Abstract
Gadolinium application in industry and medicine results in generation of large volumes of gadolinium-containing wastewater, which can present hazard for humans and environment. Adsorption proved to be an efficient way of metal removal from wastewater. Two adsorbents of different origin, yeast biomass and titanosilicate ETS-10, were applied for gadolinium removal from synthetic wastewater under different experimental conditions. Several analytical techniques, including scanning electron microscopy and X-ray diffraction were used for adsorbents characterization. The maximum removal of gadolinium by both adsorbents was achieved at pH 3.0. By studying kinetics, the applicability of the pseudo-first-order model for the description of gadolinium adsorption on analyzed adsorbents was shown. Langmuir and Freundlich adsorption isotherms were used to explain the equanimity of the sorption process. The Langmuir model showed its applicability for the explanation of the equilibrium data obtained for yeast biomass, while Freundlich model was more applicable for mesoporous material. The maximum sorption capacity of titanosilicate ETS-10 (234 mg/g) significantly overpassed the value obtained for yeast biomass (98 mg/g). The relevant thermodynamic parameters, standard free energy, enthalpy change, and entropy change were calculated to understand the nature of the adsorption process. For both adsorbents the process was spontaneous and heat-taking. Fourier transform infrared spectroscopy reaved the role of functional groups in gadolinium removal. Tested adsorbents maintained high adsorption capacity during three sorption–desorption cycles.
Similar content being viewed by others
Introduction
A collection of fifteen lanthanides (La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu) in addition with Sc and Y are rare earth elements (REEs) of particular importance for industry, medicine and agriculture1. A wide demand for REEs is attributed to their unique magnetic, chemical, light-emitting, and catalytic properties1,2. Nowadays, REEs are increasingly used in various processes, including production of computer memory, liquid crystal displays, super magnets, communication devices, rechargeable batteries, contrast agents, superconductors, catalysts, glass, fluorescent materials, fertilizers and feed additives1,3,4.
The mining, processing and extensive application of REEs finally results in their resource depletion and also lead to severe contamination of the surrounding environment with possible negative consequences for humans and other living organisms. In the review papers2,5,6 the impact of REEs on humans and aquatic organism is presented in details.
Among REEs, gadolinium used as a contrasting agent in magnetic resonance imaging is considered as a major contaminant released into the environment with unproperly treated wastewater1. Low gadolinium removal from wastewater (10–40%) and its presence in drinking water was shown in several studies performed in different countries7,8,9. Since techniques traditionally applied for wastewater treatment are insufficient or costly when metal is present at trace level10 and do not allow to achieve the desired results, it is necessary to introduce additional techniques for efficient metal ions removal. Among these techniques, adsorption is probably the most effective, economically feasible, environmentally sustainable, and technologically promising process11. Commonly applied adsorbents include activated carbon12, biochar13, metal–organic frameworks14, mesoporous materials15,16,17, biological materials18,19, and mineral materials20,21.
In recent years, much attention has been paid to application of biological adsorbents for metal removal. Biosorption has gradually become a hot topic in the field of wastewater bioremediation due to its high efficiency, affordability, high adsorption capacity, and excellent eco-compatibility22. Biosorbents are derived from renewable sources, such as microbial waste biomass, plant-based materials and animal waste and can be easily modified to increase their adsorption capacity, making them versatile and accessible for a large scale application12,13,22.
Another class of adsorbents with great potential for wastewater treatment are mesoporous materials, which are characterized by large surface area, high porosity, large pore volumes, low densities, excellent thermal, mechanical, and electrical properties17,23. In addition, mesoporous materials can be easily modified to improve their affinity for the target metal ions17.
The aim of the present study was to compare the adsoption capacity of two different types of adsorbents: biological (yeast biomass) and mesoporous (titanosilicate ETS-10) toward gadolinium ions. The effect of experimental parameters: pH, time, gadolinium concentration and temperature on adsoption capacity of tested asorbetns was assessed. Besides, the adsorption kinetics, isotherms and thermodynamics are discussed to explore the process of gadolinium adsorption in detail and finally the adsorption mechanism was investigated by Fourier transform infrared spectroscopy and using ORCA quantum chemistry program package. Both adsorbents were applied for the first time for gadolinium ions adsorption.
