Abstract
The relationship between CT-based body composition parameters and chemoradiotherapy outcomes in patients with esophageal squamous cell carcinoma (ESCC) is unclear. This study aimed to clarify the predictive value of myosteatosis and subcutaneous adipose tissue area (SATA) in ESCC patients undergoing chemoradiotherapy. The study cohort consisted of 255 ESCC patients undergoing chemoradiotherapy between January 2012 and December 2018. Body composition parameters, such as mean muscle density in Hounsfield units (HU) and adipose tissue area at the third lumbar vertebra (L3) level, were quantified on CT scans. Hazard ratios were estimated to establish the relationship between pretreatment skeletal muscle density (preSMD) and adipose tissue area with the overall survival (OS) rate. Optimal stratification was utilized to set threshold values. Kaplan–Meier plots and Cox proportional hazards models were developed to analyze survival distributions. Among 255 ESCC patients who received chemoradiotherapy, the median survival time was 24.3 months (95% CI 20.33–33.8). Multivariate analysis revealed that tumor length (HR = 1.547; 95% CI 1.115–2.145; P = 0.009), clinical stage (HR = 5.696; 95% CI 2.053–15.798; P < 0.001), and preSMD (HR = 1.528; 95% CI 1.079–2.169; P = 0.017) were independent indicators for OS. Additionally, SATA emerged as an independent predictor of preSMD (HR = 0.991; 95% CI 0.986–0.996; P = 0.038). A nomogram integrating preSMD, pretreatment subcutaneous adipose tissue area (preSATA), and independent prognostic factors effectively predicts the prognosis of ESCC patients, supplementing the TNM staging system. The study suggests that a prognostic model combining preSMD and preSATA effectively predicts the prognosis of ESCC patients undergoing chemoradiotherapy.
Similar content being viewed by others
Introduction
Esophageal cancer is a significant malignancy on a global scale, with over 570,000 new cases diagnosed annually. Particularly in China, it represents a significant health burden, accounting for 51% of global cases and 55% of deaths. These rates rank sixth and fifth among oncological diseases, respectively1,2. These statistics highlight the severity of esophageal cancer and demonstrate the geographical predominance of different tumor subtypes—adenocarcinoma in Western nations versus squamous cell carcinoma in China3,4.
While surgical resection is the treatment of choice for esophageal tumors, approximately 70% of patients are diagnosed at advanced stages, making them either ineligible for or reluctant to undergo surgery. In these cases, radical chemoradiotherapy is considered a standard care regimen5,6. This is despite the advent of novel, promising treatments, including molecular-targeted therapy and immunotherapy, which are yet to be established as the current standard of care7. Factors such as high tumor mutation burden, metabolic abnormalities, and elevated inflammation levels contribute to the worse prognosis in these patients8,9. Therefore, identifying patients who would benefit from standard treatments and developing individualized care plans to improve quality of life are considerable challenges.
Sarcopenia is a pathological condition characterized by decreased muscle strength, reduced muscle mass, and impaired physical functionality and is primarily observed in older adults10,11,12. A large-scale research has shown a significant prevalence of sarcopenia among cancer patients (12.5–72.2%), which is closely linked to the patient’s quality of life, responsiveness to treatment, and prognostic outcomes13. Myosteatosis is a pathological condition in which the fat content in skeletal muscle is abnormally increased. Imaging inspection technologies are used to quantitatively assess the density of skeletal muscle. Among various muscle-related parameters, skeletal muscle density (SMD) is one of the important indicators. Contemporary studies have demonstrated that a lower SMD has a detrimental effect on the survival rates of patients with lung, pancreatic, rectal, and ovarian cancers14,15,16,17,18.
Current studies on SMD in esophageal cancer patients undergoing chemoradiotherapy have not conclusively established the correlation between SMD and patient prognosis.
The role of adipose tissue in determining esophageal cancer outcomes has been recognized, albeit with some limitations19,20. Moreover, the prognostic value of integrated muscle density with adipose tissue content in chemoradiotherapy-treated esophageal squamous cell carcinoma (ESCC) patients remains unexplored.
