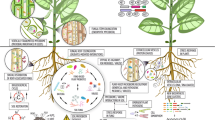
Abstract
There is a knowledge gap about the quantitative aspects of mycorrhizal fungi’s influence on ecological succession on tailings. Here, we demonstrate that inoculating mine tailings with 2% fungi yields significantly better results in terms of plant biomass and lower lipid peroxidation compared to 1% and 0%, both when growing Agrostis capillaris alone and in combination with Melilotus albus. Lipid peroxidation in the A. capillaris is positively predicted by Cu, and negatively predicted by the total Kjeldahl nitrogen in plants. The biomass of M. albus is positively predicted by the N/P ratio, and negatively by Cu concentration in the plant. This improvement was related to differences between the Technosols properties at the end of the experiment (pH, EC, N-NH4+, N-NO3−), which modulated the changes of the tailing material properties from the wet to the dry state, and to differences in the accumulation factors of Cu and Pb from substrate to plant roots, and of the transfer factors from roots to aboveground parts. This is the first time that the effects of such a slight increase in fungal inoculum percentage have been reported. Fine-tuning the fungi treatment can lead to cost-effective techniques for tailings remediation. Block diagrams of an eco-technology are proposed.
Similar content being viewed by others
Introduction
The quantity of tailings in the EU exceeds one billion tons1, and an estimated 7 billion tons are accumulated worldwide each year2. Tailings dumps and tailings ponds, which are environmentally problematic and economically unviable, form unique landscapes that pose serious environmental challenges3. In all these situations, the disposal of solid and/or liquid waste is often irregular and uncontrolled. Such activities lead to the frequent release of harmful gases and the formation of leachate with devastating effects on the environment, and implicitly, on the human population4. Given the scale and severity of the problems, managing such landscapes, including the creation of new ecosystems and the remediation of contaminated substrates, has become a key focus of various research fields. One trend that has been present for a long time in restoration ecology is targeting multiple objectives through the remediation of tailings, such as the production of various ecosystem services (ES), in addition to the simple capping of toxic materials5.
While it is clear that planning for ES benefits the social communities and enhances the acceptability of mining projects in their post-closure phase6, the eco-technologies leading to specific ES are less settled.
The revegetation strategy, which applies phytostabilization techniques, is widely recommended as a promising approach to minimize wind and water erosion while mitigating the long-term ecological risks associated with the storage of metal smelting slag7. The ES production is associated with the more mature phases of ecological succession, corresponding to a diverse vegetation structure and a more complex food web, which can, in principle, be achieved by controlling the remediation processes8. During the process, the mine tailings evolve towards a Technosol (in the nomenclature of SUITMA9).
One way to accelerate the ecological succession is by influencing the arbuscular mycorrhizal fungi (AMF) community. AMF can enter into symbiosis with the rhizosphere system of terrestrial plants, leading to the expansion of the hyphal network in the soil and an increase in the root surface area. This phenomenon leads to an increase in the resistance of plants to biotic and abiotic stress factors10,11. Some fungal species, such as the genus Rhyzophagus (R. intraradices, R. irregularis, R. aggregatus, and others), are recognized for their role in partially immobilizing heavy metals in the soil, thereby reducing their transport to plants and mitigating metal-induced phytotoxicity12,13. R. irregularis, in particular, is a species very resistant to high concentrations of copper, which are lethal for most other organisms (Tamayo et al., 2014, cited by14).
Another method to accelerate succession is to influence the vegetation cover from the very beginning. Selecting plant species that can adapt their development to such highly degraded and arid lands is critical, as conventional plant species struggle to survive in such extreme conditions12,15. To ensure long-term phytostabilization performance, using local wild plant species that are tolerant to poor soil conditions and high temperatures is preferable, as it is currently the most effective and sustainable solution16,17. These plants offer distinct advantages, as they typically exhibit high tolerance to semi-arid conditions and heavy metal pollution10,18. The interaction between plant species plays a crucial role in the dynamics of plant communities in stressful environments, such as tailings19. As the plants strongly interact with the soil microbial community, a more complex remediation approach is to manipulate both the plant community and the AMF20.
The details of the methods for accelerating ecological succession on tailings have ecotechnological consequences. In the overall budget for industrial closure, the percentage allocated to capping and revegetation is small (see16 for an analysis), therefore, performing remediation in a more complex manner would not significantly increase the total budget. Still, the remediation budget must also be optimized at the level of costly resources, such as the fungal inoculum, to make it acceptable for technological transfer to the business sector21.
In this context, the objectives of this study were:
-
1.
To test the hypothesis that inoculating mine tailings with 2% fungi yields significantly better effects than 1% and 0%, both when growing a single species (Agrostis capillaris L.) and two plant species (A. capillaris in combination with Melilotus albus Medik.). An innovative aspect of the first objective is the important decrease in the percentage of AMF used, from 7 to 10% and 7%, as recommended in the literature10,22, to 2% and 1%, respectively.
-
2.
To develop a process of accelerating the ecological succession on tailings by myco-phytoremediation. The present research was conducted on the mine tailing of a still-active pond until 2030. A novelty of the second objective is the use of fresh mine tailings sampled before the pond’s closure phase.
Materials and methods
Site description
Valea Şesei is Romania’s largest tailing repository, situated in the Metalliferous Mountains, a subgroup of the Apuseni Mountains, which are part of the Western Carpathians. The geographic coordinates are 46°22′38″N 23°14′18″E, positioned on the right tributary of the Arieș River (Fig. 1). This deposit was formed by flooding a valley with tailing and wastewater from the extraction, processing, and smelting of copper ore from Roșia Poieni. The mineralization found in this deposit consists of various minerals, including pyrite, chalcopyrite, magnetite, hematite, molybdenite, and bornite. Additionally, there are subordinate minerals such as tetrahedrite, tennantite, enargite, luzonite-stibioluzonite, goldfieldite, hessite, tellurobismutite, galena, sphalerite, covellite, chalcocite, and digenite, as well as minor occurrences of pyrrhotite. Pyrite is widely distributed throughout the entire ore body and serves as the primary sulfide mineral, particularly in the upper section of the volcanic structure, in the advanced argillic zone23.
Experimental setup
To test the hypothesis, explain the findings, and extract as much as possible information for the remediation technique we used the approach presented in Fig. 2. A gradient of fungi inoculation (from 0 to 1 to 2%) was embedded into a larger gradient of nitrogen and toxic elements availability (from raw tailing material to tailing material with increasingly complex amendments, and finally an uncontaminated topsoil (which was used also as amendment). In each treatment on the gradient, we experimented with plant growth in two variants of plant composition, either with one species or with two species. Additionally, we compared the wet raw tailing material at the time of sampling with the properties of the experimental substrates at the end of the experiments.
Schematic diagram of the experimental approach. Legend: MT t0 = tailing material at the sampling moment (wet state, at three locations on the tailing dam), different types of Technosol, in pot experiments: MT = homogenized dry tailing material, MTTS = MT with topsoil, MTTSTr = MT with topsoil and Trifolium green fertilizer, MTTSTrF1 = MTTSTr inoculated with 1% mycorrhizal fungi, MTTSTrF2 = MTTSTr inoculated with 2% mycorrhizal fungi.
