Abstract
Acute myeloid leukemia (AML) is a rare hematological disorder that has detrimental effects on human health and requires the exploration of biomarkers for diagnosis and prognostic evaluation. Essential trace elements like copper (Cu) and zinc (Zn) play diverse biological functions, however, limited data are available regarding their role in AML. The present study aimed to determine the serum levels of Cu and Zn, and assess the serum Cu/Zn ratio (SCZR) in newly diagnosed adult AML cases. This observational case-control study included 50 adult AML patients and 50 healthy controls. Blood samples were collected to analyze complete blood count (CBC), serum levels of Cu and Zn were determined by an atomic absorption spectrophotometer. The study showed significant hematological abnormalities in AML, including anemia (reduced hemoglobin/hematocrit levels), leukocytosis (elevated WBC counts), and thrombocytopenia (low platelet counts). Compared to controls, AML patients exhibited significantly higher (p = < 0.05) serum Cu levels (2.01 ± 0.87 mg/L), lower Zn levels (1.02 ± 0.54 mg/L), and an elevated SCZR (2.74 ± 2.31). Furthermore, higher blast counts (> 40%) were significantly associated (p = 0.005) with reduced Zn levels (0.82 ± 0.37 mg/L), and shorter survival episodes (9.51 ± 3.93 months; p = 0.001). A moderate-to-strong inverse correlation was observed between blast counts (> 40%) and survival duration (p = 0.001). The study demonstrated significant differences in serum Cu, Zn, and SCZR between AML patients and controls. Particularly, reduced serum Zn was linked to higher blast counts, whereas serum Cu and SCZR showed no association with blast burden. These findings highlight the prognostic relevance of blast burden and trace element dysregulation in AML.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is a group of blood and bone marrow disorders that develop due to the clonal expansion of primitive hematopoietic stem cells, known as blasts, in the bone marrow. The dysregulated growth of blast cells leads to abnormalities in cell lineages such as erythropoiesis and megakaryopoiesis. AML progresses more rapidly compared to chronic and indolent leukaemias1. Ineffective hematopoiesis and progressive marrow failure result in fatal outcomes if left untreated or inadequately managed. Although advances in AML treatment have improved survival rates in younger patients, the prognosis remains poor in older adults, with less than a 30% survival rate in the first year after diagnosis2,3. Gender-based AML incidence rates globally range from 0.9 to 2.8 per 100,000 in males to 0.4–2.2 per 100,000 in females4.
In the United States, the incidence of age-related AML is 4.3 per 100,000 people. AML pathogenesis is heterogeneous, and its definitive cause remains incompletely understood. While prior exposure to occupational or therapeutic agents, certain genetic predispositions, or environmental DNA-damaging factors (e.g., radiation, tobacco smoke, and benzene) have been implicated in some patients, most cases lack clear causation1. For Southeast Asia, demographic data on AML are highly variable: a 2020 study reported 1,733,573 annual cancer cases in the region, with Pakistan documenting the highest AML incidence (4.3 per 1,000,000). Among the 1,124,875 cancer-related deaths recorded that year, 45,707 (4.1%) were linked to leukemia5.
The European LeukemiaNet (ELN) categorizes AML based on prognostic risk groups: favorable, intermediate, and adverse groups6,7. This categorization is based on the survival and therapeutic standards determined by cytogenetics and molecular determinants. Among other clinical risk factors, older age and poor performance status are both linked with reduced rates of overall survival (OS), and complete remission (CR). In AML, the combination of diverse analytical variables is associated with a higher risk of treatment-related mortality (TRM)8. Nevertheless, these newly identified factors have not been included in the prognostic system that distinguishes AML subgroups. Consequently, it underscores the need to discover the wide-ranging and prognostic factors that influence host status, and the leukemic cells, potentially revealing novel therapeutic approaches for AML patients9.