Hence, this study endeavors to clarify the role of combined pretreatment SMD (preSMD) and the features of adipose tissue in ESCC patients receiving chemoradiotherapy. It aims to elaborate a novel prognostic scoring model based on these parameters for a more precise assessment of long-term treatment outcomes. Additionally, it seeks to evaluate the predictive role of body composition metrics at different treatment stages and their accuracy through subgroup comparative analyses. This approach is determined to employ more personalized diagnostic and therapeutic strategies for esophageal cancer patients.
Methods
Participant recruitment
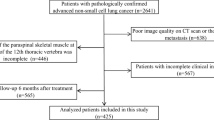
This investigation enrolled 255 patients diagnosed with ESCC who underwent radical chemoradiotherapy between January 2012 and December 2018. The inclusion criteria encompassed (1) histopathological confirmation of ESCC; (2) inoperability or refusal of surgical intervention; (3) Karnovsky Performance Status (KPS) score ≥ 70, a scale for evaluating patients’ functional status, widely used in clinical research to quantify patients’ ability to perform daily activities and self-care; (4) should have received a minimum dose of 40 Gy following radiotherapy; (5) availability of blood test results one week prior to initiating treatment; (6) absence of distant metastases; and (7) staging in accordance with the seventh edition of the American Joint Committee on Cancer (AJCC) TNM classification.
Treatment protocols
The selected participants were those undergoing radiotherapy; tumor targets and adjacent organs were delineated using spiral computed tomography (CT) scans. The treatment modalities included three-dimensional conformal or intensity-modulated radiotherapies, with a target of a prescribed dose range of 50–72 Gy distributed across 25–36 sessions and a total duration of 5–7 weeks. Dosage constraints were set to provide V20 ≤ 25% for the lungs, V30 ≤ 40% for the heart, V40 ≤ 30%, and a maximum spinal cord dose of ≤ 45.0 Gy. The administered chemotherapeutic regimens varied, covering 1–6 cycles of platinum-based treatments, either as monotherapy or in combination with other agents; the oral form of tigeol was administered to older adults, and raltitrexed was administered to those intolerant to first-line chemotherapy.
Body composition assessment
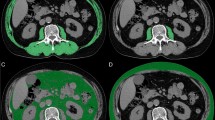
Body composition was analyzed with CT images obtained from the PACS system at the Sichuan Cancer Hospital Imaging Department. Two experienced radiologists, blinded to the clinical outcomes, utilized semiautomated SliceOmatic software (version 5.0; Tomovision, Montreal, QC, Canada) for performing measurements. The analysis was carried out by measuring the target areas on the cross-sectional images of the third lumbar vertebra (L3), including skeletal muscles (psoas major, quadratus lumborum, erector spinae, transverse abdominis, internal oblique, external oblique, and rectus abdominis), intra-muscular adipose tissue, subcutaneous adipose tissue and visceral adipose tissue. The thresholds were set as follows: − 29 to 150 Hounsfield units (HU, red), − 190 to − 30 HU (yellow), − 190 to − 30 HU (green), and − 150 to − 50 HU (blue), respectively. The total adipose tissue area (TATA) is obtained by adding the subcutaneous adipose tissue area (SATA) and the visceral adipose tissue area (VATA), which is helpful for assessing the distribution status of adipose tissue in the body (Fig. 1)21.
Quantitative assessment of body composition using computed tomography at the third lumbar vertebra (L3) level. Cross-sectional areas were measured for skeletal muscles (psoas major, quadratus lumborum, erector spinae, transverse abdominis, internal oblique, external oblique, and rectus abdominis), intramuscular adipose tissue, subcutaneous adipose tissue, and visceral adipose tissue. Tissue-specific Hounsfield unit (HU) thresholds were defined as follows: skeletal muscle (− 29 to + 150 HU, red), intramuscular adipose tissue (− 190 to − 30 HU, yellow), subcutaneous adipose tissue (− 190 to − 30 HU, green), and visceral adipose tissue (− 150 to − 50 HU, blue).
