Abstract
This study investigated the long-term stability of cytogenetic and morphological markers, including dicentric chromosomes (DC), unbalanced translocation (UT), balanced translocation (BT), and Pseudo Pelger-Huët Anomaly (PPHA), in a radiation worker exposed to an acute dose of 60Co-γ radiation. Initial dose assessment, one week after exposure via Thermoluminescent dosimeters (TLDs) and DC, yielded a physical dose of 438.8 mGy and a biological dose of 398 mGy respectively. A follow-up biodosimetry evaluation, conducted 24 years postexposure, yielded a dose estimate of 449 mGy on the basis of BT, closely matching the initial TLD measurement (+ 2.3% relative error). In contrast, the DC, UT, and micronuclei (MN) frequencies fell within the background range, confirming their instability over time. We also assessed the presence of PPHA in blood smears from the same volunteer. PPHA, a morphological marker of neutrophils originating exclusively in vivo from bone marrow progenitor cells, demonstrated a more than twofold increase in frequency compared to background levels, suggesting an association with radiation exposure. This is the first report of concurrent BT and PPHA persistence in the same individual, demonstrating the suitability of these biomarkers for retrospective detection of past radiation exposure. BT offered reliable dose reconstruction decades after exposure. We could not translate the PPHA yield into an absorbed dose, as no in-house dose‒response curve was established.
Similar content being viewed by others
Introduction
Accurate dose estimation following radiation exposure is essential for health monitoring, regulatory compliance, and medical intervention, particularly in cases of accidental, occupational, or environmental exposure1. Cumulative radiation doses pose long-term health risks, making precise dose reconstruction critical for radiation workers, radiotherapy patients, and other exposed populations2. Cytogenetic techniques such as the dicentric chromosome assay (DCA), cytokinesis blocked micronuclei (CBMN) assay, and fluorescence in situ hybridization (FISH) have been developed to address this need1. DCA, the gold standard for short-term biodosimetry, detects dicentric chromosomes (DCs) formed by ionizing radiation-induced DNA double-strand breaks. However, DCs gradually decline as damaged cells are cleared or complete their life cycle, limiting their long-term utility1,3. Similarly, MNs, extranuclear bodies formed from chromosomal fragments (or whole chromosomes), also decrease in frequency over time, further restricting their application in long-term retrospective biodosimetry1,4. More stable chromosomal markers, such as translocations, offer a solution for long-term dose estimation. Balanced translocations (BTs), in particular, are stably maintained through successive cell divisions, making them reliable markers for cumulative radiation exposure from years to decades (up to lifetime)5. Peripheral blood lymphocytes are eventually eliminated after completing their lifespan, which result in the loss of BT signals from peripheral blood. However, new lymphocytes produced from precursor bone marrow cells possess these stable aberrations (BTs are stable aberrations, involve no genetic loss, only gene rearrangements)6. These precursor cells continue to produce functional blood lymphocytes with stable aberrations and hence the frequency of peripheral blood lymphocytes with such stable aberrations remains maintained over longer periods7,8. FISH is crucial for detecting BTs, making it indispensable for long-term biodosimetry, especially in radiation workers with potential long-term exposure, and in other cumulative exposure cases, and large-scale epidemiological studies5,7.
The decay kinetics of DCs, MNs, and unbalanced translocations (UTs) have been extensively studied, with significant variability reported in their temporal dynamics9. While DCs, MNs and UTs are useful for short-term dose assessment, they exhibit rapid decay due to cell turnover (typically lasting 2–3 years), complicating long-term dose reconstruction10. Conversely, BTs demonstrate notable stability, persisting in peripheral lymphocytes for years to decades postexposure11. Although partial-body exposure and other factors can influence BT stability, their robustness makes them critical for retrospective dose estimation in long-term studies12.
