Abstract
Tisotumab vedotin (TV), the first antibody–drug conjugate (ADC) targeting tissue factor, was granted approval by the Food and Drug Administration (FDA) for the treatment of recurrent or metastatic cervical cancer. However, its adverse events (AE) are mainly recorded in clinical trials, lacking of real-world data. The FDA Adverse Event Reporting System database was searched retrospectively, and the TV-related AE reports from July 2021 to September 2024 were extracted. At the system organ class (SOC) level, only eye disorders (reporting odds ratio [ROR] = 11.53, proportional reporting ratio [PRR] = 9.53, information component [IC] = 3.25, and empirical bayes geometric mean [EBGM] = 9.53) and blood and lymphatic system disorders (ROR = 2.31, PRR = 2.26, IC = 1.18, and EBGM = 2.26) satisfied all four algorithms concurrently. The most common AE associated with TV is peripheral neuropathy (ROR = 23.03), while symblepharon (ROR = 1094.23) exhibited the strongest signal strength in the ROR algorithm. Notably, we identified several new signals, including cataract (ROR = 8.8) and Stevens-Johnson syndrome (ROR = 9.91). The median onset time for AEs was 21 days (interquartile range [IQR)]: 5–70 days), with the majority of AEs occurring within the first month of TV treatment. In addition to the AEs that are consistent with the instructions, we also identified some unreported signals. This discovery is expected to provide more precise guidance for the clinical application of TV.
Similar content being viewed by others
Introduction
Although the human papillomavirus (HPV) vaccine has been widely spread in recent years, cervical cancer continues to pose a significant public health challenge, particularly in developing countries. In 2020, it was estimated that there were approximately 604,000 new cases and 342,000 deaths globally, making cervical cancer the fourth most common cancer among women worldwide1,2. It is worth noting that recurrent or metastatic cervical cancer (r/mCC) is linked to a poor prognosis and elevated mortality rates. According to the 2025 NCCN Clinical Practice Guidelines for Cervical Cancer (first edition), platinum-based combination therapy, the angiogenesis inhibitor bevacizumab, and/or immune checkpoint inhibitors are recommended as first-line treatments for patients with r/mCC. However, a significant number of patients experience metastasis or recurrence following first-line treatment, leading to restricted therapeutic options for those with r/mCC who have previously undergone platinum-based regimens3,4. Current chemotherapy, immunotherapy, and targeted therapy drugs remain insufficient to meet clinical needs at this stage.
Tisotumab vedotin (TV) is a new antibody–drug conjugate (ADC) targeting tissue factor. By targeting tissue factor antigen on cancer cells, the microtubule-disrupting agent monomethyl auristatin E is directly delivered to cancer cells. This new anti-tumor drug not only exhibits the potent cytotoxic effects characteristic of traditional chemotherapy agents but also possesses the tumor-targeting capabilities associated with antibody therapies. This dual action can significantly enhance both the efficacy and safety of treatment5. In September 2021, TV received accelerated approval from the Food and Drug Administration (FDA) for the treatment of adult patients with r/mCC with disease progression on or after chemotherapy6. In published phase I–III clinical trials, TV has demonstrated a notable advantage in terms of objective response rate (17.8–54.4%) and progression-free survival (3.1–6.9 months) among patients who progressed after first-line standard therapy7,8. At present, it has been selected as the NCCN Clinical Practice Guide for Cervical Cancer in 2025 (1st Edition), which is the first choice for second-line or follow-up treatment of r/mCC, and the recommended level is adjusted from 2a level to 1 level. Although clinical trials have confirmed the effectiveness of TV, it may bring complex security risks due to its unique “targeted drug delivery” mechanism. The mechanism of AEs remains unclear. Since TV is a tissue factor-directed ADC containing monomethyl auristatin E, its AEs are mostly related to its main components tissue factor and monomethyl auristatin E9,10,11. The safety analysis of prior clinical trials indicates that the most frequently reported adverse events(AEs) were nausea (54%), alopecia (39%), conjunctivitis (30%), fatigue (26%), and dry eye (23%)7,8,12,13,14,15,16. Based on previous clinical studies, FDA also issued a black-box warning for TV, suggesting that TV can cause changes in the corneal epithelium and conjunctiva, leading to visual changes, including severe visual loss and corneal ulcer. In addition, the approval of the drug is accelerated based on the data of a single-arm phase II study, and its safety needs to be confirmed through post-marketing monitoring17. Thus, it is very important to explore potential AE signals by using post-marketing data.
