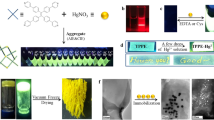
Abstract
A simple and robust colorimetric and fluorescent eugenol-based chemical sensor, namely, (E)-N’-(5-allyl-2-hydroxy-3-methoxybenzylidene)-2-hydroxybenzohydrazide (EABH) was synthesized and characterized using spectroscopic techniques such as, NMR (1H and 13C) and mass spectra. The chemosensor shows dual behavior for the colorimetric detection of Fe2+ and fluorometric detection of Pb2+ ions with high sensitivity and selectivity towards both the ions. The EABH detects Fe2+ by “naked eye” color change from lime yellow to brown and displayed fluorescence “Turn-off” response to Pb2+ ion. The binding stoichiometry of EABH based on absorption (for Fe2+) and fluorescence (for Pb2+) data along with mass spectral studies indicated 1:2 binding of metal to EABH ratio, which is further verified by Job’s plot measurement. Furthermore, the detection limits for Fe2+ and Pb2+ ions were found to be 0.166 µM and 0.095 µM, respectively proving its promising sensitivity. On the other hand, live cell imaging experiments showed that EABH has capability of penetrating through the cell membrane and exhibit significant response to the intracellular interactions when Fe2+ and Pb2+ are introduced in living cells, which reveals its effectiveness in the bioimaging applications. Furthermore, the fabricated chemical sensor EABH was successfully employed for cotton swab and test-paper applications. It is noteworthy that the developed sensor for the detection of Fe2+ and Pb2+ using colorimetric and fluorimetric assays acts as a reliable, cost-effective method compared to other conventional methods. In addition, computational studies such as Frontier Molecular Orbitals (FMOs) and Molecular Electrostatic Potential (MEP) provided a significant information on the interaction sites within EABH, giving profound insights about the chemical reactivity and various physical parameters.
Similar content being viewed by others
Introduction
Researchers are designing a new chemical sensor to identify the significant transition and heavy metal ion with high sensitivity and selectivity, in concern with industrial, health and environmental related issues. Metal toxication leads to serious health complication and cause ecosystem hazards. Hence, there is need for the development of least complicated, modest, and cost-effective sensors to detect metals1,2,3,4.
Heavy metal ions cause high toxicity due to their ability to co-ordinate with other biomolecules in the body even at extremely low quantities5. Lead ion is a second most toxic heavy metal, it is considered to be malleable, pervasive and non-degradable affecting the human health by accumulating the body, facing difficulty to excrete and seriously contaminates the ecosystem leading to pollution of air, water and soil6,7,8,9,10,11. Lead is renowned for its versatility in industrial applications due to its widespread usage in epoxy paints, radiation shielding, petroleum refining and ammunition12,13. The use of lead cannot be controlled in modern life due to its usage in cosmetic items and food preservatives14. Lead enters the environment through its use in dyes, waste plastics, pigments, batteries, gasoline and jewelry15,16. The prolonged exposure to Pb2+ even with minimal amount can cause high risks such as anemia, memory loss, digestive, neurological and cardiovascular disorders, ataxia, hypo immunity, muscle paralysis, nerve disorder, kidney disorders and mental retardation, hearing loss and Alzheimer’s disease5,17,18,19,20. It causes decreased IQs and hypertension in children11,21. According to the World Health Organization, drinking water can contain up to 10 µg of lead per liter22. Drinking lead contaminated water can cause long-term health issues including kidney problems and high blood pressure for adults, retardation of physical and mental development in infants and children23. Lead contamination in water has attracted significant attention around the world, to monitor the fast and sensitive methods for water quality assessment for the protection of human and animal health is intensified all over the world. Hence, it is very imperative to explore methods for the detection of lead that are highly convenient, reliable, good selective, rapid, quantitative and high sensitivity.
Iron is the most essential trace element and most imperative bio-active transition metal involved in living systems24,25,26. Iron exists in the form of ferrous ion (Fe2+) and ferric ion (Fe3+), which are necessary to the metabolic and other biological processes27,28,29. Iron owing a special place among variety of transition metals due to their integral role in the reactive centres of the metallo-enzymes like haemoglobin, cytochromes and biological processes. However, deficiency of iron makes serious symptoms like abnormal heart rhythms, organ damages and diseases including anaemia, Alzheimer’s and Parkinson’s disease30,31,32,33. Lack of iron weakens the immune system, allowing deadly viruses such as pneumonia and typhoid to affect the human body34. In cosmetic industry, products such as kajal, lipstick, sindoor, nail polish, Fe2+ are extensively used as colorant35. This metal ion is hazardous and toxic at higher concentrations. Iron levels in drinking water must not exceed 0.3 mg/L, as reported by China’s drinking water sanitation agencies36. Greater significance has been renowned for monitor the iron content. Although there are numerous optical and spectroscopic techniques such as atomic absorption spectroscopy (AAS), neutron activation analysis (NAA), atomic emission spectroscopy (AES), inductively coupled plasma-optical emission spectrometry (ICP-OES), inductively coupled plasma mass spectroscopy (ICP-MS), x-ray fluorescence spectrometry (XRF) is some of the methods used to detect trace levels of iron in complex matrices37,38,39,40,41,42. However, these techniques do have certain drawbacks, though, such as high costs, the requirement for skilled operators, and challenging procedures because of the preparation of several samples43. As a result, it is inevitable that low-cost, dependable, and straightforward techniques that can conduct quick and real-time analysis will be developed44. Due to its exceptional benefits, colorimetric iron chemosensors have drawn a lot of attention up to this point. However, there aren’t many reports in the literature on the colorimetric naked-eye Fe2+ detection techniques. Hence, the development of colorimetric chemosensors for ferrous ion (Fe2+) detection is taking the limelight.
The creation of chemosensors for identifying metal ions such as Pb2+ and Fe2+ has garnered significant interest because of their crucial roles in medicine, biological, and environmental systems. Among the various types of sensors, fluorescence and chromogenic “naked-eye” chemosensors are being recognized lately for metal ion detection, owing to their high sensitivity, exceptional selectivity, ease of use, quick response times, and overall simplicity. Further, single probe with numerous targets in environment and biological samples has gained increased attention due to low-cost, and high efficiency lately. The review of the literature for the detection of various metal ions using single probe by different detection methods as presented in the Table 1. Furthermore, the literature showed least detection of iron, and lead ions using the colorimetric, and fluorescence methods. In this concern the authors explored an efficient method for the detection of such metal ions using naked eye and quenching fluorescent probes.
In this study, we designed and synthesised a chemosensor utilising eugenol-based fluorophore and chromophore compound and detected for two analytes. The effectiveness of the ligand and its Fe2+ and Pb2+ complexes was examined using DFT, and the designed chemosensor was assessed for the biological practicability with real water sample analysis. Furthermore, EABH has the competence of detecting the two chief metal ions in the milieu and acts as colorimetric and fluorometric sensor response to Fe2+ and Pb2+ ions.
Experimental
Materials and methods
2-hydroxy benzohydrazide, 5-allyl-2-hydroxy-3-methoxybenzaldehyde (Eugenol-5- aldehyde), Lead (II), iron (II) and other metal salts were procured from (Merck chemicals Pvt Ltd, India). pH of the solutions was maintained by HEPES buffer. DMSO, Ethanol was procured from S.D-fine chemical Pvt Ltd. DMSO solvent system is used through-out the colorimetric and fluorescence experiments. The ligand’s (EABH) melting point and their metal complexes C1 and C2 were determined using open capillary digi apparatus. The course of the reaction is monitored using TLC technique performed with [n-hexane-ethyl acetate 8:2] medium. The m/z values of ligand (EABH) and its complexes were determined using EV LC-MS Shimadzu mass spectrometer in order to differentiate in terms of their molecular mass. The coordination admittance of the ligand (EABH) with metal ions can be captured by recording IR spectrum (400–4000 cm− 1) using Perkin-Elmer 783 spectrophotometer. 1H and 13C spectra was recorded with Bruker Advance DPX spectrometer at 400 MHz, in an ambient temperature in d6-DMSO using TMS. Sensory action of the ligand was detected using a Shimadzu spectrofluorometer (RF-6000) equipped with 150-W xenon lamp and 1 cm path length quartz cell used. Electronic absorption measurement was performed using a 3100-XE UV/vis spectrophotometer in the range 200–800 nm (Lab India).
