Abstract
Fodder production in saline environments requires salt-tolerant plants. This study investigated the potential of the halophyte Salicornia persica ecotypes as a fodder crop under seawater salinity by examining its physiological and biochemical responses. The effects of varying salinity levels [control (0.96 dS.m−1), and 10, 20, and 40 dS.m−1, achieved by diluting Persian Gulf water] on growth, yield, stomatal exchange rate, photosynthetic traits, and qualitative fodder characteristics were evaluated. Three S. persica accessions collected in Iran (Central Plateau, Urmia, and Bushehr) were included. The results showed that, among the tested ecotypes, Central Plateau and Urmia exhibited the most desirable interaction with the 10 dS.m−1 salinity treatment, highlighting a beneficial combination of ecotype and salinity level. Regarding growth characteristics, plant height and forage yield were highest at 10 dS.m−1 and lowest at 40 dS.m−1 salinity. In terms of forage quality, the Bushehr accession under non-stress conditions and the Central Plateau accession at 20 dS.m−1 exhibited the highest nitrogen and crude protein percentages. The 10 and 20 dS.m−1 salinity treatments displayed more favorable forage quality profiles, whereas the 40 dS.m−1 treatment resulted in elevated fiber and Acid Detergent Fiber (ADF) percentages, potentially reduces fodder palatability for livestock. These findings suggest that the Central Plateau and Urmia ecotypes demonstrate significant potential for forage production in saline environments. These ecotypes are a promising option for cultivation in coastal areas, particularly with irrigation using Persian Gulf seawater at a salinity of 10–20 dS.m−1.
Similar content being viewed by others
Introduction
Salinity, a pervasive environmental constraint, poses significant challenges to plant growth and development throughout their life cycle1,2. The widespread occurrence of saline soils, particularly in arid and semi-arid regions where traditional crops often struggle, coupled with diminishing freshwater resources for agricultural use, has spurred investigations into halophytes. These resilient plants, capable of thriving in high-salinity environments, are becoming increasingly valuable, and studies are focused on elucidating their tolerance and adaptive mechanisms under conditions of soil salinity or saline irrigation3,4,5.
Salicornia, recognized as one of the most salt-tolerant plants, can be successfully cultivated using seawater irrigation6,7. Globally, approximately 25 to 30 species of Salicornia have been identified, with S. persica being a native halophyte of Iran8,9. (Belonging to the Canopodiaceae family, S. persica is a succulent, annual C3 plant characterized by reduced leaves and a terminal spike-like inflorescence7,10. This species, a vital genetic resource, naturally inhabits the coastal and saline marshlands of Northwest, central, and Southern Iran11. Its diverse applications include fresh vegetable production, use in cosmetic, health, and pharmaceutical sectors, incorporation into livestock feed rations, and the extraction of high-quality seed oil6,8. Research on Salicornia in Iran indicates that the species S. persica and its ecotypes, which have adapted to the saline soils of the central regions and the saltwater of the southern parts of the country, are distinct from S. europaea and S. bigelovii12. The Bushehr ecotype of S. persica, which is primarily found in the Bushehr and Khuzestan provinces and along the Persian Gulf coast, is a diploid, annual plant adapted to the less salty waters of coastal rivers. The Urmia ecotype is also an annual plant that grows prostrate on the ground and is found among populations of S. persica and S. europaea. It is believed to have originated from a hybridization of these two species. This ecotype is capable of growing in the extremely saline soils around Lake Urmia in West and East Azerbaijan provinces. Among the S. persica ecotypes, the Central Plateau ecotype is unique in its flowering stage. Its canopy can reach approximately 80 cm. In dense stands, the plant grows upright, while in isolated plants, the stem tends to be curved. This ecotype is highly tolerant of extremely saline and marshy conditions. It is most commonly observed in the dry and very saline soils of Yazd, Isfahan, and Fars provinces13.
While Salicornia species demonstrate exceptional salinity tolerance, understanding their phenological development under both non-stressed and stressed conditions is critical for optimizing germination, establishment, and ultimately, sustainable economic yields14,15. A major obstacle in the successful cultivation of Salicornia under high-salinity conditions is the tendency for premature flowering, which curtails vegetative growth as plants grapple with the toxicity of elements such as Na+ and Cl− ions16. The transition from the vegetative to reproductive phase in Salicornia, marked by internode development and elongation, is intricately linked to environmental cues, genetics, and species-specific traits17. This shift is mediated by interactions between environmental signals and alterations in endogenous hormone levels, an attribute that varies considerably among different species9,16. Nevertheless, the interplay of genetic factors (species or ecotype) and indirect effects of salinity, such as reductions in stomatal conductance and photosynthetic capacity, inevitably leads to decreased forage yield and quality14.
Studies on S. bigelovii were conducted in Mexico from 1982 to 1988. During this six-year period, researchers found that the plant, when irrigated with seawater, produced seed yields ranging from 1.3 to 2.4 ton.ha−1. This is a comparable yield to what soybeans produce in non-saline environments. The study also documented significant biomass production for this species. Within a 200-day growing period, S. bigelovii yielded a biomass of 12.7 to 24.6 ton.ha−118. Other studies on S. herbacea and S. brachiata reveal how saline stress affects their growth. Researchers found significant changes in the plants’ phenology, including parameters such as net assimilation rate (NAR), specific leaf area (SLA), and leaf area ratio (LAR), during different growth phases. The studies also noted alterations in the plants’ shoot and root biomass and nutrient content19,20. According to research by Ranjbar et al.21, S. bigelovii’s ability to grow in high-salinity conditions is due to its effective ion management. The plant actively isolates excess Na+ by moving the ions into cell vacuoles, which helps maintain cell turgor pressure and prevents toxicity. Specialized antiporter genes also help prevent more Na+ from entering the cell, allowing the plant to thrive even when faced with high levels of Na+ and Cl− and reduced levels of K+ and Ca2+22,23. A study by Bozorgmehr et al.24 examined the plant’s biochemical response to saltwater irrigation. Their findings showed that the content of nitrogen, crude protein, fat, and ash was higher in saline-treated plants than in controls. This suggests that the increased nutrient content could be the reason for the improved fodder quality. Collectively, these findings highlight distinct physiological mechanisms governing responses to salinity and underscore ecotypic variations within Salicornia, necessitating further in-depth analysis14.
Therefore, our central hypothesis is that S. persica ecotypes, as highly salt-tolerant plants, can serve as a valuable strategic crop in coastal regions, facilitating the sustainable production of high-quality livestock fodder in arid and semi-arid landscapes. This study, therefore, aimed to examine: (1) The phenological responses of different S. persica ecotypes to salinity stress during the transition from vegetative to reproductive phases; (2) The relationship between salinity tolerance and stomatal exchange and photosynthetic characteristics among the ecotypes; and (3) The pattern of Na+ and K+ accumulation in shoots, along with the evaluation of ash and fodder quality of the ecotypes under varying salinity levels using diluted Persian Gulf seawater.
Materials and methods
Plant materials, experimental design and growing conditions of S. persica ecotypes
S. persica ecotype seeds were procured from the National Salinity Research Center of Iran during the summer of 2020. These ecotypes, originating from three distinct geographical regions (Central Plateau, Urmia, and Bushehr), were subjected to a salinity tolerance assessment on January 9, 2021, at the experimental research farm of the Department of Plant Production and Genetics, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran. To establish variable salinity concentrations, diluted seawater from the Persian Gulf was employed. The experimental design followed a split-plot arrangement within a randomized complete block design, with three replicated. Main plots encompassed four salinity treatments: a control (utilizing potable tap water with an electrical conductivity of 0.96 dS.m−1) and 10 dS.m−1, 20 dS.m−1, and 40 dS.m−1 using diluted seawater from the Persian Gulf. The three S. persica ecotypes were randomly assigned to subplots within these main plots. Figures (1, A) illustrates the geographical origins of the S. persica ecotypes studied. Figures (1, B) provides the regional and geographical details of the collection sites and the cultivation location for these S. persica ecotypes, including latitude, longitude, and altitude. In addition, Figure (1, C) shows the meteorological data collected during the research period. Table (1) outlines the physical and chemical properties of the soil at the experimental site. Lastly, Table (2) presents the quality characteristics of the irrigation water used in the experimental area.