More specifically, treatments were established with A. capillaris L. alone (labeled A1 in the figures and tables) and A. capillaris L. mixed with Melilotus albus Medik (in this case, the plants were labeled A2 and M). Each treatment had five replicates. Thus, the total number of pots along the inoculation gradient was n = 30, and along the full nitrogen and toxic elements gradient was 60.
We supplemented this with two additional treatments to verify the possible replacement of clover (Trifolium - Tr) used as a green fertilizer with alfalfa (Medicago sativa - Ms). Thus, by replacing Tr from the MTTSTr treatment with Ms and working with one and two species, all inoculated with 2% fungi, each with five replicates, an additional n = 10 pots were added to the experiment. To sum up, the full approach had 14 treatments, each with 5 replicates. The last two treatments in which we replaced Tr with Ms had a purely applicative purpose, and the data were not included in the results, but in the Supp. Inf. and supported the discussion.
Substrate analysis and details of the experimental implementation
For the physicochemical characterization of the mine tailing, 15 large samples (approximately 10 kg each) from the superficial layer (0–15 cm depth) could be individually sampled and thoroughly homogenized. Sampling was conducted at three different locations within the pond, with a focus on the most solidified areas. In contrast, the rest of the pond’s surface remained in a liquid phase due to ongoing activity. Then, the mine tailing was air dried and passed through a < 2 mm mesh size sieve. The samples were processed individually, and Table 1 reports the arithmetic means of the values of all measured variables. Among these values in Table 1, it can be observed that the pH of the tailing substrate is extremely high. A plausible explanation for this is that, as an active pond, it still contains residues from ore preparation procedures, including foaming agents, flocculants, and pH modifiers (e.g., calcium oxide), which were discharged during flotation and successive washing processes. These substances contribute to the alkaline liquid fraction of the pond, which has only partially evaporated at this stage.
In the treatments, the substrate was improved by adding topsoil (20% w/v) and green fertilizer (5% w/v). Topsoil was sampled from an area located near another tailings dam with easier access than the Valea Sesei dam, with the following geographical coordinates: 45°57’38.97"N, 22°58’55.39"E. Green fertilizers consisted of a combination of Trifolium repens L. with Trifolium pratense L., cultivated in a greenhouse of the Institute of Biology-Bucharest, Romanian Academy, or raw alfalfa (Medicago sativa L.) harvested from the Botanical Garden of the University of Bucharest, Romania. All plant species used as green fertilizer were harvested at an early developmental stage to facilitate decomposition into the Technosol. All amendments are characterized in Table 1.
For treatments of the fungi gradient, expanded clay with or without fungi was used (1% or 2% w/w of the total quantity of 500 g of Technosol with which each pot was filled). The commercial mycorrhizal inoculum was Rhizophagus irregularis species produced by INOQ GmbH, Germany. This inoculum contained 210 spores per square centimeter, sequestered in 2-mm volcanic expanded clay particles.
The species A. capillaris L. belongs to the Poaceae family (colonial bent as common name), and M. albus (Melilot clover as common name, also known as sweet clover) belongs to the Fabaceae family and is a nitrogen-fixing legume. A. capillaris is a native grass species surrounding the pond. Given its rapid growth and relatively simple management, it was selected due to its abundance and suitability for establishing vegetation cover in abandoned mine tailings10. M. albus was used to provide an additional supply of nitrogen, which was brought to the plants by the nodules on their roots. Seeds were sown for both plant species according to the supplier’s specifications, corresponding to a mass of 20 kg ha−¹ per pot. The mass of seeds in the mixture did not exceed the maximum recommended limit. The seeds (Rieger-Hofmann supplier, Germany) were added as follows:
-
In the A. capillaris single treatments, we added the optimal quantity (mass) of A. capillaris seeds, as recommended by the provider (the amount recommended is at the hectare, and we computed the corresponding proportion for the surface of a pot).
-
In the two species treatments, we added half of the optimal mass of seeds of A. capillaris and half of the optimal mass of seeds of M. albus.
All pots were randomly arranged in a growth chamber equipped with a phytotron system (London, UK), operating under a 16-hour daylight cycle (22 °C, 5000 lx) and an 8-hour darkness cycle (16 °C), with 60% humidity. The experiment was conducted over three months, during which the plants were irrigated daily with distilled water to maintain a constant substrate moisture level.
After harvesting, plants were separated into roots and shoots, and fresh biomass was recorded for each. The shoots were quickly rinsed with distilled water. The roots were washed several times with tap water until the Technosol was removed and then rinsed with distilled water. All plant material was frozen at −20 °C, lyophilized using a Martin Christ lyophilizer (Osterode am Harz, Germany), and the dry biomass (comprising both roots and shoots) was recorded. For further analysis, the dried plant material was ground using a stainless-steel mill equipped with an IKA cooling system (Wilmington, NC, USA) and stored at −20 °C until processing.
Variables measured from the technosol
The Technosol respiration was measured from wet material (in the first two hours after plant harvesting) according to the method described by24. During the first 24 h after harvesting the plants, the following variables were measured: pH (H2O) (1:2.5 v/v) and electrical conductivity EC (1:5 v/v), both using a WTW 340i multiparameter system (Weilheim, Germany) according to DIN25. Next, the Technosol moisture content was determined by pre-drying the samples at 105 °C until a constant weight was reached. Additionally, the dissolved inorganic nitrogen content was measured. Thus, wet samples were processed for nitrogen extraction by mixing 20 g of Technosol with 100 mL of 0.2 M KCl (Sigma-Aldrich, puris. p.a.) and stirring at 150 rpm for 1 h. For phosphorus extraction, 5 g of Technosol was treated with 100 mL of 0.5 M NaHCO₃ (VWR, puris. p.a.), and stirred at 150 rpm for 30 min. Subsequently, all samples were filtered using medium-porosity filter paper and analyzed spectrophotometrically (CECIL Aquarius, Milton Cambridge, UK) following the method described by26. After drying the Technosol at room temperature, protected from sunlight and sieving (< 2 mm mesh size), loss on ignition (LOI) was determined by measuring organic matter loss at 600 °C following a method adapted from27. The total metal content was analyzed using a X-ray fluorescence spectrometer, Thermo Scientific Niton GOLDD (Winchester, UK). Mineralogical analysis was conducted using a Zeiss Axio Imager A2m microscope with an attached Axiocam Erc5s 5 MP camera from Zeiss (White Plains, NY, US). Subsequently, a scanning electron microscope with energy dispersive spectroscopy (SEM-EDS) was used for further analysis. A tabletop Hitachi TM3030 SEM (Hitachi, Japan) operating at an accelerating voltage of 15 kV was used for these observations. Elemental analyses were conducted using a QUANTAX 70 EDS system from Bruker (Madison, WI, USA). A total of 45 samples were analyzed, including 15 mine tailings samples collected from three sampling points and 30 Technosol samples obtained from the pot experiment after plant harvesting (improved structure and readability). Mineral abbreviations used in this study follow the nomenclature endorsed by the Commission on New Minerals, Nomenclature, and Classification (CNMNC) of the International Mineralogical Association (IMA)28.