In addition to established cytogenetic markers, recent studies have identified radiation-induced morphological markers. Notably, Goans et al. (2015) reported that pseudo-Pelger-Huët anomaly (PPHA) is a radiation-induced morphological biomarker13. Their analysis of archival peripheral blood smears from the 1958, Y-12 criticality accident revealed that PPHA morphology in neutrophils emerged within 12 h postexposure, highlighting its potential as a marker of acute radiation exposure14. Further evidence from the Y-12 incident indicated that PPHA could persist as a long-term biomarker, with one patient exhibiting the PPHA marker for up to 16 years postexposure, suggesting a stable radiation-induced alteration14,15. The Pelger–Huët anomaly was first described by Karl Pelger (1928) and G.J. Huët (1931) as an autosomal dominant mutation16,17. PPHA is characterized by round, oval, or bilobed nuclei joined by a thin chromatin bridge. While PPHA affects all granulocytes, it is most pronounced in neutrophils. This is generally attributed to decreased expression of the lamin B receptor (LBR), a component of the nuclear lamina whose expression increases during normal granulocyte maturation. PPHA originates in the bone marrow and is caused by a dominant mutation in the lamin B receptor (LBR) gene, which is located on chromosome 1q42.1218.
This article presents a case study of a radiation worker exposed to acute 60Co-γ radiation, with initial dose assessment using DCA and a TLD. It evaluates the long-term stability of cytogenetic (DC, UT, and BT) and morphological (PPHA) markers, 24 years postexposure. Notably, this study provides the first evidence of simultaneous BT in lymphocytes and PPHA in neutrophils, demonstrating their persistence over time. These findings emphasize the potential of BT and PPHA as long-existing biomarkers for retrospective radiation dose estimation.
Materials and methods
Study design
Approximately 2 decades ago, a middle-aged industrial radiation worker was acutely exposed to 60Co-γ radiation. Initial dose estimation used physical (TLD) and biological (DCA) dosimetry (Table 1; Fig. 4-supplementary material). Recently, 24 years post-exposure, biodosimetry assessed the long-term stability of radiation-induced markers, including chromosomal aberrations (BT, UT, DC, MN) in lymphocytes and PPHA in neutrophils (Figs. 1 and 2).
This study investigated chromosomal markers, including DC, UT, BT and MN, in lymphocytes and the morphological marker PPHA in neutrophils from a willingly participating radiation worker (age-27 years, gender-male) exposed inadvertently to an acute dose of 60Co-γ radiation 24 years ago. It aimed to assess the long-term in vivo stability of these markers for accurate dose estimation. The initial dose was estimated one week after exposure, with a reassessment conducted 24 years later using blood sample from the same volunteer. The initial dose estimation was performed using DC analysis with Giemsa staining, while the follow-up assessment utilized both FISH- and Giemsa-based methods. FISH analysis measured BT, UT, and DC, whereas Giemsa staining evaluated DC and MN. Age- and sex-matched controls (positive and negative) were included in both the initial and follow-up dose estimations. The term “control” refers to blood samples obtained from unexposed healthy volunteers. The initial control blood sample was collected from one volunteer at the time of the first exposure. After 24 years, a subsequent control blood sample was collected from a different healthy, unexposed volunteer matched for age and gender. The control volunteers were not the same for the initial and later blood collections. The inclusion of age- and sex-matched positive and negative controls aimed to ensure the reliability and consistency of cytogenetic dose estimation in cases of defined radiation exposure levels. These controls served as a reference for comparison with data obtained in the exposed individual. Additionally, their inclusion helped evaluate intra-laboratory reproducibility and validate the robustness of the biodosimetry techniques used in both the initial and follow-up assessments. The negative control was not exposed, while the positive control received in vitro 0.5 Gy of 60Co-γ radiation at a dose rate of 0.4 Gy/min via a Blood Irradiator-2000 manufactured by the Board of Radiation & Isotope Technology (BRIT), DAE, India.
Irradiation was carried out following recommended guidelines1,3. A water-equivalent phantom with 3 mm buildup (polymethyl methacrylate (PMMA) plate placed on top of the phantom) was used to ensure electronic equilibrium for the samples. Both the phantom and samples were pre-warmed and maintained at 37 °C throughout the procedure. Dose and dose rate were measured using a Fricke dosimeter in the same irradiation geometry, with calibration traceable to national standards and aligned with international primary standards. All irradiations were performed on the same day.
The subject is absolutely fine and in good health conditions, with no symptoms of any oncological conditions after 24 years of exposure. A recent differential blood count was conducted and found to be within the normal range, further supporting the absence of any hematological abnormalities.