Materials and methods
Chemicals and adsorbents
All chemicals applied to produce adsorbents and in adsorption experiment were of analytical grade purchased from Sigma-Aldrich (Darmstadt, Germany).
Two materials of different origin were applied as adsorbents: titanosilicate ETS-10 and yeast Saccharomyces cerevisiae. Yeast Saccharomyces cerevisiae was obtained as a waste biomass from the beer production company. Prior to be applied as a biosorbent, the biomass was dried and homogenized. To obtain titanosilicate ETS-10 (formula 3.4Na2O:1.5K2O:TiO2:5.5SiO2:150H2O), two chemicals NaCl (1.62 g) and KCl (2.48 g) were dissolved in ultrapure water. Next, 9.41 mL of Na2SiO3 were added dropwise to the mixture to obtain Si precursor solution (solution A). To prepare the Ti precursor solution (solution B), 0.88 g of TiO2 were mixed with 7.8 mL of H2SO4 (9%). On the final stage solutions A and B were mixed, that resulted in the formation of a viscous mixture, which was transferred into a Teflon-lined stainless-steel autoclave and kept for 72 h at 230 °C in a low heat oven. Next, the obtained sorbent was cooled, centrifuged, washed several times with distilled water, and dried overnight at 50 °C.
Tested adsorbents were analyzed using several techniques. The morphology of the adsorbents was evaluated using an S3400N Hitachi microscope (Krefeld, Germany). Infrared spectra were obtained using a Shimadzu IRSpirit-X compact-ATR equipped spectrophotometer (Shimadzu, Kyoto, Japan). X-ray diffraction analysis was performed with the Empyrean Multi-Purpose Research X-Ray Diffractometer. PHREEQC 2.18 computer code was used for thermodynamic calculations, which were performed in frames of computational methodology DESTRUCT development for evaluation of engineering barrier system properties evolution.
The interactions between the gadolinium ions and titanosilicate ETS-10 surface was theoretically investigated using the ORCA quantum chemistry program package24,25. The hybrid functional B3LYP26,27 with double-zeta basis sets def228 and Density A small fragment, according to the crystalline structures from the Crystallography Open Database (COD), was used for the simulation of the adsorbent macrostructures.
Experimental design
Gadolinium working solutions were prepared by the dissolution of Gd(NO3)3·6H2O in distillated water. The effect of several parameters, including pH (2.0–6.0), time (3–120 min), gadolinium concentration (10–100 mg/L) and temperature (20–50 °C) on sorbents adsoption capacity was studied. The dosage of the applied adsorbents was 20 mg and the volume of the experimental solutions was 20 mL. Adsorption experiments were performed on a rotary Unimax 1010 shaker (Heidolph, Schwabach, Germany) at a fixed agitation speed of 200 rpm. At the end of the experiments, the sorbents were separated from the solution by filtration and gadolinium concentrations in solutions (initial and final) were measured using an ICP-OES PlasmaQuant PQ 9000 Elite spectrometer (Analytik Jena, Jena, Germany). All experiments were performed in triplicate and the avarage values were taken for calculations.
The adsorption capacity and removal efficiency of the tested adsorbents were computed from Eqs. (1–2):
where q is the adsorption capacity, mg/g; V is the volume of solution, L; Ci and Cf are initial and final metal concentrations, mg/L; and m is the weight of sorbent, g.
Sorbents regeneration
To assess the possibility of sorbents multiply use, after adsorption experiments performed at optimal experimental conditions 10 mL of 0.01 M HNO3 were added to the sorbent as eluent. After 30 min of interaction the sorbents were separated from solutions, washed firstly with 0.01 M NaOH (for neutralization) and then with distilled water, and again gadolinium solution was added. The procedure was repeated for two more cycles.
Data analysis
Two kinetic models (Eqs. 3–4) were used to characterize the gadolinium sorption29.
The pseudo-first-order model:
The pseudo-second-order model:
where qt is the amount of adsorbed gadolinium (mg/g) at time t, (mg/g); qe is the adsorption capacity at equilibrium (mg/g); k1 (1/min) and k2 (g/mg·min) are the pseudo-first-order and the second-order reaction rate equilibrium constants.