Variables measured from the plant material
Freeze-dried and ground plant materials were further processed to measure assimilatory pigments. For this analysis, 30 mg of dry shoots was homogenized at 75,000 rpm for 30 s in an extraction solution consisting of 80% acetone (Riedel-de Haën, puriss. p.a.), 15% water, and 5% ammonium hydroxide solution (Fluka puriss. p.a.) (25% v/v, in water). The homogenized samples were centrifuged at 6000 rpm for 20 min at 4 °C. The resulting supernatant was spectrophotometrically analyzed at 480, 645, 647, 663, and 664 nm to determine the concentrations of chlorophyll a, chlorophyll b, and carotenoids, following the method described by29. Lipid peroxidation (LP) was estimated by determining malondialdehyde (MDA) after homogenizing 20 mg d.w. of plant roots and shoots at 75,000 rpm for 30 s in 4 mL thiobarbituric acid (TBA) (Sigma-Aldrich puriss. p.a.) (solution containing 10% trichloroacetic acid (Riedel-de Haën, puriss. p.a.), and 0.25% thiobarbituric acid in ultrapure water. The mixture was then heated for 30 min at 95 °C, cooled for 15 min at room temperature, and centrifuged. It was subsequently spectrophotometrically analyzed at 440, 532, and 600 nm, following the method described by30. Total Kjeldahl Nitrogen (TKN) was measured as a difference between [TN] and [nitrate and nitrite (NOx)] using a Büchi AutoKjeldahl system (Flawil, Switzerland) after digestion in a block-speed digestor unit K-439 connected to a Scrubber B-415 at 550 o C, followed by subsequent titration conducted via a distillation unit KjelFlex K-360 with built-in titrator Methron, 484 Titrino plus (Flawil, Switzerland), according to a Kjeldahl method described by31. Elemental analysis was conducted using an ICP-MS Perkin-Elmer ELAN DRC-e (Concord, ON, Canada) with a single collector. Before analysis, samples underwent wet digestion in a Microwave digestor Anton Paar 3000 (Graz, Austria) using suprapure Merck 65% nitric acid. The digestion was performed following a three-step program with a progressive increase in infrared radiation (IR) up to 140 °C and pressure up to 40 bar (0.3 bar s−1) for 45 min. For quality assurance and control criteria of digestion, a standard reference material, CRM 281 of ryegrass, was digested, and the differences were found to be no more than 5%. Standard solutions were prepared for element analysis by diluting 10 µg L−1 multielement solution (Multielement ICP Calibration Standard 3, matrix 5% HNO3), Perkin-Elmer Pure Plus.
AMF root colonization rate
To estimate the rate of AMF root colonization, seven fragments of fine roots from each plant species were selected after a sequential treatment by going through the following steps: (1) clarification using 2% KOH (Merck, puris. p.a.) (w/v), (2) acidification with 2% (v/v) HCl (Sigma-Aldrich puris. p.a.), and (3) staining with lactophenol blue solution (Fluka, puris. p.a.) for fungal visualization. The stained root fragments were observed under a Carl Zeiss Axio Imager 2 microscope (Jena, Germany) at 40× magnification. Vesicles, arbuscules and hyphae were counted and the colonization was subsequently assessed as follows: the number of hyphae was assessed using intensity classes (0–5), where 0 = 0%, 1 = 1%, 2 = 10%, 3 = < 50%, 4 = > 50% and 5 = > 90%. Arbuscules were classified as A3, A2, A1, and A0, indicating abundance, frequency, low number, and absence of arbuscules, respectively. The number of vesicles was indicated according to the classifications m3 numerous, m2 frequent, m1 few, and m0 0–3 vesicles. Measurements included the frequency (%F) (Eq. 1), the intensity of mycorrhizal colonization (M%) (Eq. 2), the abundance of arbuscules in the root system (A%) (Eq. 3), and the abundance of vesicules (Eq. 4) according to32
Statistical analysis
Data analysis was performed using the Python libraries: SciPy for statistical data analysis [scipy]33, seaborn [seaborn]34, and stat annotations [statann] for statistical graphs35. The two-sided Mann-Whitney-Wilcoxon test was used to determine pairwise statistical differences. There was no p-value correction for multiple pairwise comparisons. The following symbols: ns: 5.00e−02 < p < = 1.00e+00, *: 1.00e−02 < p < = 5.00e−02, **: 1.00e−03 < p < = 1.00e−02, ***: 1.00e−04 < p < = 1.00e−03, ***: p < = 1.00e−04 were used for annotations. We performed principal component analysis (PCA) and multiple linear regression for the plant and soil variables using TIBCO Statistica version 14.0.0.15 and IBM SPSS Statistics. Before being used in the analysis, the normality of the distribution was checked, and lg normalization was performed when needed. In one case, we standardized the biomass data to a zero average and a standard deviation of 1 separately for the treatments with one species and two species, allowing for their combined inclusion in multivariate statistics. We estimated the sampling adequacy of individual and set variables for PCA by the Kaiser–Meyer–Olkin measure (> 0.50) and Bartlett’s test of sphericity (< 0.05) and removed the variables with communality values < 0.5. We checked the validity of multiple linear regression by: ANOVA of each model tested, significance of each variable included in the model, Multicollinearity by Tolerance > 0.2 and VIF < 5, a mean of residues close to zero, normality of unstandardized residues values (p > 0.05) by Shapiro-Wilk test, the existence of potential outliers by Cook’s distance < 1, and the presence of autocorrelation between regression variables by Durbin-Watson test (passed if the values was between 1 and 3). Suppl. Inf. Table 1 sums up how the measures for evaluating the adequacy of data for principal PCA and validity of MLR models were computed and interpreted. Suppl. Inf. Figure 1 shows the results of evaluating the adequacy of soil data and the validity of models for each PCA and MLR that we performed. The coefficients of the independent variables in the multiple regressions presented in the next part of the article are for the standardized input variables, allowing for easy comparison of their relative importance. We also calculated accumulation factors (ratios of toxic element concentrations in roots to those in soil), transfer factors (ratios of toxic element concentrations in aboveground parts to those in roots), and N/P ratios.
Results
In this chapter, only statistically significant differences (*: 1.00e−02 < p < = 5.00e−02) and highly statistically significant differences (**: 1.00e−03 < p < = 1.00e−02) were discussed, as a result of comparing the values of the measured variables.