Some heavy metals function as trace elements with critical biological roles in human metabolism critically. Copper (Cu) and zinc (Zn) are essential trace elements that play crucial roles in diverse enzymatic activities in cellular metabolism, maintaining DNA integrity and stability. Importantly, the Zn and Cu metals are linked to wide-ranging biological properties, and several studies have directed the potential link with the dysregulation of these micronutrients in oncogenesis by uncontrolled redox activity of enzymes10,11. In B-cell chronic lymphocytic leukemia (CLL), elevated Cu levels are identified as an effective oxidizing agent, involved in cancer progression and carrying prognostic implications. Cu level is an acute-phase reactant, which increases in tissue injury, infections, and chronic inflammations12,13. Zinc is involved in a variety of biological processes, as a structural, catalytic, and intra/extracellularsignaling component. Zinc is involved in the modulation of pro-inflammatory responses and inhibits the production of reactive oxygen species (ROS). A recent study demonstrated that decreased serum Zn levels are associated with a high mortality rate in hospitalized patients14. The relationship between the levels of these trace elements (Cu and Zn) and the prognosis of AML is well-established9,15.
Previous studies report inconsistent alterations in trace elements levels in AML. Concentrations of essential elements like selenium (Se) and Zn were significantly reduced, while Cu levels were elevated in AML patients16,17. One study concluded that chemotherapy reduces the burden of AML disease by increasing serum Zn and decreasing serum Cu18. Nonetheless, it has not yet been proved that the long-term survival of AML patients relates to the prognostic importance of these trace elements. Also, it has been suggested that the serum Cu/Zn ratio (SCZR) can be a better indication for cancer prognosis rather than serum concentrations of Cu and Zn independently19.
The study aimed to determine serum copper, zinc, and serum Cu/Zn ratio (SCZR)in AML patients, and also to associate the alterations in serum levels of trace elements (Cu and Zn levels) with the disease progression in AML patients.
Materials and methods
Study population
This comparative case-control study investigated 50 confirmed AML cases and 50 age- and sex-matched healthy controls. AML patients (diagnosed via peripheral blood, bone marrow examination, and cytochemistry/immunohistochemistry/flow cytometry) were recruited from Jinnah Hospital, Lahore, Pakistan, after obtaining ethical clearance from the ethical review committee. Participants meeting predefined inclusion/exclusion criteria were enrolled. Study procedures and potential benefits were explained, and a structured proforma collected personal history and medical data. General physical examinations were performed, and detailed family histories (particularly hematological malignancies) were documented.
Following recruitment, height was measured in centimeters, with the subjects standing in the anatomical position. Weight was measured in kilograms, with participants barefoot and wearing minimal clothing. Body mass index (BMI) was calculated by dividing the subject’s weight (kg) by the square of their height (m²). Blood pressure was recorded using a mercury sphygmomanometer. Clinical and demographic details of patients and normal controls were recorded on the provided proforma.
A 10 ml blood sample was collected by an aseptic technique from all the participants into EDTA and yellow-top serum containers. Complete blood count was performed on the EDTA vacutainer, and serum zinc and copper were measured using serum gel tubes.
Measurement of complete blood count (CBC)
Blood samples collected in EDTA vacutainers were analyzed using the Sysmex XT 1000i (Japan) hematology analyzer. The diagnostic data was reviewed by a hematologist at the University of Health Sciences, Lahore. The following parameters were measured:
-
Hemoglobin levels.
-
White Blood Cell (WBC) count.
-
Platelet count.
-
Differential count.
-
Hematocrit.
-
MCV.
-
MCH.
Determination of serum zinc and copper levels by atomic absorption spectrophotometer
The serum samples were prepared before Cu and Zn analysis on an atomic absorption spectrophotometer. About 100 µl of serum was digested with 1 ml of 10% nitric acid (HNO3). The mixture of serum and nitric acid was kept at 100 °C for 4 h. Digested samples were centrifuged at 3000 rpm for 10 min, and the supernatant was transferred into a clean Pyrex tube, and debris was removed. For analysis, the supernatant was diluted with HNO3 with a 1:5 dilution, and absorbance was taken by an atomic absorption spectrophotometer (Hitachi U2800/2900 Series) at wavelengths of 324 nm and 214 nm for Cu and Zn, respectively.