Land preparation and planting procedures
Land preparation involved primary tillage, using a moldboard plow in autumn, followed by secondary tillage utilizing a disc harrow in early January 2021. Fertilization procedures were executed based on soil test results and the nutritional requirements of Salicornia, employing a basal application of phosphorus fertilizer from superphosphate triple (100 kg.ha−1), potassium fertilizer from potassium chloride (50 kg.ha−1), and nitrogen fertilizer from urea (250 kg.ha−1 applied in four stages; 100 kg.ha−1 as a basal application and three top-dressings of 50 kg.ha−1 at 30, 60, and 90 days post-planting)25. Subsequently, experimental plots, measuring 3 m in length and 2 m in width, were delineated, with a 50 cm distance between blocks and a 1 m separation between main plots. Each plot contained 5 planting rows, spaced 30 cm apart. Given the sensitivity of Salicornia to the planting substrate, a furrow, 10 cm in depth and 5 cm in width, was prepared in each planting row and filled with washed sand. Subsequently, surface planting of 2 mg of seeds disinfected with Carbendazim fungicide was performed in each planting line at a shallow depth of 0.25 to 0.5 cm. Weed control was achieved through manual removal, performed four times during the growing season, with the intensity dependent upon the weed density.
Irrigation system design and salinity treatment application method
A drip irrigation system was implemented for this study. For the storage and supply of irrigation water, four horizontal polyethylene tanks, each with a 2000-liter capacity, and two mother tanks, with a total capacity of 12,000 L (comprising one 10000-liter and one 2000-liter tank), were utilized. Seawater from the Persian Gulf (with an EC of 77 dS.m−1), transported from Mahshahr Port (is a city in the southern district of Iran), was employed as the saline water source and stored within the mother tanks throughout the duration of the experiment. To create varied salinity levels, four distinct reservoirs were used. Each salinity level was achieved by mixing Persian Gulf seawater from the mother tanks with treated tap water within the corresponding reservoir. Salinity was monitored and adjusted using an EC-meter portable. Specifically, the first reservoir held tap water for the control treatment, while reservoirs two, three, and four were designated for salinity levels of 10, 20, and 40 dS.m−1, respectively. The drip irrigation system consisted of a main valve, a pump (a one-horsepower centrifugal pump with a maximum head of 33 m, a flow rate of 6.3 m3.h−1, and a 220-volt single-phase power supply), four screen filters installed at the inlet of each sub main line of each salinity level (with 2-inch inlet and outlet connections, capable of filtering particles larger than 130 microns), main pipelines connected to each tank, four flow meters (1-inch inlet/outlet with a measuring accuracy of 100 ml) for measuring the irrigation volume at each salinity level, valves for the sub-main lines, and five drip tapes per plot, each 3 m long (200-micron thickness, 20-cm outlet spacing, operating pressure of 0.6 to 1 atm, and a flow rate of 2 liters per hour) aligned with the planting rows. Irrigation with potable tap water commenced immediately post-planting and was maintained to ensure soil moisture until seedling establishment. Following seedling establishment, thinning of excess plants was performed using scissors at the plant collar (point of seedling emergence) to achieve a density of 17 plants/m226,27. Subsequently, the salinity treatments were introduced. To mitigate abrupt salinity shock, salinity levels were introduced gradually, with each irrigation event increasing salinity by 5 dS.m−1. At the end of this research, root-zone electrical conductivity was monitored by preparing saturated extracts from the soil substrate28. Data from the sampling are shown in Figure (10, D, and 11, J, K, and L) for each salinity level in S. persica ecotypes. The volume of irrigation water applied was calculated for each salinity treatment from seedling establishment and salinity stress initiation (45 days after planting on February 23, 2021) until the end of the growth period (harvest on May 29, 2021) based on the water requirements of Salicornia. Soil samples were collected from the root zone (30 cm depth) before irrigation using an auger to measure soil moisture by the gravimetric method. Irrigation depth and volume were calculated based on the soil moisture deficit and root depth using Eq. (1) (with an irrigation cycle of 5 days).
In the aforementioned equation, dn represents the net depth of irrigation water, quantified in mm. The gravimetric soil moisture content at field capacity is denoted by , expressed as a percentage. Similarly, i signifies the gravimetric soil moisture content immediately preceding irrigation. The soil’s bulk density is given by ρb, measured in g.cm−3. Lastly, Zr represents the effective rooting depth, expressed in mm.
Furthermore, the gross irrigation depth was determined based on the leaching needs for different salinity levels from Eqs. (2, 3, and 4).
These equations involved several factors: LF, representing the leaching fraction; ECiw, the electrical conductivity of the irrigation water, measured in dS.m−1; and ECe, the electrical conductivity of the soil saturation extract (dS.m−1), which, according to Maas et al.29, is considered the point where plant death occurs and yield reaches zero. Based on germination and early seedling development experiments15,16, this threshold value was set at 70 dS.m−1 for the studied ecotypes. Also crucial for the calculations were: dg, the gross irrigation depth (mm); V, the volume of irrigation water (liters); A, the surface area of each plot (m2); and Ea, the irrigation water application efficiency30. A 95% application efficiency for drip irrigation was used, based on sources31. Ultimately, the total volumes of irrigation water from the seedling establishment phase and onward for the control and salinity treatment levels of 10, 20, and 40 dS.m−1 were calculated to be 3167, 3726, 5175, and 9315 m3.ha−1, respectively.
Experimental traits evaluated
Phenology and growth characteristics, plant height, and forage yield
To rigorously characterize the phenological development of various S. persica ecotypes, five principal growth stages were identified and monitored. These stages, as previously defined by Izadi et al.9, included: (1) seedling emergence; (2) vegetative node formation; (3) emergence of short, fertile, segments; (4) complete flowering cones and beginning of seed development; and (5) seed maturity and drying. In each experimental treatment, the progression through these phenological stages was closely monitored daily. When 50% of the plants within a given plot had reached a specific stage, the number of days from planting was recorded. Beyond this temporal measure, growing degree days (GDD) were also calculated to quantify the advancement of phenological phases. GDD was calculated using the following formula (Eq. 5):
Where, in the aforementioned equation: Tmax represents the daily maximum temperature; Tmin denotes the daily minimum temperature; and Tb signifies the base temperature for Salicornia, which represents the threshold below which plant growth is considered negligible. For the calculation of GDD across the growing season, days where the average daily temperature exceeded the maximum threshold (35 °C) or fell below the base temperature (5 °C) for Salicornia growth were excluded from the calculations, as established in the studies by Khan and Weber32 and Abdulrahman and Williams33.
