Abstract
2. Amitriptyline, a tricyclic antidepressant, is associated with various metabolic side effects, including increased appetite and weight gain. In this study, we used the nematode C. elegans to investigate the drug’s effects on food intake, fat content, lifespan, and several genetic pathways. Amitriptyline increased food intake in a dose-responsive manner. It increased the food intake primarily through elevated on-food pharyngeal pumping. Supplementation with glucose exacerbated these effects and significantly shortened lifespan, mimicking metabolic risks seen in humans. Despite increased feeding, no changes in fat content were observed, reflecting distinct mechanisms regulating feeding and fat storage. The critical function of prdx-2 in the drug’s effect on food intake was revealed through genetic analyses. In contrast, several receptors and signaling components in the serotonergic, dopaminergic, and insulin-like pathways were not implicated. These results underscore the potential of C. elegans as a model for investigating antidepressant-induced metabolic alterations and establish the foundation for strategies to mitigate these effects.
Similar content being viewed by others
Introduction
Amitriptyline is a tricyclic antidepressant (TCA) that acts primarily as a reuptake inhibitor for the neurotransmitters serotonin and norepinephrine1. In clinical practices, prescribing amitriptyline is not only limited to mood disorders but is also used in patients suffering from neuropathic pain2. Interestingly, several studies have linked the use of TCAs, including amitriptyline, to serious adverse effects such as an increased risk of diabetes, cardiovascular diseases, and overall mortality3,4,5. Additionally, weight gain and various alterations in glucose metabolism are two of the main metabolic complaints6,7. As reported in previous clinical trials, patients treated with TCAs such as amitriptyline showed significant increases in their weight. This increase in patients’ weight was observed mostly during the first few months of treatment6,7,8. In addition, many patients on TCAs reported increased cravings for carbohydrates and sweets. These macronutrients can significantly increase fat storage due to the insulin spikes they induce9. Therefore, long-term use of the drug raises concerns about the development of obesity and type 2 diabetes8,10. This strongly indicates that modifications to the diet should be considered to mitigate the adverse metabolic effects of amitriptyline.
One of the mechanisms that might be involved in the increased appetite and weight gain induced by TCAs is their impact on neurotransmitter systems11. These drugs are known to elevate serotonin levels in the brain by blocking the reuptake of both serotonin and norepinephrine. Elevated levels of serotonin have been linked to increased appetite and carbohydrate cravings11,12,13. Additionally, TCAs may lead to increased appetite and weight gain by elevating cortisol levels through disruption of the hypothalamic-pituitary-adrenal (HPA) axis13,14. Furthermore, TCAs can cause weight gain and metabolic disturbance by impairing insulin sensitivity leading to insulin resistance and diabetes. TCAs can also influence the dopaminergic signaling pathway, potentially leading to increased food intake and reward-seeking behaviors15. Furthermore, TCAs may contribute to oxidative stress16which can further exacerbate weight gain17. While the precise mechanisms underlying the increased appetite and weight gain induced by TCAs such as amitriptyline are complex, understanding these pathways is essential for developing strategies to mitigate this adverse effect and improve patient outcomes.
In order to gain a deeper insight into the mechanisms responsible for the metabolic alterations caused by drugs such as TCAs, the nematode Caenorhabditis elegans (C. elegans) has become an effective model for testing drugs and assessing their toxicity18,19. C. elegans, with its remarkably short lifespan, serves as an excellent model for studying the effects of drugs across various stages of life. In addition, the genome of this nematode is simple, fully sequenced, and homologous to humans, which forms an excellent model for studying human diseases20. As a result, C. elegans has gained attention recently in studying the adverse effects of Psychotropic Drugs (PDs), mainly concerning feeding behavior and fat metabolism21,22,23. It provides valuable insight into the role of certain pathways in the regulation of food consumption and fat synthesis. Interestingly, previous studies in C. elegans showed that serotonin, dopamine, and insulin signaling pathways play an important role in regulating food intake24,25,26reflecting similar regulation of food intake in humans15. All of these characteristics make this model ideal for examining the metabolic effects of medications utilized in humans.
In this research, we aimed to assess the impact of amitriptyline on C. elegans to better understand the mechanisms underlying its effects on humans. Therefore, we used wild-type C. elegans and certain mutants that have defects in certain signaling pathways. The main goal was to understand the drug’s effects on several physiological parameters including food intake, fat storage, lifespan, and general metabolic health in C. elegans. Moreover, we modified the standard OP50 bacterial diet by supplementing it with glucose to establish a high-carb diet that emulates the dietary conditions associated with human metabolic disorders.
Methods
Strains and media
The reference strain used throughout the study was wild-type Bristol N2, along with additional strains including daf-2(e1370) [CB1370], daf-16(mu86) [CF1038], ser-1(ok345) [DA1814], ser-2(ok2103) [RB1690], ser-7(ok1944) [RB1585], prdx-2(gk169) [VC289], and dop-1(vs101) [LX636]. Worms were obtained from the Caenorhabditis Genetics Center (CGC). The worms are also incubated at 20 °C and maintained on standard Nematode Growth Medium (NGM) plates as previously described by Brenner (1974)27. To prevent contamination, antifungals (0.5 ml of Nystatin suspension 10,000 U/mL) and antibiotics (100 µL of 100 mg/mL Ampicillin) were added to 100 mL of NGM. All feeding-related experiments, including pharyngeal pumping, bacterial consumption, and fat staining, were conducted using live E. coli OP50 on standard NGM plates without ampicillin or antifungal agents. Antibiotics and antifungals were limited to general worm maintenance and lifespan experiments only. Worms were cultured by feeding on Escherichia coli OP50, as described by Brenner (1974)27.