The effect of increasing inoculation with fungi
In Fig. 3a, it can be seen that the root biomass of A. capillaris alone increased significantly only in the treatment with 2% fungi (MTTStrF2) compared to the treatment with 1% fungi (MTTStrF1). In A. capillaris in mixture, a significant increase was recorded only between 0% and 2% fungi, while in the species M. albus the same increase was found between 0% and 1%, and highly significant between 0% and 2% fungi. In the case of shoot biomass (Fig. 3b), a significant increase was recorded for A. capillaris alone and A. capillaris in the mixture between 0% and 2% fungi, while M. albus sp. a significant increase in biomass between treatments 1% and 2% fungi and a highly significant increase between 0% and 2% fungi. In the 2% fungal treatment, in addition to higher plant biomass, an increase in the measured assimilatory pigments was also recorded, as shown in Fig. 3c, d, and e. A higher biomass of plants grown in the treatments with 1% and 2% fungi is also supported by a statistically significant or highly significant decrease in lipid peroxidation in both roots and shoots, as shown in Fig. 3g and h. The recorded decrease in plant oxidative stress is in agreement with the highly significant increase in substrate respiration when a mixture of two plant species was grown compared to when a single species was grown, a phenomenon observed in all three treatments (Fig. 3f).
Regarding the TKN content both roots and shoots (Figs. 3i and j), the increase was highly significant between the three treatments, except for A. capillaris alonewhere between 0% and 1% and 1% − 2% fungi the increases were not significant, as is the case of A. capillaris in mixture between the treatment 0% −1% fungi. Phosphorus content had a similar pattern in roots and shoots (Figs. 3k and l) in species A. capillaris alone, where there was an not significant increase between 0% and 1% fungi, significant between 1% and 2% fungi, and only in roots between 0% and 2% fungi, while for shoots, P content was highly significant. In species A. capillaris in mixture and M. albus, the increase in P content between the three treatments was insignificant, except for shoots in A. capillaris in mixture, where the variation was highly significant between 0% and 2% fungi treatments.
Element concentrations in plant tissues remained within the acceptable limits for plant growth, except for Cu (Fig. 3m and n). The excessive levels of metals (mg kg−1) for various herbaceous species, as defined by [40], are as follows: Cu (20–100), Pb (30–300), Zn (100–400), and Ni (10–100). As can be seen in Fig. 3 from m to p, Cu and Pb had a significant or highly significant tendency to decrease in concentrations both in the 2% treatment and in the others, in roots, but also in shoots of A. capillaris alone, A. capillaris in mixture, and M. albus plant species. However, Cu and Pb concentrations did not decrease significantly for species A. capillaris alone, between 0% and 1% fungi in roots or in shoots. Also for A. capillaris alone, between 1% and 2% fungi treatments, Cu decreased significantly in roots and shoots, while Pb increased highly significant in roots and decreased also highly significant in shoots. Between 0% and 2% fungi, Cu decreased highly significant, while Pb decreased not significantly, both in roots and shoots. In species A. capillaris in mixture, between 0% and 1% fungi, Cu decreased significantly only in roots, while Pb decreased highly significant in roots and only significant in shoots. Also in A. capillaris in mixture, between 1% and 2%, both Cu and Pb increased significantly in roots and decreased highly significant in shoots, while between 0% and 2% fungi treatments, both Cu and Pb decreased insignificantly in roots and highly significant in shoots.
Box plots showing the range, the 25–75% interval, and the median of selected variables. Variation of (a) dry roots biomass, (b) dry shoots biomass, (c) chlorophyll a, (d) chlorophyll b, (e) carotenoids, (f) Technosol respiration, (g) LP in roots, (h) LP in shoots in MTTSTr, MTTSTrF1, and MTTSTrF2 treatments, (i) TKN in roots, (j) TKN in shoots, (k) P in roots, (l) P in shoots, (m) Cu in roots, (n) Cu in shoots, (o) Pb in roots, (p) Pb in shoots in MTTSTr, MTTSTrF1, and MTTSTrF2 treatments.
Explaining the effects of increasing innoculation
Correlations between plant variables
After presenting the analytical results of the measured variables on the fungi inoculation gradient, we now present the correlations between variables within the framework of the embedding gradient of nitrogen availability and element toxicity. Figure 4 illustrates this for the aboveground parts of A. capillaris and M. albus. In the PCA biplots, a positive correlation is evident between biomass, TKN, P, and pigments in the aboveground parts of A. capillaris. N/P adds to this cluster of variables in the case of M. albus, while P becomes uncorrelated. For A. capillaris, Cu, Pb, and LP are positively correlated, and all three are negatively correlated with aboveground biomass. As a result of these multiple correlations, LP in the aboveground A. capillaris is positively predicted by Cu and negatively by TKN (forward stepwise multiple regression), and the aboveground biomass of M. albus is positively predicted by N/P and negatively by Cu concentration in the plant (although Cu and Pb could not be included in the PCA for this species, as their communality was < 0.5). The scattergrams of the scores of the pots on the main extracted factors shows that the correlations are due to the clustered distribution of the experimental treatments, which smoothly distribute from the tailing material (TM) to the Technosols gradually improved with topsoil, green fertilizer and fungi, and to the topsoil, in which the plants have variables comparable with the in the most complex Technosol (MTTSTrF2).
Most of the differences between the treatments observed in the scattergrams of the pots’ scores from Fig. 4 are statistically significant (p < 0.05) when analyzed as raw data (Fig. 2 Supp. Inf), with the associated text.
After three months of plant development, a microscopic examination of the roots confirmed the symbiosis between Rhizophagus irregularis and the plant species used. Mycorrhizal colonization was detected in plant roots at varying intensities (M%) and arbuscule abundances (A%), as shown in Suppl. Inf. Figure 3 left and right. However, no statistically significant differences were observed between the 1% and 2% fungal inoculation treatments, both of which exhibited good colonization rates.
Biplots of PCA main factors (left) and scatterplots of case scores (corresponding to the pots of the experimental treatments (right) extracted for the plant variables measured in the aboveground parts of A. capillaris (above) and of M. albus (below). We have mentioned in the biplot a multiple regression equation (with standardized coefficients) that predicts the LP and biomass. The legends of the treatments are as shown in Fig. 2, and the legends of the variables are as described in the Methods section.
Differences in soil properties between treatments and in time
The results of a PCA of soil variables and total biomass of plants at the end of the experiment are presented in Fig. 5. One can notice the positive correlation between soil respiration, biomass, ammonium, and nitrate. There is no negative correlation between biomass and total Cu and Pb in the soil. However, when the topsoil is excluded from the analysis, a slight decrease in the concentrations of these elements is observed from the tailing material to the most complex Technosol (MTTSTrF2). The scattergram of the scores for the pots shows a clear separation between the topsoil (uncontaminated) and the Technosols. The scores of the pots with Technosols are distributed as expected on the gradient of enrichment with nitrogen (by the added topsoil and green fertilizer). Another effect that can be observed is the separation between the scores of pots with one species and those with two species, particularly in the case of several treatments (MT, MTTS, MTTSTr, MTTSTrF1), indicating a positive effect of on total biomass, soil respiration, and ammonium and nitrate levels in the soil.
Biplots of PCA main factors (inserted) and scatterplot of case scores (corresponding to the pots of the experimental treatments) extracted for the soil variables measured at the end of the experiment, and from the total biomass in each pot. The biomass was separately standardized for the pots containing a single species and those containing two species. We have mentioned in the inserted biplot a multiple regression equation (with standardized coefficients) that predicts Technosol respiration. The legends of the treatments are shown in Fig. 2. Triangles indicate pots with one species, and circles indicate pots with two species. The legends for the variables are described in the Methods section.