Plant height was measured by averaging the height of ten randomly selected plants within each plot at full maturity, using a standard ruler. To determine the green area (GA), total dry matter (TDM), and plant growth rate (PGR), samples were collected from a 0.2 m2 area within each plot at five different developmental stages following plant establishment. Subsequently, after each sampling, the green area (GA) was quantified using a leaf area meter (Delta-T Devices MK2, Cambridge, UK). The harvested samples were then subjected to a forced-air convection oven at 72 °C for 48 h to ensure complete desiccation. Total dry matter (TDM) was determined using a digital scale with a precision of 0.001 g. Finally, plant growth rate (PGR) and the green area were computed, following methods described by Izadi et al.9. Additionally, forage yield at full maturity (phenological stage: seed maturity and drying) was measured by harvesting a 0.5 m2 area in each plot using a quadrat. The samples were immediately taken to the laboratory, where they were dried in a fan-assisted convection oven at 72 °C for 48 h. The forage yield was then measured to the nearest 0.01 gram and converted to tons.h−113.
Physiological responses and photosynthetic characteristics
Stomatal conductance (SC) was measured in S. persica ecotypes using a porometer (model AP4, Delta-T Devices, Cambridge, UK). The porometer was calibrated prior to measurements following the manufacturer’s direct calibration protocol34. Net photosynthetic rate (Pn), transpiration rate (T), and intercellular CO2 concentration (Ci) were determined on individual S. persica shoots using a portable infrared gas analyzer (Model LCA4; ADC BioScientific Ltd., Herts, UK). To minimize diurnal variation, data collection took place between 09:00 h and 13:00 h. Readings were recorded after a 15 min acclimation period under controlled conditions of 600 µmol.m−2.s−1 irradiance, 25 °C temperature, 380 µmol.mol−1 CO2 concentration, and 60% relative humidity35. These parameters (SC, Pn, T, and Ci) were assessed during the flowering stage on five randomly selected plants within each plot, ensuring accurate sensor placement. Carboxylation efficiency (CE) and photosynthetic water use efficiency (Pwue) were also calculated according to Eqs. (6 and 7)36.
Forage quality and nutritional value
To determine the quality and nutritional value of the forage, a 100 g sample was randomly selected from each treatment group at the 50% flowering stage. These samples dried in an oven at 70 °C for 48 h. Following the drying process, the samples were ground by an electric mill and subsequently sieved through a one mm screen. The resulting powdered samples were then analyzed to measure crude protein content (CP), which was determined by quantifying nitrogen (N) levels using the Kjeldahl method (Kjeltec device, Auto Analyzer) and then multiplying the nitrogen values by 6.2537. Ash content (Ash), representing the mineral fraction, was assessed through a proximate analysis, with samples being combusted in an electric furnace at 550 °C. Additionally, fiber components were evaluated, including crude fiber (CF), neutral detergent fiber (NDF), and acid detergent fiber (ADF), with the aid of a fiber analyzer (Fibertec System 1010 Heat Extractor). These procedures adhered to the methodologies detailed by Hanušovský et al.38.
Furthermore, the concentration of sodium (Na+) and potassium (K+) in the aerial parts of the plants was assessed at full maturity. The aerial samples were dried in an oven at 70 °C for 48 h. Subsequently, a 0.1 g portion of each milled sample was mixed with 10 ml of 0.1 normal glacial acetic acid. These prepared samples were then kept at room temperature for 24 h. In the next step, the samples were placed in a 70 °C water bath for 2 h to facilitate extraction, after which the extract was obtained using Whatman filter paper. Finally, Na+ and K+ concentrations were determined using flame photometry (Flame photometer, JENWAY, UK), according to the method outlined by Asch et al.39. The values obtained from the flame photometer were then used in an Eq. (8) to compute the ionic concentrations of Na+ and K+.
In this equation, A, represents the concentration of either sodium or potassium in mg/g shoot dry weight; Y, denotes the value derived from the standard curve; and W, signifies the initial dry weight. Subsequently, the Na+/K+ ratio was obtained by dividing the sodium concentration by the potassium concentration.
Statistical performance
The effects of the independent variables (salinity levels and ecotypes) on the various measured traits were evaluated through a two-way analysis of variance (ANOVA II). All data were analyzed using SAS software (version 9.4, SAS Institute Inst., Cary, NC), employing a split-plot model structured within a randomized complete block design that included three replications. Mean comparisons were conducted using the least significant difference (LSD) at a significance level of 5%. Additionally, curve fitting and graphical representations were generated using SigmaPlot software (version 14.0, SigmaPlot Systat Software, Chicago, USA). Subsequently, to explore the relationships between the measured traits and salinity levels within each ecotype, both linear and quadratic polynomial regression models were employed using SigmaPlot software. The coefficient of determination (R²) and root mean square error (RMSE) were utilized to evaluate the quality of the fit achieved by linear and quadratic polynomial functions.
This equation uses “Yest” to represent the predicted value generated by the model, “Yabs” for the observed value, and “n” for the total number of observations.
Lastly, to determine significant relationships between growth, physiological responses, photosynthetic characteristics, and forage quality parameters in S. persica ecotypes under salinity stress treatments, Pearson correlation coefficients, principal component analysis (PCA), and hierarchical cluster analysis were calculated using Origin Pro® software (version 2021, 9.5.0.193).
Results
Phenology and growth characteristics
The two-way analysis of variance (ANOVA II) revealed that salinity levels, ecotype, sampling time, and all their double and triple interactions significantly affected green area (GA), total dry matter (TDM), and plant growth rate (PGR) (Table 3).
Phenological analysis of the studied ecotypes showed that the shortest duration, based on accumulated growing degree days (GDD), occurred between sowing and seedling emergence across all ecotypes and salinity levels. Conversely, the longest phenological period was observed during seed maturity and drying, particularly under control (0.96 dS.m−1) and 10 dS.m−1 salinity treatments across all ecotypes (Fig. 2, A, B, and C).
Map collection (Yazd, Urmia and Bushehr) and cultivation site (Ahvaz) (A), regional and geographical coordinates of the collection and cultivation site (B) and the temperature, sunny hours and precipitation during the period of Salicornia persica ecotypes cultivation site in Ahvaz for 2021 year (C).
Phenological stages (A, B and C), (GA) green area (D, E and F), (TDM) total dry matter (G, H and I), and (PGR) plant growth rate (J, K and L) of S. persica ecotypes (Central plateau, Urmia and Bushehr) under salinity by using growing degree days. Each data point represents the average of three replicates, with vertical bars (where visible) indicating standard deviation (± S.D.) within replicates.
To further examine the growth responses of S. persica ecotypes, including GA, TDM, and PGR, samples were collected at each phenological stage for growth index estimations. Subsequently, these growth parameters were plotted against accumulated growing degree days (GDD) for each ecotype under different salinity levels. Initially, no difference was observed in GA between ecotypes at different salinity levels during the seedling emergence and vegetative node formation stages. However, differences in GA became evident during the emergence of short, fertile, segments stage. At this stage, the Central Plateau ecotype under 10 dS.m−1 and the Urmia ecotype under control demonstrated the highest GA (27843.9 and 27628.7 mm2, respectively, after accumulating 1072.0 and 1139.3 GDD, respectively). These values were not significantly different from each other. The lowest GA at this stage was observed in the Bushehr ecotype under 40 dS.m−1 (6324.7 mm2 after accumulating 962.3 GDD). During the complete flowering cones and beginning of seed development stage, the Central Plateau ecotype under 10 dS.m−1 had the highest GA, increasing by 40.5% compared to the previous stage (39125.6 mm2 after accumulating 1752.7 GDD). In contrast, the Bushehr ecotype under 40 dS.m−1 exhibited the lowest GA, which increased by 82.0% compared to the previous stage (11512.8 mm2 after accumulating 1602.0 GDD). Finally, at the seed maturity and drying stage, the highest GA was observed in the Central Plateau ecotype under control (40525.0 mm2 after accumulating 2905.1 GDD), a 7.0% increase compared to the previous stage, while the lowest was recorded in the Urmia ecotype under 40 dS.m−1 (8712.2 mm2 after accumulating 1727.5 GDD), which was a 32.8% decrease compared to the previous stage (Fig. 2, D, E, and F).