Statistical analysis
The study used SPSS v 23.0 for data analysis. Categorical variables within the AML groups—such as sex distribution, clinical history, and symptoms (e.g., bone pain, splenomegaly)—were summarized. An independent student t-test was used to compare continuous variables (like age, hemoglobin, hematocrit, leukocytes, platelets, Cu, Zn, and serum Cu/Zn ratio (SCZR)) in two groups, including AML patients and controls. Linear relationships between laboratory and clinical parameters were evaluated using the Pearson Correlation Coefficient (r) to determine the correlations between patients and controls, and also between AML patients’ categories. A p-value < 0.05 was considered statistically significant.
Result
Clinical characteristics and demographic data
A total of 50 AML patients and 50 controls were included in this study. The age distribution showed a mean age of 47.38 ± 9.07 years for AML patients, while controls had a mean age of 43.16 ± 8.05 years. This difference was not statistically significant. No significant differences were found in demographic data between AML patients and controls in terms of height, weight, and BMI, suggesting that these parameters did not influence the outcomes of the study. For hematological parameters, AML patients showed significantly lower levels of hemoglobin (9.46 ± 2.78 g/dL) and hematocrit (29.84 ± 8.85%) compared to controls (12.44 ± 1.57 g/dL, 38.32 ± 3.75%), indicating the presence of anemia in the AML group (Table 1). Leukocyte counts were significantly higher in AML patients (33.51 ± 43.82 × 109/L) compared to controls (7.98 ± 1.86 × 109/L), highlighting leukocytosis in the patients. Additionally, AML patients had significantly lower platelet counts (44.10 ± 40.87 × 109/L) compared to controls (315.03 ± 61.45 × 109/L), suggesting the presence of thrombocytopenia in the disease group.
Table 2 presents the distribution frequency of demographic and clinical characteristics in AML patients. The majority of patients were male (70%), while females comprised 30% of the cohort. Consanguinity was reported in 16% of patients, and a family history of AML was present in 18%. More than half of the patients (56%) had a history of fever, while 48% reported a history of bleeding. Weight loss was observed in 40% of patients, whereas bone pain and joint pain were highly prevalent, affecting 76% and 74% of patients, respectively. Splenomegaly was noted in 34% of cases, while 42% had lymphadenopathy. A history of blood transfusion was reported in 44% of patients, and smoking was relatively uncommon, with only 12% of patients having a history of smoking.
Comparison of serum trace elements (Cu and Zn)
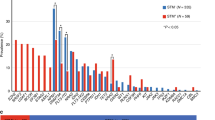
The comparison between controls and patients for copper (Cu), zinc (Zn), and serum Cu/Zn ratio (SCZR) reveals significant differences. For serum Cu levels, AML patients showed higher serum Cu levels (2.01 ± 0.87) than controls (0.88 ± 0.51433), indicating a statistical significance (p = 0.001). Serum Zn levels were found to be lower in AML patients (1.02 ± 0.54) than in controls (1.90 ± 0.79) with a significant difference (p = 0.001). Also, the mean of SCZR in AML patients was higher (2.74 ± 2.31) than in controls (0.59 ± 0.48) with a p-value of 0.001 (Table 3). Comparison between male and female determined no gender-based difference between trace elements and SCZR (Fig. 1). In AML cases, males had lower serum Cu (1.93 ± 0.83 µg/mL) than females (2.18 ± 0.96 µg/mL, p = 0.376), lower Zn (0.92 ± 0.50 vs. 1.21 ± 0.59 µg/mL, p = 0.094), and higher SCZR (3.01 ± 2.61 vs. 2.23 ± 1.55, p = 0.193). In healthy controls, no significant gender differences emerged in Cu (males 0.89 ± 0.52 µg/mL vs. 0.89 ± 0.52 µg/mL, p = 0.995), Zn (1.87 ± 0.77 vs. 1.99 ± 0.89 µg/mL, p = 0.676), or Cu/Zn ratio (0.59 ± 0.46 vs. 0.60 ± 0.57, p = 0.976). While Zn levels in AML patients showed a notable trend toward higher values in females (p = 0.094).