Chemicals
L-glutamine and Giemsa stain were obtained from Sigma‒Aldrich, USA. RPMI 1640, phytohemagglutinin-M (PHA-M), cytochalasin B (Cyto-B), fetal calf serum (FCS), streptomycin, penicillin, and colcemid were acquired from Gibco Life Technologies, USA. DPX was obtained from Merck, USA. Whole chromosome painting probes for chromosomes 1 and 2 were provided by Metasystems, Germany.
Ethical approval and blood collection
This study was approved by the Institutional Ethics Committee of BARC, Mumbai, India. Informed consent was obtained from both the subject and two control volunteers for the collection of peripheral blood samples, 5 mL and 9 mL, respectively, in lithium-heparinized vacutainers. All experiments were conducted in accordance with the guidelines and recommendations provided by the ethics committee.
Sample Preparation and analysis of cytogenetic markers
For the DC assay, eighteen sets of whole blood cultures were prepared, consisting of six cultures from the subject, six positive controls, and six negative controls, following optimized standard protocols (IAEA, ISO, and our previous study)1,3,19,20,21. The positive control consisted of isolated whole blood samples irradiated ex vivo with 0.5 Gy ⁶⁰Co-γ radiation, while negative controls comprised aliquots from the same blood collection that received no irradiation. Each culture contained 4 mL of RPMI-1640 medium, 0.5 mL of fetal bovine serum (FBS), 0.1 mL of PHA-M, and 0.5 mL of heparinized peripheral blood. The cultures were incubated at 37 °C with 5% CO₂ for 52 h. An early treatment with colcemid (0.2 µg/mL) was administered 24 h after culture setup to arrest cells in first metaphase and prevent their entry into a second division cycle. After centrifugation (186 g, 8 min), the pellet was treated with hypotonic KCl (0.075 M) and incubated for 20 min. The cells were centrifuged again and fixed in Carnoy’s solution, and the fixation was repeated three times. Fixed cells were dropped onto slides, stained with 10% Giemsa, and mounted with DPX. Five hundred to 1000 metaphases were scored for dicentrics following the IAEA and ISO guidelines.
Six whole blood cultures (two from the subject, two positive controls, and two negative controls) were prepared for the cytokinesis-block micronucleus (CBMN) assay, as per our optimized protocol in alignment with the IAEA and ISO guidelines1,4,22,23. Blood was cultured for 72 h, and Cyto-B (5 µg/mL) was added at 44 h to block cytokinesis and promote binucleated cell formation. The cultures were subsequently centrifuged (186 g, 10 min), treated with hypotonic KCl (0.075 M), and incubated for 20 min. After further centrifugation, the cells were fixed with Carnoy’s solution (methanol: acetic acid, 4:1) for three cycles24. The cell suspension was dropped onto glass slides, dried at 40 °C, stained with 10% Giemsa for 20 min, and mounted with DPX. Micronuclei were scored manually in 1000 binucleated cells under a bright-field microscope at 63X magnification.
Furthermore, three slides, one each from the subject, positive control, and negative control, each containing over 2,000 high-quality metaphases, were selected for FISH analysis. Fluorophore-labeled whole chromosome paint probes from Metasystems (Germany) were used for chromosome pairs 1 (FITC, green) and 2 (Texas Red, red)25,26. After brief pepsin treatment (using 0.005% (w/v) pepsin in 0.01 N HCl at 37 °C for 5 min), 12 µl of each probe was applied, the mixture was denatured at 75 °C for 3 min, and the mixture was hybridized overnight at 37 °C. The slide was washed in 0.4X SSC (Saline-Sodium Citrate buffer) at 72 °C and rinsed in 2X SSC with 0.05% Tween 20, followed by dehydration with ethanol (80%, 90%, 100%). Finally, DAPI (4′,6-diamidino-2-phenylindole) with antifade was applied for microscopic examination.