The trend of TDM accumulation showed similar values among all treatments at the seedling emergence stage. Subsequently, clear differences were observed across ecotypes under different salinity levels. At the vegetative node formation stage, the highest TDM accumulation was observed in the Central Plateau ecotype under 10 dS.m−1 (21.5 g.m−2 after accumulating 524.6 GDD), while the lowest values were recorded in the Bushehr ecotype under both 10 and 40 dS.m−1 (5.41 and 5.43 g.m−2 after accumulating 608.4 and 514.8 GDD, respectively) without significant difference between the two salinity levels. During the emergence of short, fertile, segments stage, the Urmia ecotype under 10 dS.m−1 exhibited the highest total dry matter (29.0 g.m−2 after accumulating 1161.4 GDD), showing a 76.8% increase compared to the previous stage. The Bushehr ecotype under 20 dS.m−1 had the lowest TDM (12.4 g.m−2 after accumulating 1102.0 GDD) at the same stage, with a 76.8% increase compared to the previous stage. TDM accumulation rate was generally maximal at the complete flowering cones and beginning of seed development stage in most treatments, except for the Urmia ecotype under 10 dS.m−1. The Central Plateau ecotype under 10 dS.m−1 showed the highest TDM at this stage (117.6 g.m−2 after accumulating 1752.7 GDD), and the Bushehr ecotype under 40 dS.m−1 recorded the lowest (33.8 g.m−2 after accumulating 1602.0 GDD). In the seed maturity and drying stage, the highest TDM was observed in the Urmia ecotype under 10 dS.m−1 (125.2 g.m−2 after accumulating 2934.2 GDD), a 14.7% increase compared to the previous stage, while the lowest TDM was recorded in the Bushehr ecotype under 40 dS.m−1 (29.4 g.m−2 after accumulating 1676.6 GDD), a 13.2% decrease compared to the previous stage (Fig. 2, G, H, and I).
Regarding changes in PGR, there was no significant difference between salinity levels for any ecotype during the seedling emergence stage. At the vegetative node formation stage, the Central Plateau and Bushehr ecotypes under 10 dS.m-1 had the highest (0.68 g.m-2.day-1 after accumulating 524.6 GDD) and lowest (0.13 g.m-2.day-1 after accumulating 608.4 GDD) PGR, respectively. During the emergence of short, fertile, segments stage, the highest PGR was observed in the Central Plateau ecotype under 20 dS.m-1 (0.55 g.m-2.day-1 after accumulating 962.3 GDD), a 105.6% increase compared to the vegetative node formation stage. The lowest PGR at this stage was found in the Bushehr ecotype under 20 dS.m-1 (0.17 g.m-2.day-1 after accumulating 1102.0 GDD), a 25.8% decrease compared to the vegetative node formation stage. In general, PGR increased until the complete flowering cones and beginning of seed development stage and then decreased. At the complete flowering cones and beginning of seed development stage, the highest PGR were obtained at 10 dS.m-1, specifically in the Central Plateau (2.81 g.m-2.day-1 after accumulating 1752.7 GDD) and Urmia (2.67 g.m-2.day-1 after accumulating 1963.6 GDD) ecotypes. The lowest PGR at this stage was observed in the Bushehr ecotype under 40 dS.m-1 (0.76 g.m-2.day-1 after accumulating 1602.0 GDD). Unlike previous stages, PGR exhibited a declining trend across all ecotypes during the seed maturity and drying stage. In this stage, the highest PGR was recorded in the Central Plateau ecotype under 40 dS.m-1 (0.56 g.m-2.day-1 after accumulating 1651.4 GDD), a 57.3% decrease compared to the previous stage. The lowest PGR was also observed in the Central Plateau ecotype under 10 dS.m-1 (−0.42 g.m-2.day-1 after accumulating 2905.1 GDD) (Fig. 2, J, K, and L).
Plant height and forage yield
The ANOVA II indicated that salinity [with the exception of carboxylation efficiency (CE) and photosynthetic water use efficiency (Pwue)], ecotype, and their interaction significantly influenced plant height (PH), forage yield (Fy), and photosynthetic parameters in S. persica ecotypes (Table 4).
Mean comparisons for S. persica PH revealed no significant differences between the Urmia and Bushehr ecotypes under control conditions and at 10 dS.m−1 salinity. Although, the Urmia ecotype exhibited the greatest PH under 10 dS.m−1 salinity stress, also significant differences between ecotypes were clearly observed with increasing salinity. Specifically, at 20 and 40 dS.m−1 salinity levels, the Urmia ecotype produced the tallest plants, while the Bushehr ecotype had the shortest plants (Fig. 3, A). Subsequently, quadratic and linear regression analyses were employed to model the relationship between salinity and plant traits across all S. persica ecotypes. The quadratic polynomial model indicated that increasing salinity up to 10 dS.m−1 had a positive effect on PH in the Central Plateau and Urmia ecotypes, resulting in a 31.8% and 8.7% increase, respectively, compared to the control treatment. Beyond this point, at 20 dS.m−1, PH decreased by 10.5% and 1.1% in the Central Plateau and Urmia ecotypes, respectively, relatine to control. At 40 dS.m−1, the reduction was 4.9% and 5.2%, respectively (R2 ≥ 0.60 and RMSE ≤ 3.83 for the Central Plateau ecotype; R2 ≥ 0.54 and RMSE ≤ 1.29 for the Urmia ecotype) (Fig. 4, A and B). In contrast, changes in PH in the Bushehr ecotype were best described by a linear function (R2 ≥ 0.96 and RMSE ≤ 1.58). PH in this ecotype decreased linearly with increasing salinity, showing reductions of 2.1%, 25.9%, and 51.8% at 10, 20, and 40 dS.m−1, respectively, compared to control (Fig. 4, C).
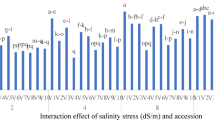
Interaction effects of salinity stress × ecotype for (PH) plant height (A), (Fy) forage yield (B), (SC) stomatal conductance (C), and (Pn) net photosynthetic rate (D) in S. persica ecotypes (Central plateau, Urmia and Bushehr). Values are mean ± S.D. based on three replicates. Different letters on top of bars indicate significant differences according to LSD test (P < 0.05).
(PH) Plant height (A, B, and C), (Fy) forage yield (D, E, and F), (SC) stomatal conductance (G, H, and I), and (Pn) net photosynthetic rate (J, K and L) of S. persica ecotypes (Central plateau, Urmia and Bushehr) under salinity stress (linear and quadratic regression models). Each data point represents the average of three replicates, with vertical bars (where visible) indicating standard deviation (± S.D.) within replicates.
In the assessment of Fy (forage yield), the Central Plateau ecotype under 10 dS.m−1 salinity stress exhibited the highest levels, showing a 20.9% increase compared to the control salinity treatment, while the Bushehr ecotype under 40 dS.m−1 salinity stress showed the lowest levels, with a 12.0% decrease (Fig. 3, B). Furthermore, the Fy response of S. persica ecotypes to different salinity levels was well-represented by a quadratic polynomial model (R2 ≥ 0.76, RMSE ≤ 2.55 for Central Plateau; R2 ≥ 0.91, RMSE ≤ 2.00 for Urmia; and R2 ≥ 0.52, RMSE ≤ 1.51 for Bushehr ecotypes, respectively). A significant difference was observed in the slopes of these regression models for Fy in response to salinity. Specifically, in the Central Plateau and Bushehr ecotypes, the slope of the polynomial regression model initially increased (0.16 and 0.20 ton.h−1, respectively) from control to 10 dS.m−1, then decreased (0.011 and 0.006 ton.h−1, respectively) at salinity levels above 10 dS.m−1. Conversely, in the Urmia ecotype, the slope of the polynomial regression model initially decreased (0.87 ton.h−1) from control to 10 dS.m−1 before increasing (0.010 ton.h−1) at higher salinity levels (Fig. 4, D, E, and F).
