Abstract
Percutaneous kyphoplasty (PKP) is one of the primary treatment options for osteoporotic vertebral compression fractures (OVCF). New vertebral compression fractures (NVCF) are common complications following PKP. This study aims to identify risk factors associated with NVCF after PKP and to develop a simple and user-friendly predictive model to assist clinicians in decision-making. A retrospective cohort study was conducted, analyzing clinical data from 340 patients with single-segment OVCF who underwent PKP at our institution between January 2020 and December 2022. We collected general clinical data and imaging findings from patients who underwent PKP at our institution. Lasso regression was employed to identify risk factors for NVCF after PKP, and the selected variables were further analyzed using an unrestricted cubic spline function. Finally, a predictive model was established using multivariate logistic regression analysis. The variables selected by Lasso regression included pre-op AVH (OR = 0.853) and vertebral height restoration rate (OR = 4.318). Restricted cubic spline function analysis demonstrated that patients with a pre-op AVH of less than 19.2 mm had a significantly increased risk of NVCF after PKP. Pre-op AVH and anterior vertebral height restoration rate are independent risk factors for NVCF after PKP. The predictive model constructed based on these two independent risk factors can effectively assess the risk of NVCF in elderly patients with OVCF undergoing PKP, providing valuable guidance for clinical decision-making.
Similar content being viewed by others
Introduction
Osteoporosis is a systemic metabolic bone disease characterized histologically by microstructural damage and the loss of bone mineral density. As an age-related condition, approximately 20% of women over the age of 50 meet the diagnostic criteria for osteoporosis based on dual-energy X-ray absorptiometry, and this prevalence increases to 30% in women aged 65 and older1. Osteoporosis leads to a decrease in bone strength, increasing the risk of fragility fractures. Among these, osteoporotic vertebral compression fractures (OVCF) are the most common type of osteoporotic fracture worldwide. Approximately 20% of individuals over the age of 70 suffer from OVCF2. OVCF often results in severe chronic pain, kyphotic deformity, and reduced mobility, significantly affecting patients’ quality of life and mental health3. The primary treatment options for OVCF include conservative management and surgical interventions. In 1987, percutaneous kyphoplasty (PKP) was first introduced as a treatment for OVCF. Due to its ability to restore vertebral body height, minimal invasiveness, and rapid recovery, PKP has gradually replaced conservative treatments and become the most widely used method in the management of OVCF, earning favor among orthopedic surgeons4,5. However, in clinical practice, some patients experience recurrent low back pain after undergoing PKP for OVCF. New vertebral compression fractures (NVCF) are a common complication following PKP, which can undermine patients’ confidence in their recovery, decrease satisfaction with the procedure, and impose a substantial economic burden on society6.
Currently, several studies have investigated the risk factors for NVCF following PKP, and researchers have developed various nomograms based on their findings, all of which demonstrate good predictive performance7,8. However, previous studies often included numerous variables, making the use of these nomograms cumbersome9,10. Our aim is to identify a smaller set of variables for inclusion in the model while still maintaining strong predictive accuracy. Furthermore, our study provides trend graphs that illustrate how changes in these variables impact the risk of re-fracture, offering clinicians valuable insights to intervene more promptly and effectively.
Patients and methods
General information
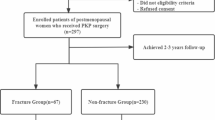
The study included patients with single-segment OVCF who underwent PKP at our medical institution between January 2018 and December 2022. The inclusion criteria were as follows:1 single-segment vertebral fractures caused by low-energy trauma (such as bending, falling, etc.) after PKP, resulting in back pain or restricted mobility;2 X-ray or CT imaging indicating vertebral compression fractures, with MRI confirming recent vertebral fractures;3 complete medical records. The exclusion criteria were:1 fractures involving two or more vertebral segments;2 vertebral fractures due to tumors, infections, or tuberculosis;3 fractures with posterior wall instability or burst fractures;4 severe spinal degeneration;5 spinal cord compression with neurological symptoms;6 poor cardiopulmonary function, unable to tolerate surgery;7 loss to follow-up or incomplete imaging data.