Drug treatments and Bacterial-Modified diet
Amitriptyline was obtained from a local pharmacy (amitriptyline hydrochloride 50 mg) and used in different concentrations (50 µM, 200 µM, 500 µM, and 1000 µM). Stock solutions of the drug were prepared for each replicate by dissolving it in distilled water containing 2% DMSO. The stock solutions were then diluted to the desired concentrations in distilled water with 2% DMSO. High-Glucose Diet (HGD) treatments were prepared by adding glucose as a 73 mM solution to the NGM plates. The treatments were introduced to the worms by spotting the solution onto the surface of the NGM plates with an OP50 lawn, in a ratio of 1:3 (OP50:treatment). Control groups received only distilled water with 2% DMSO, while HGD-only groups received distilled water with 2% DMSO and glucose supplementation.
Fat staining
Fat staining was conducted with minor modifications following a common protocol28. using 0.5% Oil-Red O (ORO). ORO stock solution was prepared by dissolving 500 mg of ORO powder in 100 mL of 100% isopropanol. Before the experiment, ORO working solution was then prepared by diluting the stock solution with water (3:2 ratio) to make 60% isopropanol. The working solution was filtered with a 0.2 μm syringe filter. Worms were treated from the L1 larval stage with various concentrations of the drugs and then stained at the L4 stage to measure body fat. Color density of the stained worms was quantified using ImageJ software (version 1.54), which was downloaded from the official ImageJ website (https://imagej.net/ij/download.html). Experiments were performed with approximately 10 animals per replicate and repeated twice.
Food intake
We used two different methods to assess the food intake throughout the experiment:
-
Pharyngeal Pumping Rates (PPRs): This method is considered as an indirect indicator of food intake. Worms were measured in L4 worms following common protocols29. Worms were exposed to the treatments starting from the L1 stage on typical NGM plates. L4 worms were deprived of food for 60 min, and their pharyngeal pumping was counted for 30 s at room temperature using a hand counter. The number of contractions was multiplied to calculate the pumping rate in pumps/minute. This process was repeated after transferring the starved worms back to food. (Food intake) was calculated as the difference between basal pumping rate (off food) and post-feeding rate (on food). The experiment was conducted with 10 worms per replicate, and each treatment was tested in three independent experiments.
-
Bacterial consumption rate: This method is considered as a direct indicator of food intake. The food intake was assessed by spectrophotometric analysis following common protocols with slight modifications30,31. Worms were exposed to the treatments from the L1 stage on typical NGM plates and fed with dead E. coli. When reaching the L4, ~ 50 worms were transferred to liquid media that contains dead E. coli desired treatments. Worms were incubated with shaking for ~ 30 h at room temperature to ensure even distribution. After the incubation period, the liquid media was collected, and the optical density (OD) at 600 nm was measured using a spectrophotometer. Food intake was determined by comparing the optical density (OD) of the bacterial suspension in different conditions. Dead E. coli alone (without worms) served as a baseline control, while an additional control included worms exposed to dead E. coli with DMSO but without treatment. The bacterial consumption by treated worms was calculated as the difference in OD after 24 h relative to the DMSO control with worms. This approach allowed for the accurate measurement of bacteria consumed by the worms. Each treatment was tested in two independent experiments.
Lifespan
This experiment, as previously described32involved monitoring an age-synchronized population of C. elegans from the larval stage until death. L1 worms were initially maintained on plates without Fluorodeoxyuridine (FUDR) until reaching the L4 stage (48 h after the L1 stage). They were then transferred to FUDR plates (33 µL of 150 mM FUDR per 100 mL NGM) with the desired treatments to inhibit reproduction. Worms were considered dead if they did not respond to stimulation with a platinum pick. The experiments were conducted in triplicate, with a sample size of ~ 30 worms per treatment group.
Statistical analysis
Data were analyzed using JASP statistics software (Version 0.19.3) downloaded from (https://jasp-stats.org/download/). All results are presented as mean ± SEM. One-way and two-way ANOVA were used to assess significant differences between groups, with Tukey’s post hoc test applied to determine specific group differences. For survival data, Kaplan-Meier (KM) survival analysis was performed. Statistical significance was set at P ≤ 0.05.
Results
Food intake (Dose-response experiment)
The experiment investigated the effects of amitriptyline, both alone and in combination with HGD, on food intake in C. elegans. As previously described in the methods section, food intake was assessed using two different methods. The indirect method was based on pharyngeal pumping rates (PPRs), while the direct method utilized spectrophotometric measurement of the consumption of E. coli by worms. Different concentrations of amitriptyline (50 µM, 200 µM, 500 µM, and 1000 µM) were tested to evaluate dose-dependent changes in feeding behavior under normal and high-glucose conditions.
The effects of amitriptyline on food intake in C. elegans measured as pharyngeal pumping rates. Panel A shows food intake in worms treated with different doses of amitriptyline under normal dietary conditions. Panel B shows the effects of amitriptyline in worms fed a high-glucose diet (HGD). Data are expressed as mean ± SEM. Significant differences compared to the DMSO control are indicated by (*), while significant differences compared to HGD alone are indicated by (†),.