Physiological responses and photosynthetic characteristics
An investigation of stomatal conductance (SC) in S. persica ecotypes revealed that the maximum rate of gas exchange through stomata was observed in the Urmia ecotype under 10 dS.m−1 salinity, with a 25.2% increase, while the minimum rate was found in the Central Plateau ecotype under 40 dS.m−1 salinity, with a 67.2% decrease, both relative to the control salinity treatment (Fig. 3, C). Furthermore, salinity significantly influenced SC in all ecotypes, and this response was well-described by a quadratic polynomial model (R2 ≥ 0.95, RMSE ≤ 17.6 for Central Plateau; R2 ≥ 0.78, RMSE ≤ 50.2 for Urmia; and R2 ≥ 0.96, RMSE ≤ 9.7 for Bushehr ecotypes, respectively) (Fig. 4, G, H, and I).
Net photosynthesis rate (Pn), a key physiological indicator, was significantly affected by the interaction of the studied treatments. The highest Pn was associated with the Urmia ecotype under control salinity treatment. Increasing salinity to 10, 20, and 40 dS.m−1 in this ecotype reduced the Pn by 24.4%, 40.0%, and 49.3%, respectively, compared to the control treatment. The lowest Pn was observed in the Bushehr ecotype under 40 dS.m−1 salinity, with a 25.3% reduction relative to the control treatment (Fig. 3, D). The responses of Pn to increasing salinity in all studied ecotypes were accurately described by a significant quadratic polynomial model (R2 ≥ 0.63, RMSE ≤ 1.87 for Central Plateau; R2 ≥ 0.99, RMSE ≤ 0.17 for Urmia; and R2 ≥ 0.64, RMSE ≤ 1.45 for Bushehr ecotypes, respectively). The quadratic polynomial function predicted maximum Pn at 11.3, 0.2, and 7.5 dS.m−1 for the Central Plateau, Urmia, and Bushehr ecotypes, respectively, with corresponding average rates of 20.93, 27.49, and 16.1 µmol.m−2.s−1 (Fig. 4, J, K, and L).
Application of 10 dS.m−1 salinity increased the transpiration rate (T) in the Central Plateau and Urmia ecotypes by 43.0% and 27.7%, respectively, compared to the control salinity treatment. However, with increasing salinity levels to 20 dS.m−1 (14.6% and 20.5% in the Central Plateau and Urmia ecotypes, respectively) and 40 dS.m−1 (38.0% and 13.7% in the Central Plateau and Urmia ecotypes, respectively), the T decreased in these ecotypes relative to the control treatment. In the Bushehr ecotype, the highest T was observed at control, and increasing salinity to 10, 20, and 40 dS.m−1 resulted in reductions of 8.2%, 32.4%, and 50.2%, respectively (Fig. 5, A). According to the quadratic polynomial regression analyses (R2 ≥ 0.59, RMSE ≤ 6.0 for Central Plateau; R2 ≥ 0.46, RMSE ≤ 3.5 for Urmia), salinity application up to 10 dS.m−1 had a positive effect on the T in the Central Plateau and Urmia ecotypes, as indicated by initial slopes of 0.263 and 0.016 µmol H2O.m−2.s−1, respectively. Conversely, salinity levels above 10 dS.m−1 had a negative effect on this parameter, with secondary slopes of −0.0171 and − 0.0056 µmol H2O.m−2.s−1, respectively (Fig. 6, A and B). In contrast, changes in the T of the Bushehr ecotype in response to increasing salinity levels were better represented by a linear function than a quadratic polynomial model (R2 ≥ 0.95 and RMSE ≤ 0.9). Specifically, for this ecotype, the T decreased linearly with a significant slope of 0.294 µmol H2O.m−2.s−1 for every 1 dS.m−1 increase in salinity (Fig. 6, C).
Interaction effects of salinity stress × ecotype for (T) transpiration rate (A), (Ci) intercellular CO2 concentration (B), (CE) carboxylation efficiency (C), and (Pwue) photosynthetic water use efficiency (D) of S. persica ecotypes (Central plateau, Urmia and Bushehr). Values are mean ± S.D. based on three replicates. Different letters on top of bars indicate significant differences according to LSD test (P < 0.05).
(T) Transpiration rate (A, B, and C), (Ci) intercellular CO2 concentration (D, E, and F), (CE) carboxylation efficiency (G, H, and I), and (Pwue) photosynthetic water use efficiency (J, K, and L) of S. persica ecotypes (Central plateau, Urmia and Bushehr) under salinity stress (linear and quadratic regression models). Each data point represents the average of three replicates, with vertical bars (where visible) indicating standard deviation (± S.D.) within replicates.
An analysis of changes in intercellular CO2 concentration (Ci) revealed that the highest value for this trait was observed in the Central Plateau ecotype under control conditions, averaging 0.49 µmol CO2.mol air−1. Increasing the salinity level to 10, 20, and 40 dS.m−1 resulted in a decrease of 26.7%, 32.8%, and 49.0%, respectively, in Ci compared to the control treatment. Conversely, the lowest Ci value was found in the Bushehr ecotype under 40 dS.m−1 salinity stress, averaging 13.2 µmol CO2.mol air−1, representing a 60.7% reduction compared to the control treatment (Fig. 5, B). Regression analysis indicated that the quadratic polynomial model effectively described the relationship between salinity and Ci (R2 ≥ 0.96, RMSE ≤ 1.7 for Central Plateau; R2 ≥ 0.92, RMSE ≤ 2.1 for Urmia; R2 ≥ 0.91, RMSE ≤ 2.6 for Bushehr). For every unit (1 dS.m−1) increase in salinity, Ci changed with initial slopes of −1.21, −1.50, and − 1.15 µmol CO2.mol air−1, and secondary slopes of 0.015, 0.026, and − 0.010 µmol CO2.mol air−1 in the Central Plateau, Urmia, and Bushehr ecotypes, respectively (Fig. 6, D, E, and F).
Unlike other photosynthetic characteristics, carboxylation efficiency (CE) exhibited a notable increase with rising stress levels across the studied ecotypes. The Bushehr ecotype displayed the highest CE under a salinity of 40 dS.m−1, averaging 0.904 mmol CO2.m−2.s−1, a substantial 88.9% increase compared to the control. Interestingly, this was not significantly different from the Urmia ecotype at 20 dS.m−1 salinity, which recorded an average of 0.859 mmol CO2.m−2.s−1, representing a 23.4% increase over its respective control. The lowest CE was observed in the Central Plateau ecotype under control conditions, with an average of 0.396 mmol CO2.m−2.s−1 (Fig. 5, C). Regression analyses revealed contrasting trends across ecotypes. A quadratic polynomial model best described the changes in CE for the Central Plateau and Urmia ecotypes with increasing salinity levels (R2 ≥ 0.59, RMSE ≤ 0.07 for Central Plateau; R2 ≥ 0.49, RMSE ≤ 0.07 for Urmia). However, a linear model provided a more accurate fit for the Bushehr ecotype (R2 ≥ 0.84, RMSE ≤ 0.07). Specifically, for the Central Plateau and Urmia ecotypes, an initial increase in CE was observed up to 20 dS.m−1 salinity, with slopes of 0.018 and 0.015 mmol CO2.m−2.s−1, respectively. Beyond this salinity threshold, the trend reversed, and CE decreased with slopes of −0.0003 and − 0.0004 mmol CO2.m−2.s−1, respectively. In contrast, the Bushehr ecotype showed a continuous linear increase in CE with a slope of 0.011 mmol CO2.m−2.s−1 (Fig. 6, G, H, and I).