Monitoring and follow-up
The patients were monitored at regular follow-up periods: quarterly in the first year, biannually for the subsequent two years, and yearly thereafter. Follow-up assessments included physical examinations, blood tests, ultrasonography, tumor marker evaluations, chest CT imaging, and esophageal barium studies. Outpatient visits and telephone interviews both facilitated continuous patient tracking.
Statistical analysis
SPSS version 27.0 and RStudio version 4.3.1 were employed to carry out statistical analyses. The comparison of continuous variables with a normal distribution was done with a t-test or ANOVA, while the χ2 test was applied for that of categorical data. The log-rank test determined optimal stratification for continuous variables at different treatment stages, thus establishing cutoff values for SMD, SATA, VATA22. Patients were categorized into low or high groups based on these thresholds. Survival analysis involved the Kaplan–Meier method and log-rank test; uni- and multi-variate analyses were carried out with the Cox proportional hazards model. A significance level of α = 0.05 was set, with p < 0.05 considered statistically significant.
Ethical conduct of research
This study was approved by the Medical Ethics Committee of Sichuan Cancer Hospital and was carried out in accordance with the principles of the Helsinki Declaration and its amendments. Further, informed consent from all participants was waived by the lRB because ofthe retrospective nature of this study. We confirm that the data have been anonymized and confidentially analyzed.
Results
Characteristics of patients
A total of 255 patients with ESCC who fulfilled the inclusion criteria were recruited. The median age in the cohort was 64 years, ranging from 34 to 87 years. Among these patients, 2.4% (n = 6) exhibited a KPS score of 70, 42% (n = 107) presented with a score of 80, and 55.7% (n = 142) had a score of 90 or above; 37.6% (n = 96) had tumors primarily located in the midthoracic region, and 60.0% (n = 153) manifested with tumors exceeding 5 cm in length. Most of these patients were in clinical stages III–IV (n = 239; 96.7%); 84.7% (n = 216) underwent chemotherapy; the prevalence of sarcopenia was 23.1% (n = 59), with the remaining 76.9% (n = 196) not having sarcopenia. The median preSMD was 36.4 (interquartile range [IQR]: 28.65, 41.975), while that of preSATA was 86.1 (IQR: 57.75, 119.75); the median preVATA was 72 (IQR: 39.25, 123.75) (Table 1).
Analysis of survival
The median survival duration for the whole study population was 24.3 months (95% CI 20.33–33.8). Univariate analyses identified KPS, tumor length, clinical stage, body mass index (BMI), chemotherapy, and Sarcopenia as the factors playing a significant role in overall survival (OS) (all p-values < 0.05). Multivariate analysis revealed that KPS (hazard ratio [HR] = 0.294; 95% confidence interval [CI] = 0.122–0.711; P = 0.007), history of alcohol consumption (HR = 1.531; 95% CI 1.101–2.128; P = 0.011), tumor length (HR = 1.547; 95% CI 1.115–2.145; P = 0.009), clinical stage (HR = 5.696; 95% CI 2.053–15.798; P < 0.001), and preSMD (HR = 1.528; 95% CI 1.079–2.165; P = 0.017) were independent indicators for prediction of outcomes in ESCC patients (Table 2, Fig. 2).
Kaplan–Meier survival analysis stratified by body composition parameters before and after treatment. (A–C) Survival curves stratified by pre-treatment skeletal muscle density (SMD), subcutaneous adipose tissue area (SATA), and visceral adipose tissue area (VATA), respectively; (D–F) survival curves stratified by post-treatment SMD, SATA, and VATA, respectively.