The Langmuir and Freundlich isotherm models (Eqs. 5 and 6) were used to characterize the equilibrium of the adsorption process30,31.
where Ce is the metal concentration at equilibrium (mg/L); qm is the maximum adsorption capacity (mg/g), b (L/mg); and KF (mg/g) and n are Langmuir and Freundlich equation constants.
Equations (7 and 8) were used to estimate the thermodynamic parameters, including the standard free energy (ΔG°), enthalpy change (ΔH°), and entropy change (ΔS°)32:
where T is the temperature, K; R is universal gas constant = 8.314 J/mol K; Kd is distribution coefficient estimated from Eq. (9) and Ca is the concentration of adsorbed gadolinium, mg/L:
Results and discussion
Adsorbents characterization
The SEM images of the yeast biomass and titanosilicate ETS-10 are presented in Fig. 1. Biological sorbent consisted of oval and smooth cells, overlapping with each other (Fig. 1a), while titanosilicate ETS-10 consisted of quasi-cubic crystals forming agglomerations (Fig. 1b).
The XRD analyses (Fig. 2) of yeast biomass showed the broad peak around 2θ = 200 that corresponds to the amorphous phase of biomass. The presence of both board and narrow reflections in the XRD spectrum of ETS-10 material indicate high crystalline, but disordered material33. More information about ETS-10 characteristics can be found in34.
The influence of experimental parameters on gadolinium ions adsorption using two tested adsorbents
The pH is one of the most important variables influencing the sorption behavior of gadolinium since it regulates the ionic species in the solution and adsorbent surface charge35,36. The pH of the experimental solution was adjusted using HNO3 or NaOH to the values in the range of 2.0–6.0 (Fig. 3a), considering gadolinium precipitation at alkaline conditions37,38. The thermodynamic speciation of gadolinium ions as a function of pH is presented in Fig. 4. In the simulation was used the database of thermodynamic data llnl.dat of calculation code PhreeqC 2.18. At the studied pH range gadolinium was present in solution in the form of Gd+3, GdNO32−, GdO2−, and GdO2H, at pHs above 6.0, the formation of hydroxo complex species such as GdO2H, GdOH+2 and GdOH+2 starts. Adsorbents showed low gadolinium removal at pH 2.0: 12% for both adsorbents. At low pH values, due to higher H+ ions concentration, these ions occupy the active sites, creating a positive charge on the sorbent, in this way limiting adsorption of gadolinium present in solution in cationic form35.
Effect of (a) pH (Ci, Gd 10 mg/L, time 60 min, temperature 22 °C), (b) time (Ci, Gd 10 mg/L, pH 3.0, temperature 22 °C), (c) temperature (Ci, Gd 10 mg/L, pH 3.0, time 60 min) and (d) gadolinium concentration (pH 3.0, time 60 min, temperature 22 °C) on gadolinium adsorption onto the studied adsorbents.
A maximum removal of gadolinium of 87% for titanosilicate ETS-10 and 62% for yeast biomass were attained at pH 3.0, when the surface of the sorbent becomes negatively charged and more active sites are available for electrostatic interaction, thus facilitating adsorption39. The decrease of adsorbents removal capacity at pHs 5.0–6.0 up to 13–22% can be explained by formation of soluble hydroxyl complexes39,40 due to the increase in concentration of OH groups in solution.
Maximum removal of gadolinium by nitrogen-doped carboxymethylated cellulose nanocrystalline carbon aerogels and kaolinite was achieved at pH 5.038,41, by spirulina at pH 3.042 and by bentonite clay at pH 6.043.
The effects of contact time on the adsorption performance of titanosilicate ETS-10 and yeast biomass are shown in Fig. 3b. Adsorption capacity of both adsorbents increased in the first 7 min of sorbent-sorbate interaction, but the adsorption capacity of titanosilicate ETS-10 was almost twice higher than that of yeast biomass. In case of titanosilicate ETS-10 in the first 7 min of sorbent–sorbate interaction, 96% of the gadolinium ions were removed from the solution and then an equilibrium was attained. For yeast biomass, in first 2 min of interaction 46% of gadolinium ions were removed from the solution and then slowly increased with time, becoming almost constant after 30 min. Fast gadolinium ions adsorption on the first stage can be attributed to the availability of large number of adsorption sites. The decrease of the adsorption rate is explained by the saturation of the binding sites and finally the adsorption equilibrium is attained39,44.