Correlations between laboratory parameters
The correlation analysis for patients and controls was carried out to observe the association between trace element levels (Cu and Zn) and AML disease. In controls, a moderate negative correlation between Cu and Zn is observed (r = −0.34, p = 0.017). In AML patients, a correlation between Cu and Zn is non-significant (r = −0.061, p = 0.67). Both controls and patients show a significant positive correlation between Cu and SCZR, with controls having a stronger correlation (r = 0.833, p = 0.001) compared to patients (r = 0.48, p = 0.001).
Additionally, both controls and patients showed a strong negative association between Zn and SCZR (r = −0.64, p = 0.001 for controls, and r = −0.64, p = 0.001 for AML patients). In AML patients, the blast count showed no correlation with Cu (r = 0.267), but weak positive trends were observed (p = 0.061). However, a significant negative association between blast count and Zn (r = −0.323, p = 0.022 was observed. The relationship between blast counts and Cu/Zn ratio also showed a weak positive correlation but was non-significant (r = 0.262; p = 0.060) (Fig. 2). Also, the association between survival (months) and blast count in AML patients showed a moderate to strong inverse correlation (r = −0.69, p = < 0.05), where higher blast counts are associated with lower survival months (Fig. 3).
Blasts category comparison
The comparison between the Blast categories (group 1, 20–40%, and group 2, > 40%) for Cu, Zn, SCZR, and survival months shows several key findings. For Cu, the 20–40 group has a mean (1.9 ± 0.71), while the > 40 group has a slightly higher mean (2.1 ± 0.99). However, the p-value of 0.40 indicated a statistically non-significant difference in Cu levels between the two groups. In contrast, Zn levels are significantly different, with the 20–40 group showing a mean (1.24 ± 0.61) and the > 40 group having a lower mean (0.82 ± 0.37), with a p-value of 0.005. This suggests that Zn levels are significantly lower in patients with higher blast counts (> 40%).
For SCZR, the 20–40 group has a mean (2.2 ± 2.4), while the > 40 group has a slightly higher mean (3.1 ± 2.2), but the p-value of 0.17 suggested that this difference is statistically non-significant. Finally, survival months are significantly different between the two groups, with the 20–40% group having a mean (16.21 ± 5.00) and the > 40% group having a much lower mean (9.51 ± 3.93), with a p-value of 0.001. This indicates that patients with a higher blast count have significantly shorter survival times. Overall, the analysis shows that serum Cu levels and SCZR did not show significant differences; Zn levels and survival months are significantly affected by the blasts category (Fig. 4).
Scatter plots depicting relationships between blast percentages and serum copper (Cu), zinc (Zn), and serum Cu/Zn ratio (SCZR) in AML patients and healthy controls. (A–C) Inverse correlations were observed between Cu and Zn and between Zn and SCZR, while a direct correlation was identified between Cu and SCZR. (D–F) Similar inverse relationships were noted between Cu and Zn and Zn and SCZR, with a positive correlation between Cu and SCZR. (G–I) Blast counts demonstrated a direct correlation with Cu levels (G) and SCZR, but an inverse association with Zn concentrations.
Discussion
Copper (Cu) and zinc (Zn)— essential trace elements implicated in cellular oxidative stress- exhibit alterations critically linked to cancer development, progression, and prognosis13,16,20. While regional variations influence trace element levels globally, meta-analyses and other studies consistently report elevated Cu and reduced Zn concentrations in acute myeloid leukemia (AML) patients9,21,22,23. To investigate this phenomenon, we compared serum Cu, Zn levels, as well as the serum Cu/Zn ratio (SCZR), between 50 acute myeloid leukemia (AML) patients and 50 healthy controls.
Results revealed significantly higher serum Cu levels in AML patients compared to controls (p = 0.001). Although blast counts showed a weak positive trend with Cu and SCZR, however, there was no statistical significance (p = 0.06), suggesting limited clinical relevance. No significant difference in Cu levels was observed between blast categories (20–40% vs. >40%), aligning with prior studies linking elevated Cu to hematological malignancies9,16,22. Elevated Cu has been associated with disease progression, whereas normalized levels correlate with remission, underscoring its prognostic utility12,24. Remarkably, excessive Cu may disrupt Zn homeostasis, exacerbating oxidative stress by impairing antioxidant defenses25.
