Abstract
Hydrogen has the potential to become a suitable alternative to the non-renewable sources like fossil fuels. The higher calorific values and ease of storage makes it better alternative to other renewable sources of energy. But the limiting factor for the usage of hydrogen is its inefficient production. To overcome this drawback, in our current work, we have developed a class of Zn substituted cobalt nanoferrites as a catalyst for producing the green and sustainable hydrogen energy. The cobalt nano catalysts are prepared through sol-gel auto-combustion (SA) method. A spinel phase is observed for the fabricated catalysts. Spherical shaped grains are found in the FESEM images of CoF1 and CoF3 catalysts. With Zn substitution, the band gap is found to vary between 1.69 to 1.91 eV which helps in the catalytic property of prepared catalysts. XPS investigation shows oxidation states of Zn, Co, Fe, and O. The magnetic measurements confirms that the introduction of dopant causes a decrease in coercivity while maintaining the saturation magnetisation, which helps in the recoverability of catalysis during the procedure. Zn0.06Co0.94Fe2O4 (x = 0.06) catalyst shows maximum photocatalytic hydrogen production of 21.51 mmol\(\:{g}_{cat}^{-1}\), with a Tafel slope of 141.88 mV/dec. Thus, the study provides the cost-effective alternative to traditionally use the catalysts which are not economically efficient for the sustainable and green H2 generation.
Similar content being viewed by others
Introduction
For the betterment of humankind, the members of the United Nations have set 17 sustainable development goals to accomplish till 2030. These goals try to tackle some of the major concerns faced by society like poverty, energy shortage, clean water availability, peace and equality. Out of these 17 goals, the SDG 7 specifically focuses on availability of clean energy source for the welfare of the society1. In order to achieve this goal, numerous studies are being conducting to enhance the efficacy of existing renewable sources. The one source which have one of the highest potential among the other sources and which is sometimes also called as the fuel of the future is the green hydrogen2,3. The green hydrogen shows the highest calorific values amongst the other renewable and non-renewable sources and it also produces energy without any carbon emissions4. The one major drawback of using hydrogen is its inefficient production5. Currently, the methods used for the hydrogen production are either non environment friendly or highly inefficient in producing the hydrogen. The methods like, gas reforming and coal reforming does yield high amount of hydrogen but they also cause the release of larger number of greenhouse gases6,7. These methods are also dependent on the fossil fuels, making them unsustainable resource. To counter these problems, the sustainable methods like photocatalysis and electrocatalysis are getting more considerations. One of the main reason for their wide spread popularity is their sustainable nature and lack of harmful emissions8,9. During these catalytic processes, the catalysts play an important part in the efficiency. The catalysts lower the activation energy required for breaking down of the water, making the process more favourable. In case of electrocatalysis, the catalysts provide a surface for the reaction to occur. Smaller catalyst’s size implies more surface area and higher quantity of active sites. This implies that in nanoscale, the catalyst will perform a lot better as compared to the bulk state catalysts10,11. Similarly for photocatalysis, the smaller size not only benefits the active sites but also helps in the formation of distinctive band gap which is not possible in the bulk state12. That’s why, the nanomaterials are often preferred as the catalysts for both of these processes. Some recent studies have shown that the spinel ferrite, a type of magnetic material, shows good catalytic abilities and stability to be used in the catalysis-based hydrogen production approaches13,14,15,16. The spinel ferrites having general formula AB2O4, could be easily prepared via using various synthesis approaches17. These ferrite materials are highly stable in acidic and in basic conditions due to their stable spinel structure and could be used in variety of scenarios like acidic or basic electrocatalysis18,19. Furthermore, spinel ferrites like cobalt ferrites shows good catalytic abilities due to readily availability of cations for the reaction. These ferrites can be modified by various dopants which further adds up to their catalytic efficiency20,21. They also show band gap within 1.5 to 2.5 eV, making them suitable for photocatalytic applications. Many studies have shown the potential of the Zn-based cobalt ferrite for the photocatalytic applications, indicating its potential for the green hydrogen production22,23,24,25. There are numerous studies indicating the effectiveness of spinel ferrites for hydrogen production26,27,28,29. Gobara et al. calculated the photocatalytic ability of NiFe2O4 spinel ferrite nanoparticles fabricated via the co-precipitation approach. The study showed crystallite size within 7 to 22 nm. The samples prepared showed high surface area with good porosity. The photocatalytic analysis showed maximum yield of 6.2 mmolh− 1 using nickel spinel ferrite30.
The recent research interest illustrates the potential of spinel ferrite for the H2-generation. But there haven’t been many studies showing the usefulness of spinel ferrite for both the photo and electrocatalytic H2 production. In the light of all these previous studies, in the current work, we are utilizing the zinc substituted cobalt ferrite-based catalysts i.e., ZnxCo1−xFe2O4 (x = 0.02, 0.04, 0.06) for hydrogen production via photo/electro catalysis. For preparing the said material, the Sol-gel autocombustion (SA) route is utilized. The SA synthesis approach is a widely utilized method for making the spinel ferrite nanomaterials. This method gives control over the structural behavior of cobalt ferrites and it is also cost efficient. In addition, the various techniques are utilized to analyze the structural, microstructural, magnetic, elementary, optical, and photo/electo catalytic traits of prepared catalysts.