A total of 340 patients who met the inclusion and exclusion criteria for OVCF treated with PKP were included in the study, with a 7:3 ratio for the modeling group and the validation group.
Ethical approval
This retrospective study was approved by the Medical Ethics Committee of Zhejiang Provincial People’s Hospital (Approval No.QT2025061) and was registered on the National Health Information Platform of China.
Observational indicators
The following parameters were recorded: sex, age, body mass index (BMI), hypertension, diabetes, bone mineral density (BMD), fracture segment, cement leakage, cement injection volume, cement distribution, cement endplate contact, anti-osteoporotic treatment, cement distribution shape, scoliosis, preoperative anterior vertebral heigh (pre-op AVH), postoperative anterior vertebral height(post-op AVH), anterior vertebral height restoration rate (AVHRR), preoperative and postoperative Cobb angles.
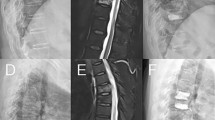
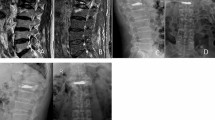
BMD was measured using a dual-energy X-ray absorptiometry scanner. A Cobb angle greater than 10° in the coronal view was defined as scoliosis. On the anteroposterior and lateral X-ray films, bone cement leakage was defined as bone cement exceeding the boundaries of the vertebral body. The distribution of bone cement was assessed by examining whether it crossed the midline and whether it was symmetrical on the postoperative anteroposterior X-ray. Crossing the midline was considered sufficient distribution, whereas not crossing it was deemed insufficient. The formula for calculating the AVHRR was as follows: AVHRR = [2 × (post-op AVH - pre-op AVH) / (upper vertebral body anterior height + lower vertebral body anterior height of the fractured vertebra)] × 100%. The anti-osteoporosis treatment regimen included oral calcium (600 mg/day) and active vitamin D (0.25 µg/dose, twice daily) as basic therapy, combined with zoledronic acid intravenous infusion (5 mg/dose, once a year) or denosumab subcutaneous injection (60 mg/dose, once every six months). Cement distribution pattern: classified into four categories:1 contact only with the upper vertebral endplate2, contact only with the lower vertebral endplate3, contact with both the upper and lower vertebral endplates, and4 contact with neither the upper nor the lower vertebral endplates (Fig. 1).
Variable handling
The continuous variables included in this study were age, BMI, BMD, cement injection volume, pre-op AVH, post-op AVH, AVHRR, preoperative Cobb angle, and postoperative Cobb angle. Non-restricted cubic spline functions were used to analyze these continuous variables to explore their relationship with the risk of NVCF following PKP. Variables showing a linear relationship with the risk of NVCF after PKP were directly included in the model. If a non-linear relationship was found, the variables were converted into binary categorical variables.
Surgical procedure
The patient was placed in the prone position. Anteroposterior fluoroscopic images were obtained using a C-arm X-ray machine to locate the bilateral vertebral pedicle projections on the skin surface of the fractured vertebra, with marker lines drawn. Routine disinfection and draping were performed. Local infiltration anesthesia with 10 g/L lidocaine was administered at the bilateral pedicle puncture sites. The procedure began on the left side. A skin incision of approximately 1 cm was made at the location corresponding to the left pedicle projection on the skin surface. The skin and subcutaneous tissue were dissected, and a needle was inserted at the 10 o’clock position of the projection, paying attention to the sagittal angle and medial tilt. Anteroposterior fluoroscopy with the C-arm confirmed the needle position at the center of the pedicle, and lateral fluoroscopy confirmed the needle was centered within the pedicle. The needle was removed, and a guidewire was inserted. A working cannula was placed approximately 0.5 cm anterior to the posterior edge of the vertebral body, and the guidewire was removed. A tamping instrument was inserted, followed by balloon insertion. Contrast medium was injected to inflate the balloon to the appropriate pressure. After balloon deflation, the contrast medium was withdrawn, and the balloon was removed. A similar procedure was performed on the right side, at the 2 o’clock position of the pedicle projection. Under fluoroscopic guidance, bone cement was slowly injected into the vertebral body, filling the anterior third of the fractured vertebral body to form an effective mechanical column. If cement leakage occurred during the procedure, cement injection was immediately stopped. Once the bone cement had solidified, the cannula was withdrawn, completing the procedure.