Chemical synthesis
Synthesis of ligand EABH
An equimolar solution of 2- hydroxy benzohydrazide (0.05 g, 0.0003 mmol) and 5-allyl-2-hydroxy-3-methoxybenzaldehyde (0.063 g, 0.0003 mmol) in ethanol were refluxed for 8 h at 60 ℃. The reaction was facilitated by adding 1 ml of glacial acetic acid. The progress of the reaction was monitored using TLC plate and UV chamber to visualize the spots. The lime yellow solid product was filtered off using Whatman filter paper. The obtained product was dried and recrystallized using hot ethanol. The pure product was subjected for further analysis. General synthetic scheme of ligand (EABH) is depicted in Fig. 1.
Yield − 86%; m. p. 215 °C, 1H-NMR(DMSO-d6, 400 MHz, ppm)-δ 12.0(br, s, 1H -OH),11.74(s,1H - OH), 10.57 (br, s, 1H -NH), 8.66 (s, 1H = C-H), 7.88 (d, J = 8 Hz, 1H, Ar-H), 7.48 (t, J = 8 Hz, 1H, Ar-H), 7.0- 6.97 (m, 3 H), 6.88 (d, J = 16 Hz, 1H), 5.98 (m, 1H), 5.13–5.08 (d, J = 20.4 Hz, 2 H), 3.81 (s, 3 H), 3.33 (d, J = 6.8 Hz, 2 H) (Figure S1 and S1a). (13C-NMR (DMSO-d6, 100 MHz, ppm)-δ164.98, 159.45, 148.84, 148.32, 145.90, 138.25, 134.39, 131, 129.01, 120.08, 119.45, 119.18, 117.73, 116.24, 116.17, 114.82 56.03, 40.08) (Figure S2). LC-MS calculated for C18H18N2O4: m/z = 326.13 and found m/z = 327.58 [M + 1] peak (Figure S3). FT-IR spectrum (v, cm− 1) 3216 − 2918 (broad, O-H), 1570 (C = N), 1624 (C = O).
Synthesis of metal complexes (C1 and C2)
To the ethanolic solution of ligand (EABH), an ethanolic solution of FeSO4.7H2O and Pb(CH3COO)2.3H2O were added separately. The reaction mixture was refluxed for 10 h at 80 ℃, precipitate was formed, the solution was filtered off and washed with ethanol for several times and kept aside for drying. The complexes of Fe(II) and Pb(II) were obtained as a grey and yellow colored solid, respectively (Fig. 2).
C1: [Fe(EABH)2], Yield-76%, m.p. 280 °C. LC-MS: m/z = 706.17 and found m/z = 707.03 [M + 1] (Figure S4). 1H NMR (400 MHz, DMSO-d6) δ 11.81 (s, 2 H), 10.58 (s, 1H), 8.68 (s, 1H), 7.89 (s, 1H), 7.46 (s, 1H), 6.94 (d, J = 18.2.0 Hz, 4 H), 5.97 (s, 1H), 5.08 (dd, J = 18.4, 12.7 Hz, 2 H), 3.81 (s, 3 H), 3.37(m, 2 H) (Figure S5 and S5a).(13C-NMR (DMSO-d6, 100 MHz, ppm)-δ 165.0, 159.4, 148.9, 148.3, 145.8, 138.2, 134.4, 131.0, 129.02, 120.06, 119.5, 119.1, 117.7, 116.2, 116.1, 114.8, 56.31, 40.47 (Figure S6).
C2: [Pb(EABH)2], Yield-72%, m.p. 260 °C. LC-MS: m/z = 858.21 and found m/z = 858.30 [M+] (Figure S7). 1H NMR (400 MHz, DMSO-d6) δ 13.89 (s, 1H), 8.41 (s, 1H), 7.84 (d, J = 7.8 Hz, 1H), 7.25 (t, J = 8.1 Hz, 1H), 6.84 (d, J = 8.1 Hz, 1H), 6.79 (d, J = 7.1 Hz, 1H), 6.74 (d, J = 2.1 Hz, 1H), 6.70 (s, 1H), 5.94 (d, J = 6.8 Hz, 1H), 5.09 (d, J = 1.9 Hz, 1H), 5.06 (m, 1H), 3.73 (s, 3 H), 3.24 (d, J = 6.7 Hz, 2 H) (Figure S8 and S8a). (13C-NMR (DMSO-d6, 100 MHz, ppm)-δ 172.10, 159.952, 157.31, 156.71, 152.28, 138.98, 131.85, 129.19, 124.96, 123.94, 120.57, 120.21, 118.21, 116.49, 115.58, 114.47, 55.84, 40.46 (Figure S9).
Cytotoxicity assay and biosensing studies
MTT (5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide) assay was used to assess the cytotoxic effect of EABH59. HeLa cells procured from BioScienceClix laboratory (Mysuru, India) were cultured in Dulbecco’s modified eagle’s medium (DMEM) containing streptomycin (100 µg/mL) under 5% CO2 atmosphere at 37 °C. The cells were then cultivated on a 36-well plate so must reach about 75% confluence before the treatment. EABH was added (25 µg/mL) to the cells in a 36-well plate and incubated for additional 24 h. To this, 25 µL of MTT (2.5 mg/mL) was added into each well and cultured for another 4 h. UV-Visible absorbance was measured at 316 nm after adding 200 µL of DMSO into the each well. In a similar way, this was performed in the case of Fe2+ and Pb2+ complexes. After the incubation period (30 min), the staining of cells was carried out. The cells were then cleaned with PBS buffer solution. The fluorescence bioimaging of cells was then viewed and captured using an Invitrogen EVOS M5000 fluorescent inverted microscope.
Density functional theory (DFT) calculations
DFT calculations for EABH, EABH-Fe2+ and EABH-Pb2+ molecules were performed using Gaussian09 software60. Geometry optimization was conducted at the B3LYP level with 6-311 + + G(d, p) and LanL2DZ basis sets.
Results and discussion
FT-IR spectral studies
Bonding nature and functional group present in EABH and its Fe (II) and Pb (II) complexes were studied by FT-IR analysis. Figure 3 shows the FTIR spectra of EABH and its Fe2+ and Pb2+ complexes. The characteristic band of stretching frequency of carbonyl (C = O) and imine group (C = N) in EABH was seen at 1624 cm− 1 and 1570 cm− 1. After complexation, the bands of the carbonyl C = O and imine (C = N) moieties were shifted to 1606 cm− 1, 1601 cm− 1 and 1549 cm− 1 and 1520 cm− 1 for Fe2+ and Pb2+, respectively. The phenolic OH stretching band in the region between 3216 and 2918 cm− 1 in free EABH were disappeared in respective complexes indicating the deprotonation of the Schiff bases due to its involvement in the coordination61. These characteristic changes in the IR spectra suggested that the imine nitrogen, carbonyl oxygen and phenolic oxygen atoms of EABH have taken part in the coordination with Fe2+ and Pb2+ ions.
UV-Visible absorption studies
To better understand the sensing ability of EABH as an efficient probe for the detection of Fe2+ ion, UV-vis absorption studies were conducted. Test solution of 60 µM of EABH (HEPES, 0.01 M pH = 7.4) in DMSO was used to carry out the experiments at room temperature. The UV-vis spectra of EABH shows a characteristic absorption peak at 281 nm due to π→π* transition of C = N group (Fig. 4a), whereas the peak at 322 nm reflected intra-molecular charge transfer (ICT) resulting due to n→π* transition, involving donor–acceptor conjugation systems47,62,63. The absorption peaks at 281 and 322 nm were blue shifted to 267 and 302 nm for Fe2+ metal ion. Furthermore, the complex formation between EABH and Fe2+/Pb2+ ions was confirmed by performing the absorbance titrations experiment, which were carried by incremental addition of Fe2+/ Pb2+ ions in a DMSO solution of EABH. It is interesting to note that the change in absorption spectra was observed at the isobestic points at 360 nm for the Fe2+ ion (Fig. 4b), and at 373 nm for Pb2+ ion (Fig. 4c). Thus, the change in the absorbance wavelength and the appearance of isobestic point indicates the formation of stable complex of EABH with Fe2+/Pb2+ ions64,65,66,67. On the other hand, the absorption band for Fe2+ ion demonstrates a strong complex formation between the EABH and Fe2+ ion. With the addition of Fe2+, Pb2+, Zn2+, Cd2+, Co2+, Fe3+, K +, Ni2+, Cu2+, Al3+ and Na+, F−, Br−, I−, HSO4− and CN− ions to the EABH solution, only Fe2+ shows brown colouration from lime yellow on addition of two equivalence of distinct metal ions, and other metal ions did not exhibit colour changes (Fig. 5). The transformation in the colour of EABH demonstrate high selectivity to Fe2+ ions due to ligand–metal change transfer (LMCT) of EABH with Fe2+68. According to the above-described experiment, the EABH in DMSO solution was used to determine Fe2+ using naked eye analysis.