Materials and methodologies
Materials used
The chemical reagents such as metal nitrates including, ferric nitrate, cobalt nitrate, zinc nitrate, and citric acid are used for making zinc doped cobalt spinel ferrites. Moreover, the ethylene glycol and ammonia are used to facilitate the reaction. All these reagents are of AR grade (\(\:\ge\:\)99%) and bought from SimSon Pvt. Ltd., India.
SA scheme for the fabrication of ZnxCo1−xFe2O4 (x = 0.02, 0.04, 0.06)
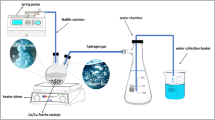
The synthesis of zinc doped cobalt nanoferrites is done via using the SA approach (Fig. 1). This synthesis approach is a chemical route which comes under the category of bottom-up approach31,32. The SA method uses metal nitrates with the assistance of a chelating agent for the better complex formation among the metal cations. At the start of the approach, aqueous mixture of nitrates and citric acid are prepared separately. The solutions are prepared by using the magnetic stirrer. After the complete dissolution, the nitrate mixture is added to citric acid. After this, the pH is kept to 7 using ammonia solution. After continuously stirring the mixed solution for half an hour, the ethylene glycol is added while keeping the temperature at 70 °C. This instigated the gel phase. Then the temperature is brought to 120 °C. This initiated the combustion of prepared gel resulting in the blackish substance. This material is ground and calcined at 900 °C for 4 h. Thus, the powder is further characterised via different techniques to analyze their different traits. The prepared catalysts are named as CoF1, CoF2, CoF3 for x = 0.02, 0.04 and 0.06.
Characterization
The structural characteristics including, crystal structure, phase formation and unit cell geometry are analyzed using the X-Ray diffractometer (Bruker D8 diffractometer; XRD). The morphology is reported through Field Emission Scanning Electron Microscope (JSM-IT800; FESEM). The characterizations facilities including, the UV-VIS spectrophotometer (Perkin Elmer, LAMBDA 750), Raman (RAM HR) and X-ray photoelectron spectroscopy (NEXSA; XPS) are being utilized for the examination of optical properties. The specific surface area of fabricated ferrite is measured using Brunauer–Emmett–Teller (BET) surface analyser. Lastly, the magnetism of the samples is analysed via the Vibrating sample magnetometer (Microsense VSM).
2.4 Photocatalytic water splitting measurements
For analysing the photocatalytic traits of the fabricated catalysts, we have employed UV light source i.e., 200 W, HgXe arc lamp (66906-200HXF-R15; Newport), which emits 180 W irradiation. The measurement is performed using photoreactor consisting of a quartz cell with bottle neck. The quartz cell is filled with the 50 mL of water along with 20 mg of photocatalysts and sacrificial agents. The sacrificial agent consisted of 500 mg of Na2S (0.128 mol/L) and Na2SO3. The sacrificial agents facilitate the photocatalysis by providing electron donor sites. The process is performed under the normal conditions of temperature and pressure. A gas chromatograph is utilized to check the hydrogen yield in 1 h interval. Lastly, the catalysts are extracted via centrifugation, drying, and then inspected for their recyclability and photocatalytic stability, indicating H2 generation over three 4-hour cycles.
Electrocatalytic water splitting measurements
The electrocatalysis is performed via 3-electrode arrangement, consisting of working (samples), reference (calomel) and counter (Pt wire) electrodes. To prepare the working electrodes from the samples, firstly the dispersion of catalysts is created via taking 6 mg of samples in 300 µL of isopropanol and 15 µL of Nafion. This dispersion is then coated by drop casting onto an ITO substrate. Before coating, the substrate is cleaned via ultrasonication. After coating, the substrate is dried and the measurements are accomplished via the chronoamperometry (CA), linear sweep voltammetry (LSV) and cyclic voltammetry (CV). CV analysis is performed within the range of 10–200 mV/s, whereas, the LSV is done at 100 mV/s. In addition, the CA is obtained at -1 V for HER.
Results and discussions
XRD investigation
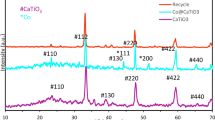
The phase development and phases purity of prepared nanomaterials is examined using XRD instrument. Figure 2 depicts the obtained plots from the XRD analysis. The figure depicts the presence of (311), (222), (400), (422), (511), (440) peaks indicating the spinel phase formation with Fd3m symmetry. No additional phases are observed in the XRD plots. Rietveld refinements of XRD were done to match the standard data (Fig. 3). The refinement parameter shows good fit with the standard data, which is evident by the chi2 values, as given in Table 1. The crystallinity is inclining with the increasing Zn doping. From XRD analysis, several key structural factors including, the crystallite size (Dnm), unit cell volume (Vcell), x-ray density (dx), hopping lengths at tetrahedral site (A-site) and octahedral site (B-site), lattice parameter (a), and strain \(\:\left(\text{ϵ}\right)\) are determined as provided in Table 1. Dnm is evaluated via the formula as given ahead33,34.