The PPRs of C. elegans were affected by amitriptyline treatment, both in the presence and absence of HGD, as illustrated in Fig. 1. As shown in Panel A, a dose-responsive increase in food intake was observed, with the most significant effect occurring at 200 µM compared to DMSO. Interestingly, the 500 µM dose exhibited a reduced effect on food intake, indicating a non-linear pattern rather than a strictly monotonic trend. This increase was driven by elevated on-food pharyngeal pumping rates, which followed a pattern similar to that of overall food intake. In contrast, off-food pumping rates showed a declining trend that did not correspond directly to food intake.
As shown in Panel B, food intake increased at 200 µM and 1000 µM amitriptyline when combined with HGD, compared to DMSO and HGD-only controls. The 500 µM dose also showed a modest increase in food intake under these conditions, which was not observed under the standard diet. While HGD alone did not significantly alter food intake, these data indicate that the feeding response to amitriptyline varies depending on the dietary background.
Optical Density (OD600) of E. coli after 24 h with C. elegans. Panel A shows the effects of amitriptyline on the density of E. coli, and Panel B shows the effects of amitriptyline combined with HGD on the density of E. coli. (*) indicates significant differences compared to the DMSO control, while (†) indicates significant differences compared to the HGD.
We also evaluated the food intake by calculating the bacterial consumption of treated worms. Consistent with PPRs, the food intake was influenced by amitriptyline, as shown in Fig. 2, both with and without a high-glucose diet. As shown in Panel A, bacterial consumption by worms increases significantly with amitriptyline treatment in a dose-dependent manner, as indicated by the decreasing optical density compared to the DMSO control. Significant reductions in OD600 are observed at 200 µM, 500 µM, and 1000 µM amitriptyline, suggesting increased bacterial consumption at these concentrations. Panel B demonstrates that the combination of HGD with amitriptyline further enhances bacterial consumption. The addition of HGD alone results in a significant reduction in OD600 compared to DMSO. Combining HGD with amitriptyline across all doses (50 µM, 200 µM, 500 µM, and 1000 µM) consistently decreases OD600, indicating increased bacterial consumption. These results suggest that both amitriptyline and HGD, individually and in combination, significantly promote bacterial consumption by C. elegans.
Role of specific genes in Amitriptyline-Induced food intake
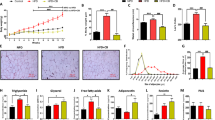
The effect of amitriptyline (200 µM) on food intake in wild type and various C. elegans mutants compared to their respective DMSO control. The figure presents pharyngeal pumping rates (PPR/min) for wild-type (A), daf-2 (B), daf-16 (C), tbh-1 (D), ser-1 (E), ser-2 (F), dop-1 (G), and (H) prdx-2 mutants. The three measured parameters include on-food pumping, food intake (difference between on- and off-food rates), and off-food pumping. Significant differences between amitriptyline-treated worms and the DMSO control are indicated by (*), with data presented as mean ± SEM.
After establishing that different concentrations of amitriptyline enhance food consumption in C. elegans, we opted for 200 µM as the main dose for subsequent experiments. This concentration was selected because of its notable impact on food intake seen in the dose-response study. In addition, we decided to explore the involvement of various relevant pathways in the effects of amitriptyline on food intake by using C. elegans mutants. Figure 3 illustrates the effects of amitriptyline treatment on food intake in wild-type and mutant C. elegans. Panel A shows that in wild-type worms, amitriptyline significantly increased food intake, with a notable rise in the on-food pumping rates and a concurrent decline in off-food pumping rates compared to the DMSO control. Panels B - G illustrate the findings for the daf-2, daf-16, tbh-1, ser-1, ser-2, and dop-1 mutants, respectively. Each of these mutants responded similarly to the wild type, with a significant increase in food intake driven by elevated on-food pumping rates, while off-food pumping rates showed no substantial changes. In contrast, Panel H shows that the prdx-2 mutant did not exhibit a significant increase in food intake following amitriptyline treatment. Both on-food and off-food pumping rates remained largely unchanged compared to the control, highlighting a distinct response compared to other strains. Overall, the patterns of on-food pumping rates closely align with the trends in food intake across all mutants, while off-food pumping rates consistently show a decreasing trend. The results highlight that amitriptyline consistently enhances feeding behavior across most genetic backgrounds, with the notable exception of the prdx-2 mutant, which did not respond to treatment in the same manner.
We also evaluated the effects of 200 µM amitriptyline on bacterial consumption by various C. elegans mutant strains. As shown in Fig. 4, each mutant is presented alongside its respective untreated control, separated by dotted lines. In most mutants, a significant reduction in OD600 is observed following treatment with amitriptyline, indicating increased bacterial consumption. However, a non-significant reduction in OD600 is seen in the prdx-2 mutant upon treatment, suggesting that the prdx-2 pathway is essential for the drug’s effect on bacterial consumption. These results suggest that amitriptyline enhances feeding in C. elegans primarily through pathways involving prdx-2 and other key regulatory genes.