Previous studies on TV have primarily originated from clinical trials conducted under controlled conditions, which often involved limited sample sizes and follow-up periods. This may have resulted in the oversight of a range of AEs. Additionally, the time of onset of some TV-related AEs is unknown. This study aimed to investigate the safety profile of TV by utilizing the FDA Adverse Event Reporting System (FAERS) database. The focus was placed on identifying the types of reported AEs and uncovering any novel or unexpected safety concerns. These insights are invaluable for healthcare providers, patients, and regulatory agencies to ensure the safe and effective clinical use of TV.
Materials and methods
Data sources and processing
We acquired the American Standard Code for Information Interchange (ASCII) data file from the FAERS database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html) covering the period from July 2021 to September 2024. FDA has established the FAERS database as a publicly accessible database that compiles global adverse event reports to facilitate post-marketing drug safety monitoring18. The database includes not only the reports of AEs submitted to the FDA, but also the reports of medication error and product quality complaint leading to adverse events, which are usually submitted by pharmacists, health care professionals, consumers and other relevant personnel. The FAERS database consists of eight distinct file types: report sources (RPSR), demographic and administrative information (DEMO), drug information (DRUG), indications for use (INDI), start and end dates for reported drugs (THER), adverse events (REAC), patient outcomes (OUTC), and invalid reports (DELETED)19. In order to obtain all relevant reports, we identify all commodity names and common names through the PROD_AI field. After searching, we used TV and its brand name Tivdak to search the drug files. We implemented the primary suspect criterion as one of the essential screening criteria.
Data mining analysis
Descriptive analyses were conducted to comprehensively gather clinical characteristics from all TV-associated reports. Given that many fields in the database are not mandatory, we accounted for the impact of missing data and prioritized the analysis of available information, which included but was not limited to gender, age, weight, indications, cumulative dose, outcomes, concomitant medications, and reporting countries. Disproportionality analyses, such as the Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Information Component (IC), and Empirical Bayes Geometric Mean (EBGM), were utilized to detect drug-associated AEs occurring more frequently than expected20,21,22,23. This was accomplished by analyzing the proportions of specific AEs between the TV and all other medications24,25. The signal detection thresholds for each algorithm were established in accordance with authoritative methodologies, and the specific formulas and thresholds are detailed in Table 1. During the data processing phase, we categorized AEs utilizing preferred terms (PTs) in accordance with the Medical Dictionary for Regulatory Activities (MedDRA, version 27.1) to ensure uniformity in terminology. Additionally, we categorized these AEs based on the System Organ Class (SOC). The data processing and statistical analyses were performed utilizing MYSQL 8.0, Navicat Premium 15, Microsoft Excel 2019(Microsoft Corp., WA, United States), and GraphPad Prism 9.5.1 (GraphPad Software, CA, United States).
Results
Descriptive analysis
We analyzed 6,037,399 reports in the FAERS database from July 2021 to September 2024. After data cleaning, a total of 506 reports were identified as suitable for comprehensive analysis. Since each report may contain one or more AEs, 506 patients reported 1100 AEs in our study. The details regarding the data collection, interpretation, and analysis process are illustrated in Fig. 1. Table 2 describes the clinical features of TV cases in detail. Among the 506 reported cases of TV-related AE, women (72.73%) were more affected than men (2.57%), which was related to the indications of the drug. From the age analysis, the proportion of 45–64 years old is relatively high (13.44%). In 2021, 2022, 2023 and 2024, we received 14 TV-related reports (2.77%), 156 reports (30.83%), 181 reports (35.77%) and 155 reports (30.63%) respectively. Most of these reports came from the United States (88.34%). A total of 340 serious AE reports were recorded, including death, life-threatening outcomes, disability and permanent injuries. Among them, 103 reports indicated hospitalization, 187 reports pointed out other serious medical events, and 32 reports were marked as deaths.