Here, λ represents wavelength, β is full width at half maxima and k represents Scherrer constant having value of 0.89. The other structural parameters are calculated using the formulas as given ahead35,36.
Here M represents molecular weight, (hkl) are miller indices, N represents Avogadro number, and V represents volume of unit cell. The strain \(\:\left(\text{ϵ}\right)\) is evaluated using the formula as given ahead35.
The “ϵ” helps in determining the crystallite behavior over the range of Zn dopant concentration. For the crystallite size, an increasing trend from 42.08 nm to 48.51 nm is obtained. The increasing crystal growth is caused via decrease in micro-strain with increasing dopant concentration. A correlation relation between the crystallite size and strain is given in Fig. 2b. The “a” shows small anomalous behaviour by first decreasing from 8.370 to 8.368 Å and then increasing to 8.374 Å when the Zn dopant content rises to 0.06. This happens because of discrepancy between the ionic radius of host cobalt cation (79 pm) and dopant zinc cation (74 pm), respectively. It has been found that the dx varies inversely with lattice parameter “a”. Therefore, the anomality of “a” is the cause for the abnormal nature of “dx”. However, the LA & LB shows a direct dependence on “a”. So, the LA and LB shows same trends as the lattice parameter. The XRD analysis confirms Zn ions incorporation within the cobalt lattice and this conclusion is being drawn from the presence of no additional phases in the XRD plots.
FESEM and EDS investigation
FESEM analysis is performed to analyse the superficial physical properties of the fabricated nanomaterials. The scans were performed at the range of 100 nm scale. Figures 4a, c depicts the FESEM illustrations of the prepared CoF1 and CoF3 ferrites. It is found that the developed catalytic materials show agglomerated surface morphologies. Moreover, the pictures are utilized to calculate the grain size using Image J software. The grain size distribution from FESEM images is shown in Figs. 4b and d. The computed grain size for CoF1 sample is found to be 96 nm while for CoF3, it is 98 nm. It is being concluded that the grain size of all the samples is within the nonorange. Moreover, the bigger grain size of CoF1 and CoF3 samples may be accredited to the aggregation of number of small crystallites37,38. The elemental composition is analysed using the EDS spectra and EDS spectra of the CoFe1 and CoFe3 are given in Fig. 5a and b. The EDS data confirms the desired elements (Co, Zn, Fe, O) within the system. The Zn peak indicates the proper incorporation of zinc doping cation within the cobalt ferrite lattice.
UV-Vis spectroscopy
This is an advance spectroscopic technique for determining the absorbance of UV and visible rays via using the sample. This technique has been utilized to determine the absorbance behavior and to estimate the band gaps of our prepared catalysts. The spectra obtained from the analysis is depicted in Fig. 6. A broad absorption region can be seen in the Fig. 6. This indicates light absorption over a broad spectrum. The estimation of Band gap is done via Tauc plots given in Eq. 739,40.
Here “α” signifies the absorption coefficient, “ν” represents photons frequency, “Eg” signifies band gap, “K” denotes the proportionality constant, and “h” is Planck’s constant. The “n” predicts the electronic transitions. If n = 2, it means direct transition happens and for n = ½, indirect transition takes place. If we take n = 2, we find a linear region, which is depicted in Fig. 6. This implies that by taking n = 2, the estimation is correct. This led to the conclusion that there exists direct band gap. The “Eg” of the samples is found to be showing uneven trend with Zn doping with the highest obtained band gap of 1.91 eV for CoF2 sample. However, With Zn doping, there is a decreasing trend in the values of Eg from 1.91 to 1.85 eV, which greatly helps in enhancing the photocatalytic efficacy of fabricated catalysts.
Raman spectroscopy
Raman spectroscopy helps in determining the vibrational modes of a material. Since these vibrational modes are unique for different materials, therefore, we could confirm the phase formation of a material by using the Raman technique. Figure 7 depicts the Raman modes of our prepared samples. As the prepared samples are of spinel symmetry, therefore, they all show the Oh7 space group. According to the group theory, the spinel symmetry is supposed to show five vibrational modes, which are A1g, Eg and 3T2g. But in case of nanomaterials, the crystallite size is generally much smaller than the wavelength of the laser used. In this case, there won’t be momentum conservation and extra Raman active modes will exist41,42,43,44,45. The observed vibrational modes of zinc doped cobalt spinel nanoferrites including, three T2g, one Eg and two A1g are given in Table 2. The stretching Fe-O and M-O bonds causes the A1g mode whereas the antisymmetric starching of these bonds is represented via the T2g(2) mode. The Eg band represents the symmetric bending of oxygen relating to Fe(M). T2g(3) shows the translational movement of MO446. The extra phonons peak appears because of comparatively large wave factor in contrary to the smaller particle size. The current type of behavior for the cobalt nanoferrites totally matches with the existing literature.