The prognostic significance of copper (Cu) and the serum Cu/Zn ratio (SCZR) in AML survival remains unclear due to outcome heterogeneity influenced by patient- and disease-specific factors. A recent study highlights the prognostic potential of trace elements in AML26. In this study, serum Cu correlated with disease-free survival (DFS) and overall survival (OS), while SCZR was linked to OS in younger AML patients. SCZR also correlated with European LeukemiaNet (ELN) risk stratification and complete remission (CR), reinforcing its prognostic value.
Serum zinc (Zn) levels were significantly lower in AML patients (p = 0.001), and showed an inverse correlation with blast counts (p = 0.022). Patients with > 40% blasts exhibited reduced Zn levels (p = 0.005) and shorter survival (p = 0.001). While Zn and SCZR showed negative correlations in controls (p = 0.017), this relationship was absent in AML patients (p = 0.675). These findings align with the previous studies9,22,27, though conflicting data from the U.S. suggest no significant Zn reduction in AML26.
Copper and zinc levels are generally stable under normal nutritional conditions, though they fluctuate during severe deficiency or supplementation28. Antioxidant properties of Zn enhance enzymes like glutathione reductase and catalase, while its deficiency exacerbates inflammation mediated by NF-κB-. In vitro studies demonstrate the role of Zn in suppressing pro-inflammatory cytokines (e.g., TNF-α, IL-1β) and upregulating anti-inflammatory proteins like A20, and PPAR-α29.
AML patients exhibited a significantly elevated serum Cu/Zn ratio (SCZR) compared to healthy controls (p = 0.001), indicative of oxidative stress—a key contributor to leukemia pathogenesis30. While the ratio was higher in patients with > 40% blasts, the difference was non-significant (p = 0.17). Serum Cu/Zn ratio also serves as an important nutritional biomarker, with elevated ratios suggesting zinc deficiency31. Furthermore, SCZR correlates with inflammation, immune dysfunction, and infection risk32, potentially contributing to non-relapse mortality in AML9. Disrupted Cu and Zn homeostasis in leukemia stems from diet, genetics, and environment. Zn deficiency (low seafood/nuts/legumes) impairs immunity and genomic stability, while excess Cu (high red meat) may fuel AML progression oxidatively; high serum Cu and Cu/Zn ratio predict poorer survival. Genetic variants in transporters (SLC30A, and SLC31A) and metallothioneins alter metal handling, susceptibility, resistance, and ferroptosis. Environmental toxins (benzene, arsenic) further dysregulate Cu and Zn balance via metal-binding proteins33,34. Overall, serum copper, zinc, and their ratio may serve as biomarkers for oxidative stress, nutritional status, and prognosis in AML patients undergoing chemotherapy, offering insights into disease monitoring and therapeutic strategies.
Conclusion
The study showed a significant difference in serum Cu, Zn, and SCZR between AML patients and controls; however, an association was observed between reduced serum Zn and blast counts. On the other hand, serum Cu and SCZR were not linked to the blast counts. Furthermore, higher blast count was inversely associated with reduced serum Zn and patient survival rates. This may highlight the prognostic influence of blast burden and trace element dysregulation in AML.
Limitations
-
The are some limitations to our study that should be addressed in large-scale studies. The small sample size of 50 AML patients and 50 healthy controls is very small and may restrict the appropriate findings, which necessitates multi-center studies.
-
The possibility of dietary habits, genetics, and environmental factors may contribute to regional alterations in Cu and Zn levels.
-
Additional longitudinal studies are required to assess various interfering factors in Cu/Zn ratio measurement in leukemia.
-
Furthermore, although the serum Cu/Zn ratio shows potential as a biomarker, its clinical utility in prognosis and treatment monitoring requires validation through larger, standardized studies.