To investigate the thermodynamic behavior of the tested adsorbents, the experiments were performed at temperatures 20 °C, 30 °C, 40 °C, and 50 °C. Change of the temperature almost did not affect gadolinium removal by yeast biomass (Fig. 3c), which was on the level of 47–55%. This suggests that gadolinium adsorption process is not strictly related to its endothermic nature and is a physical adsorption45. The adsorption of gadolinium ions onto titanosilicate ETS-10 was affected by the temperature: its rise up to 40 °C resulted in an increase of adsorbent removal capacity to 85%, while further temperature increase resulted in the significant decrease of removal efficiency (from 9.2 to 5.4 mg/g or from 85 to 50%). An increase of the gadolinium adsorption on the temperature range of 20–40 °C is explained by the enhancement of the rate of diffusion of adsorbate molecules within the pores and across the adsorbent’s boundary layer44. Its decrease at higher temperatures can be associated with the weakening of the structure of the adsorbent46. An increase of the temperature in the same range resulted in a decrease of gadolinium removal by Arthospira platensis42.
The effect of initial gadolinium concentration of the adsorbents’ adsorption capacity is presented in Fig. 3d. An increase of gadolinium concentration in the solution resulted in the growth of the sorbents’ adsorption capacity from 8.2 to 97 mg/g for titanosilicate ETS-10 and from 8.1 to 33 mg/g for yeast biomass. In case of yeast biomass, at gadolinium concentration of 75 mg/L the saturation of the biosorbent, due to filling of adsorption sites was observed. Adsorption capacity of titanosilicate ETS-10 increased continuously at studied gadolinium concentrations range. According to47 an increase of metal concentration in solution provides a driving force to overcome all mass transfer resistances of metal ions between the aqueous and solid phase, facilitating metal uptake.
Kinetics, equilibrium and thermodynamics of the gadilinium adsorption
The ability to predict the kinetics of metal ions adsorption is critically important for the design of a wastewater treatment systems48. Two kinetic models, namely pseudo-first order and pseudo-second order in their non-linear forms were applied to describe experimentally obtained data (Fig. 5, Table 1).
Both models showed a good fit of the experimental data for titanosilicate ETS-10, with the correlation coefficient values of 0.99. Since both models showed high values of coefficient of correlation, on the next stage, the Akaike information criterion (AIC) test was applied, to highlight the more applicable model.
According to AIC results the pseudo-first-order model, assumes the gadolinium adsorption is a relationship between the rate of occupied adsorption sites by the metal ions and the unoccupied sites available on the surface of adsorbents44, fits better the experimental data. The same model was more consistent with the adsorption of gadolinium onto yeast biomass, indicating that the adsorption process may be of physical nature36.
The equilibrium of the sorption was evaluated applying two commonly used adsorption isotherms, Langmuir and Freundlich. The non-linear plots of the models are presented in Fig. 6 and the results of the fitting are given in Table 2.
Based on the high values of the correlation coefficients (Table 2) both models are reasonably suitable for experimental data explanation. According to AIC, Freundlich model described better data obtained for titanosilicate ETS-10, suggesting a chemical adsorption process44. In case of yeast biomass, Langmuir was the proper model to describe experimental data, suggesting formation of a monolayer by gadolinium ions at the surface of the sorbent43. The good fit of the experimental data to the Langmuir isotherm indicates a more physical sorption mechanism49. The theoretical maximum adsorption capacity obtained from Langmuir model was 234 mg/g for titanosilicate ETS-10 and 98 mg/g for yeast biomass, showing high affinity of mesoporous material for gadolinium ions.
The maximum adsorption capacity of two tested investigated adsorbents for the removal of gadolinium ions was compared with the capacity values reported for other adsorbents (Table 3). As can be seen from the presented values, adsorption capacity of the tested adsorbents was in line or even higher than reported values.