XPS investigation
The XPS spectroscopy is useful for determining the state of elements present within the system. This method is based on the phenomenon of photoelectric emission, and during this process, a high energetic beam of electrons penetrates the target and causes the ejection of electrons present at inner subshells. From the energy of beam and ejected electrons, we could calculate the B.E. of electrons. Since the electrons of different subshells shows unique binding energy which allows to find the state of the atom within the system. In case of spinel ferrite, this approach is helpful for determining the occupancies of the cations. An overall XPS survey of the CoF1 and CoF3 samples are given in the Figs. 8a and 9a. This confirms the presence of Co2p, Fe2p, Zn2p, O1s and C1s elements in the developed CoF1 and CoF3 samples. A detailed analysis on the individual peaks is also done in order to specify the cation occupancies. The indexing of the individual peaks is done from the existing literature and the standard binding energy data. Figures 8b and 9b shows the detailed peak analysis for the Zn dopant. It is found that for lower dopant content, the Zn dopant i.e. x = 0.02, there exist one Zn peak for Zn2p3/2 while for the higher doping content i.e. for x = 0.06, two peaks are obtained for Zn dopant which are Zn2p3/2 and Zn2p1/2. The absence of Zn2p1/2 peak for the CoF1 sample is because of its higher B.E. and lower intensity as compared to Zn2p3/2. It means that at lower doping content, there aren’t enough Zn cations which show significant Zn2p1/2 peak. The peaks for Co2p peaks for the CoF1 and COF3 samples is shown in Figs. 8c and 9c. The Co2p peaks for both the samples has been split into two consecutive peaks that is Co2p3/2 and Co2p1/2 and it may be attributed to the spin orbital coupling. Here, we have used the Co2p3/2 peak for deconvolution, because of its high intensity and easily distinguishable broadening. The Co2p3/2 peak is deconvoluted into Co2+(Oh) and Co2+(Th), where the former represents the Co2+ taking up the octahedral sites while the latter represents the cobalt cations taking up the tetrahedral sites. Furthermore, the peaks are assigned to Co(II) sate of cobalt cations as suggested by the previous studies47. The presence of cobalt cations in both the sites indicates that the mixed spinel structure is formed for both the samples47,48. Figures 8d and 9d shows the oxygen peak analysis for both the samples. The oxygen peaks for both samples are deconvoluted into three peaks which represents the presence of oxides, hydroxide defects, and water within the system. Figures 8e and 9e shows the Fe2p peak analysis for the CoF1 and CoF3 samples. As in the case of cobalt peaks, the Fe peaks have also been split into two peaks i.e., 1/2 and 3/2 due to the spin orbital coupling. Also, the smaller amplitude of 1/2 peak makes it difficult to accurately deconvolute this peak. That’s why, we have chosen the 3/2 peak for the deconvolution. The deconvoluted peaks are indexed using the existing literature, implying that the Fe cation is present in 3 + state at both the octa and tetrahedral sites49. The Figs. 8f and 9f shows the detailed deconvolution of carbon peaks for both the sample50,51. All of the calculated peak position and the estimated elemental distribution is shown in Table 3. Overall, the XPS analysis suggested that the mixed cobalt spinel nanoferrite is formed for all the composition with smaller number of defects, which are present due to the incorporation of Zn dopant species.
BET investigation
To analyse the surface area of prepared catalysis, the BET study is done via nitrogen isotherms. The Fig. 10 a, b gives the BET isotherms for CoF1 and CoF3, indicating the hysteresis loops with the relative pressure (P/Po) approaching to one. This represents the mesopores and macropores within the structure and comes under the type IV isotherm category52,53. The BET implies that CoF1 sample has specific surface area of 8.00 m2/g and for CoF3, it is 6.403 m2/g. So, the BET analysis indicated that the prepared Zn doped Co nanomaterials possessed high specific surface area and porosity making them suitable catalysts.
VSM investigation
VSM analysis is useful for determining the magnetism in a material. This analysis is performed to check the magnetic nature of the fabricated samples. Figure 11(a) represents the magnetic hysteresis for fabricated catalysts. The M-H loop suggest that the developed samples have soft ferromagnetic nature. Saturation magnetization (Ms), coercivity (Hc), retentivity (Mr), and magnetic moment (nB) are obtained from M-H loops as tabulated in Table 4. Here “nB” is evaluated via using the Eq. 854,55.
Here M.W. denotes molecular weight, the term “5585” represents constant called conversion factor. Squareness ratio (S.R.) is also obtained for all samples using the given formula56,57.
