism caused by glyceraldehyde 3-phosphate dehydrogenase inactivation3. Excessive gamma-aminobutyric acid (GABA) production by reactive astrocytes1 or conversion of extracellular adenosine triphosphate to adenosine by microglia1 might suppress neuronal activity due to the inhibitory effects of these neurotransmitters. However, we found no difference in the proportion of c-Fos positive neurons between ICH and control animals in the ipsilateral piriform region upon presentation of an odor stimulus, indicating that the remaining neuronal cells in the hypometabolic region can still be activated.
Besides neuronal loss or dysfunction, other possible mechanisms have been postulated for the occurrence of alterations in metabolism following ICH. A mouse study by Han et al. revealed that post-ICH glucose hypometabolism shown by PET was associated with decreased GLUT1 expression and with damage to striatal blood vessels7. Since GLUT1 is mainly present on endothelial and astrocytic cells, bloodbrain barrier damage in the early stages post ICH may account for decreased GLUT1 and, possibly, hypometabolism. They also found decreased astrocytic GLUT1 mRNA at day 1 and hypothesized that increased astrocyte reactivity over time may accompany increased GLUT1 in astrocyte end-feet during metabolic recovery and subsequent hypermetabolism.
In our study, increased perihematomal diffusion was found, which was most pronounced in the BG, PC and CC, and followed a similar time course as the metabolic changes, i.e. reaching its maximum at day 1 post ICH induction and partially recovering by day 7. Increased ADC values have previously been shown to indicate the presence of vasogenic edema9,10. We did not find perihematomal regions of restricted diffusion which would indicate cytotoxic edema or ischemia. The slightly large voxel size of our DWI images (333 × 333 × 500 μm³) and strict thresholding at │Z│>3 may have limited the finding of (possibly less pronounced) regions of hypoperfusion. Thus, cytotoxic edema may be present in ICH to a lesser degree than vasogenic edema34. Little is known about a possible relationship between edema formation and hypometabolism following ICH onset5. There seemed to be some spatial overlap of the hypometabolic regions and increased diffusion regions, since both were commonly present in the PC and BG, but we found no correlation between the volumes of these regions or between the mean PET and ADC Z-score in the delineated hypometabolic volume. Evidence of secondary ischemia following ICH, which could be caused by arterial compression and vasoconstriction, is contradictory35,36. While transient ischemia could explain the presence and recovery of hypometabolism, the absence of regions with decreased ADC values in our study does not support that idea.
While the CC displayed increased diffusion after ICH, little to no hypometabolism was observed in that region. As the CC primarily consists of myelinated axons, it displays an inherently low baseline glucose metabolism, in contrast to gray matter where glucose demand and uptake are highest. This could explain our finding of little to no hypometabolism in the CC.
To further investigate changes in diffusion, analyzing fractional anisotropy (FA) values could be of interest. While ADC values reflect the overall extent of diffusion within a tissue, FA values provide insight into its directional properties. However, since our study primarily focused on changes in glucose metabolism and did not reveal significant correlations between hypometabolism and increased diffusion in the PHR, we did not further explore perfusion-related changes.
Unlike initially planned, no control animals were included in the analysis beyond day 2 post-surgery. During experiments, a small group of three animals was included until the last timepoint, but the exclusion of one of these animals (due to the presence of a lesion) further reduced that number. Consequently, control animals were omitted from imaging analysis on days 3 and 7, and histology on day 8. Instead, baseline scans and contralateral tissue from ICH animals served as controls at these timepoints. The findings in control animals included for imaging at day 1 (see Fig. 5b-c) and for histological analysis (see Fig. 7a-c) on day 2 indicate that the surgical procedure and saline injection do not induce changes in cerebral glucose metabolism, ADC values, and cell counts.
To reduce variance in the data and thus increasing statistical power while minimizing sample size, the present study only included male animals. In terms of cerebral [18F]FDG uptake, there is evidence that female rats display greater variance compared to male counterparts37. Data regarding differences between male and female ICH populations in terms of incidence, pathophysiology, and outcome are scarce and findings are contradicting38,39,40,41,42. Studies in murine ICH models have suggested a positive relation between female sex and recovery overall, which can be attributed to different effects of gonadal hormones39,43,44,45. To further investigate these sex-related differences and to achieve a better representation of the human ICH population, one should consider including both sexes in future studies.