Fat staining
Given the observed increase in food intake, we aimed to investigate whether this was accompanied by a corresponding increase in body fat. As shown in Fig. 5, the fat content of C. elegans was evaluated in response to different treatment conditions, including the control group (DMSO), HGD, 200 µM amitriptyline, and the combination of 200 µM amitriptyline with HGD. The HGD group exhibits a significant increase in fat content compared to the control group. In contrast, treatment with 200 µM amitriptyline alone does not significantly alter fat content. Similarly, the combination of 200 µM amitriptyline and HGD does not result in any significant changes in fat content compared to the control. These results suggest that while HGD increases fat accumulation, amitriptyline, either alone or in combination with HGD, does not significantly affect fat storage in C. elegans.
Lifespan
We also wanted to examine the effect of amitriptyline on the lifespan of C. elegans. As shown in Fig. 6, the lifespan of worms was evaluated in response to various treatments, including HGD, 200 µM amitriptyline, and their combination on the survival of C. elegans. As shown in Panel A, the addition of glucose to the worms’ diet significantly reduces their lifespan compared to the control group (DMSO). Panel B shows that treatment with 200 µM amitriptyline alone has no significant effect on lifespan. In Panel C, the combination of 200 µM amitriptyline with HGD results in a significant reduction in lifespan compared to the control. However, statistical comparisons revealed no significant difference between worms treated with HGD alone and those treated with amitriptyline plus HGD. These results suggest that while amitriptyline alone does not impact survival, HGD alone or in combination with amitriptyline significantly decreases the lifespan of C. elegans. Table 1 also provides an additional descriptive summary of lifespan data.
Discussion
The present study investigates the metabolic effects of the antidepressant amitriptyline on some physiological parameters in the model organism C. elegans. The group led by Michael Petrascheck previously showed that amitriptyline stimulates food intake in C. elegans23. In this work, we further extended this observation by characterizing the drug’s impact on food intake, body fat composition, and lifespan. We also identified some of the mechanisms and genetic pathways that might mediate these effects.
To evaluate food intake throughout the experiment, we employed two different methods. The first method was calculating the pharyngeal pumping rates. This method is considered one of the most reliable methods to assess food intake in C. elegans33. It also has many advantages, as it can provide insights into the general health and behavior of the worms34. However, this method does not measure the exact amount of bacteria ingested by worms. On the other hand, measuring food intake using the OD600 technique is considered more direct as it calculates the difference in the density of dead E. coli after and before treatments30,31. Although this technique generates accurate data on consumption rate, it requires a significant amount of time and specialized equipment. The discrepancies observed in our study between these two assays, particularly at intermediate doses, underscore the importance of using complementary approaches to assess feeding behavior in C. elegans. Nonetheless, pharyngeal pumping remains the most reliable method for evaluating food intake as it evaluates the general feeding behaviors and overall health of C. elegans33,34.
Beyond examining food intake under normal conditions, we also explored how glucose, a known modulator of lifespan in C elegans35,36interacts with amitriptyline’s effects. By adding 73 mM glucose to the diet, we aimed to mimic the high-carb diet prevalent in Western societies37. These excessive carbs and sugar consumption have been linked to reduced human longevity38,39,40. Furthermore, patients taking TCAs, such as amitriptyline, often demonstrate an increased craving for carbohydrates and sugars9. Hence, experimental conditions have been designed to resemble those in humans.
Our findings reveal that amitriptyline increases food intake in a dose-responsive manner, with the most pronounced effect observed at 200 µM compared to DMSO. This increase is primarily mediated by elevated (on-food) pharyngeal pumping rates, accompanied by a decrease in basal pumping rates (off-food). These results highlight the ability of amitriptyline to modulate feeding behavior in C. elegans under normal dietary conditions. The results of increased food intake in response to treatment with amitriptyline mirror the drug’s known effects on humans8,41. This strongly suggests that C. elegans could be a useful model to study the metabolic effects of amitriptyline and other TCAs.
As previously mentioned, increased cravings for sweets are a regular complaint of patients on TCAs9. These cravings are likely due to the behavioral effects of these drugs on certain neurotransmitters, especially serotonin and dopamine9. These neurotransmitters are well-known to modulate appetite and feeding behavior in humans15,25,42. Thus, we wanted to further investigate the effects of amitriptyline on food intake in C. elegans in the presence or absence of HGD. Interestingly, glucose stimulated a more robust effect of amitriptyline on the food consumption of C. elegans. This indicates that there could be possible interactions between the mechanisms of amitriptyline and glucose metabolism. In contrast, our results demonstrate that the introduction of glucose alone had no effect on the food intake of C. elegans. This result is consistent with previous reports in the literature43.
Given the known risks of premature death and cardiovascular issues in patients taking TCAs3,4,5we investigated whether amitriptyline similarly impacts lifespan in C. elegans. Our results show that 200 µM amitriptyline does not significantly impact the lifespan of worms fed a typical bacterial diet. However, adding glucose at 73 mM concentration to the diet significantly shortened the lifespan of the treated worms in the presence and absence of amitriptyline. These effects of glucose align with several previous studies showing the negative impact of high-glucose diets on C. elegans longevity35,36. Moreover, statistical comparisons between glucose-only and glucose-plus-amitriptyline groups showed no significant difference, indicating that glucose alone may be the primary driver of lifespan reduction in our data.