The values of Ms show an overall increase with Zn doping from 70.88 to 75.19 emu/g. With the rising Zn content, the Zn replaces Co at B-site, which could shift the Co from the B to the A-site. This means that AB exchange interaction takes place within the cobalt ions at B- and A-sites which are more intense than the A-A interactions and B-B interactions. It explains the overall increase in the saturation magnetisation. But with the further increase in the doping content, the Zn cations can occupy both sites which could decrease the cobalt concentration at both sites. This could cause the overall decrease in the values of the saturation magnetisation. The nB of the samples also shows similar trend to Ms because the overall magnetic moment shows dependency on the Ms. The Mr and Hc both show declining trend with rising Zn cations. This could be due to the non-magnetic behavior of zinc dopant cations which results in the decrease of coercivity and retentivity for the fabricated catalysts. Moreover, the values of squareness ratio are below 0.5, which could define the multi-magnetic domain formation for the zinc doped cobalt catalysts13. Thus, it is found that the high magnetic nature of zinc doped cobalt nanoferrite based catalysts are highly beneficial for the recoverability during the photocatalysis process.
Photo/electro catalytic water splitting investigation
Photocatalytic water splitting investigation
The synthesised catalysts are tested for the H2-production capabilities via water splitting through photocatalysis. Figure 12a represents the H2 evolution within 4 h with time interval of one hour. The evolution rates have been measured for all the prepared samples. Figure 12b presents the overall H2-production over the period of 8 h for all of the samples. Total apparent H2-evolution for CoF1, CoF2 and CoF3 samples are 11.76, 16.97 and 21.51 mmol\(\:{\text{g}}_{\text{c}\text{a}\text{t}}^{-1}\). This indicates that there is a net increase in H2-evolution with the rising Zn dopant content. The average H2-production has also been evaluated, as given in Fig. 12c. The average H2-production for CoF1, CoF2 and CoF3 samples is found to be 2.76, 3.15 and 5.55 mmol\(\:{\text{g}}_{\text{c}\text{a}\text{t}}^{-1}\)h1, respectively (Table 5. It is observed that the CoF3 showed maximum H2-production amid the other samples. The highest active sample is further subjected for the stability analysis using the gas chromatographer. By subjecting the samples for three successive cycles, the, repeatability, of the catalysts are analysed (Fig. 10d). The 3rd cycle indicated slight decrease in the H2 evolution (21.04 mmol\(\:{\text{g}}_{\text{c}\text{a}\text{t}}^{-1}\)) as compared to the first cycle. This indicates that the photocatalysts shows good reusability up-to three cycles and moreover, this may also be due to the high magnetic property of doped cobalt catalysts.
Electrocatalytic water splitting investigation
The electrocatalytic measurement is performed to check the H2-production using the prepared electrocatalysts. The electrocatalytic measurements are performed using a 3-electrode setup in 0.5 M of H2SO4 electrolyte. The reaction is performed under the ambient conditions. The HER for the samples is investigated by the LSV and CV investigation as represented in Fig. 13(a, b). The rising Zn dopant content causes an upsurge in overpotential at 10 mA/cm− 2, which indicate that the CoF3 sample show the highest HER. The overpotential values of CoF1, CoF2, and CoF3 samples at 10 mA cm− 2 are − 1.11, -1.09 and − 1.08 V (Table 5). Tafel examination is performed by making use of HER responses. Figure 13(c) gives Tafel polarization plots of all the electrocatalysts. The calculated HER responses is given in Table 5. The decline in resistance at applied potential is monitored between 10 and 200 mV. By utilizing the CV analysis of optimum HER, the electrocatalysis of CoF3 is also performed as depicted in Fig. 13(d). The chronoamperometric analysis is done to test the durability of CoF3 sample operating at – 1 V run up to 8000s. For a period of 8000s, little to now changes has been observed in the current density, as given Fig. 14. A comparative analysis of recent studies and our study has also been shown in the Table 6.
Conclusion
The current study shows the fabrication of ZnxCo1−xFe2O4 (x = 0.02–0.06) catalysts employing the SA approach. The structural studies show the cubic phase structure. The phase confirmation is done via the Rietveld refinement. The FESEM images show the formation of agglomerated morphologies with grain size of between 96 and 98 nm. The fabricated samples also show all of the expected Raman modes implying the pure phase formation. The elemental purity is confirmed using the EDS analysis, which show the all of the desired (Co, Zn, Fe, O) elements. The magnetic nature is also studied by the VSM which indicates that the developed samples inherited the soft magnetic nature with Ms in between 70.88 and 75.19 emu/g. The computed band gaps for the samples falls within the range of 1.69–1.91 eV. The study show that the highest photocatalytic H2-production is 21.51 mmol\(\:{\text{g}}_{\text{c}\text{a}\text{t}}^{-1}\) within 8 h via the CoF3 sample. The electrocatalytic study indicates that the CoF3 sample had Tafel slope of 141.88 mV/dec, which is lowest amongst the other developed samples. Therefore, the current study concludes that the synthesised samples have good potential for the green H2 production and could be used as a potential economic catalyst for large scale H2-production.
Figures and Tables.