The thermodynamic behavior of the developed adsorbent is shown in Fig. 1S as Van’t Hoff plot, and the calculated parameters are presented in Table 4.
The negative ΔG° values exhibit the spontaneity of the gadolinium adsorption onto tested adsorbents. The negative value of ΔH° shows that adsorption is a heat-taking process, while positive values of ΔS° indicate an increase in randomness at the solid/solution interface during adsorption44.
Gadolinium adsorption mechanisms
The FTIR was applied in order to understand the possible mechanisms of gadolinium ions adsorption. The FTIR spectra of gadolinium- loaded and control yeast biomass are presented in Fig. 7a.
The spectra show distinct peaks in three regions of 500–1000 cm−1, 1030–1630 cm−1 and 1900–3300 cm−1. The peak at 516 cm−1 is attributed to a PO43 band. At 1033.8 cm−1, an intense peak characteristic of a C–O stretch vibration is observed51,52,53. There is a shift of a peak at 1226 cm−1 characteristic of C–N stretch in aliphatic amines to 1219 cm−1 of C–O for esters and carboxylic acids after adsorption. The peak at 1396 cm−1 resulting from a C–H bend; C–N stretch of amides; NO2 symmetric stretch and N–O stretch disappeared after biosorption. It can also be observed that, there was a peak at 1527 cm−1 which shifted to 1519 cm−1 indicating an N–H in plane bend from secondary protein amides, which overlaps with the C–N and NO2 asymmetric stretches52. At 1627.9 cm−1 a peak attributed to carbonyl groups of proteins (C=O) is observed which complements the C–N, N–H bonds observed earlier. There are weak C–H aromatic overtones around 1900–2000 cm−151. Small multiple weak peaks observed between 2036–2368 cm−1 representing C≡N and C≡C stretches, undergo significant signal changes. Some become weaker, stronger, disappear and some re-appear after gadolinium adsorption53.
These changes indicate that these bonds contribute to the biosorption process. A peak at 2916.3 cm−1 results from the stretching vibrations of aliphatic C–H indicating the presence of sp3 hybridized carbons to hydrogens. Finally, a strong and broad hydroxyl peak representing the symmetric stretching vibration of the O–H bonds becomes weaker at 3255 cm−1. Therefore, the presence of N–H, O–H and the C=O peaks indicates that the yeast biomass contains the CONH and COOH functional groups, taking part in hydrogen bonding. As a result, there is reduction in peak intensity after metal biosorption. These changes indicate the contribution of amide and carboxylic acid groups to gadolinium adsorption and the potential role of protein N that could be located in the yeast cell wall on element binding.
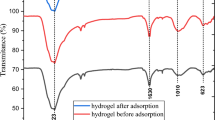
The FTIR spectra of titanosilicate ETS-10 before and after gadolinium adsorption are shown in Fig. 7b. Significant peaks are observed in the fingerprint range of 430–1024 cm−1, which indicate the presence of Si–O and Ti–O stretching and bending bonds. The peak at 424 cm−1 is attributed to Ti–O rocking and Si–O–Ti bending groups33,54. A Si–O rocking and O–Si–O bend is observed from a peak at 578 cm−1 with a Ti–O stretch and O–Ti–O at 779 cm−133. A peak at 848 cm−1 characteristic of Si–O–and Ti–O– terminal bonds disappeared after adsorption indicating the participation of these terminal groups in gadolinium adsorption. At 1033 cm−1, a common stretching vibration of the C–O group is observed. The Ti–O–Ti at 1504 cm−1 moved to 1512 cm−1 after adsorption. Bending vibration of water molecules are seen at 1666 cm−1. Therefore, the changes in intensities and shifts of the peaks observed after adsorption indicate a successful loading of the metal onto sorbent via adsorption or ion exchange. In the case of the ETS-10 structure, the formation of three Gd–O-type bonds with the adsorbent surface is very difficult, because in this configuration, the distance between the oxygen atoms on the surface is greater. Between the hydration water molecules and O atoms on the surface of the adsorbent structure, physical interactions with the hydrogen-bond-type are formed, which contribute to the facilitation of adsorption and the binding of the Gd atom to the adsorbents’ structure (Fig. 8).