Another fact is that statistically significant differences existed between the wet tailing substrate and Technosol at the end of the experiment, after harvesting the plants (see the measured variables Suppl. Inf. in Tables 1 and 2, and boxplots in Fig. 6 and Suppl. Inf. Figure 4). There is a strong decrease in pH, an increase in the EC, and an increase in ammonium (Fig. 6). The change in nitrate is more complex, with a decrease in the case of the dry tailing material and a further increase in the Technosols. One can notice that the amendments and the fungi have significantly influenced the outcome of drying the wet tailing material.
Adding topsoil and green fertilizers to the tailing material decreased the concentrations of elements such as Cu, Pb, Zn, and Ni (see Tables 1 and 1 Suppl. Inf). Consequently, the concentrations of these elements were lower in the amendments than those in Technosol (see Table 1). This was associated with a reduced transfer of toxic elements in the aboveground parts, coupled with their immobilization in the roots (Fig. 5 Suppl. Inf., which also shows the influence of inoculation on these patterns).
Box plots showing the range, the 25–75% interval, and the median of selected variables in the sampled tailing material (MT0, n = 15) and the experimental substrates (n = 10, including both the pots with one and with two species). Letters show the significant differences (p < 0.05) with the Mann-Whitney test. The legend of the treatments is as in Fig. 2.
We also investigated changes in the mineralogy of the tailing material and Technosols, but did not observe any changes at the timescale of our experiment. The Technosol included the following categories of mineral species: sulfides, silicates, oxides-hydroxides, sulfates, phosphates, and carbonates. Silicates are the most prevalent class of minerals in the studied samples, constituting over 85% of the total. Quartz, sodium feldspar (albite), and potassium feldspar (orthoclase/microcline) are the most dominant silicates. Albite crystals often exhibit zoning, and some show incipient kaolinization (transformation into kaolinite). Potassium feldspar frequently occurs in association with albite, forming perthitic textures. Most copper sulfides were found to be associated with potassium feldspar. Biotite and muscovite are present in all the studied samples, often occurring as long prismatic crystals, sometimes containing Ti oxides (Suppl. Inf. Figure 6 left). The fixed metal-containing forms in the Technosol primarily consisted of non-oxidized sulfides, which make up the mineralogical composition of the exploited deposits. During the short-term duration of this experiment, these minerals remained unchanged. However, oxidation processes may occur over time, potentially increasing the bioavailability of metals for plant uptake. A detailed description of the minaralogy is provided in the Suppl. Inf. Figure 6 and the associated text.
Discussions
The effect of increasing inoculation with fungi
The total biomass was significantly higher when we introduced a mixture of plant species, A. capillaris and M. albus (Fig. 3). This occurred due to the biomass of M. albus, despite the relative decrease of the biomass of A. capillaris under interspecific competition. The most substantial decrease of A. capillaris biomass as a result of competition was in the topsoil (by 55%), and the smallest in the mine tailing (by 11%), with intermediate percentages in the amended mine tailing treatments (by 25–38%, Fig. 3). Soil nutrient conditions influence the competitiveness of A. capillaris. In their works36, Minden and Venterink found that A. capillaris was a poor competitor under P-limitation conditions. We did not observe such effects in our toxic environment; however, A. capillaris performed better in stress-tolerant conditions, which aligns with the results of Liu and his colleagues37. In a study by38, the authors combined A. capillaris with Trifolium pratense in a unique experiment, demonstrating that the leguminous species outcompeted A. capillaris, particularly by accessing nitrogen, rather than through competition for light. A similar mechanism might be responsible for the success of M. albus in our experiment. M. albus is an annual or at most biennial nitrogen-fixing legume39 with a taproot and a well-branched stem, which can reach a height of 75–200 cm40, being thus characterized by high productivity. We can hence conclude that, in the present experiment, these specific characteristics of the M. albus led to an increase in total biomass production. The use of this species had a dual role: on the one hand, it released N from the root nodules, and on the other hand, it enriched the soil with organic matter resulting from the decomposition of a large amount of plant material. The development of A. capillaris species in mixtures (as indicated by chlorophyll a and b, carotenoids, lipid peroxidase, total Kjeldahl nitrogen, and phosphorus, Fig. 3, Suppl. Inf. Figure 2) was defective, both in treatments with topsoil only, with clover used as fertilizer, and with fungi, as well. developed spectacularly better in the substrate in which M. albus was initially sown.
Previous experiments reported significant differences in mycorrhizal colonization between 1% and 7% fungal inoculation10, but differences were not observed under the specific conditions of the present study (Supp. Inf. Figure 3). It is well established that the physicochemical properties of the soil have a significant effect on the AMF community, which in turn affects the germination of AMF spores and the infection of hyphae, ultimately altering the processes of element absorption and accumulation in plants41. Nitrogen-rich green fertilizers generally decrease soil pH and increase electrical conductivity42, a pattern also observed in this study. However, unlike chemical fertilizers, nitrogen-rich biofertilizers do not reduce AMF colonization, although soil pH remains the primary environmental factor affecting AMF community composition43. Acidic soils inhibit the growth and spore germination of AMF, thereby reducing their functionality37, whereas the effects of mycorrhizae are more pronounced in alkaline soils44. Given the alkaline pH of the tailing material (Fig. 6, Suppl. Inf. Table 2), it is likely that pH was the primary factor preventing significant differences in mycorrhization between the 1% and 2% AMF inoculation treatments, although to some extent different accumulation factors and transfer factors of Cu and Pb between the treatments exist (Suppl. Inf. Figure 5), suggesting the complexity of the phenomena in the rhizosphere.
It is known that excess copper in plants can affect photosynthesis by disrupting pigment composition and chloroplast structure, which in turn leads to changes in thylakoid membrane composition45. From the perspective of heavy metal pollution in plants, the two forms of metals - mobile and fixed - represent the danger of both short-term and long-term pollution that plants suffer46,47. In this study, Cu was absorbed in excessively high concentrations, leading to specific adaptive responses in plants. Plants employ various biochemical mechanisms to counteract oxidative stress induced by reactive oxygen species (ROS)45. Significant differences were observed between experimental treatments in terms of assimilatory pigment content, TKN, and LP (Fig. 3, Suppl. Inf. Figure 2). In microbial-assisted phytoremediation, the existing microbial pool in the mine tailings and amendments can interact with the inoculated microorganisms, potentially influencing soil biodiversity and plant-microbe interactions48.
Correlations between plant variables
Oxidative stress is typically increased by toxic elements and decreased by improvements in the nutritional status of plants49, which aligns with the findings reported here (Fig. 4). Additionally, inoculation with fungi is known to potentially decrease the transfer of elements in aboveground biomass10. LP in plants was correlated with soil pollution gradients in the field50, but such patterns have not been investigated in Technosols until now, particularly in the context of tailing dam remediation. Fertilization of tailings may mitigate the effects of toxic elements on plant oxidative stress51. We found, as demonstrated by the multiple regression predicting LP and biomass based on the full gradient (Fig. 4), and by the significant differences between treatments on the inoculation gradient, that both nitrogen and Cu control plant performance.