Correlation between preSMD and pathological characteristics
This study compared the levels of oreSMD and preSATA across subgroups based on age, KPS, tumor length, and clinical stage, stratified by gender at different treatment stages, showing statistical significance (P < 0.05, Fig. 3). To assess the relationship between pathological characteristics and preSMD, univariate and multivariate logistic regression analyses were performed. Univariate analysis showed that gender, age, alcohol consumption history, clinical stage, BMI, SATA, and VATA were positively correlated with preSMD. Multivariate analysis revealed that age (HR = 0.962; 95% CI 0.940–0.986; P < 0.001), alcohol consumption history (HR = 2.255; 95% CI 1.297–3.917; P = 0.004), tumor length (HR = 1.518; 95% CI 1.030–2.236; P = 0.035), clinical stage (HR = 3.234; 95% CI 1.374–7.612; P = 0.007), BMI (HR = 0.890; 95% CI 0.805–0.984; P = 0.022), and preSATA (HR = 1.725; 95% CI 1.072–2.775; P = 0.025) were independent predictors of preSMD (Table 3, Fig. 4).
Comparative analysis of SMD and SATA distribution across different subgroups. (A,B) Box-and-whisker plots showing the distribution of skeletal muscle density (SMD) across subgroups before and after treatment, respectively; (C,D) Distribution of subcutaneous adipose tissue area (SATA) across subgroups before and after treatment, respectively. KPS: Karnofsky performance status score.
Correlation analysis between pre-treatment SMD and clinical parameters. (A) Hierarchical clustering heat map depicting correlations among all variables; (B–H) Pearson correlation analyses between pre-treatment SMD and skeletal muscle index (SMI), visceral adipose tissue area (VATA), subcutaneous adipose tissue area (SATA), total adipose tissue area (TATA), hemoglobin levels, body mass index (BMI), and age, respectively.
Development of a prognostic score model based on preSMD and preSATA
To further segregate patients based on treatment outcomes, we incorporated levels of preSMD and preSATA in the model to create four subgroups. In model 1, patients with preSMD < 37.3 and preSATA < 65 manifested the shortest survival rate, whereas in model 4, patients with preSMD ≥ 37.3 and preSATA ≥ 65 were characterized by the longest survival rate (p = 0.004, Fig. 4). Thus, we combined these two metrics to form the preSMD-SATA index, defined as the product of preSMD and preSATA. A log-rank test for optimal stratification resulted in a cutoff value of 2,609.82, with high preSMD-SATA ≥ 2,609.82 (n = 103; 40.4%) and low preSMD-SATA < 2,609.82 (n = 152; 59.6%). Clinicopathological factors significantly associated with preSMD-SATA included age, BMI, chemotherapy, and Skeletal muscle index (SMI) (all p-values < 0.05, Table 4). Additionally, survival analysis based on preSMD-SATA classification demonstrated significantly better outcomes for the high preSMD-SATA group compared to the low preSMD-SATA group (both p-values < 0.05, Fig. 5).
Impact of pre-treatment SMD-SATA combinations on overall survival. (A) Kaplan–Meier survival curves stratified by four distinct SMD-SATA phenotypes: Model 1 (pre-treatment SMD < 37.3 HU and SATA < 65 cm2), Model 2 (pre-treatment SMD ≥ 37.3 HU and SATA < 65 cm2), Model 3 (pre-treatment SMD < 37.3 HU and SATA ≥ 65 cm2), and Model 4 (pre-treatment SMD ≥ 37.3 HU and SATA ≥ 65 cm2); (B-C) Survival curves comparing pre- and post-treatment SMD-SATA combinations, respectively.
Construction of a risk score prognostic model and prediction nomogram
We developed a prognostic model based on multifactorial Cox regression to enhance the quantitative analysis of treatment outcomes. The risk scores were positively associated with poorer prognoses. Analysis of the prognostic heatmap and survival curves, including time-dependent survival curves for various risk scores, revealed significant differences (p < 0.05), indicating higher risk scores in patients were linked to lower survival rates. The prognostic receiver operating characteristic curves demonstrated that the 1-, 3-, and 5-year area under the curve (AUC) values were 68.8, 70.5, and 73.1, respectively, thus substantially outperforming the conventional TNM staging system. Furthermore, for the purpose of precisely forecasting the prognosis of ESCC patients subjected to chemoradiotherapy, we constructed a nomogram.Through the multivariate analysis implemented by the COX risk regression model, we ascertained independent and prominently significant prognostic determinants, namely KPS, Alcohol history, Tumor length, Clinical stage, and preSMD, with a P-value < 0.05. These factors were then integrated into the nomogram as cardinal variables and and serve as an effective complement to the TNM staging system (Fig. 6).