Regarding photosynthetic water use efficiency (Pwue), the results indicated that, with the exception of the control condition where the Urmia ecotype exhibited the highest Pwue (averaging 1.044 µmol CO2/mmol H2O), the Bushehr ecotype consistently demonstrated superior Pwue at all other salinity levels. Specifically, under salinity treatments of 10, 20, and 40 dS.m−1, the Bushehr ecotype recorded average Pwue values of 0.907, 0.897, and 1.116 µmol CO2/mmol H2O, respectively. Conversely, the Central Plateau ecotype exhibited the lowest Pwue across all salinity levels, with averages of 0.596, 0.519, 0.669, and 0.792 µmol CO2/mmol H2O at the control, 10, 20, and 40 dS.m−1 salinity treatments, respectively (Fig. 5, D). Linear regression analyses revealed that the trends in Pwue for the Central Plateau and Bushehr ecotypes (R2 ≥ 0.77, RMSE ≤ 0.05 for Central Plateau; R2 ≥ 0.91, RMSE ≤ 0.04 for Bushehr) increased with rising salinity, with slopes of 0.0061 and 0.0092 µmol CO2/mmol H2O increase, respectively (Fig. 6, J and L). In contrast, a quadratic polynomial model best described the Pwue response in the Urmia ecotype (R2 ≥ 0.54, and RMSE ≤ 0.12). This model showed a decreasing trend up to 20 dS.m−1, with a slope of −0.0231 µmol CO2/mmol H2O per one dS.m−1 increase. However, beyond this salinity level, the trend reversed, exhibiting an increasing slope of 0.0004 µmol CO2/mmol H2O per one dS.m−1 increase (Fig. 6, K).
Forage quality and nutritional value
An investigation into the fodder quality of S. persica ecotypes subjected to varying salinity regimes revealed significant interaction effects between salinity and ecotype on the majority of assessed qualitative characteristics, as determined through two-way analysis of variance (ANOVA II). The notable exceptions were acid detergent fiber (ADF) and neutral detergent fiber (NDF) fractions, for which only the effect of ecotype was statistically significant (Table 5).
The study discerned a positive relationship between increasing salinity levels and both forage nitrogen (N) and crude protein (CP) percentage up to a threshold of 10 dS.m−1. Specifically, under an imposed salinity of 10 dS.m−1, the Central Plateau ecotype exhibited the highest average of these traits, registering 5.42% for N and 33.9% for CP. Conversely, the Bushehr ecotype, when exposed to 40 dS.m−1, manifested the lowest average values at 2.43% and 15.2%, respectively. Importantly, these minimal readings did not significantly differ from the Urmia ecotype under 20 dS.m−1 salinity conditions, which recorded an average of 2.45% and 15.3% for N and CP respectively (Fig. 7, A and B). The implementation of regression modeling demonstrated that the quadratic polynomial model afforded an enhanced representation of N percentage (R2 ≥ 0.49, RMSE ≤ 0.47 for Central Plateau; R2 ≥ 0.80, RMSE ≤ 0.17 for Urmia), and CP percentage (R2 ≥ 0.49, RMSE ≤ 2.96 for Central Plateau; R2 ≥ 0.80, RMSE ≤ 1.04 for Urmia) fluctuations in both the Central Plateau and Urmia ecotypes across increasing salinity levels. Analysis of the Central Plateau ecotype revealed that, up to 10 dS.m−1, initial upward slopes of 0.0104% and 0.065% were evident for N and CP, respectively. In contrast, salinity increases beyond this level induced a reduction in the percentage of these attributes, marked by secondary slopes of −0.0009% and − 0.006% (Fig. 8, A and D). The Urmia ecotype, on the other hand, demonstrated initial downward slopes of −0.0615% and − 0.038% for N and CP respectively, up to a salinity of 20 dS.m−1. Above this threshold, subsequent enhancements in salinity coincided with positive secondary slopes of 0.0010% and 0.0061% in forage N and CP (Fig. 8, B and E). However, a linear model offered a more suitable fit for the Bushehr ecotype when characterizing these compositional changes (R2 ≥ 0.97, RMSE ≤ 0.16 for N percentage; and R2 ≥ 0.97, RMSE ≤ 0.99 for CP percentage). Lastly, the Bushehr ecotype exhibited a linear decline in both N and CP percentage, with respective slopes of −0.067% and − 0.0423% (Fig. 8, C and F).
Interaction effects of salinity stress × ecotype for (N) nitrogen (A), (CP) crude protein (B), (Ash) ash percent (C), and (CF) crude fiber (D) of S. persica ecotypes (Central plateau, Urmia and Bushehr). Values are mean ± S.D. based on three replicates. Different letters on top of bars indicate significant differences according to LSD test (P < 0.05).
(N) Nitrogen (A, B, and C), (CP) crude protein (D, E, and F), (Ash) ash percent (G, H, and I), and (CF) crude fiber (J, K and L) of S. persica ecotypes (Central plateau, Urmia and Bushehr) under salinity stress (linear and quadratic regression models). Each data point represents the average of three replicates, with vertical bars (where visible) indicating standard deviation (± S.D.) within replicates.
An analysis of ash content (Ash) revealed a consistent trend of escalation across all three ecotypes under examination in response to heightened salinity levels. Specifically, the introduction of saline stress, escalating from the control treatment (non-stress) to 10, 20, and 40 dS.m−1, induced an increase in Ash. This increase was quantified at 2.62%, 6.80%, and 10.30% for the Central Plateau ecotype, 2.10%, 5.10%, and 11.80% for the Urmia ecotype, and 1.20%, 4.40%, and 2.80% for the Bushehr ecotype, respectively (Fig. 7, C). Regression model fitting demonstrated that a linear regression model effectively captured the variation in Ash percentage in response to increasing salinity for both the Central Plateau and Urmia ecotypes (R2 ≥ 0.96, RMSE ≤ 0.74 for Central Plateau; R2 ≥ 0.99, RMSE ≤ 0.32 for Urmia). This linear relationship was characterized by slopes in Ash by 0.266% and 0.306% for every unit increase in salinity (dS.m−1) in the Central Plateau and Urmia ecotypes, respectively (Fig. 8, G, and H). Conversely, a quadratic polynomial model offered a superior fit for characterizing the trend in the Bushehr ecotype (R2 ≥ 0.86, and RMSE ≤ 0.62). In this ecotype, the Ash exhibited an increase up to a salinity level of 20 dS.m−1, with a slope of 0.353%. However, beyond this threshold, a secondary slope of −0.0065% indicated a decrease in Ash with further increases in salinity (Fig. 8, I).