Postoperative follow-up
All patients received oral calcium and active vitamin D as basic treatment during their hospital stay, in combination with zoledronic acid or denosumab. A lumbar brace was worn when patients were out of bed. All patients underwent spinal X-ray examination 24 h postoperatively. It was recommended that patients return for outpatient follow-up every month for health education and review of anti-osteoporotic treatment. X-ray examinations were scheduled at 1, 3, 6 months, and at 1 and 2 years postoperatively. If patients failed to attend scheduled follow-ups, a phone inquiry was made to assess their condition. The endpoint of the follow-up was 2 years after PKP surgery or the occurrence of a new vertebral fracture.
Diagnosis of vertebral refracture
1. Patients who experience recurrent back pain and limited mobility, particularly during activities such as turning or rising, after undergoing PKP during the follow-up period. 2.Spinal imaging, including X-rays or MRI, indicating a new vertebral fracture. MRI was used to exclude other causes of vertebral fractures.
Statistical analysis
Statistical analysis was performed using SPSS version 26 and R software version 4.22. The Kolmogorov-Smirnov test was used to assess the normality of the data. Normally distributed continuous variables are presented as mean ± standard deviation, and group comparisons were conducted using the independent two-sample t-test. Non-normally distributed continuous variables are expressed as median (Q1, Q3), and group comparisons were performed using the Mann-Whitney U test. Categorical data are presented as frequencies and/or percentages, with group comparisons conducted using the χ² test.
Continuous variables were preprocessed using R. Restricted cubic spline functions were applied to examine the relationship between continuous variables and the risk of re-fracture after PKP. Variables with a linear association with post-PKP re-fracture risk were directly included in the analysis, while those showing nonlinear associations were dichotomized before inclusion. The Restricted cubic spline analysis was conducted using the rsm package.
Lasso regression was used for variable selection, and the selected variables were then included in a multivariate logistic regression analysis to identify independent risk factors for re-fracture after PKP. Lasso regression was conducted using the glmnet package. receiver operating characteristic curves (ROC), calibration curves, and decision curve analysis (DCA) curves were plotted using the pROC, glmnet, and rsm packages, respectively, to evaluate the model’s discrimination ability, accuracy, and clinical applicability. A p-value of < 0.05 was considered statistically significant.
All methods were carried out in accordance with relevant guidelines and regulations.
Results
A total of 340 patients who met the inclusion criteria were enrolled in this study. Of these, 240 patients were included in the modeling cohort to develop and build the model, and 100 patients were included in the validation cohort to assess the model’s predictive performance. In the modeling cohort, 31 patients developed NVCF after PKP, while in the validation cohort, 14 patients experienced NVCF after PKP. Details are provided in Tables 1 and 2.
Key variables
Categorical variables were directly included in the analysis, while continuous variables were assessed for linearity with postoperative PKP refracture. Restricted cubic spline (RCS) functions were employed to evaluate the relationships between continuous variables and PKP refracture. The results demonstrated linear associations (P > 0.05) between the following variables and NVCF after PKP: age, BMI, BMD, bone cement dosage, preoperative vertebral height, postoperative vertebral height, preoperative Cobb angle, and postoperative Cobb angle. In contrast, AVHRR exhibited a nonlinear relationship (P < 0.001) and was incorporated into the model using a cutoff value of 16.8 %. Lasso regression was used to select variables, yielding the coefficient variations of each variable (Fig. 2A). Through iterative analysis using ten-fold cross-validation, the optimal model with the best performance and the minimal number of variables was obtained when λ = 0.081 (Logλ = -2.51) (Fig. 2B). The selected variables included pre-op AVH and AVHRR. Patients with a pre-op AVH of less than 13.4 mm had a significantly increased risk of developing NVCF after PKP. Moreover, when the AVHRR exceeded 16.8%, the risk of NVCF after PKP progressively increased. Notably, the risk of NVCF peaked when the AVHRR reached 25.2% (Fig. 3).