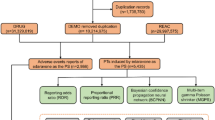
Flowchart of the screening process for tisotumab vedotin (TV)-related adverse events (AEs). A total of 6,037,399 demographic and administrative information (DEMO) was obtained from the FAERS database. After removing 812,645 duplicate reports, there are still 5,224,754 reports left. 506 suspicious drug AE reports were screened out from 20,472,084 drug AE reports, with tisotumab vedotin as the primary suspect drug (PS). From 15,745,197 preferred terminology for adverse events (REAC), 1100 AEs were screened using tisotumab vedotin as the PS. Finally, 506 AE reports were included in the analysis. DEMO, demographic and administrative information; DRUG, drug information; REAC, preferred terminology for adverse event; PS, primary suspect drug; AEs, adverse events.
Disproportionality analysis
SOC level
A total of 25 SOCs were recorded in AE reports related to TV. Figure 2 visually represents the distribution of AE reports frequencies across the various SOCs. It can be seen from Table 3 that, the SOC with the highest number of AEs and highest ROR values was eye disorders (209 reports, ROR 11.53, PRR 9.53, IC 3.25, and EBGM 9.53), besides, eye disorders and blood and lymphatic system disorders (42 reports, ROR 2.31, PRR 2.26, IC 1.18, and EBGM 2.26) are the SOCs that simultaneously meet all four criteria.
PT level
Among the 506 reports submitted, the disproportionality analysis identified 30 significant PTs that simultaneously satisfied all four calculation criteria. These 30 PTs were categorized into 10 SOCs; the distribution of AEs at the SOC level is presented in Table 4. It can be seen that, consistent with the previous clinical trial results and instructions, eye disorders are the most common system organs of AEs caused by TV treatment, with 15 PTs showing significant signals, among which symblepharon (ROR 1094.23) and ocular toxicity (ROR 889.82) have the strongest correlation. There is a strong correlation between anaemia (ROR 4.99) and febrile neutropenia (ROR 7.85) and TV in blood and lymphatic system disorders; In addition, there is a strong correlation between bleeding and TV, including vaginal haemorrhage (ROR 15.38), haematuria (ROR 11.2), cystitis haemorrhagic (ROR 42.46) and epistaxis (ROR 10.53); Among nervous system disorders, neuropathy peripheral (ROR 23.03) and peripheral motor neuropathy (ROR 201.08) are the main diseases that are significantly related to TV treatment.
Among these important PTs, 5 are not listed in the drug instructions, mainly focusing on eye disorders, blood and lymphatic system disorders, skin and subcutaneous tissue disorders. We have revealed some important PTs with high ROR, which are not listed in the specification, including cataract (ROR 8.8), febrile neutropenia (ROR 7.85), Stevens-Johnson syndrome (ROR 9.91), muscular weakness (ROR 5.44), liver function test increased (ROR 8.53).
As shown in the Fig. 3, except for peripheral motor neuropathy (ROR 201.08), the eye disorders are the most closely related to TV Furthermore, cystitis haemorrhagic(ROR 42.46), neuropathy peripheral (ROR 23.03), vaginal haemorrhage (ROR 15.38) and haematuria (ROR 11.20) also have strong correlation with TV.
Time-to-onset analysis
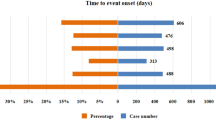
As shown in Fig. 4, We analyzed TV-induced 125 AEs using the Time-to-event onset (TTO) reported after excluding instances of missing data and reporting inaccuracies. The median time to onset was found to be 21 days, with an interquartile range (IQR) spanning from 5 to 70 days. it can be seen that most AEs in these patients(n = 79, percentage = 15.61%) happened within the initial month of TV usage in Fig. 4. Secondly, the proportions of reporting AEs in the second, third, and fourth months after the use of TV were 2.31%, 2.17%, and 1.98% respectively. This trend shows that with the passage of time, the possibility of AE after using TV will gradually decrease. It is worth noting that after 4 months of TV treatment, the risk of AE decreased, approaching a negligible level. We also analyzed onset time of SOCs individually in Fig. 5. It has been observed that the top five SOCs of AEs following treatment with TV are: general disorders and administration site conditions (median = 6.5), respiratory, thoracic and mediastinal disorders (median = 6.5), gastrointestinal disorders (median = 9.5), skin and subcutaneous tissue disorders (median = 11), and Injury, poisoning and procedural complications (median = 17). After administration of TV, the median onset time for the most frequently occurring AE-related eye disorders was 41.5 days, with an IQR from 18.75 to 127 days. Notably, AEs associated with nervous system disorders and hepatobiliary disorders occurred later, with median onset times of 83 and 99.5 days, respectively.