-
This study only measured serum copper and zinc levels in AML patients, without estimating contributing factors like diet, environment, or genetics. Hence, the underlying mechanisms of Cu and Zn imbalance remain undetermined.
Data availability
The data collected during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Shallis, R. M., Wang, R., Davidoff, A., Ma, X. & Zeidan, A. M. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 36, 70–87 (2019).
Meyers, J., Yu, Y., Kaye, J. A. & Davis, K. L. Medicare fee-for-service enrollees with primary acute myeloid leukemia: an analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl. Health Econ. Health Policy. 11 (3), 275–286 (2013).
Shah, A., Andersson, T. M., Rachet, B., Björkholm, M. & Lambert, P. C. Survival and cure of acute myeloid leukaemia in england, 1971–2006: a population-based study. Br. J. Haematol. 162 (4), 509–516 (2013).
Miranda-Filho, A. et al. Epidemiological patterns of leukaemia in 184 countries: a population-based study. Lancet Haematol. 5 (1), e14–e24 (2018).
Rifat, R. H. et al. Incidence, mortality, and epidemiology of leukemia in South asia: an ecological study. Open. J. Epidemiol. 13 (1), 73–82 (2022).
Döhner, H. et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 140 (12), 1345–1377 (2022).
Herold, T. et al. Validation and refinement of the revised 2017 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia 34 (12), 3161–3172 (2020).
Ramos, F. et al. Survival outcomes and health-related quality of life in older adults diagnosed with acute myeloid leukemia receiving frontline therapy in daily practice. J Pers Med 13 (12), (2023).
Li, T., Shi, L., Wei, W., Xu, J. & Liu, Q. The trace that is valuable: serum copper and copper to zinc ratio for survival prediction in younger patients with newly diagnosed acute myeloid leukaemia. BMC Cancer. 23 (1), 14 (2023).
Bafaro, E., Liu, Y., Xu, Y. & Dempski, R. E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal. Transduct. Target. Ther. 2, 17029 (2017).
Bian, C. et al. Copper homeostasis and Cuproptosis in tumor pathogenesis and therapeutic strategies. Front. Pharmacol. 14, 1271613 (2023).
Labib, H. A., Hassanein, M. & Etewa, R. L. Serum copper is a simple but valuable prognostic marker in B-cell chronic lymphocytic leukemia. Int. J. Hematol. 100 (6), 575–581 (2014).
Shanbhag, V. C. et al. Copper metabolism as a unique vulnerability in cancer. Biochim. Biophys. Acta Mol. Cell. Res. 1868 (2), 118893 (2021).
Rodic, S., McCudden, C. & van Walraven, C. The prognostic value of serum zinc levels in acutely hospitalized patients: a systematic review. Biol. Trace Elem. Res. 199 (12), 4447–4457 (2021).
Rasool, M. et al. Assessment of Circulating biochemical markers and antioxidative status in acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) patients. Saudi J. Biol. Sci. 22 (1), 106–111 (2015).
Valadbeigi, S., Javadian, S., Ebrahimi-Rad, M., Khatami, S. & Saghiri, R. Assessment of trace elements in serum of acute lymphoblastic and myeloid leukemia patients. Exp. Oncol. 41 (1), 69–71 (2019).
Zuo, X. L., Chen, J. M., Zhou, X., Li, X. Z. & Mei, G. Y. Levels of selenium, zinc, copper, and antioxidant enzyme activity in patients with leukemia. Biol. Trace Elem. Res. 114 (1–3), 41–53 (2006).
Abulimiti, M. et al. Exploring and clinical validation of prognostic significance and therapeutic implications of copper homeostasis-related gene dysregulation in acute myeloid leukemia. Ann. Hematol. 103 (8), 2797–2826 (2024).
Zabłocka-Słowińska, K. et al. Serum and whole blood Cu and Zn status in predicting mortality in lung cancer patients. Nutrients 13 (1), 60 (2020).
Ullah, M. I. et al. Biological role of zinc in liver cirrhosis: an updated review. Biomedicines 11(4), (2023).