Risk relationship chart of model variables with the occurrence of NVCF after PKP. (A) Relationship between preoperative anterior vertebral heigh pre-op AVH and the risk of NVCF after PKP. (B) Relationship between vertebral height recovery rate and the risk of NVCF after PKP. NVCF new vertebral compression fracture, PKP Percutaneous kyphoplasty, Pre-op AVH preoperative anterior vertebral heigh.
Model development and performance
A nomogram was developed based on the independent risk factors selected by Lasso regression, including pre-op AVH and AVHRR (Fig. 4; Table 2). The concordance indices (C-index) for the modeling and validation cohorts were 0.833 and 0.771, respectively, which indicate that the prediction model possesses good discriminative ability in distinguishing between patients who will and will not experience re-fracture after PKP. To further evaluate the model’s performance, ROC curves were generated for both cohorts. The area under the ROC curve (AUC) demonstrated strong classification performance, reinforcing the robustness of the model. In the modeling cohort (Hosmer and Lemeshow (HL) test: χ² = 5.4419, P = 0.7095) and the validation cohort (HL test: χ² = 8.8594, P = 0.3543), calibration curves were constructed to assess the agreement between the predicted probabilities of re-fracture and the actual observed outcomes. The calibration plots showed that the predicted risks closely matched the observed incidences, suggesting that the model is well-calibrated and provides accurate individualized risk estimations (Fig. 6). DCA was conducted to evaluate the clinical usefulness of the prediction model by quantifying the net benefit across a range of threshold probabilities. For the modeling cohort, the DCA demonstrated that the model provided a positive net benefit when the threshold probability ranged from 0.01 to 0.52, while for the validation cohort, the effective threshold range was between 0.01 and 0.42 (Fig. 7). These ranges suggest that the model can support clinical decision-making over a broad spectrum of risk thresholds (Figs. 4, 5, 6 and 7).
Discussion
OVCF are common in patients with osteoporosis, particularly among the elderly who often have comorbidities. Their overall health status frequently limits surgical options. Minimally invasive procedures such as PKP are widely used to treat OVCF due to their advantages of reduced trauma, faster recovery, rapid pain relief, and partial restoration of vertebral height11,12.PKP is typically the preferred option for patients who may not be suitable candidates for open surgery, as these surgeries generally carry higher risks. For elderly or frail patients, minimizing surgical trauma and achieving faster recovery are critical for improving prognosis and quality of life13,14,15. However, PKP can lead to complications such as pulmonary embolism, cement leakage, anaphylactic shock, and NVCF16,17. NVCF is a common and severe long-term complication that can significantly reduce patients’ quality of life, damage their confidence in recovery, and create a substantial economic burden on society. The risk factors for NVCF following PKP remain controversial18,19. Previous studies have established predictive models for NVCF, but the inclusion of too many variables has reduced the practicality and ease of use of these models. A simplified model is more beneficial for broader clinical application. In this study, we used two easily obtainable radiographic factors to assess the likelihood of NVCF following PKP and provided a graphical representation of the relationship between variable changes and the risk of recurrent NVCF. This approach aims to assist clinicians in making timely and effective preventive interventions.