The slides were scanned using the system Metafer 5 (MetaSystems, Germany), assembled on the base of the ZEISS Axio Imager.Z2 microscope, equipped for fluorescence imaging with a motorized stage and a high-resolution CCD camera (CoolCube 4). The software modules ISIS and Ikaros of the Metafer system were used, respectively, for fluorescence image acquisition and processing, and for karyotyping and chromosome analysis. Scans at 10X magnification identified high-quality metaphases, followed by final image capture at 63X using AutoCapt mode and appropriate filter sets. For two-color FISH, metaphases were captured via FITC, Texas Red, and DAPI filters, and images were overlaid. A total of 1,000 metaphases per sample were analyzed for BT, UT, and DC in accordance with the IAEA and ISO guidelines.
Initially, 24 years ago, DC analysis was performed manually using Giemsa-stained metaphases under a bright-field microscope at 1000× magnification. In the recent phase of the study, DC and MN scoring were again conducted manually using Giemsa staining under bright-field microscopy (1000X). For the FISH-based analysis, metaphases were captured at 100X magnification using Metafer 5 software. The scoring of BT, UT, and DC was performed manually from the captured images using the ISIS module.
To estimate radiation doses for the samples analyzed in this study, dose–response calibration curves specific to DCs and BTs were applied, as previously established and validated in our laboratory. The dose–response relationship followed the linear-quadratic model, (Y = αD + βD²). For DCs, the linear (α) and quadratic (β) coefficients were 0.027 DCs Cell− 1 Gy− 1 and 0.065 DCs Cell− 1 Gy− 2, respectively. For BTs (involving chromosomes 1 or 2 only), the corresponding α and β values were 0.01377 BTs Cell− 1 Gy− 1 and 0.01303 BTs Cell− 1 Gy− 2, respectively27,28.
Sample Preparation for PPHA scoring
Thin blood smears were prepared on glass slides from blood samples from the subject and two age- and sex-matched control volunteers. The slides were air-dried in a laminar flow hood and fixed in 100% chilled methanol for 5 min. After fixation, the smears were stained with Wright‒Giemsa stain for 15 min, washed twice with distilled water, and subsequently mounted with DPX. Microscopic analysis was conducted with a brightfield microscope under a 63X objective to estimate the frequency of PPHA. Neutrophils with incomplete nuclear segmentation and bilobed nuclei were characterized as PPHA13,14,15.
Statistical analysis
The 95% confidence intervals for the aberration yield and radiation dose estimates were calculated on the basis of a Poisson distribution, following IAEA (2011) and ISO 19,238 recommendations1,3. The doses were estimated via CABAS software29. Papworth U test values, dispersion index, standard errors, and the applicability of Poisson statistics were employed to analyze our datasets.
Results
Patient history, initial dose evaluation, and long-term follow-up assessment 24 years after exposure
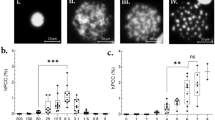
Table 1 lists the doses estimated soon after exposure and 24 years postexposure via various markers and techniques, along with the relative errors. The negative control presented background levels of aberrations for all types of markers. Statistical analysis confirmed that all data points (shown in Table 1), for DC, UT and BT exhibited, Papworth’s u-test values within the acceptable range of ± 1.96, the dispersion index (σ2/Y) approximated 1, and adhering to a Poisson distribution.
The TLD measurement indicated a physical dose of 438.8 mGy. Metaphase analysis showed cells containing solely one dicentric chromosome, with no instances of cells exhibiting two or more dicentrics was observed. The frequency of UT and DC fell within three standard deviations (3σ) of the background frequency, indicating that they were below the detection limits of the assay1,3. Both the DC yields and MN yields were also within the background frequency. BT dose aligns with TLD and DCA’s, suggesting stability1.
Additionally, it is well-documented that the frequency of balanced translocations tends to increase slightly with age, which may contribute to the slightly elevated dose estimations observed in the follow-up assessment.
Representative images of FISH-processed metaphases, Giemsa-stained metaphases, and binucleated cells. (A) FISH-Control metaphase with no aberrations. (B) FISH-Metaphase with one BT between red and black chromosomes. (C) FISH-Metaphase with one UT between the green and black chromosomes. (D) Giemsa stained control metaphase without DCs. (E) Giemsa stained metaphase with one DC accompanied by an acentric fragment. (F) Giemsa stained control binucleated cell without MNs. (G) Giemsa stained binucleated cell containing one MN.