Kim, S., Freeland-Graves, J. H., Babaei, M., Sachdev, P. K. & Beretvas, S. N. Quantifying the association between acute leukemia and serum zinc, copper, and selenium: a meta-analysis. Leuk. Lymphoma. 60 (6), 1548–1556 (2019).
Siddiqui, A. J. et al. Serum metallomics reveals insights into the associations of elements with the progression of preleukemic diseases toward acute leukemia. Biology Methods Protocols. 9 (1), bpad027 (2024).
Zekavat, O. R. et al. Trace elements in children with acute lymphoblastic leukemia. Asian Pac. J. Cancer Prev. 22 (S1), 43–47 (2021).
Kaiafa, G. D. et al. Copper levels in patients with hematological malignancies. Eur. J. Intern. Med. 23 (8), 738–741 (2012).
Bremner, I. & Beattie, J. H. Copper and zinc metabolism in health and disease: speciation and interactions. Proc. Nutr. Soc. 54 (2), 489–499 (1995).
Ohanian, M. et al. A heavy metal baseline score predicts outcome in acute myeloid leukemia. Am. J. Hematol. 95 (4), 422–434 (2020).
Wang, W. et al. Serum copper level and the copper-to-Zinc ratio could be useful in the prediction of lung cancer and its prognosis: A Case-Control study in Northeast China. Nutr. Cancer. 73 (10), 1908–1915 (2021).
Malavolta, M. et al. Serum copper to zinc ratio: relationship with aging and health status. Mech. Ageing Dev. 151, 93–100 (2015).
Jarosz, M., Olbert, M., Wyszogrodzka, G., Młyniec, K. & Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 25 (1), 11–24 (2017).
Dong, C., Zhang, N. J. & Zhang, L. J. Oxidative stress in leukemia and antioxidant treatment. Chin. Med. J. (Engl). 134 (16), 1897–1907 (2021).
Bahi, G. A. et al. Assessments of serum copper and zinc concentration, and the cu/zn ratio determination in patients with multidrug resistant pulmonary tuberculosis (MDR-TB) in Côte d’ivoire. BMC Infect. Dis. 17 (1), 257 (2017).
Laine, J. T., Tuomainen, T. P., Salonen, J. T. & Virtanen, J. K. Serum copper-to-zinc-ratio and risk of incident infection in men: the Kuopio ischaemic heart disease risk factor study. Eur. J. Epidemiol. 35 (12), 1149–1156 (2020).
Zhu, B. et al. Decoding the implications of zinc in the development and therapy of leukemia. Adv. Sci. (Weinh) 12(9), (2025).
Martins, A. C. et al. Association between heavy metals, metalloids and metabolic syndrome: new insights and approaches. Toxics 11(8), 670 (2023).
Acknowledgements
We are grateful to the participants of this study. We are also thankful to Jouf University, Sakaka, Saudi Arabia, for providing the funding for this research.
Funding
This work was funded by the Deanship of Graduate Studies and Scientific Research at Jouf University under grant No. (DGSSR-2023-01 -02369).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final version of the manuscript and agree to be responsible for all parts of the research. Conceptualization, M.I.U, and M.A.N; Data curation, M.S, S.H, and A.N.A; Formal analysis, E.M, S.H, Shahid. H, and A.F; Funding acquisition, M.I.U; Investigation, M.S, S.H, A.N.A, and M.A; Methodology, A.A.M.A and M.A; Project administration, M.I.U; Software, Shahid.H and A.F; Supervision, M.A.N; Writing – original draft, E.M, Shahid.H, A.A.M.A, A.F and M.A; Writing – review & editing, M.A.N.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
The study obtained Ethical approval from the Ethical Review Committee, University of Health Sciences Lahore, Pakistan (UHS/EAPC-22/ERC/2) dated April 28, 2022. Helsinki guidelines (modified 2013) were followed for the collection of human subjects, and written informed consent was obtained from each participant.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ullah, M.I., Manni, E., Shakil, M. et al. Association of serum copper, zinc and copper to zinc ratio in patients with acute myeloid leukemia. Sci Rep 15, 34091 (2025). https://doi.org/10.1038/s41598-025-14812-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14812-4