Pre-op AVH is an independent risk factor for NVCF following PKP Our study found that when pre-op AVH is less than 13.4 mm, the risk of NVCF after PKP increases. A lower pre-op AVH typically indicates more severe vertebral compression deformity, which may involve the vertebral endplates and even the anterior and posterior vertebral walls. When vertebral integrity is compromised, bone cement is more likely to leak during its dispersion, increasing the risk of NVCF by 4.9 times20. When bone cement leaks into the intervertebral disc, it can affect disc metabolism, accelerate degenerative changes, and reduce the disc’s ability to buffer external forces, further increasing the risk of NVCF after PKP21,22. Excessive vertebral compression results in tighter compression of the vertebral cortical porosity and trabecular bone, and bone cement cannot restore the microstructural deformities, which compromises vertebral stability and increases the likelihood of NVCF after PKP.
AVHRR is also an independent risk factor for NVCF after PKP. Our study found that a AVHRR greater than 16.8% increases the likelihood of NVCF after PKP, with the highest risk occurring when the AVHRR is 25.2%. Excessive restoration of vertebral height may increase paravertebral soft tissue tension, leading to increased vertebral load and bone necrosis23,24. The greater the vertebral height restoration, the larger the amount of bone cement required, which increases the vertebral stiffness and strength, thereby raising the stress on the facet joints and affecting spinal stability, thus increasing the risk of NVCF25. Iida et al.26also found that excessive restoration of vertebral height could cause expansion of the vertebral endplates, disrupting the slight concave shape of the vertebral endplate and increasing the stress load on adjacent vertebrae. Moreover, in our study, the risk of NVCF showed a slight decrease when the AVHRR exceeded 25.2%. Upon reviewing the patients’ imaging data, we found that some patients had severely compressed pre-op AVH, which affected spinal stability. In these cases, restoring a small amount of vertebral height was sufficient to achieve a significant increase in vertebral height and improve spinal stability. The primary goal of PKP is to relieve pain and prevent further vertebral collapse, rather than to restore vertebral height. Therefore, clinicians should not overly focus on restoring vertebral height.
This study incorporated the two identified risk factors to develop a predictive model for NVCF after PKP. Compared to previous studies, this model simplifies the risk factors, making it more convenient for clinicians to use. Since the model is based on a complex regression equation, we visualized it and created a nomogram for easier clinical application. In the nomogram, each independent risk factor corresponds to a specific score, and the total score can be used to calculate the probability of developing NVCF after PKP. ROC and calibration curves confirm that the nomogram developed in this study has good discriminatory ability and accuracy. The DCA further indicates that this model has strong clinical utility.
This study has several limitations. First, as a retrospective study, the results may be subject to bias due to case selection and data loss. Second, this is a single-center study with a relatively small sample size, and the external validity of the model needs further verification.
Conclusion
Pre-op AVH and VHRR are independent risk factors for NVCF after PKP. The predictive model developed in this study, based on these two independent risk factors, can be used to assess the risk of NVCF in elderly patients with OVCF undergoing PKP. For high-risk patients, early notification to the patient and their family members, along with timely intervention, can help reduce the incidence of NVCF and alleviate both the patient and societal economic burden.
Data availability
The datasets used and analysed during the current study available from the corresponding author(kongmingxiang@hmc.edu.cn) on reasonable request.
Abbreviations
- OCVF:
-
Osteoporotic vertebral compression fracture
- PKP:
-
Percutaneous kyphoplasty
- NVCF:
-
New vertebral compression fractures
- BMI:
-
Body mass index
- BMD:
-
Bone mineral density
- AVHRR:
-
Anterior vertebral height restoration rate
- ROC:
-
Operating characteristic curves
- DCA:
-
Decision curve analysis
- HL:
-
Hosmer and Lemeshow
References
Ballane, G., Cauley, J. A., Luckey, M. M. & El-Hajj Fuleihan, G. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos. Int. 28 (5), 1531–1542 (2017).
Long, Y., Yi, W. & Yang, D. Advances in Vertebral Augmentation Systems for Osteoporotic Vertebral Compression Fractures. Pain Res Manag. 2020: 3947368. (2020).