Data availability
The datasets used and analysed during the current study are available from the corresponding author (R. J.) on reasonable request.
References
Halkos, G. & Gkampoura, E. C. Where do we stand on the 17 sustainable development goals?? An overview on progress. Econ. Anal. Policy. 70, 94–122 (2021).
Hassan, N. S. et al. Recent review and evaluation of green hydrogen production via water electrolysis for a sustainable and clean energy society. Int. J. Hydrog Energy. 52, 420–441 (2024).
Hosseini, S. E. & Wahid, M. A. Hydrogen production from renewable and sustainable energy resources: promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 57, 850–866 (2016).
Tiwari, A. Hydrogen leading the green energy future. Development 15, 16 (2022).
Ma, N., Zhao, W., Wang, W., Li, X. & Zhou, H. Large scale of green hydrogen storage: opportunities and challenges. Int. J. Hydrog Energy. 50, 379–396 (2024).
Rostrup-Nielsen, J. R., Sehested, J. & Nørskov, J. K. Hydrogen and synthesis gas by steam-and C02 reforming, (2002).
LeValley, T. L., Richard, A. R. & Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies–A review. Int. J. Hydrog Energy. 39, 16983–17000 (2014).
Aydemir, M. et al. Photocatalytic–electrocatalytic dual hydrogen production system. Int. J. Hydrog Energy. 41, 8209–8220 (2016).
Singh, W. M. et al. Electrocatalytic and photocatalytic hydrogen production in aqueous solution by a molecular Cobalt complex. Angew Chem. 124, 6043–6046. https://doi.org/10.1002/ange.201200082 (2012).
Jia, Y., Jiang, K., Wang, H. & Yao, X. The role of defect sites in nanomaterials for electrocatalytic energy conversion. Chem 5, 1371–1397 (2019).
Xu, H., Shang, H., Wang, C. & Du, Y. Low-Dimensional metallic nanomaterials for advanced electrocatalysis. Adv. Funct. Mater. 30, 2006317. https://doi.org/10.1002/adfm.202006317 (2020).
Thongam, D. D. & Chaturvedi, H. Advances in nanomaterials for heterogeneous photocatalysis. Nano Express. 2, 012005 (2021).
More, P. et al. Effect of sintering temperature on the structural, morphological, and the magnetic properties of Ni0. 25Cu0. 55 Zn0. 20 Fe2O4 nano ferrite. J. Magn. Magn. Mater. 586, 171192 (2023).
Malaie, K., Heydari, Z. & Ganjali, M. R. Spinel nano-ferrites as low-cost (photo) electrocatalysts with unique electronic properties in solar energy conversion systems. Int. J. Hydrog Energy. 46, 3510–3529 (2021).
Sundararaj, S. B. & Thangavelu, S. Insights into the Electrocatalytic Oxygen Evolution Reaction and Photocatalytic Methylene Blue Degradation of Mixed Spinel Ni x Cu 1– x Fe 2 O 4 Nanocomposites Anchored at Sulfur-Doped g-C 3 N 4. J. Phys. Chem. C. 127, 4953–4966. https://doi.org/10.1021/acs.jpcc.2c08600 (2023).
Farhan, A. et al. Metal ferrites-based nanocomposites and nanohybrids for photocatalytic water treatment and electrocatalytic water splitting. Chemosphere 310, 136835 (2023).
Patil, K., Kadam, S., Lokhande, P., Balgude, S. & More, P. The effects of Cobalt and magnesium co-doping on the structural and magnetic properties of ZnFe2O4 synthesized using a sonochemical process. Solid State Commun. 337, 114435 (2021).
Reddy, D. H. K. & Yun, Y. S. Spinel ferrite magnetic adsorbents: alternative future materials for water purification? Coord. Chem. Rev. 315, 90–111 (2016).
Mathew, D. S. & Juang, R. S. An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions. Chem. Eng. J. 129, 51–65 (2007).
Qin, H. et al. Spinel ferrites (MFe2O4): synthesis, improvement and catalytic application in environment and energy field. Adv. Colloid Interface Sci. 294, 102486 (2021).
Amiri, M., Eskandari, K. & Salavati-Niasari, M. Magnetically retrievable ferrite nanoparticles in the catalysis application. Adv. Colloid Interface Sci. 271, 101982 (2019).
Patil, K. et al. Influence of Cu–Mg substituted ZnFe2O4 ferrite as a highly efficient nanocatalyst for dye degradation and 4-nitrophenol reduction. J. Phys. Chem. Solids. 167, 110783 (2022).
Jangam, K., Patil, K., Balgude, S., Patange, S. & More, P. Magnetically separable Zn1- xCo0.5 xMg0.5 xFe 2 O 4 ferrites: stable and efficient sunlight-driven photocatalyst for environmental remediation. RSC Adv. 10, 42766–42776 (2020).
Arade, S. et al. Zn0. 5Ni0. 5MnxFe2-xO4 magnetically separable nano ferrite: A highly efficient photocatalyst for environmental remediation. Inorg. Chem. Commun. 170, 113170 (2024).