This study comes with some limitations. One limitation is that histological analyses were performed one day after PET and DWI scanning due to practical reasons. Since brain metabolism and edema development are dynamic processes5,46we cannot rule out that this could have influenced our results. Furthermore, it is not clear if and to what extent single-housing might have influenced our findings. It is well known that isolation induces stress in social animals like rats, but the severity and effects of that induced stress caused by isolation periods less than two weeks in adult rats are understudied47,48. We chose for individual housing to avoid the animals from injuring each other after surgery, promoting recovery, and to facilitate video-monitoring of post-ICH epileptic seizures (data not included in this manuscript). Due to the short duration of a maximum of ten days of isolated housing we expect little to no influence of this housing condition on our results. Another limitation is related to the use of an external brain template and atlas28 to define the volumes of interest for region-based image analysis. While both study cohorts included only male animals, they differed in weight (250 g vs. 317 g on average) and strain (Wistar vs. Sprague-Dawley). Subtle anatomical differences between these cohorts lead to suboptimal co-registration and delineation, such as overestimation of the amygdala, which included parts of the preoptic nucleus and the interstitial nucleus of the posterior limb of the anterior commissure. However, since the regions of interest were generally large, the impact of these inconsistencies is expected to be negligible. Finally, our results in the rat collagenase model may not be fully extrapolatable to the human situation. The collagenase model mimics human ICH through the presence of blood vessel rupture and the possibility of hematoma expansion. However, collagenase in itself has been suggested to cause an inflammatory reaction, although no hard evidence exists to support this theory49,50.
To summarize, we described perihematomal [18F]FDG PET and ADC changes over time in the rat collagenase model, combined with a quantitative histological analysis. We found perihematomal decreased glucose metabolism, increased diffusion, loss of neurons and increased numbers of microglia and astrocytes the first days after ICH, extending to brain structures surrounding the lesion such as the PC and BG. While [18F]FDG uptake and ADC values partially recovered over time, changes in the number of neurons, astrocytes and microglia did not. Thus there seems to be no direct correlation between hemorrhage induced changes in glucose uptake or diffusion and changes in the absolute number of neurons/glial cells.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Li, X., Zhang, L., Wolfe, C. D. A. & Wang, Y. Incidence and Long-Term survival of spontaneous intracerebral hemorrhage over time: A systematic review and Meta-Analysis. Front Neurol 13 (2022).
Gross, B. A., Jankowitz, B. T. & Friedlander, R. M. Cerebral intraparenchymal hemorrhage: A review. JAMA 321, 1295 (2019).
Ikram, M. A., Wieberdink, R. G. & Koudstaal, P. J. International epidemiology of intracerebral hemorrhage. Curr. Atheroscler Rep. 14, 300–306 (2012).
Zazulia, A. R., Videen, T. O. & Powers, W. J. Transient focal increase in perihematomal glucose metabolism after acute human intracerebral hemorrhage. Stroke 40, 1638–1643 (2009).
Lin, X. et al. Cerebral glucose metabolism: influence on perihematomal edema formation after intracerebral hemorrhage in Cat models. Acta Radiol. 51, 549–554 (2010).
Ardizzone, T. D. et al. Glutamate receptor Blockade attenuates glucose hypermetabolism in perihematomal brain after experimental intracerebral hemorrhage in rat. Stroke 35, 2587–2591 (2004).
Han, X., Ren, H., Nandi, A., Fan, X. & Koehler, R. C. Analysis of glucose metabolism by 18F-FDG-PET imaging and glucose transporter expression in a mouse model of intracerebral hemorrhage. Sci. Rep. 11, 10885 (2021).
Puy, L. et al. Intracerebral Hemorrhage-Induced cognitive impairment in rats is associated with brain atrophy, hypometabolism, and network dysconnectivity. Front. Neurosci. 16 (2022).
Huisman, T. A. G. M. Diffusion-weighted imaging: basic concepts and application in cerebral stroke and head trauma. Eur. Radiol. 13, 2283–2297 (2003).