Differences in soil properties between treatments and over time
The differences in the properties of the Technosols at the end of the experiment (Figs. 5 and 6, Suppl. Inf. Figure 4) are typical of those observed after amending them with topsoil and green fertilizers10,16. We are not aware of previous work using wet tailings as input material in such experiments. Existing literature, for instance, examines the effect of wet-dry cycles on mechanical properties52 and the disposal of wet tailings53. It is reasonable to assume that the decrease in pH (Fig. 6) was due, in our case, to the weathering of sulfides54, which are present in the tailing material, although we did not observe quantitative differences in the mineralogy when we compared the wet tailings with the dry tailing material at the end of the experiment (Suppl. Inf. Figure 6). The short timescale of the experiment precluded a further decrease in pH, which is usually associated with acid mine drainage production55.
Applied issues
The costs associated with biotic and abiotic materials used in phytoremediation techniques are recognized technological challenges in any eco-technology that uses microorganisms48. In the myco-phytostabilization technique applied in the present study, an attempt was made to optimize the growth conditions of the fungi by applying inoculations in tiny quantities. Thus, although the cost per pot was low, the approach is scalable for field applications without prohibitive expenses, ensuring the technology’s long-term efficacy. As with natural attenuation, the evaluation of phytoremediation effectiveness is specific to each contaminated site, with the eventual correction of the remediation technology used taking into account the characteristics of the substrates and contaminants cited by5. The present study was conducted using an eco-technology previously tested on two tailings ponds of similar geochemistry and mineralogy56, and now applied at the pot scale on a third, still-active pond. The cost-effectiveness of the technique at a very large scale (e.g., one square kilometer, as is the case of the still active Valea Seșei dam) will depend on the cost-effectiveness of the method for producing the inoculum (Fig. 7). One can notice that the approach of increasing the production of ES is nested, one level targeting the acceleration of ecological succession at vegetation patch scale (as reported in this article), and another level working on the distribution of vegetation patches on the surface of the whole tailing dam57.
The further maturation of technology, currently being developed to technology readiness level 4 (TRL4, Suppl. Inf. Figure 7), will involve optimizing critical resources and processes (in orange in Fig. 7), including the fungi inoculum. Current providers offer lower prices as the quantity of inoculum increases; however, they have not yet secured contracts for mining remediation at this scale, leaving the pricing issue unresolved. An alternative option is to internalize the production inoculum for the project in-house58, which may be particularly interesting when native strains from the area are used. These engineering problems of upscaling are to be solved at the TRL5-6 scale by a business-academic cooperation.
An attempt at cost optimization involved using raw alfalfa instead of clover as a green fertilizer (Suppl. Inf. Figure 8); however, biomass production was significantly lower. This reduction was likely due to the excessive release of nutrients from the decomposition of alfalfa, which may have diminished the mycorrhizal benefits of AMF. This, in turn, prevented mycorrhizal symbiosis and reduced its ability to limit metal uptake, as reported by59. To optimize alfalfa as a green fertilizer, further studies are necessary to determine the appropriate amount that can be effectively applied. Another potential optimization, to be investigated, could be to use successive M. albus and A. capillaris crops instead of seeding them together60.
To extrapolate the entire eco-technology to sites with different geochemical, mineralogical, climatic, and ecological “fingerprints,” one would have to reiterate the technological development processes from experimental pot work to field plots and the corresponding field investigations. However, the template of the block diagrams from Suppl. Inf. Figure 7 and the process diagram from Fig. 7 can offer inspiration.
Conclusions
The inoculation of mine tailings with 2% fungi leads to significantly better effects in terms of plant biomass and lower LP than 1% and 0%, both when growing one species, and two plant species. The explanation relies on improving the nutrition of the plant, in our case, mostly related to nitrogen (as TKN in plants), as well as the N/P ratio and the decrease of Cu and Pb in plants. This improvement was related to significant differences between the Technosols properties at the end of the experiment (pH, EC, N-NH4+, N-NO3−), which modulated the changes of the tailing material properties from the wet to the dry state, and to differences in the accumulation factors of Cu and Pb from substrate to plant roots, and of the transfer factors from roots to aboveground parts.
Due to the strong interaction between the physicochemical properties of the mining substrate and AMF, fungal colonization can differ from one contaminated area to another, even when the same plant species and amendments are used. Therefore, additional laboratory research is required for each contaminated site when initiating a phytoremediation technology. This approach facilitates a deeper understanding and optimization of the conditions within mine tailings substrates, ensuring that AMF inoculation results in maximal mycorrhizal colonization and a more substantial reduction in plant elemental concentrations. Additionally, assessing the physicochemical properties of the mine tailing enables the evaluation of pH correction requirements and the determination of appropriate amendment quantities, taking into account the initial nutrient composition of the mining substrate.
The present study confirms that the myco-phytotechnology previously developed by our research team has been successfully applied at the laboratory scale under the experimental conditions presented in this article. Moreover, both 1% and 2% fungal inoculations produced similar results, with no statistically significant difference between the two treatments. This finding is particularly advantageous, as using 1% fungal inoculation reduces overall costs without compromising effectiveness. The results can be extrapolated to other tailing dams with similar geochemistry, mineralogy, and ecological settings. The entire technological process can be adapted to tailing dams with varying characteristics using the suggested block diagrams.
Data availability
The data is available for download in Excel format at https://zenodo.org/records/15847780.
References
EUROSTAT. (European Commission. (2021). https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics
Araujo, F., Taborda-Llano, I., Nunes, E. & Santos, R. Recycling and reuse of mine tailings: A review of advancements and their implications. Geosciences 12 https://doi.org/10.3390/geosciences12090319 (2022).
Gairola, S. U., Bahuguna, R. & Bhatt, S. S. Native plant species: a tool for restoration of mined lands. J. Soil. Sci. Plant. Nutr. 23, 1438–1448. https://doi.org/10.1007/s42729-023-01181-y (2023).
Borbón-Palomares, D. B., González-Méndez, B., Loredo-Portales, R. & Tinoco-Ojanguren, C. Molina-Freaner, F. Phytostabilization alternatives for an abandoned mine tailing deposit in Northwestern Mexico. Plant. Soil. 497, 199–218. https://doi.org/10.1007/s11104-023-06095-3 (2023).
Wang, J. & Aghajani Delavar, M. Techno-economic analysis of phytoremediation: A strategic rethinking. Sci. Total Environ. 902, 165949. https://doi.org/10.1016/j.scitotenv.2023.165949 (2023).
Rosa, J. C. S., Morrison-Saunders, A., Hughes, M. & Sánchez, L. E. Planning mine restoration through ecosystem services to enhance community engagement and deliver social benefits. Restor. Ecol. 28, 937–946. https://doi.org/10.1111/rec.13162 (2020).