Development and validation of ECSS prognostic risk score model incorporating pre-treatment SMD-SATA parameters. (A) Forest plot depicting risk factors in the prognostic model with hazard ratios and 95% confidence intervals; (B) time-dependent receiver operating characteristic (ROC) curves with area under the curve (AUC) values; (C) risk-stratified survival curves based on the derived risk score; (D–F) decision curve analyses for 1-year, 3-year, and 5-year survival predictions, respectively; (G) prognostic nomogram incorporating independent predictive factors; (H) calibration plots comparing predicted versus observed survival probabilities.
Discussion
Since Rosenberg et al. first proposed the concept, sarcopenia has been universally recognized as an age-related pathological condition with decreased muscle mass and strength. These changes may lead to grave consequences, including falls and disabilities10,23. Recent investigations have emphasized the high incidence of sarcopenia among cancer patients, its negative impact on quality of life, and its significant association with the adverse effects of anticancer treatments and overall prognosis24,25,26. Our study demonstrates a comprehensive analysis of the prognostic significance of preSMD and adipose tissue in predicting the clinical outcomes of esophageal cancer patients undergoing chemoradiotherapy. The results showed that body composition parameters significantly correlated with OS (P < 0.05). Through multivariate analysis, preSMD was outlined as an independent predictor of survival in patients with ESCC. Additionally, preSATA was found to be significantly associated with preSMD. A prognostic model was developed by combining preSMD and preSATA and visualized using a nomogram to enhance the differentiation of patient groups based on their prognostic prospects.
To the best of our knowledge, existing literature primarily focuses on the prognostic implications of SMI in ESCC patients, thus revealing a lack of standardized criteria for prognostic evaluations of SMI and SMD in ESCC patients27,28,29. An observational study found that 16.5% of patients who underwent radical esophagectomy had skeletal muscle atrophy, while 51.8% had musculoskeletal lipolysis, further confirming that skeletal muscle atrophy and intramuscular fat deposition are intricately connected with diminished OS rates27. The results of this research reflected a correlation between SMI and OS in survival analysis (p = 0.013); however, in the multivariate analysis, SMI was not found to be an independent prognostic factor. Conversely, SMD was shown to be a significant prognostic determinant for ESCC patients undergoing radiotherapy and chemotherapy. While skeletal muscle atrophy and intramuscular fat deposition serve as indicators of sarcopenia, these conditions exhibit distinct features. Skeletal muscle atrophy primarily leads to a decrease in muscle mass, adversely affecting a patient’s physical endurance, disease resistance, and recuperative capacity. Patients with low SMD exhibit significant myosteatosis within skeletal muscles and structural degeneration, which leads to the disorder of muscle fiber arrangement and mitochondrial dysfunction, ultimately resulting in systemic metabolic disorders. In addition, this condition can trigger chronic low-grade inflammatory responses, manifested as the continuous elevation of pro-inflammatory factors accompanied by an increase in oxidative stress, which accelerates protein degradation, ultimately leading to a decline in the quality of life, poor treatment tolerance, and poor prognosis of patients30,31.