Fainardi, E. et al. Temporal changes in perihematomal apparent diffusion coefficient values during the transition from acute to subacute phases in patients with spontaneous intracerebral hemorrhage. Neuroradiology 55, 145–156 (2013).
Nam, M. H. et al. Excessive astrocytic GABA causes cortical hypometabolism and impedes functional recovery after subcortical stroke. Cell. Rep. 32, 107861 (2020).
Badimon, A. et al. Negative feedback control of neuronal activity by microglia. Nature 586, 417–423 (2020).
Shang, H. et al. The radical scavenger Edaravone improves neurologic function and perihematomal glucose metabolism after acute intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 24, 215–222 (2015).
Joo, J. Y., Schaukowitch, K., Farbiak, L., Kilaru, G. & Kim, T. K. Stimulus-specific combinatorial functionality of neuronal c-fos enhancers. Nat. Neurosci. 19, 75–83 (2016).
Van Der Staay, F. J., Arndt, S. S. & Nordquist, R. E. Evaluation of animal models of neurobehavioral disorders. Behav. Brain Funct. 5, 11 (2009).
Webb, E., Yuan, M., Lemoine, R., Wang, Y. & N. & Imaging in animal models. Integr. Cancer Sci. Th. 3, 428–431 (2016).
Rosenberg, G. A., Mun-Bryce, S., Wesley, M. & Kornfeld, M. Collagenase-induced intracerebral hemorrhage in rats. Stroke 21, 801–807 (1990).
Liu, C. & Qiu, J. Ordered subset expectation maximum algorithms based on symmetric structure for image reconstruction. Symmetry 10, 449 (2018).
Germonpré, C. et al. Seizures and interictal epileptiform activity in the rat collagenase model for intracerebral hemorrhage. Front. Neurosci. 15, 682036 (2021).
Illig, K. R. & Haberly, L. B. Odor-evoked activity is spatially distributed in piriform cortex. J. Comp. Neurol. 457, 361–373 (2003).
Bamji-Stocke, S., Biggs, B. T. & Samuelsen, C. L. Experience-dependent c-Fos expression in the primary chemosensory cortices of the rat. Brain Res. 1701, 189–195 (2018).
Larsen, L. E. et al. Hippocampal seizures differentially modulate locus coeruleus activity and result in consistent time-locked release of noradrenaline in rat hippocampus. Neurobiol. Dis. 189, 106355 (2023).
Schmitz, C. & Hof, P. R. Design-based stereology in neuroscience. Neuroscience 130, 813–831 (2005).
Proesmans, S. et al. Voxel-Based analysis of [18F]-FDG brain PET in rats using Data-Driven normalization. Front. Med. 8, 744157 (2021).
López-González, F. J. et al. Intensity normalization methods in brain FDG-PET quantification. NeuroImage 222, 117229 (2020).
Tournier, J. D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 202, 116137 (2019).
Leemans, A., Jeurissen, B., Sijbers, J. & Jones, D. ExploreDTI: a graphical toolbox for processing. Proc. Intl. Soc. Mag. Reson. Med. (2009).
Johnson, G. A., Calabrese, E., Badea, A., Paxinos, G. & Watson, C. A multidimensional magnetic resonance histology atlas of the Wistar rat brain. NeuroImage 62, 1848–1856 (2012).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition (Elsevier, 2006).
Matsushita, K. et al. Evidence for apoptosis after intracerebral hemorrhage in rat striatum. J. Cereb. Blood Flow. Metab. 20, 396–404 (2000).
Qureshi, A. I. et al. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery 52, 1041–1048 (2003).
Li, Q. et al. Ultrastructural characteristics of neuronal death and white matter injury in mouse brain tissues after intracerebral hemorrhage: coexistence of ferroptosis, autophagy, and necrosis. Front. Neurol. 9, 581 (2018).
Malkov, A. et al. Seizure-induced reduction in glucose utilization promotes brain hypometabolism during epileptogenesis. Neurobiol. Dis. 116, 28–38 (2018).