Fluorescence studies
The selectivity of probe was determined by fluorescence emission spectra in the range of 200–900 nm. The addition of Fe2+, Pb2+, Zn2+, Cd2+, Co2+, Fe3+, K +, Ni2+, Cu2+, Al3+, Na+, F−, Br−, I−, HSO4− and CN− (40 µM) metal ions to the sensor EABH (60 µM, HEPES 0.01 M pH = 7.4) in DMSO with an excitation wavelength 326 nm (Fig. 6a). Only Pb2+ showed a significant fluorescence quenching at 498 nm, while the remaining metal ions did not show discernible changes in the spectra of EABH. Further, a decrease in fluorescence intensity upon the addition of Pb2+ ion due to the complexation between EABH and Pb2+ ion69. “Turn-off” mechanism was noticed for Pb2+ ion addition into the EABH, while the sensor failed to sense other metal ions (Fig. 6b). The above results indicated that the probe EABH is highly selective for Pb2+ and the sensor EABH functions as an exceptional selective fluorescence “on-off” sensor. The prepared complex follows static quenching, since the fluorescence intensity diminishes on increasing the metal ion concentration. Further, the metal complex acting as non-fluorophore on coordination with metal ion, due to the electrostatic interaction stabilizing the complex and the ligand-metal ions interaction. Hence, the complex does not exhibit fluorescence70. However, the quantum yield (QY) of EABH was calculated using the standard reference Quinine sulphate (Methanol, QY = 0.54) at 326 nm excitation wavelength using below mentioned in Eq. (1):
Where, R and S represent reference and sample, I is the intensity, A is the absorbance value and η is refractive index of the solvent. The relative quantum yield of EABH in DMSO was evaluated to be 0.04 However, addition of Pb2+ ions to EABH solution obtained quantum yield was 0.00959.
Competitive titration
To verify the practicability of EABH as a selective probe for the specific recognition of Pb2+, metal cation competition experiment was conducted. The effect of competing ions on the coordination relationship between the probe EABH and Pb2+ was investigated by adding Pb2+ to EABH solutions containing different metal ions (Fe2+, Zn2+, Cd2+, Co2+, Fe3+, K +, Ni2+, Cu2+, Al3+, Na+, F−, Br−, I−, HSO4− and CN− ions), respectively. By comparing the fluorescence intensity of EABH-Pb2+ complexes after the addition of competing ions, from the experiments we determined that the addition of competing ions doesn’t alter the selectivity of the sensor EABH with Pb2+ and the probe EABH was still quenched by Pb2+ and remained unchanged on adding competing metal ions. The results indicate that the specific response of the probe EABH with Pb2+ is largely uninterrupted by the competing metal ions. The response of Pb2+ ions and the probe EABH can be attributed to the chelation of EABH with Pb2+71 and the fluorescence is quenched but in the case of other competing ions, there is no significant changes in the fluorescence spectra because they did not form stable complexes. The fluorescence intensity of EABH responds only to Pb2+ ions, this indicates the high sensitivity and selectivity of EABH towards Pb2+ detection, while the latter metal ions offered no- interference as presented in bar diagram (Fig. 7).
Binding stoichiometric studies (Job’s plot)
The EABH with the Fe2+ and Pb2+ binding ratios were obtained with Job’s plot method of continuous variation operated at ambient temperature72. The plot of absorbance/fluorescence vs. mole fraction of ferrous ions at 326 nm/ Pb2+ at 498 nm were used to assess the binding ability of complex with EABH (60 µM) and metal ions at 40 µM. From jobs plot, maximum absorbance and fluorescence intensity for Fe2+ and Pb2+ were observed at a molar ratio 0.3, which suggests that the binding stoichiometry between the sensor EABH and the metal ions Fe2+ and Pb2+ is 1:2 [M: L], which was further confirmed by LC-MS, stoichiometry with respect to the [probe + M2+]73. Accordingly, we proposed the possible structures shown in (Fig. 2) between the sensor EABH and the metal ions (Fe2+/Pb2+) (Fig. 8).
Solvent effect
The solvent dependency of the EABH was assessed to estimate the fluorescence property to comprehend its interaction with Fe2+/Pb2+ ions. Which was examined in various solvents viz., DMF, DMSO, acetonitrile, THF, ethanol, and methanol. Figure 9 depicts the experiment performed by using EABH (60 µM) solution in different solvents. EABH displayed maximum fluorescence intensity in DMSO, compared to various solvents.
Stern-Volmer analysis
The equivalence relationship between EABH, and Pb2+ and the metal quenching efficiency of fluorophore and quencher was investigated by fluorescence titration up to 40 mM concentrations of Pb2+ with 5 mM intervals and maintaining the constant EABH concentration (60 µM) in DMSO, 0.01 M HEPES buffer at 326 nm excitation (Fig. 10a). The EABH intensity in DMSO at 498 nm decreases continuously with increasing Pb2+ concentration (Fig. 10b). Fluorescence quenching mechanism of the sensor probe EABH can be understood by applying Stern-Volmer Eqs.2,74.
The fluorescence intensities of the sensor in the absence and presence of different concentrations of Pb2+ ion is denoted as F0 and F, respectively. Ksv is the Stern-Volmer constant, and the total concentration of Pb2+ is denoted by [Q]. The plot of F0 /F against [Q], gives 2.1 × 105 M− 1 Ksv of the Pb2+ (Fig. 10c).
(a) Fluorescence spectrum of EABH (60 µM), (b) Changes in Fluorescence intensity in DMSO solution (HEPES 0.01 M, pH 7.4) with different concentration of Pb2+ (0–40 µM), λexc, and emission at 326 nm, 498 nm, respectively. The intensity bar graphs for EABH (pink bar) and with Pb2+ (blue bar), (c) stern volmer plot of EABH with different Pb+ 2 ion concentration.
Limit of detection
The limit of detection of EABH was determined by maintaining a constant concentration of EABH and altering the concentrations of Fe2+ and Pb2+ ions. The absorption and fluorescence spectra were obtained using 60 µM of EABH in DMSO the results show a linear rise in absorbance for Fe2+ and decrease in fluorescence intensity for Pb2+ ions. LOD of the sensor molecule for Fe2+ and Pb2+ ion was calculated using Eq. (3):
where σ is the standard deviation and k is the slope of the calibration curve. The limit of detection for Fe2+ and Pb2+ ions was found to be 0.166 × 10− 6 M and 0.095 × 10− 6 M based on plotting the absorbance against their concentrations of Fe2+, and fluorescence intensity vs. Pb2+ concentration, respectively as shown in Fig. 11 are lower than the WHO guidelines for drinking water. Furthermore, the comparison of EABH with the reported chemosensors is shown in Table 2. The key characteristics of the developed chemosensor was its simplicity, usefulness, and affordable synthesis along with its ease in high sensitivity, selectivity, and naked eye detection of Pb2+/Fe2+ ions with lower detection limit compared to reported chemosensors. Similarly, the previously reported hydrazide-based Schiff base and their LOD are tabulated in Table 3.
Reversible EDTA Titration
Amplified its regeneration and reversible properties of the developed sensor are the substantial features for its real time applicability using sodium salt of EDTA86. The solutions of EABH-Fe2+ and EABH-Pb2+ with the chelating ligand EDTA acting as complexing agent (Fig. 12a and b). Addition of one equivalence of Pb2+ ion solution, exhibts decreased fluorescence emission intensity at (498 nm), and the regeneration of free EABH was noticed when 1.0 eq of EDTA was added to the EABH-Pb2+ complex. When EDTA was added to EABH-Fe2+ complex, the initial brown colour of the solution changes to lime yellow indicating the formation of Fe-EDTA complex, affirming the release of EABH as a free ligand and reversal of colour change was noticed (Fig. 12c and d). The above results greatly potential for its use in logic gate construction.