A prospective clinical study conducted on patients with advanced esophageal and gastric cancers receiving first-line palliative chemotherapy showed an association between SMD and an increased occurrence of grade 3–4 toxicities. However, no significant correlation between low SMD and survival outcomes was detected (p = 0.787)25. To elucidate the prognostic significance of SMD in esophageal cancer, we examined several potentially involved factors. First, tumor burden variability was assessed. Contrary to prior studies, which focused on advanced esophageal and gastric cancer patients who typically exhibited compromised physical conditions, we found that the extent of tumor burden and therapeutic efficacy were pivotal points in determining survival rates. Second, the histological subtype of esophageal cancer proved to be a critical determinant: most patients in the referenced studies had adenocarcinoma (83%), whereas our research exclusively covered squamous cell carcinoma cases (100%). Esophageal adenocarcinoma is strongly associated with obesity-related dietary habits and gastroesophageal reflux disease, whereas ESCC is more commonly linked to smoking, alcohol consumption, and elevated intake of hot beverages32. Lastly, the selection of SMD cutoff values appears to be a crucial factor in explaining outcome disparities. Because of the variations in body composition, the threshold values for SMI and SMD differ. For instance, a study by Tamandl et al. on postsurgical esophageal cancer patients indicated significantly poorer OS rates for those with SMD < 40 HU33. Considering the generally lower involvement of Asian populations in our study compared to that of European and American cohorts, this investigation adopted SMI cutoffs adjusted to Asians: 40.8 cm2/m2 for men and 34.9 cm2/m2 for women, with a pretreatment sarcopenia incidence of 23.1% (n = 59)26. The selected SMD cutoff composed 37.3 HU, revealing a musculoskeletal liposis prevalence of 54.1% that aligned with prior findings30. Nonetheless, these cutoff values warrant further validation across broader populations, including ESCC patients of diverse ethnic backgrounds and exhibiting various pathological types, to ascertain the most effective thresholds.
In terms of adipose tissue analysis, a retrospective examination of 485 advanced gastric cancer patients identified that a reduction in preoperative visceral adipose tissue served as an independent prognostic postsurgical factor19. Han et al.34 applied a deep learning algorithm to differentiate subcutaneous adipose tissue (SAT) and visceral adipose tissue in lumbar spine CT scans. They discovered that the SAT index in cachexic patients had significantly lower values than in their noncachexic counterparts and that this correlation was associated with shorter survival rates among cachexia patients with low SAT indices. This is consistent with our findings displaying that higher levels of visceral and subcutaneous fat relate to higher OS. Moreover, preSATA appeared to be a predictive marker for preSMD, suggesting that integrating SMD with SATA enhances prognostic accuracy, yet another study mentioned no significant impact of subcutaneous fat area index and visceral fat area index on OS and progression-free survival rates28. While obesity is known to be involved in the development of various cancers35, the underlying mechanisms remain poorly understood. Notably, esophageal cancer is frequently observed to occur concomitantly with malnutrition; adipose tissue serves as a crucial energy reserve, reflecting nutritional status. Persistent energy underconsumption exacerbates malnutrition risk, causing progressive depletion of muscle and fat mass36,37. Based on the above research results, it is postulated that preSMD holds the potential to serve as a cardinal reference index for pre-treatment risk stratification in cancer patients. Specifically, quantitative determination of skeletal muscle density by local CT images before radiotherapy can help identify high-risk patients, thereby facilitating the optimization of treatment strategies. For example, for patients with reduced preSMD, preoperative nutritional supplementation, anti-inflammatory treatment, and exercise rehabilitation can be performed to improve skeletal muscle condition and enhance patients’ tolerance to subsequent treatment methods. In addition, patients with lower preSMD may require more stringent monitoring during radiotherapy and personalized treatment and rehabilitation blueprints to reduce the incidence of complications and improve long-term survival outcomes. Future studies can further analyze the specific advantages of interventions in improving the prognosis of patients with reduced preSMD.
Nonetheless, this study is subject to limitations. Being a retrospective study design, it primarily relied on imaging assessments of body composition without concurrent evaluation of muscle function. Additionally, potential biases are present due to the limited sample size and single-center nature of the study. Future validation through multicenter, prospective studies employing larger cohorts is essential. Furthermore, the specific interplay between sarcopenia and muscle density requires deeper analysis.