Persistence of PPHA, 24 years postexposure
Although the data on PPHA for initial exposure were unavailable, PPHA was assessed in the blood smear of the subject, which was collected 24 years after radiation exposure (Fig. 2). A microscopic analysis of 4,500 neutrophils from the subject revealed a PPHA incidence of 5.22%, a twofold increase compared with that observed in two age- and sex-matched controls. (Table 2). These results provide evidence of the long-term in vivo persistence of PPHA, emphasizing its potential as a biomarker for radiation biodosimetry over extended periods. Similar findings have also been reported in few other studies13,14,15. This study, for the first time, demonstrates the simultaneous in vivo presence of elevated yield of BT in lymphocytes and PPHA in neutrophils in the peripheral blood of the same individual 24 years postexposure. These findings establish a correlation between BT and PPHA in terms of their stability and present a valuable tool for cumulative and long-term biodosimetry in individuals exposed to radiation in the distant past.
Representative images of neutrophils from the subject’s blood smear at 24 years postexposure. Panels A-G display neutrophils exhibiting the PPHA, whereas panel H shows a morphologically normal neutrophil. Panel I present a microscopic field view capturing both a rare PPHA-affected neutrophil and a normal neutrophil within the same field. Images were obtained via bright-field microscopy.
Discussion
Accurate dose estimation in radiation workers is crucial for regulatory and health monitoring purposes. Dose assessment is typically required within weeks to months after exposure but may also be necessary years or even decades later, especially for retrospective cumulative dose estimation and for health effects and other epidemiological studies 1,2,3,4,7. Various radiation-induced cytogenetic markers have been identified for dose estimation, each with varying stability over time. DC and UT, for example, have a relatively short persistence, typically lasting 2–3 years30. In contrast, the only known chromosomal marker with long-term stability, spanning years to decades, is BT, which can persist in peripheral lymphocytes over an extended period (Fig. 3)1,7.
This case study illustrates the biological dose reconstruction of a radiation worker who was exposed to acute 60Co-γ radiation. The biological dose estimated soon after exposure via DCA was closely aligned with the physical dose measured by the TLD of the subject. However, this correlation declined over time, as expected. The follow-up biodosimetry assessment, conducted 24 years later using multiple markers, including DCA, CBMN, BT, and UT, revealed that BT was the most reliable marker for dose reconstruction. In contrast, the other markers were found to be within 3σ of the background frequency, which is consistent with their known instability due to cellular turnover and elimination of unstable chromosomal aberrations 1,2,3,4,30,31. The results demonstrate the long-term in vivo stability of radiation-induced BT, supporting their use for retrospective dose estimation even decades post-exposure. This stability aligns with findings from previous studies32,33,34.
The relative errors in dose estimates, shown in Table 1, are with reference to the TLD measurement, not the actual reconstruction of the physical dose, as the exact physical information is not available. Many physical factors, such as nonuniformity of the radiation field, the body acting as a partial shield for the physical dosimeter, and the dose distribution or profile within the body, either due to the distance factor or attenuation or due to possible surrounding structural materials, can lead to uncertainties in the physical dose35,36. While consciously accepting these limitations, it is also important to note that there was very close agreement with the biological dose and TL measurements. Hence, the compared deviations are referred with reference to the TL measured dose.
The neutrophil-based morphological marker PPHA was assessed in the blood smear of the exposed subject 24 years after radiation exposure. The analysis adhered to a strict definition of PPHA cells, characterized by a bilobed nucleus with two round segments of nearly equal size connected by a thin chromatin strand13,14,15,16,17. A previous report by Goans et al. (2015) documented the in vivo persistence of PPHA cells up to 16 years postexposure in blood smears from eight individuals involved in the 1958 Y-12 criticality accident at Oak Ridge, TN, USA13. In our study, we extend these findings, demonstrating the persistence of PPHA cells up to 24 years postexposure. For the first time, we present evidence of the coexistence of PPHA with BT in the same individual, providing novel insight into the long-term stability of hematopoietic markers. These results validate the long-term persistence of PPHA cells and emphasize their potential correlation with radiation exposure. However, as a dose‒response curve for PPHA has not yet been established in our laboratory, we were unable to correlate the yield of PPHA with the biological dose precisely. Nevertheless, the observed yield of PPHA falls within the dose range of 0.29–0.86 Gy, as reported by Goans et al. (2015)13. This range closely aligns with the initial dose estimates derived from TLD and DC analysis following exposure, as well as the dose estimated from BT, 24 years postexposure.