10. Logic gate application
Molecular logic gate was constructed based on the photochemical behaviour of fluorescence probes under consideration87. The reversibility of fluorescence study for the EABH probe was estimated by constructing the logic gate of INHIBIT type, based on the ON/OFF characteristics of the fluorescence probe88,89, as shown in (Fig. 13). The reversibility of EABH’S detection of Pb2+ ions by adding EDTA was framed using the Boolean logic function. EABH emission at 498 nm was used as the output of the logic gate function90. Addition of Pb2+ to strong fluorescent solution of free ligand, weak fluorescence was observed, whereas EDTA added to EABH-Pb2+ shows strong fluorescence. The “ON” (output = 1) for strong and “OFF” (output = 0) for weak emission, respectively. For this particular truth table, the presence and absence of the Pb2+ or EDTA corresponds to the signals ‘1’ and ‘0’, respectively. Addition of Pb2+ (input 1) to EABH, quenching of fluorescence was observed. With input 2, mere addition of EDTA had no effect on the intensity of EABH. The addition of Pb2+ and EDTA turned on the EABH fluorescence. The output signal was noted with the shift in emission at 498 nm. The 2 input can be credited to Pb2+ and EDTA, showing outputs state fluorescence “OFF” for A and B (Table 4). The input of Pb2+ was represented by a NOT gate. The OR gate function was used to represent the input – output combination of EABH-Pb2+-EDTA.
Practical applications in real water sample
Figure 14 depicts UV-visible and fluorescence spectra for the detection of the Fe2+/Pb2+ in real specimens using EABH probe in both tap water and lake water is a crucial aspect. Real water samples were gathered, and standard addition approach is used to analyse the samples using UV-visible and Fluorescence spectroscopic techniques91,92,93. Lake water from kukkaralli lake, Mysuru and laboratory tap water was collected. The different sample concentrations of Fe2+/Pb2+ metal ions (0–25 µM) were added to the probe EABH (30 µM), and spiked samples were obtained. The results represent sensitive, and selectiveness monitored in environmental samples of the probe towards the Fe2+/Pb2+ ions.
Cell viability and bio-imaging studies
The cell viability was determined using MTT assay to assess the cytotoxicity of EABH. The HeLa cells were treated with the different concentrations of EABH (5, 10, 15, 20 and 25 µM) and incubated for 24 h. More than 91% of cells remained viable, indicating their non-toxic nature. Further, the sensing property of EABH was further studied in the biological system using in vitro cell imaging experiments. The biosensing property of EABH was performed in the presence of Fe2+ and Pb2+ ions. As shown in Fig. 15, in the presence of Fe2+ and Pb2+ ions, no fluorescence was observed. Thus, it is evident that EABH can be utilized in the biomedical applications.
Frontier molecular orbitals and reactivity indices
Frontier Molecular Orbitals (FMOs) theory is essential for understanding chemical reactivity and various physical parameters94. It involves the molecular orbital energies of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). These orbitals affect molecular interactions, with HOMO indicating electron donation ability and LUMO representing electron acceptance. The HOMO-LUMO energies and the energy gap (ΔE) play a key role in determining molecular stability95. A small ΔE signifies high polarizability, reduced kinetic stability, and increased chemical reactivity, categorizing the molecule as soft. The ΔE values for EABH and its complexes, EABH-Fe2+ and EABH-Pb2+, were found to be 4.275 eV, 2.004 eV, and 3.040 eV, respectively. Figure 14 illustrates the encapsulation of the HOMO and LUMO maps in the molecules. The significant reduction in the HOMO-LUMO gap upon EABH complexation with Fe2+ (2.004 eV) and Pb2+ (3.040 eV), compared to free EABH (4.275 eV), is due to metal-ligand interactions. The stronger d-orbital hybridization and ligand-to-metal charge transfer (LMCT) in EABH-Fe2⁺ leads to greater gap reduction, while weaker p-orbital interactions and relativistic effects in EABH-Pb2+ result in a smaller decrease96. This electronic modulation influences fluorescence properties, making these complexes potential candidates for sensing applications97.
Figure 16 demonstrates that the HOMOs are localized on the methoxybenzylidene and acetohydrazide moieties, and partially localized on the methyl moieties, excluding the phenol ring in the EABH structure. In contrast, LUMOs are mainly distributed across the entire molecule, except for the methyl moiety with the allyl group in the EABH structure. In the bidentate structures of EABH-Fe2+ and EABH-Pb2+, the HOMO is primarily localized on the methoxybenzylidene and acetohydrazide moieties, with additional localization on the Fe atom in EABH-Fe2+, while excluding the phenol ring and allyl group in both complexes. The LUMO is distributed across the entire molecule, with the exception of the allyl group in EABH-Fe2+ and both the allyl group and Pb atom in EABH-Pb2+. The chemical reactivity parameters of the compounds were evaluated based on the HOMO and LUMO energy values (Table 5). EABH has an ionization potential (I) of 5.812 eV, which indicates its strong electron-donating ability. Hardness (η), which reflects resistance to changes in the electronic environment, was calculated as 2.137 eV for EABH, and 1.002 eV and 1.520 eV for EABH-Fe2+ and EABH-Pb2+, respectively, suggesting a soft character along with strong polarizability and reactivity98. The chemical potential (µ), which represents the tendency of electrons to escape from an equilibrium system, was calculated as -3.674 eV for EABH, -2.988 eV for EABH- Fe2+, and − 3.263 eV for EABH-Pb2+. The global electrophilicity index (ψ) measures how stable the energy is when the system gets an extra electronic charge from the environment. The calculated values were 3.157 eV for EABH, and 4.455 eV and 3.502 eV for the EABH-Fe2+ and EABH-Pb2+ complexes, respectively.
Molecular electrostatic potential
The Molecular Electrostatic Potential (MEP) reflects electron density and serves as a crucial tool for identifying electrophilic and nucleophilic interaction sites, as well as hydrogen bonding regions99. In the MEP map, red and yellow represent negatively charged areas associated with electrophilic reactivity, while blue indicates positively charged areas linked to nucleophilic reactivity, as shown in Fig. 17. The MEP visualization indicates that the EABH molecule has several possible sites for electrophilic attack, primarily around oxygen and nitrogen atoms. The negative regions are concentrated near these electronegative atoms, suggesting electrophiles would preferentially target these positions. The MEP map further reveals that the negative potential sites correspond to oxygen and nitrogen atoms, while the positive potential sites are located around hydrogen atoms. These regions provide information about possible noncovalent interactions within the molecule.
Sensing mechanism
Figure 18 depicts the sensing mechanism of the chemosensor EABH towards the detection of Fe2+ and Pb2+ ions. In the case of metal complexes, the metal ion form coordination bonds with imine nitrogen, carbonyl oxygen and phenolic oxygen atoms of EABH. Addition of Fe2+ to the sensor EABH, Fe2+ ion establishes ligand–metal change transfer (LMCT) with EABH ligand, thereby hinders photoinduced electron transfer (PET). On the other hand, the addition of lead centrals quenching in the fluorescence intensity of the EABH due to the excited-state intramolecular proton transfer (ESIPT) process explains the fluorescence quenching mechanism100,101.
Test-kit application
To find the practical applicability of the probe, cotton swabs and a paper strip test applications were performed for Fe2+/Pb2+ determination in DMSO solution. The test-kit studies were performed with the cotton swabs and Whatman filter strips saturated with the EABH solution. When different amounts of Fe2+/Pb2+ (8.33–125µM) were dipped with the cotton swab distinct brown coloration changes for Fe2+ were clearly seen under daylight and becoming non- fluorescent for Pb2+ under irradiation with UV under 365 nm (Fig. 19a and b). Whereas, for paper strip experiment test Whatman filter strips were immersed into solution of EABH for duration of 5 min and then left to dry. the dried strips were immersed into Fe2+/Pb2+ ion solution, for Fe2+ change in brown coloration was noticed and become non-fluorescent on paper strips after addition of Pb2+ ion (Fig. 19c and d) hence, these applications could be used for semi-quantitative identifying Fe2+/Pb2+ ions102,103.