Discussion
TV is the first and only ADC approved by the FDA for second-line treatment of patients with r/mCC. It is also the first ADC targeting tissue factor in the world. In the past, the research on TV mainly focused on its mechanism of action, clinical trials and literature reviews, but the research on the real world was very limited. Therefore, the aim of this study is to interpret the potential AEs associated with TV, to inform the summary of product characteristics, and to delineate the safety profile of TV as a reference for clinical medication. In order to improve the accuracy of the analysis, four algorithms are used in this study, and the results are verified from multiple angles. Only when these four algorithms are satisfied can we think that a signal has been generated, so as to detect a more reliable safety signal26,27,28.
Our research found that female patients were dominant. Secondly, compared with other age groups, the 45–64 age group has a higher risk of AE, which is consistent with the established clinical indications of TV. It is worth noting that pharmacists and doctors (39.92 and 47.43%) submit the most AE reports, not the consumers themselves. This could be attributed to pharmacists’ and doctors’ greater familiarity with the reporting procedures, the clinical significance of AEs and the potential side effects of medications. Moreover, patients might fail to identify certain symptoms as AEs or may lack awareness of the reporting process. In addition, most of the reports are from the United States (88.34%), which may be because TV was first introduced in that country. Regarding the results of AEs, hospitalization and other serious AEs are the most common, except for unknown events. This is very important because it may be related to the indications of TV, because patients with r/mCC usually have poor prognosis.
According to the disproportional analysis, we determined 25 SOCs and 30 signals. Eye disorders are the most common SOC, and the most common PT are dry eye, eye disorder, ocular toxicity, keratitis, cataract, visual impairment, ocular congestion and ulcerative keratitis, which are consistent with previous studies. In the Innovate 204 trial, there were 138 secondary ocular AEs (53%) in 101 patients with r/mCC, most of which were Grade 1–2, and were confined to the ocular surface. Among them, 26% patients reported conjunctivitis, 23% patients reported dry eyes, 11% patients showed keratitis, and 2 (2%) patients were of grade 38. The same eye AEs were found in Innovate 206 trial13. Most eye AEs are not serious, which can be improved by reducing the dose or stopping treatment. At present, the mechanism of TV-related ocular toxicity is still unclear. Preclinical studies show that tissue factor is expressed in the conjunctiva, which may make eyes more sensitive to the potential toxicity of TV9,10. Secondly, some studies have found that TV-related ocular toxicity may be due to the cytotoxic load of aurastatin, such as monomethyl aurastatin E, which can cause off-target injury of corneal epithelial cells29. Therefore, it may be necessary to further study the mechanism. In addition, through consulting the previous clinical studies and instructions, it is found that cataract have not been reported, so we can focus on these AE in the future treatment.
In addition, besides eye disorders, blood and lymphatic system disorders are the only SOC that meets the criteria of the four algorithm. The common PT in blood and lymphatic system disorders are anemia and febrile neutropenia. Febrile neutropenia was identified as a new signal by four algorithms, which indicated that it had potential correlation with TV. However, according to the results of the study, no grade 4 neutropenia was observed in patients receiving TV+carboplatin treatment. On the contrary, in the GOG 240 trial, 26% of patients treated with cisplatin+paclitaxel had neutropenia above grade 430,31,32. Therefore, whether febrile neutropenia is related to the use of TV still needs further study.
Our study found that there is a strong correlation between bleeding and TV, including vaginal haemorrhage, epistaxis, haematuria and cystitis haemorrhagic, which is consistent with previous clinical studies and instructions. In the innovaTV 204 study, bleeding-related AEs were reported in 39 patients (39%) and were predominantly grades 1 or 2, with 34 patients (34%) experiencing grade 1 events, 3 patients (3%) experiencing grade 2 events, and 2 patients (2%) experiencing grade 3 events8. According to the TV instructions, 62% of cervical cancer patients treated with TV had bleeding events. The most common AEs were epistaxis (44%), haematuria (10%) and vaginal haemorrhage (10%), and 5% patients had third-degree bleeding. The higher incidence of epistaxis compared to other forms of bleeding associated with TV may be attributed to tissue factor expression in the nasal epithelium and the rich vascularization of the nose33,34,35. Vaginal hemorrhage and hematuria occurrences might be linked to underlying conditions such as local tumor growth or prior pelvic radiotherapy. In clinical use, standard intervention measures should be taken as needed to control any form of bleeding during the treatment of TV.