Glucose is known to shorten the lifespan of C. elegans by increasing oxidative stress44raising the possibility that the lifespan reduction observed with amitriptyline and glucose co-treatment was primarily driven by glucose-induced oxidative damage. Future studies could address this directly by measuring reactive oxygen species (ROS) levels or antioxidant enzyme activity. In addition, indirect approaches such as co-treatment with natural compounds that counteract the effects of glucose may be useful. Many natural compounds possess antioxidant properties and are known to extend lifespan in C. elegans45,46,47. Therefore, their inclusion as co-treatments may help clarify whether the reduction in lifespan reflects a true pharmacological interaction or merely results from glucose-induced oxidative stress.
Interestingly, our results of the possible combined effects of amitriptyline and glucose may indicate that a low-carb diet could lower the risk associated with TCAs in humans, especially when considering that such diets have been shown to benefit individuals with mental health disorders. Previous reports have shown that reduced sugar and carbohydrate intake is associated with improvements in blood glucose levels48,49which could help in reducing the risk of anxiety and depression50,51. Diets low in carbohydrates could also cause elevated ketone bodies in the blood, which have been linked to improved cognitive function, mood enhancement, and minimal brain inflammation52,53,54. More research is required, but it is worth considering that low-carbohydrate diets could theoretically serve as adjunctive measures along with standard treatments for mental disorders.
To further examine the effects of amitriptyline on C. elegans, we evaluated its effect on the fat content in the presence and absence of glucose. Interestingly, the changes in body fat content in C. elegans in response to amitriptyline were inconsistent with the increase in food intake. This outcome suggests that treatment with amitriptyline may influence food intake without altering the mechanisms involved in the synthesis of fat. These findings align with previous studies, emphasizing that food intake and fat regulation are governed by distinct mechanisms in C. elegans24,43. On the other hand, our results showed that the addition of glucose alone was associated with a significant increase in body fat, a result consistent with previous reports43.
Our study also investigated the role of specific pathways hypothesized to be involved in amitriptyline’s effect on food intake in C. elegans. First, we targeted the serotonergic pathway, which is known to regulate food intake and feeding behavior in humans and C. elegans15,24,42. To achieve this, we used mutants with some defective receptors in this pathway. Ser-1, ser-2, and ser-7 mutants exhibited responses similar to those seen in the wild type. Thus, this result indicates that these receptors may not participate in the action of amitriptyline on food consumption. It also indicates that the role of the serotonergic pathway might be limited in regulating the effects of amitriptyline on feeding in C. elegans. However, the findings do not completely rule out a possible involvement of the serotonergic pathways in the observed effect of amitriptyline. Hence, further studies should focus on other receptors and alternative signaling components of the serotonergic pathway to clarify the amitriptyline’s effect on feeding behavior in C. elegans.
The insulin signaling pathway is also involved in feeding regulation in humans and C. elegans55,56. To meet the aim of the study, food intake was examined in daf-16 and daf-2 mutants to evaluate its role in the observed effect of the drug. We found that amitriptyline resulted in increased feeding in daf-16 and daf-2 mutants, with similar results seen in the wild type. Hence, it is unlikely that this pathway contributed to the drug’s action on food intake. Similarly, we examined the role of the dopaminergic signaling pathways in the drug’s action on food intake. This pathway also contributes to the regulation of food intake in humans and C. elegans15,25,57,58. For this, we used dop-1 mutants that showed an increase in food intake similar to the result observed in the wild-type. This suggests that the dop-1 receptor may not be involved in mediating the drug’s effect on food intake. It is worth mentioning that only the dop-1 mutants were tested in this study to assess the involvement of the dopamine signaling pathway. The observed effects suggest that other dopamine receptors or pathways may also play a role. Therefore, further research is needed to evaluate the contributions of additional dopamine receptors to the effect of amitriptyline on food intake.
Oxidative stress has been linked to various metabolic changes in humans and other models. Given that some TCAs have been reported to induce oxidative stress and increase ROS production16,59we used prdx-2 mutants, known to protect C. elegans from oxidative damage60to explore whether redox regulation contributes to the metabolic effects of amitriptyline. Interestingly, the prdx-2 mutants had a different response to the treatment, as their food intake was not significantly affected compared to the other strains. This might suggest that the actions of the drug depend on the function of prdx-2. The prdx-2 gene encodes peroxiredoxin, an antioxidant enzyme involved in redox homeostasis and the regulation of oxidative stress61,62,63. This suggests that redox-sensitive signaling pathways may mediate the effects of amitriptyline on food intake. Amitriptyline may affect oxidative stress or redox signaling, influencing feeding behavior. The prdx-2 gene plays a key role in maintaining this balance60. Without functional prdx-2, worms cannot trigger the feeding response caused by the drug, suggesting that prdx-2 is a crucial link between managing oxidative stress and the behavioral effects of amitriptyline. However, our study did not include direct measurements of oxidative stress, such as reactive oxygen species (ROS) levels or redox-sensitive reporters, which limits our ability to confirm this mechanistic link. Furthermore, our conclusions are based on a single prdx-2 allele, and we did not perform rescue experiments with a wild-type transgene, which should be considered when interpreting the results. Therefore, it is important to interpret the prdx-2 results cautiously and avoid overstating mechanistic conclusions. While the data support a role for redox signaling, they remain correlative in the absence of direct functional validation. Future work should aim to address these gaps to clarify the contribution of oxidative stress and prdx-2 to amitriptyline’s metabolic effects.