Conclusion
Herein, we have synthesized and characterized a new and robust colorimetric and fluorimetric sensor, EABH based on eugenol moiety, which shows a good sensitivity and selectivity towards both Fe2+ and Pb2+ ions in DMSO (0.01 M HEPES buffer, pH = 7.4). The imine nitrogen, phenolic oxygen and carbonyl oxygen atom of the EABH co-ordinated with lead and iron ions, forming a Pb2+ and Fe2+ complex with the EABH ligand, which was confirmed by FTIR. The job’s plot and LC-MS techniques, signifies the co-ordination at the ratio of 2:1 (L: M) stoichiometry. The chemosensor EABH detected Fe2+ by “naked eye” colour change from lime yellow to brown, this confirms the effective and selective sensor for Fe2+ detection by UV-visible spectroscopic method and displayed fluorescence quenching for Pb2+. The chemosensor EABH can detect the Fe2+and Pb2+ ions with the LOD of 0.166 µM and 0.095 µM, respectively. The established chemosensor acts as an intriguing compound for the quantification of Fe2+/Pb2+ ions even in the live cells, revealing its biosensor potential. In addition, the frontier molecular orbitals, reactivity indices and molecular electrostatic potential calculations revealed that the electronic modulation influences fluorescence properties, thus making the ligand as a potential candidate for sensing applications. Furthermore, the significant sensing nature of EABH was demonstrated by employing it in test-kit applications.
Data availability
All the data generated or analyzed during this study are included within the article and supporting information file.
References
Shruthi, B. et al. Highly selective fluorescent and colorimetric methylphenyl-based sensor towards Zn2 + ion detection: Synthesis, X-ray crystallography and selectivity studies. Inorgan. Chim. Acta. 556 https://doi.org/10.1016/j.ica.2023.121614 (2023).
Bravo, V. et al. A new phenanthrene-based bis-oxime chemosensor for Fe(III) and Cr(III) discrimination. Tetrahedron 68, 4882–4887. https://doi.org/10.1016/j.tet.2012.03.089 (2012).
Wei, T. B. et al. A highly selective chemosensor for colorimetric detection of Fe 3 + and fluorescence turn-on response of Zn2+. Dyes Pigm. 97, 297–302. https://doi.org/10.1016/j.dyepig.2012.12.025 (2013).
Rahimi, H., Hosseinzadeh, R. & Tajbakhsh, M. A new and efficient pyridine-2,6-dicarboxamide-based fluorescent and colorimetric chemosensor for sensitive and selective recognition of Pb2 + and Cu2+. J. Photochem. Photobiol Chem. 407 https://doi.org/10.1016/j.jphotochem.2020.113049 (2021).
Sadia, M. et al. Trace level detection of Pb2 + ion using organic ligand as fluorescent-on probes in aqueous media. Heliyon 11 https://doi.org/10.1016/j.heliyon.2024.e41125 (2025).
Rout, K. et al. Triazole-based novel Bis schiff base colorimetric and fluorescent turn-on dual chemosensor for Cu2 + and Pb2+: application to living cell imaging and molecular logic gates. RSC Adv. 9, 25919–25931. https://doi.org/10.1039/c9ra03341f (2019).
Sun, T., Niu, Q., Guo, Z. & Li, T. A simple highly sensitive and selective turn-on fluorescent chemosensor for the recognition of Pb2+. Tetrahedron Lett. 58, 252–256. https://doi.org/10.1016/j.tetlet.2016.12.022 (2017).
Xu, H., Qin, W., Zhu, J. & Liu, Y. G. A smartphone-assisted chromogenic and fluorogenic dual-channel sensor for specific determination of toxic Pb2 + ions with multifaceted applications. Tetrahedron 168 https://doi.org/10.1016/j.tet.2024.134316 (2024).
Zhang, Y. et al. A biomass-derived schiff base material composited with polylactic acid nanofiber membrane as selective fluorescent ‘turn off/on’ platform for Pb2 + quantitative detection and characterization. Int. J. Biol. Macromol. 214, 414–425. https://doi.org/10.1016/j.ijbiomac.2022.06.089 (2022).
Zhang, Z., Lu, S., Sha, C. & Xu, D. A single thiourea-appended 1,8-naphthalimide chemosensor for three heavy metal ions: Fe3+, Pb2+, and Hg2+. Sens. Actuators B Chem. 208, 258–266. https://doi.org/10.1016/j.snb.2014.10.136 (2015).
Wu, G. et al. A highly sensitive and selective turn-on fluorescent probe for Pb(II) ions based on a coumarin-quinoline platform. RSC Adv. 6, 100696–100699. https://doi.org/10.1039/c6ra19734e (2016).
David, C. I. et al. A reversible fluorescent chemosensor based on a naphthalene dyad for Pb(II) ions: applications in food, water, and bio-imaging. J. Food Compos. Anal. 133 https://doi.org/10.1016/j.jfca.2024.106448 (2024).
R, K. et al. Quinoline-quinoline schiff-base as an effective chromogenic, fluorogenic, and smartphone assisted RGB detection of Pb2 + ion in near aqueous medium. Environ. Res. 250 https://doi.org/10.1016/j.envres.2024.118530 (2024).
Tamizhselvi, R. & Arumugam Napoleon, A. Ninhydrin and Isatin appended 2-Hydrazinobenzothiazole based simple schiff bases for colorimetric selective detection of Cr3 + and Pb2 + ions. Inorg. Chem. Commun. 145 https://doi.org/10.1016/j.inoche.2022.109983 (2022).
Shaily, A. et al. Highly selective and sensitive coumarin–triazole-based fluorometric ‘turn-off’ sensor for detection of Pb2 + ions. Luminescence 33, 713–721. https://doi.org/10.1002/bio.3468 (2018).
Sharma, V. et al. A new quinoline-based fluorescent-colorimetric chemosensor for sensitive and selective on–off detection of Pb 2+ ions. RSC Adv. 15, 4236–4249. https://doi.org/10.1039/D4RA08193E (2025).
Akther Rupa, S., Emmanuel Ghann, W., Abdul Majed Patwary, M. & Uddin, J. Highly selective naked eye pyridine-2,6-dicarbohydrazide based colorimetric and fluorescence chemosensor for Pb2 + with AIE activity. Inorg. Chem. Commun. 144, 109937. https://doi.org/10.1016/j.inoche.2022.109937 (2022).
Ahmad, I. et al. Crystal structure of N′-[(E)-(2-hydroxynaphthalen-1-yl) methylidene] Benzenesulfonohydrazide (HNMBSH) and its application as Pb2 + ion sensor by its fabrication onto glassy carbon electrode. Inorganica Chim. Acta. 467, 297–306. https://doi.org/10.1016/j.ica.2017.08.028 (2017).
Jiang, C. et al. A simple and rapid fluorescent approach for Pb2 + determination and application in water samples and living cells. Spectrochim Acta Mol. Biomol. Spectrosc. 263 https://doi.org/10.1016/j.saa.2021.120168 (2021).
Zhao, J., Li, C., Wei, S., Lü, C. & Zou, L. W. A multifunctional fluorescent probe based on schiff base with AIE and ESIPT characteristics for effective detections of Pb2+, Ag + and Fe3+. Spectrochim Acta Mol. Biomol. Spectrosc. 300 https://doi.org/10.1016/j.saa.2023.122904 (2023).
Velmurugan, K. et al. Quinoline based reversible fluorescent probe for Pb2+; applications in milk, bioimaging and INHIBIT molecular logic gate. Food Chem. 348 https://doi.org/10.1016/j.foodchem.2021.129098 (2021).
Paul, S., Barman, P., Dey, N. & Watkinson, M. Recent developments in pyrene-based fluorescence recognition and imaging of Ag + and Pb2 + ions: synthesis, applications and challenges. Sens. Diagnostics. 3, 946–967. https://doi.org/10.1039/d3sd00289f (2024).
Kuang, H. et al. Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sens. (Switzerland). 13, 4214–4224. https://doi.org/10.3390/s130404214 (2013).