In our research, the nervous system disorders related to TV therapy are mainly neuropathy peripheral and peripheral motor neuropathy. Neuropathy peripheral occurred in 42% of patients with cervical cancer treated with TV across instructions. In the interim analysis of Innovate 301 presented at ESMO meeting in 2023, the proportion of AE related to peripheral neuropathy was 36% after TV treatment, of which 27% was peripheral sensory neuropathy, 3% was sensory abnormality, 2% was myasthenia, and 2% was peripheral sensory motor neuropathy. Monomethyl aurastatin E can induce peripheral neuropathy, which is a well-proved side effect of tubulin binding agents36. In terms of mechanism, monomethyl aurastatin E binds to tubulin with high affinity, which inhibits microtubule dynamics and destroys microtubule function by blocking tubulin polymerization37. In addition, monomethyl aurastatin E-bound tubulin inhibits microtubule assembly, leading to cell cycle arrest and subsequent apoptosis in G2-M phase38. The inhibition of microtubule-dependent axonal transport mediated by monomethyl aurastatin E increases the susceptibility to peripheral neuropathy, mainly due to the extensive length of axonal projections and the key role of the microtubule network in maintaining long-distance axonal transport between neuronal cell bodies and distal nerve endings39. The development of peripheral neuropathy may lead to prolonged infusion time or decreased dosage, which will have a negative impact on the treatment effect and the quality of life of patients. In severe cases, peripheral neuropathy may even endanger the life of patients. Therefore, when using TV, we should closely monitor the new symptoms or worsening symptoms of peripheral neuropathy in all patients treated with TV. Peripheral neuropathy related to TV can be managed by dose adjustment and dose reduction.
Skin and subcutaneous tissue disorders are also common AEs in the process of TV therapy. Our study found that skin rash, alopecia and Stevens-Johnson syndrome have a strong correlation with TV treatment. In previous clinical trials, alopecia was the most frequently reported AE related to skin and subcutaneous tissue disorders. Chemotherapies, particularly microtubule-disrupting agents, are known to cause high rates of alopecia40. Alopecia was observed in innovaTV 201 (treatment emergent, 22 patients, 40%) and innovaTV 204 (treatment related, grade 1, 13 patients; grade 2, 25 patients8,12; Stevens-Johnson syndrome has not been mentioned in previous clinical studies, and whether it is related to the treatment of TV needs further study.
The results of this study show that the median time of TV-related AE onset is 21 (IQR, 5–70) days, and most of them occur within the first month of TV treatment (n = 79,15.61%), but some AE may still occur within one year (n = 46,9.09%). Therefore, in future clinical trials, a longer follow-up period is needed to observe TV-related AEs. In the innovaTV 204 trial, the median time for the onset of eye disorder AEs was 1.4 months, peripheral neuropathy was 3.1 months, and bleeding-related AEs was 0.3 months8. These findings align with our research results, which reported a median time of 41.5 days for eye disorders and 83 days for nervous system disorders. Collectively, these results emphasize the importance for clinicians and pharmacists to closely monitor both labeled and potential AEs in patients, as they can be life-threatening, particularly during the first month of treatment.
Similar to prior studies utilizing pharmacovigilance databases, several limitations of the present study warrant acknowledgment. First of all, given the voluntary nature of reporting to the FAERS database, it is impossible to calculate the incidence or prevalence of AEs, and underreporting remains a significant concern. Secondly, the existence of reports in the FAERS database does not imply causal relationships; thus, the findings of this study merely indicate potential AEs, underscoring the need for vigilance among clinicians and pharmacists. Thirdly, many unmeasurable confounding factors, such as drug interactions, comorbidity and drug combinations, may affect AEs and are not considered in the data analysis. Fourthly, the AE report of TV may be affected by the processing before the TV is used, which may lead to misjudgment of the results. Fifthly, the long-term follow-up of AE was not analyzed in this study, which might be a limitation. Sixthly, this study only depends on the FAERS database in the United States. The sources of its reports and the results may vary according to race and region. Seventh, although this study provided information on the outcomes of AEs, it did not evaluate the severity of AEs. Therefore, the AEs can not be classified and analyzed according to their severity. In addition, the disproportional analysis did not quantify risk or determine the causal relationship, but estimates the signal strength, which is related to clinic. Consequently, prospective clinical trials remain essential to confirm causal associations.