Griffoni, C. et al. Percutaneous vertebroplasty and balloon kyphoplasty in the treatment of osteoporotic vertebral fractures: a prospective randomized comparison. Eur. Spine J. 29, 1614–1620. https://doi.org/10.1007/s00586-020-06434-3 (2020).
Lin, M. et al. A nomogram for predicting residual low back pain after percutaneous kyphoplasty in osteoporotic vertebral compression fractures. Osteoporos. Int. 34 (4), 749–762 (2023).
Galibert, P., Deramond, H., Rosat, P. & Le Gars, D. [Preliminary note on the treatment of vertebral Angioma by percutaneous acrylic vertebroplasty]. Neurochirurgie 33 (2), 166–168 (1987).
Nieuwenhuijse, M. J., Putter, H., van Erkel, A. R. & Dijkstra, P. D. New vertebral fractures after percutaneous vertebroplasty for painful osteoporotic vertebral compression fractures: a clustered analysis and the relevance of intradiskal cement leakage. Radiology 266 (3), 862–870 (2013).
Ma, Y. et al. Establishment and validation of a nomogram for predicting new fractures after PKP treatment of for osteoporotic vertebral compression fractures in the elderly individuals. BMC Musculoskelet. Disord. 24, 728. https://doi.org/10.1186/s12891-023-06801-3 (2023).
Gao, W. et al. Establishment and verification of a predictive nomogram for new vertebral compression fracture occurring after bone cement injection in Middle-Aged and elderly patients with vertebral compression fracture. Orthop Surg. 15:961–972. (2023). https://doi.org/10.1111/os.13655
Zheng, J. et al. Development and validation of a nomogram for predicting new vertebral compression fractures after percutaneous kyphoplasty in postmenopausal patients. J. Orthop. Surg. Res. 18, 914. https://doi.org/10.1186/s13018-023-04400-5 (2023).
Bian, F. et al. Risk factors for recollapse of new vertebral compression fractures after percutaneous kyphoplasty in geriatric patients: establishment of a nomogram. BMC Musculoskelet. Disord. 23, 458. https://doi.org/10.1186/s12891-022-05409-3 (2022).
Hinde, K. et al. Mortality outcomes of vertebral augmentation (Vertebroplasty and/or balloon Kyphoplasty) for osteoporotic vertebral compression fractures: A systematic review and Meta-Analysis. Radiology 295, 96–103. https://doi.org/10.1148/radiol.2020191294 (2020).
Mao, Y., Wu, W., Zhang, J. & Ye, Z. Prediction model of adjacent vertebral compression fractures after percutaneous kyphoplasty: a retrospective study. BMJ Open. 13, e064825. https://doi.org/10.1136/bmjopen-2022-064825 (2023).
Chen, A. T., Cohen, D. B. & Skolasky, R. L. Impact of nonoperative treatment, vertebroplasty, and kyphoplasty on survival and morbidity after vertebral compression fracture in the medicare population. J. Bone Joint Surg. Am. 95, 1729–1736. https://doi.org/10.2106/JBJS.K.01649 (2013).
Voggenreiter, G. Balloon kyphoplasty is effective in deformity correction of osteoporotic vertebral compression fractures. Spine (Phila Pa. 1976). 30, 2806–2812. https://doi.org/10.1097/01.brs.0000190885.85675.a0 (2005).
Lu, Y., Cai, X., Shen, J. & Luo, R. Development and validation of a prediction model for vertebral recompression and adjacent vertebral fracture after kyphoplasty in geriatric patients. Eur. Spine J. https://doi.org/10.1007/s00586-024-08485-2 (2024).
Ma, X. et al. Risk factors for new vertebral compression fractures after percutaneous vertebroplasty: qualitative evidence synthesized from a systematic review. Spine (Phila Pa. 1976. 38, E713–E722. https://doi.org/10.1097/BRS.0b013e31828cf15b (2013).