Our findings on the possible role of prdx-2 in the drug’s effects on feeding behavior in C. elegans highlight the broader significance of oxidative stress in metabolic health. Oxidative stress is regarded as one of the key factors in the regulation of obesity in humans64. Excess fat, specifically visceral fat, is associated with significantly elevated reactive oxygen species (ROS)65,66. These factors cause fat accumulation and disrupt metabolism, leading to problems with appetite control and energy balance, which ultimately leads to overeating and weight gain65,67. Moreover, it has been suggested that oxidative stress may play a significant role in the weight gain induced by the use of PDs68. By understanding the connection between oxidative stress and obesity, we can explore new ways to reduce the impact of amitriptyline on food intake and body weight in humans.
Despite these interesting findings in our study, several limitations need to be acknowledged. One limitation of this study is the use of commercially available amitriptyline tablets, which contain pharmaceutical excipients that may potentially influence physiological responses. Although this approach reflects real-world clinical formulations, future studies should aim to replicate the findings using purified, laboratory-grade amitriptyline to ensure reproducibility and eliminate confounding effects from non-active ingredients. A further limitation relates to the pharyngeal pumping assay. In this assay, the post-starvation refeeding response may independently enhance pumping rates due to hyperphagic behavior. While our design aimed to quantify the net effect of amitriptyline by subtracting baseline activity, we acknowledge that starvation itself could modulate serotonergic pathways and influence results. Future studies may consider including continuously fed controls to further disentangle these variables.
Another limitation of our study is that we only assessed a subset of insulin, serotonin, and dopamine receptor mutants, along with tbh-1, to probe octopaminergic involvement in feeding regulation. While these genes have been previously implicated in modulating food intake in C. elegans24,56,58,69We acknowledge that this selection does not capture the full spectrum of neurotransmitter signaling. Notably, key synthesis genes such as tph-1 (serotonin) and cat-2 (dopamine), as well as other relevant receptors like ser-5, were not tested in this study. Future work should incorporate these additional targets to better define the neurochemical mechanisms underlying amitriptyline-induced feeding responses.
Conclusion
This study offers valuable insights into the metabolic effects of amitriptyline and highlights its capacity to enhance food consumption in C. elegans in a dose-responsive manner. Our results also emphasize the importance of nutritional components in affecting the effectiveness of the medication. Even with an increased food intake, the absence of notable changes in fat storage underscores the distinct regulation between feeding and fat metabolism in C. elegans. Genetic studies revealed prdx-2 to be a potential player in how amitriptyline affects eating behaviors, highlighting the importance of controlling oxidative stress. The lack of substantial engagement of serotonergic, dopaminergic, and insulin-like pathways elucidates the uniqueness of these systems. These findings confirm C. elegans as a reliable model for examining the metabolic impacts of TCAs and provide a basis for future research focused on alleviating their negative metabolic effects in humans.
Data availability
The datasets generated and analyzed during this study are available from the corresponding author upon reasonable request.
References
Bryson, H. M., Wilde, M. I. & Amitriptyline A review of its Pharmacological properties and therapeutic use in chronic pain States. Drugs Aging. 8, 459–476 (1996).
Moore, R. A., Derry, S., Aldington, D., Cole, P. & Wiffen, P. J. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst. Rev. CD008242 (2015). (2015).
Jang, H. Y. et al. Antidepressant use and the risk of major adverse cardiovascular events in patients without known cardiovascular disease: A retrospective cohort study. Front Pharmacol 11, 3 (2020).
Barnard, K., Peveler, R. C. & Holt, R. I. G. Antidepressant medication as a risk factor for type 2 diabetes and impaired glucose regulation: systematic review. Diabetes Care. 36, 3337–3345 (2013).
Maslej, M. M. et al. The mortality and myocardial effects of antidepressants are moderated by preexisting cardiovascular disease: A Meta-Analysis. Psychother. Psychosom. 86, 268–282 (2017).
Fernstrom, M. H. & Kupfer, D. J. Antidepressant-induced weight gain: a comparison study of four medications. Psychiatry Res. 26, 265–271 (1988).
Berken, G. H., Weinstein, D. O. & Stern, W. C. Weight gain. A side-effect of tricyclic antidepressants. J. Affect. Disord. 7, 133–138 (1984).
Ranjbar, S., Pai, N. & Deng, C. The association of antidepressant medication and body weight gain. Online J. Health Allied Sci. 12, 1–9 (2013).
Yeragani, V. K. et al. Carbohydrate craving and increased appetite associated with antidepressant therapy. Can. J. Psychiatry Rev. Can. Psychiatr. 33, 606–610 (1988).
Chadwick, W., Wilson, G., van de Venter, M., Oelofsen, W. & Roux, S. Shifts in metabolic parameters surrounding glucose homoeostasis resulting from tricyclic antidepressant therapy: implications of insulin resistance? J. Pharm. Pharmacol. 59, 95–103 (2007).
Gill, H. et al. Antidepressant medications and weight change: A narrative review. Obes. Silver Spring Md. 28, 2064–2072 (2020).
Nihalani, N., Schwartz, T. L., Siddiqui, U. A. & Megna, J. L. Weight gain, obesity, and psychotropic prescribing. J. Obes. 893629 (2011). (2011).
Barden, N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J. Psychiatry Neurosci. JPN. 29, 185–193 (2004).
Bose, M., Oliván, B. & Laferrère, B. Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr. Opin. Endocrinol. Diabetes Obes. 16, 340–346 (2009).