Jangam, K., Patil, K., Balgude, S., Patange, S. & More, P. Synthesis and characterization of magnetically separable Zn1-xCoxFeMnO4 nanoferrites as highly efficient photocatalyst for degradation of dye under solar light irradiation. J. Phys. Chem. Solids. 148, 109700 (2021).
Ma, H. & Liu, C. A mini-review of ferrites-based photocatalyst on application of hydrogen production. Front. Energy. 15, 621–630. https://doi.org/10.1007/s11708-021-0761-0 (2021).
Benlembarek, M. et al. Synthesis, physical and electrochemical properties of the spinel CoFe2O4: application to the photocatalytic hydrogen production. Int. J. Hydrog Energy. 47, 9239–9247 (2022).
Dillert, R., Taffa, D. H., Wark, M., Bredow, T. & Bahnemann, D. W. Research Update: Photoelectrochemical water splitting and photocatalytic hydrogen production using ferrites (MFe2O4) under visible light irradiation, APL Mater. 3 (2015). https://pubs.aip.org/aip/apm/article/3/10/104001/121371 (accessed August 16, 2024).
Rekhila, G., Bessekhouad, Y. & Trari, M. Visible light hydrogen production on the novel ferrite NiFe2O4. Int. J. Hydrog Energy. 38, 6335–6343 (2013).
Gobara, H. M., Nassar, I. M., El Naggar, A. M. & Eshaq, G. Nanocrystalline spinel ferrite for an enriched production of hydrogen through a solar energy stimulated water splitting process. Energy 118, 1234–1242 (2017).
Bokov, D. et al. Nanomaterial by Sol-Gel Method: Synthesis and Application, Adv. Mater. Sci. Eng. (2021) 5102014. (2021). https://doi.org/10.1155/2021/5102014
Parashar, M., Shukla, V. K. & Singh, R. Metal oxides nanoparticles via sol–gel method: a review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 31, 3729–3749. https://doi.org/10.1007/s10854-020-02994-8 (2020).
Mahmood, M. et al. Spinel ferrite magnetic nanostructures at the surface of graphene sheets for visible light photocatalysis applications. Phys. B Condens. Matter. 550, 317–323 (2018).
Jasrotia, R. et al. Sol-gel fabricated Cu0.6Zn0.4-xCoxFe2O4 spinel ferrite based nano catalysts for green hydrogen generation. J. Sol-Gel Sci. Technol. https://doi.org/10.1007/s10971-025-06809-2 (2025).
Sonia, M. M. L. et al. Effect of lattice strain on structure, morphology and magneto-dielectric properties of spinel NiGdxFe2- xO4 ferrite nano-crystallites synthesized by sol-gel route. J. Magn. Magn. Mater. 466, 238–251 (2018).
Nha, T. T. N., Toan, D. N., Nam, P. H., Khan, D. T. & Phong, P. T. Determine elastic parameters and nanocrystalline size of spinel ferrites MFe2O4 (M = Co, fe, mn, Zn) through X-ray diffraction and infrared spectrum: comparative approach. J. Alloys Compd. 996, 174773 (2024).
Agale, P., Salve, V., Arade, S., Balgude, S. & More, P. Tailoring structural and chemical properties of zno@ g-C3N4 nanocomposites through Sr doping: insights from multi technique characterization. Solid State Sci. 107960. (2025).
Salve, V. et al. Enhanced photocatalytic activity of SnO 2@ gC 3 N 4 heterojunctions for methylene blue and bisphenol-A degradation: effect of interface structure and porous nature. RSC Adv. 15, 15651–15669 (2025).
Almessiere, M. A. et al. Sonochemical synthesis and physical properties of Co0. 3Ni0. 5Mn0. 2EuxFe2- xO4 nano-spinel ferrites. Ultrason. Sonochem. 58, 104654 (2019).
Almessiere, M. A. et al. Structural, magnetic, optical properties and cation distribution of nanosized Co0. 7Zn0. 3TmxFe2- xO4 (0.0 ≤ x ≤ 0.04) spinel ferrites synthesized by ultrasonic irradiation. Ultrason. Sonochem. 58, 104638 (2019).
Bououdina, M. & Manoharan, C. Dependence of structure/morphology on electrical/magnetic properties of hydrothermally synthesised Cobalt ferrite nanoparticles. J. Magn. Magn. Mater. 493, 165703 (2020).
Sumalatha, M. et al. Raman and in-field 57Fe Mössbauer study of cation distribution in indium (In) substituted phase pure Cobalt ferrite (CoFe2- xInxO4). J. Magn. Magn. Mater. 523, 167561 (2021).
Chandramohan, P., Srinivasan, M. P., Velmurugan, S. & Narasimhan, S. V. Cation distribution and particle size effect on Raman spectrum of CoFe2O4. J. Solid State Chem. 184, 89–96 (2011).