Conclusion
TV was approved by the FDA in September, 2021, and was fully approved in April of 2024. During this transition period, it is necessary to use FAERS to continuously monitor AE. Based on the real world data in FAERS database, this study conducted a survey and determined the AE highly related to TV through disproportional analysis. The AEs detected in this study are largely consistent with those documented in the manual, and some potential AEs, such as cataract, febrile neutropenia, Stevens-Johnson syndrome, muscular weakness and liver function test increased have also been uncovered. Furthermore, this paper reports the median time for common systemic diseases, providing valuable references for clinicians to optimize drug usage and enhance the safety management of TV. Given the increasing use of TV, pharmacovigilance studies may play an important role in facilitating risk–benefit assessment through large real-world databases, especially for unanticipated AEs that are not documented by the label. In conclusion, our findings and management recommendations may improve clinicians/researchers awareness of TV-associated toxicity and help reduce risk.
Data availability
The original contributions put forward in this study is included in the article. Further inquiries can be made directly to the corresponding author(Name: Mei Guo; Email: 774208647@qq.com;Institution: Department of Obstetrics and Gynecology, Guangyuan Central Hospital, Guanyuan, China)
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
Zamorano, A. S. et al. Repeating platinum/bevacizumab in recurrent or progressive cervical cancer yields marginal survival benefits. Gynecol. Oncol. Rep. 22, 48–51 (2017).
McLachlan, J. et al. The impact of systemic therapy beyond first-line treatment for advanced cervical cancer. Clin. Oncol. 29(3), 153–160 (2017).
Khongorzul, P. et al. Antibody-drug conjugates: A comprehensive review. Mol. Cancer Res. 18(1), 3–19 (2020).
Arn, C. R., Halla, K. J. & Gill, S. Tisotumab vedotin safety and tolerability in clinical practice: Managing adverse events. J. Adv. Pract. Oncol. 14(2), 139–152 (2023).
de Bono, J. S. et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): A first-in-human, multicentre, phase 1–2 trial. Lancet Oncol. 20(3), 383–393 (2019).
Coleman, R. L. et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 22(5), 609–619 (2021).
Ando, R. et al. Tissue factor expression in human pterygium. Mol. Vis. 8(17), 63–69 (2011).
Cho, Y. et al. Evidence for enhanced tissue factor expression in age-related macular degeneration. Lab Invest. 91(4), 519–526 (2011).
Zhu, Y. et al. Treatment-related adverse events of antibody-drug conjugates in clinical trials: A systematic review and meta-analysis. Cancer 129(2), 283–295 (2023).
Hong, D. S. et al. Tisotumab vedotin in previously treated recurrent or metastatic cervical cancer. Clin. Cancer Res. 26(6), 1220–1228 (2020).
Yonemori, K. et al. Tisotumab vedotin in Japanese patients with recurrent/metastatic cervical cancer: Results from the innovaTV 206 study. Cancer Sci. 113(8), 2788–2797 (2022).
Vergote, I. et al. Tisotumab vedotin in combination with carboplatin, pembrolizumab, or bevacizumab in recurrent or metastatic cervical cancer: Results from the innovaTV 205/GOG-3024/ENGOT-cx8 study. J. Clin. Oncol. 41(36), 5536–5549 (2023).
Camarda, F. et al. Antibody drug conjugates in recurrent or metastatic cervical cancer: A focus on tisotumab vedotin state of art. Ther. Adv. Med. Oncol. 16, 17588359241277648 (2024).
U.S. Food and Drug Administration. NDA/BLA Multi-Disciplinary Review and Evaluation: BLA 761208; Tivdak, Tisotumab vedotin-tftv. Accessed Jul 2025. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/761208Orig1s000MultidisciplineR.pdf.
Markham, A. Tisotumab vedotin: First approval. Drugs 81(18), 2141–2147 (2021).