The age dependency of PPHA in healthy human populations requires further investigation, as current data are insufficient to establish a clear relationship. While PPHA is linked to ionizing radiation, it lacks exclusivity, as it can also be induced by myelodysplastic syndromes, certain genetic abnormalities, and rare mutagenic agents not typically encountered by the general public. The dose-response relationship for PPHA in humans is less defined than for other biodosimetric markers. A linear trend is assumed, supported by macaque studies37, but limited human data from controlled exposure studies hinder precise characterization.
Peripheral blood lymphocytes with BT and neutrophils displaying PPHA both originate from bone marrow precursor cell populations, which can persist over extended periods, even a lifetime5,11,38. Unlike BT, which can also be generated in peripheral blood cells, PPHA is exclusively produced in the bone marrow in response to in vivo radiation exposure18. This specificity to bone marrow makes in vitro induction of PPHA and, consequently, the creation of an in vitro calibration curve unfeasible. The codetection of BT and PPHA in samples strengthens the potential of PPHA as a robust marker for retrospective and cumulative exposure assessment.
In the present study, we utilized a single age- and gender-matched control sample for background frequency assessment, in which no BT were detected. While this approach provided a baseline for our current analysis, we acknowledge that spontaneous BT frequency has been reported to exhibit an age-dependent increase in the general population. Consequently, incorporating a larger cohort of matched control volunteers in future studies might yield a more refined background frequency estimate, potentially leading to some minor adjustments in dose estimation.
Conclusion
This study provides the first evidence of the simultaneous in vivo persistence of PPHA and BT 24 years after radiation exposure. These findings highlight the stability of both biomarkers, offering a powerful tool for long-term retrospective biodosimetry. The association between PPHA and BT highlights their potential as reliable indicators for cumulative radiation dose estimation, shaping future approaches to radiation monitoring and health risk assessment decades after exposure.
Data availability
All data generated in this study are included in the article. Additional data supporting the findings are available from the corresponding author (Email ID: bsapra@barc.gov.in), upon reasonable request.
References
IAEA, C. D. Applications in Preparedness for and Response To Radiation Emergencies (EPR-Biodosimetry. International Atomic Energy Agency, 2011).
Mu, H. et al. Ionizing Radiation Exposure: Hazards, Prevention, and Biomarker Screening25pp.15294–15306 (Environmental Science and Pollution Research, 2018).
ISO. ISO 19238:2023 Radiological protection - Performance Criteria for Service Laboratories Performing Biological Dosimetry by cytogenetics — Dicentric Assay (International Organization for Standardization, 2023).
ISO. ISO 17099:2024 Radiological protection — Performance Criteria for Laboratories Using the cytokinesis-block Micronucleus (CBMN) Assay in Peripheral Blood Lymphocytes for Biological Dosimetry (International Organization for Standardization, 2024).
Lucas, J. N. & Deng, W. Our views on issues in radiation biodosimetry based on chromosome translocations measured by FISH. Radiat. Prot. Dosimetry. 88 (1), 77–86 (2000).
Roukos, V. & Misteli, T. The biogenesis of chromosome translocations. Nat. Cell Biol. 16 (4), 293–300 (2014).
ISO 20046:2019(en. ) Radiological protection — Performance criteria for laboratories using Fluorescence In Situ Hybridization (FISH) translocation assay for assessment of exposure to ionizing radiation.
Chaurasia, R. K. & Sapra, B. K. Effects of low dose ionizing radiation on human health: evidence for revisiting radiation protection policies. In Handbook on Radiation Environment Vol. 1 (ed. Aswal, D. K.) Sources, Applications and Policies, 417–442 (Singapore, Springer Nature Singapore, 2024).
de Lemos Pinto, M. M. P., Santos, N. F. G. & Amaral, A. Current status of biodosimetry based on standard cytogenetic methods. Radiat. Environ. Biophys. 49, 567–581 (2010).