Meguid, M. M. et al. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutr. Burbank Los Angel Cty. Calif. 16, 843–857 (2000).
Abdel-Salam, O. M. E., Morsy, S. M. Y. & Sleem, A. A. The effect of different antidepressant drugs on oxidative stress after lipopolysaccharide administration in mice. EXCLI J. 10, 290–302 (2011).
McMurray, F., Patten, D. A. & Harper, M. E. Reactive oxygen species and oxidative stress in Obesity-Recent findings and empirical approaches. Obes. Silver Spring Md. 24, 2301–2310 (2016).
Hunt, P. R. The C. elegans model in toxicity testing. J. Appl. Toxicol. JAT. 37, 50–59 (2017).
Leung, M. C. K. et al. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol. Sci. Off J. Soc. Toxicol. 106, 5–28 (2008).
Strange, K. An overview of C. elegans biology. Methods Mol. Biol. Clifton NJ. 351, 1–11 (2006).
Dwyer, D. S., Awatramani, P., Thakur, R., Seeni, R. & Aamodt, E. J. Social feeding in caenorhabditis elegans is modulated by antipsychotic drugs and calmodulin and May serve as a protophenotype for asociality. Neuropharmacology 92, 56–62 (2015).
Almotayri, A. et al. Metabolic and behavioral effects of olanzapine and Fluoxetine on the model organism caenorhabditis elegans. Saudi Pharm. J. SPJ Off Publ Saudi Pharm. Soc. 29, 917–929 (2021).
Perez-Gomez, A. et al. A phenotypic caenorhabditis elegans screen identifies a selective suppressor of antipsychotic-induced hyperphagia. Nat. Commun. 9, 5272 (2018).
Srinivasan, S. et al. Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms. Cell. Metab. 7, 533–544 (2008).
Hills, T., Brockie, P. J. & Maricq, A. V. Dopamine and glutamate control area-restricted search behavior in caenorhabditis elegans. J. Neurosci. Off J. Soc. Neurosci. 24, 1217–1225 (2004).
Dillon, J., Holden-Dye, L., O’Connor, V. & Hopper, N. A. Context-dependent regulation of feeding behaviour by the insulin receptor, DAF-2, in caenorhabditis elegans. Invertebr Neurosci. IN. 16, 4 (2016).
Brenner, S. The genetics of caenorhabditis elegans. Genetics 77, 71–94 (1974).
Stuhr, N. L. et al. Rapid lipid quantification in caenorhabditis elegans by oil red O and nile red staining. Bio-Protoc 12, e4340 (2022).
Raizen, D., Song, B. M., Trojanowski, N. & You, Y. J. Methods for measuring pharyngeal behaviors. WormBook Online Rev. C Elegans Biol. 1–13. https://doi.org/10.1895/wormbook.1.154.1 (2012).
Quantifying Food Intake in Caenorhabditis Elegans by Measuring Bacterial Clearance. United States, (2024). https://doi.org/10.3791/66422
Gomez-Amaro, R. L. et al. Measuring food intake and nutrient absorption in caenorhabditis elegans. Genetics 200, 443–454 (2015).
Measuring Caenorhabditis Elegans Life Span on Solid Media. United States, (2009). https://doi.org/10.3791/1152
Avery, L. & You, Y. J. C. elegans feeding (May 21, 2012), wormbook. ed. C Elegans Res. Community WormBook https://doi.org/10.1895/wormbook.1.150.1
Huang, C., Xiong, C. & Kornfeld, K. Measurements of age-related changes of physiological processes that predict lifespan of caenorhabditis elegans. Proc. Natl. Acad. Sci. U S A. 101, 8084–8089 (2004).
Almotayri, A., Thomas, J., Munasinghe, M. & Jois, M. The effect of Mianserin on lifespan of caenorhabditis Elegan is abolished by glucose. Curr. Aging Sci. 14, 118–123 (2021).
Choi, S. S. High glucose diets shorten lifespan of caenorhabditis elegans via ectopic apoptosis induction. Nutr. Res. Pract. 5, 214–218 (2011).
Shan, Z. et al. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999–2016. JAMA 322, 1178–1187 (2019).
Seidelmann, S. B. et al. Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public. Health. 3, e419–e428 (2018).
Qin, P. et al. Dietary carbohydrate quantity and quality and risk of cardiovascular disease, all-cause, cardiovascular and cancer mortality: A systematic review and meta-analysis. Clin. Nutr. Edinb. Scotl. 42, 148–165 (2023).
Darjoko, S. T., Wahyuningsih, T. & Sudikno, S. High carbohydrate intake increases risk of coronary heart disease in adults: a prospective cohort study. Universa Med. 38, 90–99 (2019).
Gracious, B. L. & Meyer, A. E. Psychotropic-induced weight gain and potential Pharmacologic treatment strategies. Psychiatry Edgmont Pa. Townsh. 2, 36–42 (2005).
van Galen, K. A., Horst, T., Serlie, M. J. & K. W. & Serotonin, food intake, and obesity. Obes. Rev. Off J. Int. Assoc. Study Obes. 22, e13210 (2021).
Lu, Z. & Qiu, Z. High glucose concentration restricts fat consumption in caenorhabditis elegans. Int. J. Clin. Exp. Med. 10, 10554–10559 (2017).
Schlotterer, A. et al. C. elegans as model for the study of high glucose- mediated life span reduction. Diabetes 58, 2450–2456 (2009).