This research represents a pioneering effort to consolidate preSMD and preSATA as combined body composition parameters in the evaluation of ESCC patients receiving chemoradiotherapy. It emphasizes the necessity for meticulous assessment and early intervention for patients falling into the high-risk category according to these parameters.
Conclusions
These findings highlight the need for additional clinical studies to elucidate the impact of body composition metrics on ESCC prognosis, facilitating the development of more personalized and effective therapeutic strategies.
Data availability
The data supporting the findings of this study will be available upon reasonable written request approved by the corresponding author.
Abbreviations
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CT:
-
Computed tomography
- ESCC:
-
Esophageal squamous cell carcinoma
- HU:
-
Hounsfield units
- KPS:
-
Karnofsky performance status
- OS:
-
Overall survival
- preSMD:
-
Pretreatment skeletal muscle density
- SMD:
-
Skeletal muscle density
- SMI:
-
Skeletal muscle index
- SATA:
-
Subcutaneous adipose tissue area
- TATA:
-
Total adipose tissue area
- VATA:
-
Visceral adipose tissue area
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
Han, B. et al. Cancer incidence and mortality in China, 2022. J. Natl. Cancer Center. 1, 1 (2024).
Castro, C. et al. Patterns and trends in esophageal cancer mortality and incidence in Europe (1980–2011) and predictions to 2015. Ann. Oncol. 25(1), 283–290 (2014).
Kamangar, F. et al. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet Gastroenterol. Hepatol. 5(6), 582–597 (2020).
van Hagen, P. et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 366(22), 2074–2084 (2012).
Lordick, F., Mariette, C., Haustermans, K., Obermannová, R. & Arnold, D. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27, v50–v57 (2016).
Triantafyllou, T. & Wijnhoven, B. P. L. Current status of esophageal cancer treatment. Chin J. Cancer Res. 32(3), 271–286 (2020).
Xie, H. et al. Comprehensive comparison of the prognostic value of systemic inflammation biomarkers for cancer cachexia: A multicenter prospective study. Inflamm. Res. 71(10–11), 1305–1313 (2022).
Allen, B. M. et al. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat. Med. 26(7), 1125–1134 (2020).
Cruz-Jentoft, A. J. et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 48(1), 16–31 (2019).
Chen, L.-K. et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Directors Assoc. 21(3), 300–307 (2020).
Yuan, S. & Larsson, S. C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 155, 533 (2023).
Shachar, S. S., Williams, G. R., Muss, H. B. & Nishijima, T. F. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur. J. Cancer 57, 58–67 (2016).
Aleixo, G. et al. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 145, 102839 (2020).
van Dijk, D. P. et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J. Cachexia. Sarcopenia Muscle 8(2), 317–326 (2017).
Blauwhoff-Buskermolen, S. et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J. Clin. Oncol. 34(12), 1339–1344 (2016).
Sjoblom, B. et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin. Nutr. 35(6), 1386–1393 (2016).
van der Zanden, V. et al. Low preoperative skeletal muscle density is predictive for negative postoperative outcomes in older women with ovarian cancer. Gynecol. Oncol. 162(2), 360–367 (2021).
Matsui, R., Inaki, N. & Tsuji, T. Impact of visceral adipose tissue on long-term outcomes after gastrectomy for advanced gastric cancer. Nutrition 97, 111619 (2022).
Devericks, E. N., Carson, M. S., McCullough, L. E., Coleman, M. F. & Hursting, S. D. The obesity-breast cancer link: A multidisciplinary perspective. Cancer Metastasis Rev. 41(3), 607–625 (2022).
Kazemi-Bajestani, S. M. R., Mazurak, V. C., & Baracos, V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Seminars in cell & developmental biology. Academic Press 54, 2–10 (2016).
Zhuang, C. L. et al. Associations of low handgrip strength with cancer mortality: A multicentre observational study. J. Cachexia. Sarcopenia Muscle 11(6), 1476–1486 (2020).
Rosenberg, I. H. Sarcopenia: Origins and clinical relevance. J. Nutr. 127(5), 990S-S991 (1997).