Kwiatkowski, A. L. et al. Cobalt ferrite nanorods synthesized with a facile green method in a magnetic field. Nanomaterials 14, 541 (2024).
Jacintho, G. V. M., Brolo, A. G., Corio, P., Suarez, P. A. Z. & Rubim, J. C. Structural investigation of MFe 2 O 4 (M = Fe, Co) magnetic fluids. J. Phys. Chem. C. 113, 7684–7691. https://doi.org/10.1021/jp9013477 (2009).
Nandan, B., Bhatnagar, M. C. & Kashyap, S. C. Cation distribution in nanocrystalline Cobalt substituted nickel ferrites: X-ray diffraction and Raman spectroscopic investigations. J. Phys. Chem. Solids. 129, 298–306 (2019).
Yuan, C. et al. Facile template-free synthesis of ultralayered mesoporous nickel Cobaltite nanowires towards high-performance electrochemical capacitors. J. Mater. Chem. 22, 16084–16090 (2012).
Yang, W. W. P. H., Xian, T. & JL, J. XPS and magnetic properties of CoFe2O4 nanoparticles synthesized by a polyacrylamide gel route. Mater. Trans. 53, 1586–1589 (2012).
Fantauzzi, M. et al. Nanostructured spinel Cobalt ferrites: Fe and Co chemical state, cation distribution and size effects by X-ray photoelectron spectroscopy. RSC Adv. 9, 19171–19179 (2019).
Baruwati, B., Reddy, K. M., Manorama, S. V., Singh, R. K. & Parkash, O. Tailored conductivity behavior in nanocrystalline nickel ferrite. Appl. Phys. Lett. 85, 2833–2835 (2004).
Sutka, A., Pärna, R., Mezinskis, G. & Kisand, V. Effects of Co ion addition and annealing conditions on nickel ferrite gas response, Sens. Actuators B Chem. 192, 173–180 (2014).
Manohar, A., Vijayakanth, V., Vattikuti, S. P. & Kim, K. H. Structural, BET and EPR properties of mixed zinc-manganese spinel ferrites nanoparticles for energy storage applications. Ceram. Int. 49, 19717–19727 (2023).
Somvanshi, S. B., Khedkar, M. V., Kharat, P. B. & Jadhav, K. M. Influential diamagnetic magnesium (Mg2+) ion substitution in nano-spinel zinc ferrite (ZnFe2O4): thermal, structural, spectral, optical and physisorption analysis. Ceram. Int. 46, 8640–8650 (2020).
Xue, L. C. et al. Magnetic moment directions and distributions of cations in cr (Co) substituted spinel ferrites Ni0. 7Fe2. 3O4, AIP Adv. 5 (2015).
Liu, S. R. et al. Estimation of cation distribution in spinel ferrites Co1 + xFe2- xO4 (0.0⩽ x⩽ 2.0) using the magnetic moments measured at 10 K. J. Alloys Compd. 581, 616–624 (2013).
Trier, S. H. & Abdali, M. S. The structural, magnetic, and optical properties of Cu1-xCoxFe2O4 spinel ferrite and its applications. Al-Qadisiyah J. Pure Sci. 25, 1–15 (2020).
Hossain, M. D., Hossain, M. A. & Sikder, S. S. Hysteresis loop properties of rare Earth doped spinel ferrites: A review. J. Magn. Magn. Mater. 564, 170095 (2022).
Chamani, S. et al. Photocatalytic hydrogen evolution performance of metal ferrites/polypyrrole nanocomposites. Int. J. Hydrog Energy. 47, 32940–32954 (2022).
Zhu, C. C. et al. ZnFe2O4 nanoparticles with iron-rich surfaces for enhanced photocatalytic water vapor splitting. Appl. Surf. Sci. 636, 157842 (2023).
Mahmoud, W. E., Al-Ghamdi, A. A., Al-Turki, Y. A. & Al-Amri, A. M. The physical and photocatalytic properties of thallium doped Cobalt ferrites for efficient hydrogen fuel production. Ceram. Int. 48, 15082–15089 (2022).
Dai, F. et al. Magnetic ZnFe2O4@ ZnSe Hollow nanospheres for photocatalytic hydrogen production application. Compos. Part. B Eng. 173, 106891 (2019).
Acknowledgements
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Funding
Open access funding provided by Parul University. This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Author information
Authors and Affiliations
Contributions
J. P. wrote the whole paper and performed the synthesis; A. A. C., H. A. R., N. R., M. R., and S. provided the characterization facilities; A. S. performed the software; B. L., and K. R. provided the resources; M. F., and T. A, performed the catalytic measurements; R.J performed the investigation, writing paper, supervision, conceptualization, and review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have declared that no conflicts of interest exist in relation to the publication of this research article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Prakash, J., Chaudhary, A.A., Somvanshi, A. et al. Boosting green hydrogen generation using Zn-substituted CoFe2O4 catalysts prepared by sol-gel technique for water splitting applications. Sci Rep 15, 30119 (2025). https://doi.org/10.1038/s41598-025-14902-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14902-3