Sorbents regeneration
During three adsorption–desorption experiments both adsorbents showed high adsorption capacity and desorption efficiency (Fig. 9). In three cycles, the desorption efficiency of titanosilicate ETS-10 toward gadolinium was on the level of 87–80%, and of yeast biomass −62 to 48%, that indicates on the possibility of their multiply use.
Conclusions
The adsorption capacity of two adsorbents of different origin toward gadolinium was tested. For both adsorbents the process was quick and pH dependent with maximum metal removal being attained at pH 3.0 in less than 30 min. The kinetics of the adsorption was better described by pseudo-first order model, suggesting its physical nature. The Langmuir model fit well the experimental data for yeast biomass, while Freundlich model was more applicable for titanosilicate ETS-10. The maximum adsorption capacity calculated for titanosilicate ETS-10 of 234 mg/g was considerable higher compared to values obtained for yeast biomass (98 mg/g). The process of gadolinium adsorption was spontaneous, temperature-independent for yeast biomass and affected by high temperatures in case of titanosilicate ETS-10. FTIR analysis showed active involvement of amide and carboxylic groups in gadolinium adsorption by yeast biomass, and of Si–O– and Ti–O– in case of titanosilicate ETS-10. High adsorption and desorption capacities were obtained for both materials, that allow their multiply use on the industrial scale. Both adsorbents showed to be good candidates for gadolinium removal, however, for practical application yeast biomass is more preferable due to its low cost.
Since most often wastewater are solutions with complex composition in the future studies it is necessary to investigate the effect of accompanying heavy metals and rare earth elements on the efficiency of gadolinium adsorption.
Data availability
All data are presented in the manuscript.
References
Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 10, 1285–1303 (2019).
Malhotra, N. et al. An updated review of toxicity effect of the rare earth elements (REEs) on aquatic organisms. Animals 10, 1–27. https://doi.org/10.3390/ani10091663 (2020).
Tommasi, F. et al. Review of rare earth elements as fertilizers and feed additives: A knowledge gap analysis. Arch. Environ. Contam. Toxicol. 81, 531–540. https://doi.org/10.1007/s00244-020-00773-4 (2021).
Atinkpahoun, C. N. H., Pons, M. N., Louis, P., Leclerc, J. P. & Soclo, H. H. Rare earth elements (REE) in the urban wastewater of Cotonou (Benin, West Africa). Chemosphere 251, 126398 (2020).
Brouziotis, A. A. et al. Toxicity of rare earth elements: An overview on human health impact. Front. Environ. Sci. 10, 948041. https://doi.org/10.3389/fenvs.2022.948041 (2022).
Wang, W., Yang, Y., Wang, D. & Huang, L. Toxic effects of rare earth elements on human health: A review. Toxics 12, 317. https://doi.org/10.3390/toxics12050317 (2024).
Birka, M., Wehe, C. A., Hachmöller, O., Sperling, M. & Karst, U. Tracing gadolinium-based contrast agents from surface water to drinking water by means of speciation analysis. J. Chromatogr. A. 1440, 105–111 (2016).
Telgmann, L. et al. Speciation and isotope dilution analysis of gadolinium-based contrast agents in wastewater. Environ. Sci. Technol. 46, 11929–11936 (2012).
Laczovics, A. et al. Relationship between gadolinium-based MRI contrast agent consumption and anthropogenic gadolinium in the influent of a wastewater treatment plant. Sci. Total Environ. 877, 162844 (2023).
Islam, M. A., Angove, M. J. & Morton, D. W. Recent innovative research on chromium (VI) adsorption mechanism. Environ. Nanotechnol. Monit. Manag. 12, 100267. https://doi.org/10.1016/j.enmm.2019.100267 (2019).
Jang, E. H., Pack, S. P., Kim, I. & Chung, S. A systematic study of hexavalent chromium adsorption and removal from aqueous environments using chemically functionalized amorphous and mesoporous silica nanoparticles. Sci. Rep. 10, 1–20 (2020).
Duan, C., Ma, T., Wang, J. & Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process Eng. 37, 101339. https://doi.org/10.1016/j.jwpe.2020.101339 (2020).