Zhan, Y., Jiang, J., Liao, H., Tan, H. & Yang, K. Risk factors for cement leakage after vertebroplasty or kyphoplasty: A Meta-Analysis of published evidence. World Neurosurg. 101, 633–642. https://doi.org/10.1016/j.wneu.2017.01.124 (2017).
Dai, C., Liang, G., Zhang, Y., Dong, Y. & Zhou, X. Risk factors of vertebral re-fracture after PVP or PKP for osteoporotic vertebral compression fractures, especially in Eastern asia: a systematic review and meta-analysis. J. Orthop. Surg. Res. 17, 161. https://doi.org/10.1186/s13018-022-03038-z (2022).
Feng, L., Feng, C., Chen, J., Wu, Y. & Shen, J. M. The risk factors of vertebral refracture after kyphoplasty in patients with osteoporotic vertebral compression fractures: a study protocol for a prospective cohort study. BMC Musculoskelet. Disord. 19, 195. https://doi.org/10.1186/s12891-018-2123-6 (2018).
Sun, Y. C. et al. Risk of post-vertebroplasty fracture in adjacent vertebral bodies appears correlated with the morphologic extent of bone cement. J. Chin. Med. Assoc. 74, 357–362. https://doi.org/10.1016/j.jcma.2011.06.008 (2011).
Kim, M. H., Lee, A. S., Min, S. H. & Yoon, S. H. Risk factors of new compression fractures in adjacent vertebrae after percutaneous vertebroplasty. Asian Spine J. 5, 180–187. https://doi.org/10.4184/asj.2011.5.3.180 (2011).
Zhang, Z., Zhang, J., He, B., Dong, Q. & Hao, D. Effect of bone cement distribution on adjacent disc degeneration after vertebral augmentation for osteoporotic vertebral compression fractures in aging patients. Front. Surg. 10, 1256401. https://doi.org/10.3389/fsurg.2023.1256401 (2023).
Yu, W., Xu, W., Jiang, X., Liang, D. & Jian, W. Risk factors for recollapse of the augmented vertebrae after percutaneous vertebral augmentation: A systematic review and Meta-Analysis. World Neurosurg. 111, 119–129. https://doi.org/10.1016/j.wneu.2017.12.019 (2018).
Heo, D. H., Chin, D. K., Yoon, Y. S. & Kuh, S. U. Recollapse of previous vertebral compression fracture after percutaneous vertebroplasty. Osteoporos. Int. 20, 473–480. https://doi.org/10.1007/s00198-008-0682-3 (2009).
Mills, E. S. et al. Secondary fracture rate after vertebral osteoporotic compression fracture is decreased by Anti-Osteoporotic medication but not increased by cement augmentation. J. Bone Joint Surg. Am. 104, 2178–2185. https://doi.org/10.2106/JBJS.22.00469 (2022).
Iida, K., Kumamaru, H., Saito, T. & Harimaya, K. Overcorrection of fractured vertebrae increases the incidence of adjacent fractures after balloon kyphoplasty: A retrospective study. J. Orthop. 24, 194–200. https://doi.org/10.1016/j.jor.2021.02.035 (2021).
Acknowledgements
We sincerely appreciate the support and valuable contributions of all authors and patients who participated in this study.
Author information
Authors and Affiliations
Contributions
AZ and MK designed the study. AZ and YF gathered the data and wrote the manuscript. JS and XX designed the figures and provided valuable feedback. AZ and MK revised the manuscript. All authors agreed on the final manuscript. The article was composed by all of the authors, and the final version was approved by all of them.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
Due to the retrospective nature of the study, the medical ethics committee of the Zhejiang Provincial People’s Hospital waived the need of obtaining informed consent(QT2025061).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, A., Feng, Y., Xie, X. et al. Development and validation of a prediction model for new vertebral fracture after percutaneous kyphoplasty. Sci Rep 15, 31249 (2025). https://doi.org/10.1038/s41598-025-14931-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-14931-y