Kaland, D. A. et al. U.S. food and drug administration analysis of newly identified adverse events with lurbinectedin: Extravasation, rhabdomyolysis, and tumor lysis syndrome. Clin. Lung Cancer 23(8), e556–e562 (2022).
Yang, Y. et al. A real-world pharmacovigilance study of FDA adverse event reporting system (FAERS) events for venetoclax. PLoS ONE 17(12), e0278725 (2022).
Bate, A. et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54(4), 315–321 (1998).
Evans, S. J., Waller, P. C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 10(6), 483–486 (2001).
Szarfman, A., Machado, S. G. & O’Neill, R. T. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 25(6), 381–392 (2002).
van Puijenbroek, E. P. et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 11(1), 3–10 (2002).
Hu, Y. et al. Colitis following the use of immune checkpoint inhibitors: A real-world analysis of spontaneous reports submitted to the FDA adverse event reporting system. Int. Immunopharmacol. 84, 106601 (2020).
Yan, Y. D. et al. Toxicity spectrum of immunotherapy in advanced lung cancer: A safety analysis from clinical trials and a pharmacovigilance system. EClinicalMedicine 50, 101535 (2022).
Sakaeda, T. et al. Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10(7), 796–803 (2013).
Noguchi, Y. et al. Signals of gastroesophageal reflux disease caused by incretin-based drugs: A disproportionality analysis using the Japanese adverse drug event report database. J. Pharm. Health Care Sci. 4, 15 (2018).
Zhou, Q. et al. Adverse events of epidiolex: A real-world drug safety surveillance study based on the FDA adverse event reporting system (FAERS) database. Asian J. Psychiatr. 90, 103828 (2023).
Chen, H. et al. Ocular adverse events associated with antibody-drug conjugates: A comprehensive pharmacovigilance analysis. Front. Immunol. 15, 1495137 (2024).
Tewari, K. S. et al. Improved survival with bevacizumab in advanced cervical cancer. N. Engl. J. Med. 370(8), 734–743 (2014).
Tewari, K. S. et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (gynecologic oncology group 240). Lancet 390(10103), 1654–1663 (2017).
Sugiyama, T. et al. A single-arm study evaluating bevacizumab, cisplatin, and paclitaxel followed by single-agent bevacizumab in Japanese patients with advanced cervical cancer. Jpn. J. Clin. Oncol. 47(1), 39–46 (2017).
Shimizu, S. et al. Tissue factor and tissue factor pathway inhibitor in nasal mucosa and nasal secretions of chronic rhinosinusitis with nasal polyp. Am. J. Rhinol. Allergy 29(4), 235–242 (2015).
Ahmed, J. et al. Management of epistaxis: A guide for junior doctors. Br. J. Hosp. Med. 82(7), 1–8 (2021).
Manfredini, R., Gallerani, M. & Portaluppi, F. Seasonal variation in the occurrence of epistaxis. Am. J. Med. 108(9), 759–760 (2000).
Dumontet, C. et al. Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 22(8), 641–661 (2023).
Best, R. L. et al. Microtubule and tubulin binding and regulation of microtubule dynamics by the antibody drug conjugate (ADC) payload, monomethyl auristatin E (MMAE): Mechanistic insights into MMAE ADC peripheral neuropathy. Toxicol. Appl. Pharmacol. 15(421), 115534 (2021).
Younes, A. et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 363(19), 1812–1821 (2010).
Mariotto, S. et al. Brentuximab vedotin: Axonal microtubule’s Apollyon. Blood Cancer J. 5(8), e343 (2015).
Rossi, A. et al. Chemotherapy-induced alopecia management: Clinical experience and practical advice. J. Cosmet. Dermatol. 16(4), 537–541 (2017).
Author information
Authors and Affiliations
Contributions
All authors were involved in the study. Study design: JJL, MG, and YZ; Extraction data: MG; Analysis and interpretation of data: YZ, XQY, JZ, TY and QT. All authors participated in the interpretation of the results and contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Zhao, Y., Yang, X. et al. Signal mining and risk analysis of tisotumab vedotin adverse events based on the FAERS database. Sci Rep 15, 30212 (2025). https://doi.org/10.1038/s41598-025-14710-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-14710-9
Keywords
This article is cited by
-
Tisotumab vedotin: potential safety signals identified based on FAERS data
Reactions Weekly (2025)