Wan, C. F. et al. A new multifunctional schiff base as a fluorescence sensor for Fe2 + and F – ions, and a colorimetric sensor for Fe3+. J. Lumin. 178, 115–120. https://doi.org/10.1016/j.jlumin.2016.05.039 (2016).
Immanuel David, C. et al. Experimental and theoretical studies on a simple S-S-Bridged dimeric schiff base: selective Chromo-Fluorogenic chemosensor for nanomolar detection of Fe2+ & Al3 + Ions and its varied applications. ACS Omega. 5, 3055–3072. https://doi.org/10.1021/acsomega.9b04294 (2020).
Kim, K. B. et al. A cap-type schiff base acting as a fluorescence sensor for zinc(ii) and a colorimetric sensor for iron(ii), copper(ii), and zinc(ii) in aqueous media. Dalton Trans. 42, 16569–16577. https://doi.org/10.1039/c3dt51916c (2013).
Zhou, Y., Yang, X., Jang, W. J., Yan, M. & Yoon, J. Binding- and activity-based small molecule fluorescent probes for the detection of Cu+, Cu2+, Fe2 + and Fe3 + in biological systems. Coord. Chem. Rev. 522 https://doi.org/10.1016/j.ccr.2024.216201 (2025).
Jyothi Priya, M. et al. A simple fluorescent Turn-Off schiff base sensor for Cu2 + and Fe2 + ions and its applications in real water sample analysis and logic gate construction. Polyhedron 260 https://doi.org/10.1016/j.poly.2024.117110 (2024).
Bhalla, P., Malhotra, K., Tomer, N. & Malhotra, R. Binding interactions and sensing applications of Chromone derived schiff base chemosensors via absorption and emission studies: A comprehensive review. Inorg. Chem. Commun. 146 https://doi.org/10.1016/j.inoche.2022.110026 (2022).
Umare, M., Patel, D. A., Bhardwaj, V., Sk, A. K. & Sahoo, S. K. Pyridoxal derived AIEgen for fluorescence Turn-off sensing of Cu2 + and Fe2 + Ions and fluorescence imaging of latent fingerprints. J. Fluoresc. 33, 601–611. https://doi.org/10.1007/s10895-022-03109-8 (2023).
Santhoshkumar, S., Velmurugan, K., Prabhu, J., Radhakrishnan, G. & Nandhakumar, R. A naphthalene derived schiff base as a selective fluorescent probe for Fe2+. Inorganica Chim. Acta. 439, 1–7. https://doi.org/10.1016/j.ica.2015.09.030 (2016).
Kouser, R. et al. Turn–on benzophenone based fluorescence and colorimetric sensor for the selective detection of Fe2 + in aqueous media: validation of sensing mechanism by spectroscopic and computational studies. Spectrochim Acta Mol. Biomol. Spectrosc. 247 https://doi.org/10.1016/j.saa.2020.119156 (2021).
Rasin, P., Mathew, M. M., Manakkadan, V., Palakkeezhillam, V. N. V. & Sreekanth, A. A highly fluorescent Pyrene-Based sensor for selective detection of Fe3 + Ion in aqueous medium: computational investigations. J. Fluoresc. 32, 1229–1238. https://doi.org/10.1007/s10895-022-02940-3 (2022).
Basha, S. B., Charles, I. D., Raju, N., Manokaran, S. & Kuzhandaivel, H. An efficient 2-aminothiazolesalicylaldehyde fluorescent chemosensor for Fe2 + ion detection and a potential inhibitor of NUDT5 signaling hormone for breast cancer cell and molecular keypad lock application. Chem. Pap. 76, 7061–7073. https://doi.org/10.1007/s11696-022-02373-z (2022).
Khatun, S. et al. Highly chemoselective turn-on fluorescent probe for ferrous (Fe2+) ion detection in cosmetics and live cells. J. Photochem. Photobiol B. 209 https://doi.org/10.1016/j.jphotobiol.2020.111943 (2020).
Zhang, C., Pan, G. & He, Y. Conjugated microporous organic polymer as fluorescent chemosensor for detection of Fe3 + and Fe2 + ions with high selectivity and sensitivity. Talanta 236 https://doi.org/10.1016/j.talanta.2021.122872 (2022).
Punia, P., Bharti, M. K., Dhar, R., Thakur, P. & Thakur, A. Recent advances in detection and removal of heavy metals from contaminated water. ChemBioEng Reviews. 9, 351–369. https://doi.org/10.1002/cben.202100053 (2022).
March, G., Nguyen, T. & Piro, B. Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosens. (Basel). 5, 241–275. https://doi.org/10.3390/bios5020241 (2015).
Evans, E. H. et al. Atomic spectrometry update. Advances in atomic emission, absorption and fluorescence spectrometry, and related techniques. J. Anal. Spectrom. 20, 562. https://doi.org/10.1039/b505062f (2005).
Manousakas, M., Papaefthymiou, H., Eleftheriadis, K. & Katsanou, K. Determination of water-soluble and insoluble elements in PM2.5 by ICP-MS. Sci. Total Environ. 493, 694–700. https://doi.org/10.1016/j.scitotenv.2014.06.043 (2014).
Pendleton, M. W., Washburn, D. K., Ann Ellis, E. & Pendleton, B. B. Comparing the Detection of Iron-Based Pottery Pigment on a Carbon-Coated Sherd by SEM-EDS and by Micro-XRF-SEM, (2014).
Väisänen, A., Laatikainen, P., Ilander, A. & Renvall, S. Determination of mineral and trace element concentrations in pine needles by ICP-OES: evaluation of different sample pre-treatment methods. Int. J. Environ. Anal. Chem. 88, 1005–1016. https://doi.org/10.1080/03067310802308483 (2008).
Gumpu, M. B., Sethuraman, S., Krishnan, U. M. & Rayappan, J. B. B. A review on detection of heavy metal ions in water – An electrochemical approach. Sens. Actuators B Chem. 213, 515–533. https://doi.org/10.1016/j.snb.2015.02.122 (2015).
Nayan Kumar, H. N. et al. Recent advances in on-site monitoring of heavy metal ions in the environment. Microchem. J. 182, 107894. https://doi.org/10.1016/j.microc.2022.107894 (2022).
Wang, J., Wei, T., Ma, F., Li, T. & Niu, Q. A novel fluorescent and colorimetric dual-channel sensor for the fast, reversible and simultaneous detection of Fe3 + and Cu2 + based on terthiophene derivative with high sensitivity and selectivity. J. Photochem. Photobiol Chem. 383 https://doi.org/10.1016/j.jphotochem.2019.111982 (2019).
Hu, Y., Chen, A., Kong, Z. & Sun, D. A reversible colorimetric and fluorescence turn-off chemosensor for detection of Cu2 + and its application in living cell imaging. Molecules 24 https://doi.org/10.3390/molecules24234283 (2019).
Rupa, S. A. et al. Synthesis of a novel hydrazone-based compound applied as a fluorescence turn-on chemosensor for iron(iii) and a colorimetric sensor for copper(ii) with antimicrobial, DFT and molecular Docking studies. RSC Adv. 13, 23819–23828. https://doi.org/10.1039/d3ra04364a (2023).
Pang, Y. et al. Schiff base compounds as fluorescent probes for the highly sensitive and selective detection of Al3 + Ions. Molecules 28 https://doi.org/10.3390/molecules28073090 (2023).
Tomer, N. & Malhotra, R. Schiff base as a fluorescent sensor derived from Chromone moiety for the effective detection of Zn (II) ions. J. Mol. Struct. 1252 https://doi.org/10.1016/j.molstruc.2021.132124 (2022).
Yang, J. Y., Han, J. H., Bin Shang, Z., Wang, Y. & Shuang, S. M. New schiff base probe for the fluorometric turn-on sensing of Cd2 + ions and bio-imaging application. J. Lumin. 249 https://doi.org/10.1016/j.jlumin.2022.119017 (2022).
Chethanakumar, M. et al. Luminescent pyrene-based schiff base receptor for hazardous Mercury(II) detection demonstrated by cell imaging and test strip. J. Fluoresc. 33, 539–551. https://doi.org/10.1007/s10895-022-03066-2 (2023).
Bhorge, Y. R. et al. A new pyrene-based Schiff-base: A selective colorimetric and fluorescent chemosensor for detection of Cu(II) and Fe(III). Spectrochim Acta Mol. Biomol. Spectrosc. 130, 7–12. https://doi.org/10.1016/j.saa.2014.03.110 (2014).