Luo, Y., Xing, R., Wan, Z. & Chen, Y. Vertical distribution of nutrients, enzyme activities, microbial properties, and heavy metals in zinc smelting slag site revegetated with two herb species: implications for direct revegetation. Sci. Total Environ. 879, 163206. https://doi.org/10.1016/j.scitotenv.2023.163206 (2023).
Iordache, V. In Plant Microbiome Paradigm 191–230 (Springer, 2020).
Morel, J. L. & Heinrich, A. B. SUITMA—soils in urban, industrial, traffic, mining and military areas. J. Soils Sediments. 8, 206–207. https://doi.org/10.1007/s11368-008-0023-3 (2008).
Neagoe, A. et al. Effects of arbuscular mycorrhizal fungi on agrostis capillaris grown on amended mine tailing substrate at pot, lysimeter, and field plot scales. Environ. Sci. Pollut Res. Int. 21, 6859–6876. https://doi.org/10.1007/s11356-013-1908-2 (2014).
Ahmed, N. et al. Symbiotic synergy: how arbuscular mycorrhizal fungi enhance nutrient uptake, stress tolerance, and soil health through molecular mechanisms and hormonal regulation. IMA Fungus. 16, e144989. https://doi.org/10.3897/imafungus.16.144989 (2025).
Zhang, M., Shi, Z., Lu, S. & Wang, F. A. M. F. Inoculation alleviates molybdenum toxicity to maize by protecting leaf performance. J. Fungi (Basel). 9. https://doi.org/10.3390/jof9040479 (2023).
Ahmed, N. et al. Optimizing the dual role of Biochar for phosphorus availability and arsenic immobilization in soils. Sci. Total Environ. 957, 177810. https://doi.org/10.1016/j.scitotenv.2024.177810 (2024).
Sabra, M. et al. Beneficial root endophytic fungi increase growth and quality parameters of sweet Basil in heavy metal contaminated soil. Front. Plant. Sci. 9, 1726. https://doi.org/10.3389/fpls.2018.01726 (2018).
Ziqiang, A. et al. Application of phytostabilization technology in remediation of metal mine wasteland. J. Landsc. Res. 10, 65 (2018).
Constantinescu, P. et al. Implications of Spatial heterogeneity of tailing material and time scale of vegetation growth processes for the design of phytostabilisation. Sci. Total Environ. 692, 1057–1069. https://doi.org/10.1016/j.scitotenv.2019.07.299 (2019).
Zine, H. et al. Wild plants for the phytostabilization of phosphate mine waste in Semi-Arid environments: A field experiment. Minerals 11 https://doi.org/10.3390/min11010042 (2020).
Parraga-Aguado, I., Gonzalez-Alcaraz, M. N., Alvarez-Rogel, J., Jimenez-Carceles, F. J. & Conesa, H. M. The importance of edaphic niches and pioneer plant species succession for the phytomanagement of mine tailings. Environ. Pollut. 176, 134–143. https://doi.org/10.1016/j.envpol.2013.01.023 (2013).
Zedníková, P., Kukla, J. & Frouz, J. The growth, competition, and facilitation of grass and legumes in Post-mining soils. J. Soil. Sci. Plant. Nutr. 23, 3695–3704. https://doi.org/10.1007/s42729-023-01290-8 (2023).
Sun, C., Liu, G. & Xue, S. Interaction between plant competition and rhizospheric bacterial community influence secondary succession of abandoned farmland on the loess plateau of China. Front. Plant. Sci. 9, 898. https://doi.org/10.3389/fpls.2018.00898 (2018).
Wang, L., Ji, B., Hu, Y., Liu, R. & Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 184, 594–600. https://doi.org/10.1016/j.chemosphere.2017.06.025 (2017).
Von Alten, H., Blal, B., Dodd, J., Feldmann, F. & Vosatka, M. In Mycorrhizal Technology in Agriculture: from Genes To Bioproducts281–296 (Springer, 2002).
Cioacă, M. E., Munteanu, M., Qi, L. & Costin, G. Trace element concentrations in porphyry copper deposits from metaliferi mountains, romania: A reconnaissance study. Ore Geol. Rev. 63, 22–39. https://doi.org/10.1016/j.oregeorev.2014.04.016 (2014).
Schinner, F., Öhlinger, R., Kandeler, E. & Margesin, R. Methods in Soil Biology (Springer Science & business media, 2012).
ISO11260:2018. Soil quality—determination of effective cation exchange capacity and base saturation level using barium chloride solution (2nd ed). International Organization for Standardization (ISO), Geneva, Switzerland. AFNOR Paris, (2018).
Neagoe, A., Ebenå, G. & Carlsson, E. The effect of soil amendments on plant performance in an area affected by acid mine drainage. Geochemistry 65, 115–129. https://doi.org/10.1016/j.chemer.2005.06.009 (2005).
Van Reeuwijk, M. & Sc, M. Efficient simulation of non-hydrostatic free-surface flow, thesis, Delft Univ. of Technology, Delft, Netherlands, (2002).
Warr, L. N. IMA–CNMNC approved mineral symbols. Mineral. Mag. 85, 291–320. https://doi.org/10.1180/mgm.2021.43 (2021).
Schopfer, P. Experimentelle PflanzenphysiologieVol. 2 (Springer-, 1989).
Hodges, D. M., DeLong, J. M., Forney, C. F. & Prange, R. K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207, 604–611 (1999).
Hudek, C., Sturrock, C. J., Atkinson, B. S., Stanchi, S. & Freppaz, M. Root morphology and Biomechanical characteristics of high altitude alpine plant species and their potential application in soil stabilization. Ecol. Eng. 109, 228–239. https://doi.org/10.1016/j.ecoleng.2017.05.048 (2017).
Trouvelot, A., Kough, J.L. & Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methods d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae (eds. Gianinazzi-Pearson, V. & Gianinazzi, S.) INRA, Paris, 217–221 (1986).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in python. Nat. Methods. 17, 261–272. https://doi.org/10.1038/s41592-019-0686-2 (2020).
Charlier, F. et al. trevismd/statannotations: v0.6 (v0.6.0). Zenodo. (2023). https://doi.org/10.5281/zenodo.8396665
Waskom, M. Seaborn: statistical data visualization. J. Open. Source Softw. 6 https://doi.org/10.21105/joss.03021 (2021).
Minden, V., Venterink, O., Stevens, C. & H. & Plant traits and species interactions along gradients of N, P and K availabilities. Funct. Ecol. 33, 1611–1626. https://doi.org/10.1111/1365-2435.13387 (2019).
Liu, X., Feng, Z., Zhao, Z., Zhu, H. & Yao, Q. Acidic soil inhibits the functionality of arbuscular mycorrhizal fungi by reducing arbuscule formation in tomato roots. Soil. Sci. Plant. Nutr. 66, 275–284 (2020).