Crude fiber content (CF) analysis revealed that the Urmia ecotype consistently exhibited the highest levels of this trait across all salinity treatments. Specifically, under control conditions and at 10, 20, and 40 dS.m−1, the Urmia ecotype displayed average CF percentages of 34.8%, 35.3%, 35.9%, and 39.8%, respectively. Conversely, the Central Plateau ecotype recorded the lowest crude fiber content, averaging 12.7% at a salinity level of 20 dS.m−1 (Fig. 7, D). Quadratic polynomial models effectively characterized the trends in CF across all three ecotypes (R2 ≥ 0.71, RMSE ≤ 2.55 for Central Plateau; R2 ≥ 0.99, RMSE ≤ 0.13 for Urmia; R2 ≥ 0.95, RMSE ≤ 0.27 for Bushehr). According to the model outputs, the initial slopes of change in CF for the Central Plateau, Urmia, and Bushehr ecotypes were estimated at −0.160%, −0.006%, and − 0.309%, respectively, with secondary slopes of 0.0098%, 0.0033%, and 0.0072% (Fig. 8, J, K, and L).
Regarding the traits of acid detergent fiber (ADF) and neutral detergent fiber (NDF) percentages, given the significant influence of ecotype on these characteristics, only the means of the ecotype effect are presented in Fig. 9. An analysis of these mean values revealed that the Urmia ecotype exhibited the highest ADF and NDF percentages, averaging 42.39% and 44.16%, respectively. Conversely, the lowest ADF percentage was observed in both the Bushehr and Central Plateau ecotypes, with respective means of 36.54% and 36.80% (Fig. 9, A). The Central Plateau ecotype also displayed the lowest NDF percentage, averaging 29.85% (Fig. 9, B).
A comparative analysis of sodium (Na+) and potassium (K+) ion concentrations, and their resultant ratio (Na+/K+), within Salicornia forage across three ecotypes under varying salinity regimes, demonstrated a consistent trend of increased Na+ accumulation with elevated salinity. Specifically, forage Na+ content exhibited a direct positive relationship with salinity, reaching peak levels at 40 dS.m−1 (averaging 78.43, 105.32, and 89.19 mg/g dry weight for the Central Plateau, Urmia, and Bushehr ecotypes, respectively). Conversely, the lowest Na+ concentrations were observed in control (non-saline) conditions, measuring 58.82, 21.17, and 29.39 mg/g dry weight for the respective ecotypes (Fig. 10, A). Regression analyses revealed ecotype-specific responses to salinity. A non-linear quadratic polynomial model best described Na+ accumulation patterns in the Urmia (R2 ≥ 0.94, RMSE ≤ 7.72) and Bushehr (R2 ≥ 0.94, RMSE ≤ 5.55) ecotypes. This model illustrated an initial rapid increase in Na+ content, with slopes of 6.64 and 4.55 mg/g dry weight per dS.m−1, up to 20 dS.m−1, followed by decreasing slopes of −0.113 and − 0.076 mg/g dry weight per dS.m−1, respectively. In contrast, the Central Plateau ecotype exhibited a linear increase in Na+ content (slope of 0.941 mg/g dry weight per dS.m−1; R2 ≥ 0.99, RMSE ≤ 0.55) (Fig. 11, A, B, and C). Conversely, forage K+ concentrations decreased with increasing salinity levels. Maximum K+ levels were found in control conditions (67.00, 52.75, and 61.30 mg/g dry weight for Central Plateau, Urmia, and Bushehr ecotypes, respectively), with reductions of 74.6%, 67.4%, and 75.5%, respectively at 40 dS.m−1 (Fig. 10, B). A quadratic polynomial model effectively captured this decline in K+ content across all ecotypes (R2 ≥ 0.99, RMSE ≤ 1.28 for Central Plateau; R2 ≥ 0.98, RMSE ≤ 1.55 for Urmia; R2 ≥ 0.94, RMSE ≤ 4.17 for Bushehr) (Fig. 11, D, E, and F). The Na+/K+ ratio, in turn, demonstrated a positive linear relationship with increasing salinity. The highest ratios were recorded at 40 dS.m−1 (4.677, 6.146, and 5.938, respectively) and the lowest in the absence of salt stress (0.876, 0.402, and 0.486, respectively) (Fig. 10, C). The linear regression models accurately described the change in Na+/K+ ratio in response to salinity increase, exhibiting slopes of 0.098, 0.141, and 0.133 for the Central Plateau, Urmia, and Bushehr ecotypes, respectively (R2 ≥ 0.99, RMSE ≤ 0.05 for Central Plateau; R2 ≥ 0.94, RMSE ≤ 0.50 for Urmia; R2 ≥ 0.96, RMSE ≤ 0.37 for Bushehr) (Fig. 11, G, H, and I).
Interaction effects of salinity stress × ecotype for (Na+) shoot sodium concentration (A), (K+) shoot potassium concentration (B), (Na+/K+) shoot sodium to potassium ratio (C), and (EC) soil electrical conductivity (D) of S. persica ecotypes (Central plateau, Urmia and Bushehr). Values are mean ± S.D. based on three replicates. Different letters on top of bars indicate significant differences according to LSD test (P < 0.05).
(Na+) Shoot sodium concentration (A, B, and C), (K+) shoot potassium concentration (D, E, and F), (Na+/K+) shoot sodium to potassium ratio (G, H, and I), and (EC) soil electrical conductivity (J, K, and L) of S. persica ecotypes (Central plateau, Urmia and Bushehr) under salinity stress (linear and quadratic regression models). Each data point represents the average of three replicates, with vertical bars (where visible) indicating standard deviation (± S.D.) within replicates.
Electrical conductivity (EC) of soil
The two-way analysis of variance (ANOVA II) results regarding the electrical conductivity (EC) of saturated soil extracts revealed a statistically significant interaction effect between salinity and ecotype at a 1% probability level (Table 5). Subsequent mean comparisons of the salinity-ecotype interactions demonstrated that the highest levels of saturated soil extract EC were observed in plots subjected to 40 dS.m−1 salinity, particularly within the Urmia and Bushehr ecotypes. Conversely, control plots across all three ecotypes exhibited the lowest EC levels (Fig. 10, D). Furthermore, a direct relationship between salinity levels and soil extract EC was established; increases in salinity corresponded to increases in EC across all three studied ecotypes, following a linear model (R2 ≥ 0.99, RMSE ≤ 1.16 for Central Plateau; R2 ≥ 0.99, RMSE ≤ 0.79 for Urmia; R2 ≥ 0.97, RMSE ≤ 2.88 for Bushehr). Specifically, the model output indicated that for each unit increase in salinity (one dS.m−1) above the control level, saturated soil extract EC increased by slopes of 1.11, 1.27, and 1.44 dS.m−1 for the Central Plateau, Urmia, and Bushehr ecotypes, respectively. These findings underscore the substantial impact of escalating salinity on the EC of saturated soil extracts within the investigated ecotypes (Fig. 11, J, K, and L).
Pearson correlation coefficients, principal component analysis (PCA), and hierarchical cluster analysis
As the culmination of this investigation, Pearson’s correlation matrix, principal component analysis (PCA), and hierarchical cluster analysis was utilized to assess the interrelationships between the studied attributes in S. persica ecotypes under varying salinity stress conditions. Specifically, Pearson’s correlation matrix visualization was shown the PH displayed a positive and significant correlation with some of the photosynthetic characteristics, including SC (r = 0.74**), Pn (r = 0.61*), T (r = 0.62*), and NDF (r = 0.60*), and it had a negative and significant correlation only with EC of soil (r = −0.62*). Similarly, Fy also showed a positive and significant correlation with SC (r = 0.74**), Pn (r = 0.88**), T (r = 0.88**), and Ci (r = 0.68*), and a negative and significant correlation with shoot Na+/K+ ratio (r = −0.60*). Pn, as a key trait affecting forage growth and quality, showed a positive and significant correlation with SC (r = 0.80**), T (r = 0.75**), and Ci (r = 0.68*), and a negative and significant correlation with Ash (r = −0.63*), shoot Na+ concentration (r = −0.61*), shoot Na+/K+ ratio (r = −0.73**), and EC of soil (r = −0.67*). In contrast, the shoot Na+/K+ ratio exhibited a unique and consistently statistically significant negative correlation with majority of the traits examined: Fy (r = −0.60*), SC (r = −0.65*), Pn (r = −0.73**), Ci (r = −0.85**), N (r = −0.70*), CP (r = −0.70*), and shoot K+ concentration (r = −0.90**) as illustrated in Fig. 12.