Ricoul, M., Gnana-Sekaran, T., Piqueret-Stephan, L. & Sabatier, L. Cytogenetics for Biological Dosimetrypp.189–208 (Methods and Protocols, 2017).
Hsieh, W. A., Lucas, J. N., Hwang, J. J., Chan, C. C. & Chang, W. P. Biodosimetry using chromosomal translocations measured by FISH in a population chronically exposed to low dose-rate 60 Co γ-irradiation. Int. J. Radiat. Biol. 77 (7), 797–804 (2001).
Rodriguez, P. et al. Analysis of translocations in stable cells and their implications in retrospective biological dosimetry. Radiat. Res. 162 (1), 31–38 (2004).
Goans, R. E., Iddins, C. J., Christensen, D., Wiley, A. & Dainiak, N. Appearance of pseudo-Pelger Huet anomaly after accidental exposure to ionizing radiation in vivo. Health Phys. 108 (3), 303–307 (2015).
Goans, R. E., Iddins, C. J., Ossetrova, N. I., Ney, P. H. & Dainiak, N. The pseudo-Pelger Huet cell—a new permanent radiation biomarker. Health Phys. 112 (3), 252–257 (2017).
Goans, R. E. et al. The pseudo-Pelger Huët cell as a retrospective dosimeter: analysis of a radium dial painter cohort. Health Phys. 117 (2), 143–148 (2019).
Pelger, K. Demonstrate Van Een Paar Zeldzaam voorkomende typen Van Bloedlichaampjes En bespreking der patienten. Ned Tijdschr Geneeskd. 72, 1178 (1928).
Huët, G. J. Ueber Eine Bisher Unbekannte Familiaere Anomalie Der Leukocyten11pp.1264–1266 (Klinische Wochenschrift, 1932).
Colella, R. & Hollensead, S. C. Understanding and recognizing the Pelger-Huët anomaly. Am. J. Clin. Pathol. 137 (3), 358–366 (2012).
Chaurasia, R. K. et al. Establishment and multiparametric-cytogenetic Validation of 60Co-gamma-ray Induced, phospho-gamma-H2AX Calibration Curve for Rapid Biodosimetry and Triage Management during Radiological Emergencies866p.503354 (Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2021).
Chaurasia, R. K., Shirsath, K. B., Desai, U. N., Bhat, N. N. & Sapra, B. K. Establishment of in vitro calibration curve for 60Co-γ-rays induced Phospho-53BP1 foci, rapid biodosimetry and initial triage, and comparative evaluations with γH2AX and cytogenetic assays. Frontiers in Public Health, 10, p.845200. (2022).
Ganguly, B. B., Ganguly, S. & Kadam, N. N. Spectrum of stable and unstable rearrangements in lymphocytic chromosomes investigated in Bhopal population 30 years post MIC disaster amid co-exposure to lifestyle, living, and occupational hazards. Environ. Sci. Pollut. Res. 30 (1), 1997–2019 (2023).
Vijayalakshmi, J. et al. Establishment of ex vivo calibration curve for X-ray induced dicentric + ring and micronuclei in human peripheral lymphocytes for biodosimetry during radiological emergencies, and validation with dose blinded samples. Heliyon, 9(6) e16738 (2023).
Liu, Q. et al. Chromosomal Aberrations and DNA Damage in Human Populations Exposed To the Processing of Electronics Waste16pp.329–338 (Environmental science and pollution research, 2009).
Chaurasia, R. K., Shirsath, K. B. & Sapra, B. K. Protocol for one-step selective lysis of red blood cells and platelets with long-term preservation of white blood cells (human) at ambient temperature. STAR protocols, 2(4), p.100834. (2021).
Ha, J. et al. Cytogenetic testing by fluorescence in situ hybridization is improved by plasma cell sorting in multiple myeloma. Scientific reports, 12(1), p.8287. (2022).
Lin, P. P., Gires, O., Wang, D. D., Li, L. & Wang, H. Comprehensive in situ co-detection of aneuploid circulating endothelial and tumor cells. Scientific reports, 7(1), p.9789. (2017).