Bayar, J. et al. Biochar-based adsorption for heavy metal removal in water: A sustainable and cost-effective approach. Environ. Geochem. Health 46, 1–25. https://doi.org/10.1007/s10653-024-02214-w (2024).
Lin, G. et al. A systematic review of metal organic frameworks materials for heavy metal removal: Synthesis, applications and mechanism. Chem. Eng. J. 460, 141710. https://doi.org/10.1016/j.cej.2023.141710 (2023).
Da’na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Microporous Mesoporous Mater. 247, 145–157. https://doi.org/10.1016/j.micromeso.2017.03.050 (2017).
Kim, B. C. et al. Magnetic mesoporous materials for removal of environmental wastes. J. Hazard. Mater. 192, 1140–1147 (2011).
Grozdov, D. & Zinicovscaia, I. Mesoporous materials for metal-laden wastewater treatment. Materials 16, 5864. https://doi.org/10.3390/ma16175864 (2023).
Wang, J. & Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 27, 195–226. https://doi.org/10.1016/j.biotechadv.2008.11.002 (2009).
Razzak, S. A. et al. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ. Adv. 7, 100168 (2022).
Esmaeili, A., Mobini, M. & Eslami, H. Removal of heavy metals from acid mine drainage by native natural clay minerals, batch and continuous studies. Appl. Water Sci. 9, 1–6 (2019).
Xie, S. et al. Application of clay minerals as adsorbents for removing heavy metals from the environment. Green Smart Min. Eng. 1, 249–261 (2024).
Hussain, M. K. et al. Unleashing the power of bio-adsorbents: Efficient heavy metal removal for sustainable water purification. J. Water Process Eng. 64, 105705. https://doi.org/10.1016/j.jwpe.2024.105705 (2024).
Fadillah, G. & Saleh, T. A. Advances in mesoporous material for adsorption and photoconversion of CO2 in environmental pollution: Clean environment and clean energy. Sustain. Chem. Pharm. 29, 100812. https://doi.org/10.1016/j.scp.2022.100812 (2022).
Neese, F. Software update: The ORCA program system, version 4.0.. Wiley Interdiscip. Rev. Comput. Mol. Sci. 8, e1327 (2018).
Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput Mol. Sc.i 2, 73–78 (2012).
Becke, A. D. Density-functional thermochemistry III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 37, 785 (1988).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Wang, J. & Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard Mater. 390, 122156 (2020).
Makul, N. Adsorption Isotherms. Dict. Concr. Technol. https://doi.org/10.1007/978-981-97-2998-2_25 (2025).
Wang, J. & Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 258, 127279 (2020).
Lima, E. C., Hosseini-Bandegharaei, A., Moreno-Piraján, J. C. & Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 273, 425–434 (2019).
Zinicovscaia, I. et al. Adsorption capacity of silica SBA-15 and titanosilicate ETS-10 toward indium ions. Materials 16, 3201 (2023).
Zinicovscaia, I. et al. Sorption of Ce(III) by silica SBA-15 and titanosilicate ETS-10 from aqueous solution. Water 13, 3263 (2021).
Maslova, M., Mudruk, N., Ivanets, A., Shashkova, I. & Kitikova, N. The effect of pH on removal of toxic metal ions from aqueous solutions using composite sorbent based on Ti–Ca–Mg phosphates. J. Water Process Eng. 40, 101830 (2021).
Pang, Y. et al. Cadmium adsorption performance and mechanism from aqueous solution using red mud modified with amorphous MnO2. Sci. Rep. 12, 1–18 (2022).
Sasikumar, P., Narasimhan, S. V. & Velmurugan, S. Development of a modified ion exchange resin column for removal of gadolinium from the moderator system of PHWRs. Sep. Sci. Technol. 48, 1220–1225 (2013).
Xu, T., Zheng, X., Li, A. & Ji, B. Selective adsorption of gadolinium by nitrogen-doped carboxymethylated cellulose nanocrystalline carbon aerogels functionalized in the ammonia-urea system. Molecules 28, 7965 (2023).
Alghamdi, A. A. et al. Efficient adsorption of lead (II) from aqueous phase solutions using polypyrrole-based activated carbon. Materials 12, 2020 (2019).