Zamberlan, D. C. et al. Rosmarinus officinalis L. increases caenorhabditis elegans stress resistance and longevity in a DAF-16, HSF-1 and SKN-1-dependent manner. Braz J. Med. Biol. Res. Rev. Bras. Pesqui Medicas E Biol. 49, e5235 (2016).
Xu, T. et al. Longevity-promoting properties of ginger extract in caenorhabditis elegans via the insulin/IGF-1 signaling pathway. Food Funct. 13, 9893–9903 (2022).
Munasinghe, M., Almotayri, A., Thomas, J., Heydarian, D. & Jois, M. Early exposure is necessary for the lifespan extension effects of cocoa in C. elegans. Nutr. Metab. Insights. 14, 11786388211029443 (2021).
Gannon, M. C. & Nuttall, F. Q. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes 53, 2375–2382 (2004).
Cai, L. et al. Low-carbohydrate diets lead to greater weight loss and better glucose homeostasis than exercise: a randomized clinical trial. Front. Med. 15, 460–471 (2021).
Calabrese, L., Frase, R. & Ghaloo, M. Complete remission of depression and anxiety using a ketogenic diet: case series. Front Nutr 11, 3 (2024).
Murphy, P., Likhodii, S., Nylen, K. & Burnham, W. M. The antidepressant properties of the ketogenic diet. Biol. Psychiatry. 56, 981–983 (2004).
Chinna-Meyyappan, A. et al. Effects of the ketogenic diet on cognition: a systematic review. Nutr. Neurosci. 26, 1258–1278 (2023).
Rong, L. et al. Effects of ketogenic diet on cognitive function of patients with alzheimer’s disease: a systematic review and meta-analysis. J. Nutr. Health Aging. 28, 100306 (2024).
Ota, M. et al. Effect of a ketogenic meal on cognitive function in elderly adults: potential for cognitive enhancement. Psychopharmacol. (Berl). 233, 3797–3802 (2016).
Schur, E. A. & Tong, J. Insulin action to inhibit food intake: is it all in your head?? J. Clin. Endocrinol. Metab. 107, e874–e876 (2022).
Avery, L. & You, Y. J. C. elegans feeding. WormBook Online Rev. C Elegans Biol. 1–23. https://doi.org/10.1895/wormbook.1.150.1 (2012).
Sawin, E. R., Ranganathan, R. & Horvitz, H. R. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26, 619–631 (2000).
Suo, S., Culotti, J. G. & Van Tol, H. H. M. Dopamine counteracts octopamine signalling in a neural circuit mediating food response in C. elegans. EMBO J. 28, 2437–2448 (2009).
El-Demerdash, E. & Mohamadin, A. M. Does oxidative stress contribute in tricyclic antidepressants-induced cardiotoxicity? Toxicol. Lett. 152, 159–166 (2004).
Oláhová, M., Veal, E. A. & A peroxiredoxin PRDX-2, is required for insulin secretion and insulin/IIS-dependent regulation of stress resistance and longevity. Aging Cell. 14, 558–568 (2015).
Sadowska-Bartosz, I. & Bartosz, G. Peroxiredoxin 2: an important element of the antioxidant defense of the erythrocyte. Antioxid Basel Switz 12, 3 (2023).
Li, H., Yang, H., Wang, D., Zhang, L. & Ma, T. Peroxiredoxin2 (Prdx2) reduces oxidative stress and apoptosis of myocardial cells induced by acute myocardial infarction by inhibiting the TLR4/Nuclear factor kappa B (NF-κB) signaling pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 26, e926281 (2020).
Oláhová, M. et al. A redox-sensitive Peroxiredoxin that is important for longevity has tissue- and stress-specific roles in stress resistance. Proc. Natl. Acad. Sci. U S A. 105, 19839–19844 (2008).
Marseglia, L. et al. Oxidative stress in obesity: a critical component in human diseases. Int. J. Mol. Sci. 16, 378–400 (2014).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114, 1752–1761 (2004).
Manna, P., Jain, S. K., Obesity, O. & Stress Adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 13, 423–444 (2015).
Drougard, A., Fournel, A., Valet, P. & Knauf, C. Impact of hypothalamic reactive oxygen species in the regulation of energy metabolism and food intake. Front. Neurosci. 9, 56 (2015).
An, H. et al. Obesity, altered oxidative stress, and clinical correlates in chronic schizophrenia patients. Transl Psychiatry. 8, 258 (2018).
Churgin, M. A., McCloskey, R. J., Peters, E. & Fang-Yen, C. Antagonistic serotonergic and octopaminergic neural circuits mediate Food-Dependent locomotory behavior in caenorhabditis elegans. J. Neurosci. Off J. Soc. Neurosci. 37, 7811–7823 (2017).
Author information
Authors and Affiliations
Contributions
A.M.A, K. E, A.H.A, N.M. A, conceived and designed the study. A.M.A, K. E, conducted the experiments, analyzed the data, and prepared the figures. A.M.A, wrote the main manuscript text. All authors reviewed, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Almotayri, A.M., Alghamdi, A.A.A., Almimoni, N.M. et al. Amitriptyline increases food intake via prdx-2 without altering other physiological parameters in C. elegans under a modified bacterial diet. Sci Rep 15, 29428 (2025). https://doi.org/10.1038/s41598-025-14773-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14773-8