Rout, K., Manna, A. K., Sahu, M. & Patra, G. K. A guanidine based Bis schiff base chemosensor for colorimetric detection of Hg(II) and fluorescent detection of Zn(II) ions. Inorganica Chim. Acta. 486, 733–741. https://doi.org/10.1016/j.ica.2018.11.021 (2019).
Gopalakrishnan, A. K. et al. A novel imidazole bound schiff base as highly selective Turn-on fluorescence sensor for Zn2 + and colorimetric kit for Co2+. J. Fluoresc. 32, 189–202. https://doi.org/10.1007/s10895-021-02839-5 (2022).
Wang, S., Men, G., Zhao, L., Hou, Q. & Jiang, S. Binaphthyl-derived Salicylidene schiff base for dual-channel sensing of cu, Zn cations and integrated molecular logic gates. Sens. Actuators B Chem. 145, 826–831. https://doi.org/10.1016/j.snb.2010.01.060 (2010).
Tang, L., Li, F., Liu, M. & Nandhakumar, R. Single sensor for two metal ions: colorimetric recognition of Cu 2 + and fluorescent recognition of Hg2+. Spectrochim Acta Mol. Biomol. Spectrosc. 78, 1168–1172. https://doi.org/10.1016/j.saa.2010.12.072 (2011).
Bhalla, P., Goel, A., Tomer, N. & Malhotra, R. Multi responsive chemosensor for the determination of metal ions (Co2+, Cu2+, and Zn2 + ions). Inorg. Chem. Commun. 136 https://doi.org/10.1016/j.inoche.2021.109181 (2022).
Tomer, N., Goel, A., Bhalla, P., Bhagat, P. & Malhotra, R. Chromone derived effective probe for the detection of metal ion (Cu2+) and chemical explosive (p-nitrotoluene). J. Photochem. Photobiol Chem. 427 https://doi.org/10.1016/j.jphotochem.2022.113823 (2022).
Gowda, B. G. et al. A highly selective schiff base chemosensor for rapid colorimetric detection of Fe2 + and fluorometric detection of Al3 + ions: synthesis, characterization, real water sample analysis and DFT studies. J. Mol. Struct. 1339 https://doi.org/10.1016/j.molstruc.2025.142378 (2025).
Frisch, M. J. et al. Gaussian, Inc., (2009). (n.d.).
Priya, N. P., Arunachalam, S., Manimaran, A., Muthupriya, D. & Jayabalakrishnan, C. Mononuclear Ru(III) schiff base complexes: synthesis, spectral, redox, catalytic and biological activity studies. Spectrochim Acta Mol. Biomol. Spectrosc. 72, 670–676. https://doi.org/10.1016/j.saa.2008.10.028 (2009).
Divyashree, N. R. et al. Highly selective and sensitive fluorescent TURN-ON furan-based schiff base for zinc(ii) ion probing: chemical synthesis, DFT studies, and X-ray crystal structure. New J. Chem. 47, 17420–17433. https://doi.org/10.1039/d3nj02466k (2023).
Liu, B., Zhuang, J. & Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano. 7, 2195–2213. https://doi.org/10.1039/d0en00449a (2020).
Hadi, H. et al. Design, synthesis, and density functional theory studies of a new selective chemosensor for Pb2+. Heliyon 9 https://doi.org/10.1016/j.heliyon.2023.e20206 (2023).
Goel, A., Tomer, N., Bhalla, P. & Malhotra, R. Pyranone based probe for the selective and specific recognition of zinc ions. Inorganica Chim. Acta. 534 https://doi.org/10.1016/j.ica.2022.120828 (2022).
Bhalla, P. et al. Chemoselective detection based on experimental and theoretical calculations of Cu2 + ions via deprotonation of Chromone derived probe and its application. J. Mol. Struct. 1264 https://doi.org/10.1016/j.molstruc.2022.133251 (2022).
Bhalla, P., Tomer, N., Bhagat, P. & Malhotra, R. Chromone functionalized pyridine chemosensor for cupric ions detection. Spectrochim Acta Mol. Biomol. Spectrosc. 264 https://doi.org/10.1016/j.saa.2021.120279 (2022).
Bhalla, P. & Malhotra, R. Optical chemosensor as a sensitive and selective tool for the detection of thiocyanate ions via Cu2 + Induced sensor and its practical application. J. Fluoresc. https://doi.org/10.1007/s10895-024-03943-y (2024).
Khairnar, N. et al. A highly selective fluorescent turn-on chemosensor for Zn2 + based on a benzothiazole conjugate: their applicability in live cell imaging and use of the resultant complex as a secondary sensor of CN-. Dalton Trans. 44, 2097–2102. https://doi.org/10.1039/c4dt03247k (2015).
De Costa, M. D. P. & Jayasinghe, W. A. P. A. Detailed studies on complexation behaviour and mechanism of fluorescene quenching of naphthalene linked hydroxmic acid Wirth transition metal ions by UV-visible and fluorescene spectra. J. Photochem. Photobiol Chem. 162, 591–598. https://doi.org/10.1016/S1010-6030(03)00419-2 (2004).
Chen, X. et al. Synthesis and application of purine-based fluorescence probe for continuous recognition of Cu2 + and glyphosate. Spectrochim Acta Mol. Biomol. Spectrosc. 304 https://doi.org/10.1016/j.saa.2023.123291 (2024).
Alorabi, A. Q., Zabin, S. A., Alam, M. M. & Abdelbaset, M. Schiff base ligand 3-(-(2-Hydroxyphenylimino) Methyl)-4H-Chromen-4-One as colorimetric sensor for detection of Cu2+, Fe3+, and V5 + in aqueous solutions. Genet. Res. (Camb). 2022. https://doi.org/10.1155/2022/4899145 (2022).
Alici, O. & Aydin, D. A Schiff-base receptor based on phenolphthalein derivate appended 2-furoic hydrazide: highly sensitive fluorogenic turn on chemosensor for Al3+. J. Photochem. Photobiol Chem. 404 https://doi.org/10.1016/j.jphotochem.2020.112876 (2021).
Divyashree, N. R. et al. Turn-ON’ furfurylamine-based fluorescent sensor for Cd2 + ion detection and its application in real water samples. Polyhedron 238 https://doi.org/10.1016/j.poly.2023.116411 (2023).
Sharma, V. et al. A new quinoline-based fluorescent-colorimetric chemosensor for sensitive and selective on-off detection of Pb2 + ions. RSC Adv. 15, 4236–4249. https://doi.org/10.1039/d4ra08193e (2025).
So, H., Lee, M. & Kim, C. A unique Thiosemicarbazide-Based colorimetric chemosensor for Fe2 + in pure aqueous solution with the lowest detection limit. ChemistrySelect 5, 10521–10525. https://doi.org/10.1002/slct.202002062 (2020).
Nagarajan, R., Kamaraj, E., Kim, C. H. & Lee, K. H. Novel Bis naphthalene-2-ol based colorimetric chemosensor for the detection of Fe2 + in physiological pH and its DFT calculation studies. Talanta Open. 6 https://doi.org/10.1016/j.talo.2022.100143 (2022).
Jung, J. M., Lee, S. Y. & Kim, C. A novel colorimetric chemosensor for multiple target metal ions Fe2+, Co2+, and Cu2 + in a near-perfect aqueous solution: experimental and theoretical studies. Sens. Actuators B Chem. 251, 291–301. https://doi.org/10.1016/j.snb.2017.05.055 (2017).
Kim, Y. S., Lee, J. J., Lee, S. Y., Jo, T. G. & Kim, C. A highly sensitive benzimidazole-based chemosensor for the colorimetric detection of Fe(II) and Fe(III) and the fluorometric detection of Zn(II) in aqueous media. RSC Adv. 6, 61505–61515. https://doi.org/10.1039/c6ra10086d (2016).
Luo, R. et al. A multifunctional off–on fluorescence probe for Al3+, Zn2 + and La3 + detection and cellular imaging applications. J. Iran. Chem. Soc. 20, 361–369. https://doi.org/10.1007/s13738-022-02672-y (2023).