Nipp, R. D. et al. Sarcopenia is associated with quality of life and depression in patients with advanced cancer. Oncologist 23(1), 97–104 (2018).
Dijksterhuis, W. P. M. et al. Association between body composition, survival, and toxicity in advanced esophagogastric cancer patients receiving palliative chemotherapy. J. Cachexia Sarcopenia Musc. 10(1), 199–206 (2019).
Chen, X.-Y. et al. Sarcopenia is an effective prognostic indicator of postoperative outcomes in laparoscopic-assisted gastrectomy. Eur. J. Surg. Oncol. 45(6), 1092–1098 (2019).
Srpcic, M., Jordan, T., Popuri, K. & Sok, M. Sarcopenia and myosteatosis at presentation adversely affect survival after esophagectomy for esophageal cancer. Radiol. Oncol. 54(2), 237–246 (2020).
Qian, J. et al. Sarcopenia is associated with prognosis in patients with esophageal squamous cell cancer after radiotherapy or chemoradiotherapy. BMC Gastroenterol. 22(1), 211 (2022).
Mallet, R. et al. Prognostic value of sarcopenia in patients treated by radiochemotherapy for locally advanced oesophageal cancer. Radiat. Oncol. 15(1), 116 (2020).
Henin, G., Loumaye, A., Leclercq, I. A. & Lanthier, N. Myosteatosis: Diagnosis, pathophysiology and consequences in metabolic dysfunction-associated steatotic liver disease. JHEP Rep. 100, 963 (2023).
Zoico, E. et al. Myosteatosis and myofibrosis: Relationship with aging, inflammation and insulin resistance[J]. Arch. Gerontol. Geriatr. 57(3), 411–416 (2013).
Rustgi, A. K. & El-Serag, H. B. Esophageal carcinoma. N. Engl. J. Med. 371(26), 2499–2509 (2014).
Tamandl, D. et al. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur. Radiol. 26(5), 1359–1367 (2016).
Han, J. et al. Subcutaneous, but not visceral, adipose tissue as a marker for prognosis in gastric cancer patients with cachexia. Clin. Nutr. 40(9), 5156–5161 (2021).
Colditz, G. A. & Peterson, L. L. Obesity and cancer: Evidence, impact, and future directions. Clin. Chem. J. Am. Assoc. Clin. Chem. 64(1), 154–62 (2018).
Kiss, N. et al. Low muscle mass, malnutrition, sarcopenia, and associations with survival in adults with cancer in the UK Biobank cohort. J. Cachexia Sarcopenia Musc. 1, 1 (2023).
Movahed, S. et al. Comprehensive assessment of nutritional status and nutritional-related complications in newly diagnosed esophageal cancer patients: a cross-sectional study. Clin. Nutr. 40(6), 4449–4455 (2021).
Acknowledgements
We would like to thank Enago (http://www.enago.cn) for the manuscript review and editing support.
Funding
This work was supported by grants from the Project of Wu Jieping Medical Foundation, 320.6750
Author information
Authors and Affiliations
Contributions
L.X., Y.L., H.L. and T.L. spearheaded the study’s conceptualization and design, oversaw data collection and analysis, and were instrumental in drafting and revising the manuscript. XZ and XN significantly contributed to the assessment of body composition and the recording of data for the manuscript. TL was responsible for securing project funding. All authors have reviewed and given their approval to the final manuscript version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was approved by the Medical Ethics Committee of Sichuan Cancer Hospital. Since data were evaluated retrospectively, pseudonymously and were solely obtained for treatment purposes, a requirement of informed consent was waived by the Ethics Committee of Sichuan Cancer Hospital. All patient data were handled in compliance with privacy protection regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiao, L., Liu, Y., Zhang, X. et al. Predictive value of myosteatosis and subcutaneous adipose tissue on the prognosis of ESCC patients undergoing chemoradiotherapy. Sci Rep 15, 30529 (2025). https://doi.org/10.1038/s41598-025-14769-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14769-4