Cruz-Lopes, L. P., Macena, M., Esteves, B. & Guiné, R. P. F. Ideal pH for the adsorption of metal ions Cr6+, Ni2+, Pb2+ in aqueous solution with different adsorbent materials. Open Agric. 6, 115–123 (2021).
Kautenburger, R. & Beck, H. P. Influence of geochemical parameters on the sorption and desorption behaviour of europium and gadolinium onto kaolinite. J. Environ. Monit. 12, 1295–1301 (2010).
Yushin, N. et al. Cyanobacteria Arthospira platensis as an effective tool for gadolinium removal from wastewater. Clean Technol. 5, 638–651 (2023).
Dotto, G. L. et al. Selective adsorption of gadolinium from real leachate using a natural bentonite clay. J. Environ. Chem. Eng. 11, 109748 (2023).
Mahmood, U. et al. Adsorption of lead ions from wastewater using electrospun zeolite/MWCNT nanofibers: Kinetics, thermodynamics and modeling study. RSC Adv. 14, 5959–5974 (2024).
Kul, Z. E., Nuhoğlu, Y., Kul, S., Nuhoğlu, Ç. & Torun, F. E. Mechanism of heavy metal uptake by electron paramagnetic resonance and FTIR: Enhanced manganese(II) removal onto waste acorn of Quercus ithaburensis. Sep. Sci. Technol. 51, 115–125 (2016).
Zinicovscaia, I., Yushin, N., Grozdov, D., Rodlovskaya, E. & Khiem, L. H. Yeast—As bioremediator of silver-containing synthetic effluents. Bioengineering 10, 398 (2023).
Chowdhury, S., Mishra, R., Kushwaha, P. & Saha, P. Removal of safranin from aqueous solutions by NaOH-treated rice husk: Thermodynamics, kinetics and isosteric heat of adsorption. Asia Pac. J. Chem. Eng. 7, 236–249 (2012).
Sayago, U. F. C., Castro, Y. P., Rivera, L. R. C. & Mariaca, A. G. Estimation of equilibrium times and maximum capacity of adsorption of heavy metals by E. crassipes (review). Environ. Monitor. Assess. 192, 1–16. https://doi.org/10.1007/s10661-019-8032-9 (2020).
Zeng, W., Xu, L., Wang, Q., Chen, C. & Fu, M. Adsorption of indium(III) ions from an acidic solution by using UiO-66. Metals 12, 579 (2022).
Kuila, S. K. & Kundu, T. K. Adsorption studies of gadolinium ion on graphitic carbon nitride. In: IOP Conference Series: Materials Science and Engineering vol. 338 (Institute of Physics Publishing, 2018).
De Rossi, A. et al. Chromium (VI) biosorption by saccharomyces cerevisiae subjected to chemical and thermal treatments. Environ. Sci. Pollut. Res. 25, 19179–19186 (2018).
Smith, B. Infrared Spectral Interpretation: A Systematic Approach (CRC Press, 2018). https://doi.org/10.1201/9780203750841.
Stathatou, P. M. et al. Lead removal at trace concentrations from water by inactive yeast cells. Commun. Earth Environ. 3, 1–9 (2022).
Zhao, G. X. S., Lee, J. L. & Chia, P. A. Unusual adsorption properties of microporous titanosilicate ETS-10 toward heavy metal lead. Langmuir 19, 1977–1979 (2003).
Acknowledgements
The authors would like to thanks Dr. Maria Ignat for mesoporous adsorbents synthesis.
Funding
This research was supported by Joint Institute for Nuclear Research-South Africa project no. 37 from 3.01.2025 item 14.
Author information
Authors and Affiliations
Contributions
Conceptualization, I.Z., and N.Y.; methodology, I.Z., D.H., N.Y.; investigation N.Y, D.H. and D.S. software, N.Y.; validation, I.Z. and N.Y.; formal analysis, I.Z., D.S. and N.Y.; writing—original draft preparation, I.Z and D.S.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zinicovscaia, I., Yushin, N., Sifelani, D. et al. Efficiency of biological versus mesoporous adsorbents for gadolinium removal from wastewater. Sci Rep 15, 30500 (2025). https://doi.org/10.1038/s41598-025-14970-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-14970-5