Ironside, N., Chen, C. J., Ding, D., Mayer, S. A. & Connolly, E. S. Perihematomal edema after spontaneous intracerebral hemorrhage. Stroke 50, 1626–1633 (2019).
Wilkinson, D. A. et al. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology 134, 240–248 (2018).
Zazulia, A. R. et al. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J. Cereb. Blood Flow. Metab. 21, 804–810 (2001).
Sijbesma, J. W. A. et al. Test-Retest stability of cerebral 2-Deoxy-2-[18F]Fluoro-D-Glucose ([18F]FDG) positron emission tomography (PET) in male and female rats. Mol. Imaging Biol. 21, 240–248 (2019).
Puy, L. et al. Intracerebral haemorrhage. Nat. Rev. Dis. Primers. 9, 1–18 (2023).
Gokhale, S., Caplan, L. R. & James, M. L. Sex differences in incidence, pathophysiology, and outcome of primary intracerebral hemorrhage. Stroke 46, 886–892 (2015).
van Asch, C. J. et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 9, 167–176 (2010).
Roquer, J. et al. Sex-related differences in primary intracerebral hemorrhage. Neurology 87, 257–262 (2016).
Umeano, O. et al. Gender and age interact to affect early outcome after intracerebral hemorrhage. PLOS ONE. 8, e81664 (2013).
Chen, Z., Xi, G., Mao, Y., Keep, R. F. & Hua, Y. Effects of progesterone and testosterone on ICH-Induced brain injury in rats. In Intracerebral Hemorrhage Research: from Bench To Bedside (eds Zhang, J. & Colohan, A.) 289–293 (Springer, 2011). https://doi.org/10.1007/978-3-7091-0693-8_48.
Li, H. et al. Sex differences in cell death. Ann. Neurol. 58, 317–321 (2005).
Hsieh, J. T. et al. Sex-Specific effects of progesterone on early outcome of intracerebral hemorrhage. Neuroendocrinology 103, 518–530 (2016).
Hua, Y. et al. Behavioral tests after intracerebral hemorrhage in the rat. Stroke 33, 2478–2484 (2002).
Manouze, H. et al. Effects of Single Cage Housing on Stress, Cognitive, and Seizure Parameters in the Rat and Mouse Pilocarpine Models of Epilepsy. eNeuro 6 (2019).
Mumtaz, F., Khan, M. I., Zubair, M. & Dehpour, A. R. Neurobiology and consequences of social isolation stress in animal model—A comprehensive review. Biomed. Pharmacother. 105, 1205–1222 (2018).
MacLellan, C. L., Paquette, R. & Colbourne, F. A. Critical appraisal of experimental intracerebral hemorrhage research. J. Cereb. Blood Flow. Metab. 32, 612–627 (2012).
Manaenko, A., Chen, H., Zhang, J. H. & Tang, J. Comparison of different preclinical models of intracerebral hemorrhage. In Intracerebral Hemorrhage Research: from Bench To Bedside (eds Zhang, J. & Colohan, A.) 9–14 (Springer, 2011). https://doi.org/10.1007/978-3-7091-0693-8_2.
Acknowledgements
The authors would like to thank Joris Van Asselberghs and Veerle Baeckeland for the help and guidance during the stereological analysis. Graphs were created using GraphPad Prism version 10.4 (GraphPad Software, Boston, Massachusetts USA, www.graphpad.com).
Author information
Authors and Affiliations
Contributions
S.P., R.R., P.B., B.D., K.V., C.V., and V.D.H. designed the study. C.G. developed the animal model. S.P. performed the experimental work and data collection. S.P., M.L., R.R., C.V., and V.D.H. analyzed the data. S.P., M.L., R.R., and V.D.H. interpreted the results and wrote the initial manuscript. All authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
S.P. was supported by the Special Research Fund of Ghent University (BOF, 01D27418). M.L. was supported by the Flemish Research Foundation (FWO, G027422N). The other authors declare no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Proesmans, S., Lajtos, M., Raedt, R. et al. Perihematomal changes in glucose metabolism and cell counts in the rat collagenase model for intracerebral hemorrhage. Sci Rep 15, 29338 (2025). https://doi.org/10.1038/s41598-025-14689-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14689-3