Bhat, N. N., Anjaria, K. B. & Rao, B. S. Dose rate effect and its implications in biodosimetry using chromosomal aberration analysis. Radiation Prot. Environ. 26 (3–4), 536–540 (2003).
Chaurasia, R. K. et al. FISH unveils a unified method for multi-marker biodose assessment. Sci. Rep. 15 (1), 1–15 (2025).
Deperas, J. et al. CABAS: a freely available PC program for fitting calibration curves in chromosome aberration dosimetry. Radiat. Prot. Dosimetry. 124 (2), 115–123 (2007).
Ludovici, G. M. et al. Cytogenetic bio-dosimetry techniques in the detection of dicentric chromosomes induced by ionizing radiation: A review. The European Physical Journal Plus, 136(5), p.482. (2021).
Kaddour, A. et al. M., Transmission of Induced Chromosomal Aberrations through Successive Mitotic Divisions in Human Lymphocytes after In Vitro and In Vivo Radiation. Scientific reports, 7(1), p.3291. (2017).
Balajee, A. S. & Hadjidekova, V. Retrospective cytogenetic analysis of unstable and stable chromosome aberrations in the victims of radiation accident in Bulgaria. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 861, p.503295. (2021).
McKenna, M. J. et al. Chromosome translocations, inversions and telomere length for retrospective biodosimetry on exposed US atomic veterans. Radiat. Res. 191 (4), 311–322 (2019).
Tawn, E. J. & Whitehouse, C. A. Persistence of translocation frequencies in blood lymphocytes following radiotherapy: implications for retrospective radiation biodosimetry. Journal of Radiological Protection, 23(4), p.423. (2003).
Kron, T. Thermoluminescence dosimetry and its applications in medicine–Part 1: physics, materials and equipment. Australasian Phys. Eng. Sci. Med. 17 (4), 175–199 (1994).
Ghomeishi, M., Mahdiraji, G. A., Adikan, F. M., Ung, N. M. & Bradley, D. A. Sensitive fibre-based thermoluminescence detectors for high resolution in-vivo dosimetry. Scientific Reports, 5(1), p.13309. (2015).
Hayes, J. M. et al. Pseudo Pelger-Huët anomalies as potential biomarkers for acute exposure radiation dose in rhesus macaques (Macaca mulatta). Int. J. Radiat. Biol. 98 (5), 913–923 (2022).
Reilly, A. et al. Lamin B1 deletion in myeloid neoplasms causes nuclear anomaly and altered hematopoietic stem cell function. Cell. Stem Cell. 29 (4), 577–592 (2022).
Acknowledgements
The authors would like to express their sincere gratitude to Mr. Shrikant Jagtap and other technical staffs from our lab for their valuable assistance and technical support.
Funding
Open access funding provided by Department of Atomic Energy. This work was supported by the institutional fund of the host institute (BARC, Mumbai, India). No external funding was involved in this study.
Author information
Authors and Affiliations
Contributions
Rajesh Kumar Chaurasia, Ranjana S. Pathak and Kapil B. Shirsath: Conceptualization, Methodology, Data Curation and Writing; Balvinder K. Sapra, Nagesh N. Bhat, Arshad Khan, Anjana Goel: Review, Editing, Supervision and Resources. Rajesh Kumar Chaurasia and Ranjana S. Pathak has contributed equally to the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The research proposal for this study was approved by the Institutional Ethics Committee of the Bhabha Atomic Research Centre (BARC) Mumbai, India. The project was conducted in accordance with the ethical guidelines outlined in the declaration. Ethical consent was obtained from all human volunteers participating in this study, and the project details were thoroughly explained to them prior to their involvement.
Consent for publication
All authors have agreed to publish the manuscript in its present form.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chaurasia, R.K., Pathak, R.S., Goel, A. et al. First evidence of coexistence of Pseudo Pelger Huet anomaly and balanced translocation in a two decades retrospectively exposed human subject. Sci Rep 15, 29292 (2025). https://doi.org/10.1038/s41598-025-14858-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14858-4
Keywords
This article is cited by
-
Pan centromeric FISH enhances precision in radiation biodosimetry
Scientific Reports (2026)