Miranda-Apodaca, J., Mena-Petite, A., Lacuesta, M. & Munoz-Rueda, A. Perez-Lopez, U. A physiological approach to study the competition ability of the grassland species trifolium pratense and agrostis capillaris. J. Plant. Physiol. 254, 153284. https://doi.org/10.1016/j.jplph.2020.153284 (2020).
Bozhanska, T., Mihovski, T., Naydenova, G., Knotová, D. & Pelikán, J. Comparative studies of annual legumes. Biotechnol. Anim. Husb. 32, 311–320. https://doi.org/10.2298/bah1603311b (2016).
Annaeva, M. et al. in IOP Conference Series: Earth and Environmental Science. 012170 (IOP Publishing).
Garcia, K. G. V., de Souza Oliveira Filho, J. & de Araújo Pereira, A. P. Mendes filho, P. F. Can inoculation of native arbuscular mycorrhizal fungi from a mining area attenuate stress of acacia mangium willd. To excess manganese? J. Soils Sediments. 24, 3252–3264. https://doi.org/10.1007/s11368-024-03874-y (2024).
Liu, L. et al. Unraveling fertilization effects on the dynamics of arbuscular mycorrhizal fungal community in the Qinghai-Tibet alpine meadow. Soil. Ecol. Lett. 6 https://doi.org/10.1007/s42832-024-0248-0 (2024).
Xiao, D., Tang, Y., Zhang, W., Hu, P. & Wang, K. Lithology and niche habitat have significant effect on arbuscular mycorrhizal fungal abundance and their interspecific interactions. Sci. Total Environ. 919, 170774. https://doi.org/10.1016/j.scitotenv.2024.170774 (2024).
Farghaly, F. A., Nafady, N. A. & Abdel-Wahab, D. A. The efficiency of arbuscular mycorrhiza in increasing tolerance of triticum aestivum L. to alkaline stress. BMC Plant. Biol. 22, 490. https://doi.org/10.1186/s12870-022-03790-8 (2022).
Mir, A. R., Pichtel, J. & Hayat, S. Copper: uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometals 34, 737–759. https://doi.org/10.1007/s10534-021-00306-z (2021).
Jianu, D. et al. in Bio-Geo Interactions in Metal-Contaminated Soils Soil Biology Ch. Chapter 3, 35–79 (2012).
Wang, N., Liu, Z., Sun, Y., Lu, N. & Luo, Y. Analysis of soil fertility and toxic metal characteristics in open-pit mining areas in Northern Shaanxi. Sci. Rep. 14, 2273. https://doi.org/10.1038/s41598-024-52886-8 (2024).
Mahabub, M. S., Alahi, F. & Al Imran, M. Unlocking the potential of microbes: biocementation technology for mine tailings restoration - a comprehensive review. Environ. Sci. Pollut Res. Int. 30, 91676–91709. https://doi.org/10.1007/s11356-023-28937-4 (2023).
Kovačević, M., Jovanović, Ž., Andrejić, G., Dželetović, Ž. & Rakić, T. Effects of high metal concentrations on antioxidative system in phragmites australis grown in mine and flotation tailings ponds. Plant. Soil. 453, 297–312. https://doi.org/10.1007/s11104-020-04598-x (2020).
Dazy, M. et al. Changes in plant communities along soil pollution gradients: responses of leaf antioxidant enzyme activities and phytochelatin contents. Chemosphere 77, 376–383. https://doi.org/10.1016/j.chemosphere.2009.07.021 (2009).
Cruz, F., Gomes, M. P., Bicalho, E. M. & Garcia, Q. S. Fertilization assures mineral nutrition but does not overcome the effects of Fe accumulation in plants grown in iron ore tailings. Environ. Sci. Pollut Res. Int. 29, 18047–18062. https://doi.org/10.1007/s11356-021-16989-3 (2022).
Wu, L. et al. Mechanical properties of tailings sample with different moisture contents under dry and wet cycles. Adv. Civil Eng. 2019 https://doi.org/10.1155/2019/9079754 (2019).
Oldecop, L. & Rodari, G. Unsaturated mine tailings disposal. Soils Rocks. 44, 1–12. https://doi.org/10.28927/sr.2021.067421 (2021).
Fausak, L. et al. Effect of wetting and drying processes on ultramafic and mafic tailing minerals amended with topsoil. Environ. Chem. 21 https://doi.org/10.1071/en23037 (2024).
Dold, B. Evolution of acid mine drainage formation in sulphidic mine tailings. Minerals 4, 621–641. https://doi.org/10.3390/min4030621 (2014).
Neagoe, A. Contributions to plant ecophysiology under heavy metals stress and to scaling for phytoremediation in contaminated areas (2024). https://doi.org/10.5281/zenodo.13684611 Habilitation thesis, University of Bucharest.
Iordache, V. in Invited Conference at Florida Gulf Coast University, (2025). https://doi.org/10.5281/zenodo.14833097 (10501 FGCU Boulevard South, Fort Myers, Florida 33965 – 6565, 5 February 2025.
Khan, A. & Mohammad, A. In Nanotechnology for Environmental Pollution Decontamination 469–484 (Apple Academic, 2022).
Ma, X. et al. Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality. New. Phytol. 229, 2957–2969. https://doi.org/10.1111/nph.17077 (2021).
Kumari, S. & Maiti, S. K. Nitrogen recovery in reclaimed mine soil under different amendment practices in tandem with legume and non-legume revegetation: A review. Soil Use Manag. 38, 1113–1145. https://doi.org/10.1111/sum.12787 (2022).
Acknowledgements
We thank the reviewers and the editor for their constructive criticism and insightful recommendations. This experiment was supported by a grant from the Romanian Ministry of Education and Research, CCCDI - UEFISCDI, project number PN-III-P2-2.1-PED-2019-2584, within PNCDI III RESET (https://resetbiogeochemistry.wordpress.com/), and data processing and interpretation were done within the project number 156 METTELFLUX (https://mettelflux.com/) PN-III-P4 -ID-PCE-2020-0494 funded by UEFISCDI, Romania, and the project number RO1567-IBB01/2025 of the Institute of Biology Bucharest of the Romanian Academy.
Author information
Authors and Affiliations
Contributions
A.N. established the experimental design, participated in the conduct of the experiment, interpreted the results, prepared the original draft, and participated in the acquisition of funding and technological development; M.M. participated in the running of the experiment, evaluated mycorrhizal colonization, and participated in writing and revising of the manuscript; M.O. participated in the monitoring of the experiment, and the revision of the article. L. S.M. participated in the experiment’s running, performed physicochemical analyses, and edited the references chapter. G.D. performed BSE and SEM analyses, participated in mineralogy description; D.J. participated in drafting the mineralogy participated in mineralogy description and technological development. S.I. processed the data and revised the article. V. I. sampled the mining substrate, acquired funding with Aurora Neagoe, and participated in processing data, writing, and revising the manuscript and technological development.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Neagoe, A., Manu, M., Onete, M. et al. Increasing the fungal inoculation of mine tailings from 1 to 2% decreases plant oxidative stress and increases the soil respiration rate. Sci Rep 15, 30030 (2025). https://doi.org/10.1038/s41598-025-14973-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14973-2