Arabahmadi, R. & Kamali, S. Two-input combinatorial logic circuit as a Hydrazide schiff base fluorescence chemosensor based on excited state intramolecular proton transfer (ESIPT) for the detection of Al3+, and CN – in water sample: DFT calculations, and molecular Docking studies. J. Water Process. Eng. 76 https://doi.org/10.1016/j.jwpe.2025.108230 (2025).
Joshi, P.et al. Fluorescence modulation of naphthalene containing Salicyl hydrazide-based receptor through aggregation-induced emission enhancement approach: dual detection of lanthanum and cyanide ions in semi-aqueous medium. Luminescence 36, 986–994. https://doi.org/10.1002/bio.4025 (2021).
Dhanasekaran, K. & Napoleon, A. A. Pyrene derived Imine functionalized moiety for highly selective colorimetric detection of Cu2 + ion real time sample with supportive DFT studies. Inorg. Chem. Commun. 151 https://doi.org/10.1016/j.inoche.2023.110481 (2023).
Kumar, M. et al. N-diethylaminosalicylidene based turn-on fluorescent schiff base chemosensor for Al3 + ion: synthesis, characterisation and DFT/TD-DFT studies. J. Mol. Struct. 1247 https://doi.org/10.1016/j.molstruc.2021.131257 (2022).
Sharma, S., Chayawan, A., Jayaraman, J., Debnath, K. S. & Ghosh 2-Hydroxy-naphthalene hydrazone based dual-functional chemosensor for ultrasensitive colorimetric detection of Cu2 + and highly selective fluorescence sensing and bioimaging of Al3+. J. Photochem. Photobiol Chem. 437 https://doi.org/10.1016/j.jphotochem.2022.114408 (2023).
Musikavanhu, B. et al. Dual detection of Hg2 + and Pb2 + by a coumarin-functionalized schiff base in environmental and biosystems. Spectrochim Acta Mol. Biomol. Spectrosc. 313 https://doi.org/10.1016/j.saa.2024.124101 (2024).
Santra, A., Mishra, S., Panda, S. K. & Singh, A. K. ESIPT and PET-based easy-to-synthesize unsymmetrical ligand in the reversible fluorimetric sensing of Al3 + and relay sensing of inorganic and biological phosphates. Inorganica Chim. Acta. 537 https://doi.org/10.1016/j.ica.2022.120933 (2022).
Gupta, V. K., Jain, A. K. & Shoora, S. K. New on-off optical probe based on schiff base responding to Al3 + ions: logic gate application. Sens. Actuators B Chem. 219, 218–231. https://doi.org/10.1016/j.snb.2015.05.026 (2015).
Ghosh, K., Tarafdar, D., Samadder, A. & Khuda-Bukhsh, A. R. Piperazine-based simple structure for selective sensing of Hg2 + and glutathione and construction of a logic circuit mimicking an INHIBIT gate. New J. Chem. 37, 4206–4213. https://doi.org/10.1039/c3nj00855j (2013).
Sharma, S. et al. Method for highly selective, ultrasensitive fluorimetric detection of Cu2 + and Al3 + by schiff bases containing o-phenylenediamine and o-aminophenol. Methods 217, 27–35. https://doi.org/10.1016/j.ymeth.2023.06.013 (2023).
Sahoo, S. K. & Crisponi, G. Recent advances on iron(III) selective fluorescent probes with possible applications in bioimaging. Molecules 24 https://doi.org/10.3390/molecules24183267 (2019).
Zhang, X. et al. A simple turn-off schiff base fluorescent sensor for copper (II) ion and its application in water analysis. Molecules 26 https://doi.org/10.3390/molecules26051233 (2021).
Gupta, N., Singhal, D. & Singh, A. K. Highly selective colorimetric and reversible fluorometric turn-off sensors based on the pyrimidine derivative: mimicking logic gate operation and potential applications. New J. Chem. 40, 641–650. https://doi.org/10.1039/c5nj02118a (2016).
Fukui, K. Role of Frontier Orbitals in Chemical Reactions, Science 218 (1982) 747–754. (1979). https://doi.org/10.1126/science.218.4574.747
Sun, Y. X. et al. Experimental and density functional studies on 4-(3,4-dihydroxybenzylideneamino)antipyrine, and 4-(2,3,4-trihydroxybenzylideneamino)antipyrine. J. Mol. Struct. (Thoechem). 904, 74–82. https://doi.org/10.1016/j.theochem.2009.02.036 (2009).
Ardizzoia, G. A., Colombo, G., Therrien, B. & Brenna, S. Tuning the fluorescence emission and HOMO-LUMO band gap in homoleptic Zinc(II) complexes with N, O ‐Bidentate (Imidazo[1,5‐ a ]pyrid‐3‐yl)phenols. Eur. J. Inorg. Chem. 2019, 1825–1831. https://doi.org/10.1002/ejic.201900067 (2019).
Swamynayaka, A. et al. Third-order nonlinear optical studies of Bis(4-methylbenzylammonium) tetrachloridocuprate metal-organic crystal with optical limiting behavior: experimental and theoretical investigations. J. Mol. Struct. 1269, 133827. https://doi.org/10.1016/j.molstruc.2022.133827 (2022).
Ananda, S. et al. Structural, thermal, dielectric, nonlinear optical properties and DFT investigations of a novel material 2-(6-chloropyridin-3-yl)-N’-(2,3-dihydro-1,4-benzodioxin-6-ylmethylidene)acetohydrazide for optoelectronic applications. J. Mater. Sci.: Mater. Electron. 32, 14677–14702. https://doi.org/10.1007/s10854-021-06025-y (2021).
Huang, Y., Rong, C., Zhang, R. & Liu, S. Evaluating frontier orbital energy and HOMO/LUMO gap with descriptors from density functional reactivity theory. J. Mol. Model. 23, 3. https://doi.org/10.1007/s00894-016-3175-x (2017).
Gowda, B. G. et al. Novel pyrene-based schiff base ligand as a fluorescent sensor for Al3 + and colorimetric sensor for Ni2 + ions detection: synthesis, photo-physical and cell imaging studies. Inorg. Chem. Commun. 167 https://doi.org/10.1016/j.inoche.2024.112814 (2024).
Gowda, B.G. et al. Turn-off pyrene-based ligand as a fluorescent sensor for the detection of Cu2+ and Fe2+ ions: synthesis and application in real water samples, logic gate construction, and Bio-Imaging. Chemosensors 12 https://doi.org/10.3390/chemosensors12060091 (2024).
Genc, H. N. et al. Naked-eye colorimetric and switch-on fluorescence chemosensor based on a Rhodamine derivative for Hg2+: smartphone device, test-kit and food sample applications. J. Photochem. Photobiol Chem. 438 https://doi.org/10.1016/j.jphotochem.2023.114558 (2023).
Bhalla, P., Bhagat, P. & Malhotra, S. A simple naphthaldehyde based sensor as optical and colorimetric for the detection of Hg2+/Cr3 + in real samples. J. Mol. Struct. 1282 https://doi.org/10.1016/j.molstruc.2023.135130 (2023).
Acknowledgements
One of the authors, Jyothi Priya M is thankful to the “Karnataka Science and Technology Promotion Society (KSTePS)”, Department of Science and Technology (DST), Government of Karnataka for the award of fellowship. The authors sincerely acknowledge the Director, Amrita Vishwa Vidyapeetham, Mysuru Campus for providing infrastructure facilities. The authors extend their appreciation to the Ongoing Research Funding Program (ORF-2025-734), King Saud University, Riyadh, Saudi Arabia for their support.
Funding
Declaration.
Research Funding Program (ORF-2025-734), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
J.P.M., H.D.R. S.P.K.: Conceptualization, Methodology, Investigation, Formal analysis; J.P.M., A.S., B.J., M.M., S.P.K.: Data curation, Investigations, Formal analysis, Writing- Original draft preparation. M.I, K.S.A., C.S., R.G.A., V.S., E.S., S.P.K.: Visualization, Project administration, validation. H.D.R., S.P.K.: Resources, Supervision, Writing- Reviewing and Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Priya, M.J., Revanasiddappa, H.D., Jayalakshmi, B. et al. A reversible eugenol derived colorimetric and fluorescence “turn-off” probe for Fe2+ and Pb2+ ions detection. Sci Rep 15, 31264 (2025). https://doi.org/10.1038/s41598-025-14725-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14725-2