Pearson correlation analysis was performed to assess the relationships between S. persica ecotypes traits (PH = plant height; Fy = forage yield; SC = stomatal conductance; Pn = net photosynthetic rate; T = transpiration rate; Ci = intercellular CO2 concentration; CE = carboxylation efficiency; Pwue = photosynthetic water use efficiency; N = nitrogen; CP = crude protein; Ash =ash percent; CF = crude fiber; ADF = acid detergent fiber; NDF = neutral detergent fiber; Na+ = shoot sodium concentration; K+ = shoot potassium concentration; Na+/K+ = shoot sodium to potassium ratio; and EC = soil electrical conductivity) under salinity stress. Correlation strength is visually represented by ellipse size (larger ellipse = weaker correlation and smaller ellipse = stronger correlation), with positive and negative correlations indicated by pink and green, respectively (n = 12, and p ≤ 0.01).
Principal Component Analysis (PCA) was employed to investigate the relationships between measured traits and salinity treatments in S. persica ecotypes, with the objective of identifying salinity tolerance mechanisms associated with favorable traits. In the resulting three-dimensional (3D) plot, S. persica ecotypes under varying salinity stress levels, exhibiting similar responses for a given variable, clustered together along the corresponding principal component (PC) vector. This allowed for the discrimination of treatments exhibiting improved fodder quality and production traits, such as reduced crude fiber (CF), shoot sodium (Na+) content, and shoot Na+/K+ ratio, alongside enhanced photosynthetic characteristics and forage production. Eigenvalues greater than 2.093 were used as a criterion for determining the number of significant principal components. The resulting PCA 3D plot revealed three principal components (PCs) that collectively accounted for 83.37% of the total variance within the dataset. Specifically, PC1 and PC2, visualized on the horizontal axes (left and right, respectively), explained the largest proportions of the variance, 48.30% and 23.44% respectively. PC3, displayed on the vertical axis, accounted for an additional 11.63% of the total variance. Based on the highest vector loadings, strong positive correlations were identified: with PC1, CE, Pwue, Ash, CF, ADF, NDF, Na+, Na+/K+, and EC; with PC2, PH, Fy, SC, Pn, T, CE, Pwue, CF, ADF, NDF, Na+, and Na+/K+; and with PC3, CE, Pwue, NDF, and K+, respectively (Fig. 13, A).
Principal component analysis (A), and hierarchical cluster analysis (B) were performed to assess the relationships between traits of S. persica ecotypes under salinity stress. The PCA 3D-plot displays the loadings of various growth, physiological responses, photosynthetic characteristics, forage quality, and nutritional value on S. persica ecotypes under salinity stress (E1-0.96, E1-10, E1-20, and E1-40 = Central plateau ecotype under control, 10, 20, and 40 dS.m−1; E2-0.96, E2-10, E2-20, and E2-40 = Urmia ecotype under control, 10, 20, and 40 dS.m−1; and E3-0.96, E3-10, E3-20, and E3-40 = Bushehr ecotype under control, 10, 20, and 40 dS.m−1). Loading of variables (PH = plant height; Fy = forage yield; SC = stomatal conductance; Pn = net photosynthetic rate; T = transpiration rate; Ci = intercellular CO2 concentration; CE = carboxylation efficiency; Pwue = photosynthetic water use efficiency; N = nitrogen; CP = crude protein; Ash = ash percent; CF = crude fiber; ADF = acid detergent fiber; NDF = neutral detergent fiber; Na+ = shoot sodium concentration; K+ = shoot potassium concentration; Na+/K+ = shoot sodium to potassium ratio; and EC = soil electrical conductivity) on the first, two and three principal components (PC1, PC2 and PC3), showing the percentage variance explained by each. Vector length represents the variable’s discriminatory power, while proximity to the origin (intersection of PC1, PC2, and PC3) indicates similarity between variables. In the hierarchical cluster chart S. persica ecotypes under varying salinity treatment groups are plotted based on their correlation values for each variable, with closer grouping indicating greater similarity within all given traits.
Graphical hierarchical cluster analysis was implemented to facilitate a comprehensive assessment of traits and to select the optimal combinations of salinity treatments and ecotypes, based on all measured traits in the salinity experiment using irrigated Persian Gulf seawater. The resulting cluster diagram, which uses a variety of colors to distinguish groups (Fig. 13, B), demonstrated a substantial range of variation in the studied characteristics of S. persica ecotypes across the different levels of salinity. The study classified the combined effects of ecotype and salinity stress into four separate clusters. The control groups (0.96 dS.m−1), from all three ecotypes, formed a single cluster due to the subtle differences and comparable reactions in their measured characteristics. Although there was a difference in forage yield between the salinity levels of 20 and 40 dS.m−1, the three ecotypes subjected to 40 dS.m−1, along with the Bushehr ecotype under 20 dS.m−1, showed similar patterns in photosynthesis, physiological functions, and forage quality, leading to their grouping within one cluster. The remaining combinations of salinity and ecotype treatment were grouped as follows: the Central Plateau and Urmia ecotypes under 10 and 20 dS.m−1 formed their own group. Finally, the Bushehr ecotype under 10 dS.m−1 salinity and the Urmia ecotype under 20 dS.m−1 salinity were grouped together, as displayed by the graphical hierarchical cluster analysis (Fig. 13, B).
Discussion
Phenology and growth characteristics
This study explored the effects of varying salinity levels, achieved by diluting Persian Gulf seawater, on the phenology, growth, physiology, forage yield, and nutritional value of three S. persica ecotypes. The results revealed significant variations in responses among ecotypes and across different salinity levels, underscoring the complex interaction between genetic background and salt stress.
The research demonstrated that salinity accelerates plant phenological development, prompting faster life cycle completion, potentially to avoid stress. As Ayala and O’Leary35 noted, the most rapid response to high salinity in Salicornia is a reduction in photosynthetic green area; they further indicated that increasing stress curtails ongoing photosynthesis, leading to reduced growth, biomass, and economic yields. However, growth curves suggest that optimal growth doesn’t occur in the absence of salinity; instead, a certain level appears necessary for optimal development, with stress manifesting beyond that. Tracking growth parameters using growing degree days (GDD) revealed key differences in the growth strategies of three S. persica ecotypes. The Bushehr ecotype completed its growth stages more quickly and with fewer GDDs, which led to lower production of total dry matter (TDM) and green area (GA). Conversely, the Central Plateau and Urmia ecotypes needed more GDDs to reach their peak GA and TDM, indicating a longer growth period and a greater investment in photosynthetic tissues. The Bushehr ecotype of Salicornia originates from the Bushehr and Khuzestan provinces and the southern coasts of the Persian Gulf. In these areas, the final stages of the plant’s growth cycle often coincide with the intense heat of early summer. To avoid these harsh conditions, this ecotype completes its life cycle quickly, with minimal heat exposure. This is why it finishes its growth cycle sooner than the other two ecotypes, which come from the central and northwestern parts of the country. The longer growing period of the Central Plateau and Urmia ecotypes makes them less suitable for the Ahvaz climate. In this region, high temperatures at the end of the season can negatively impact the plants by reducing photosynthesis and increasing respiration, ultimately hurting the production of GA and TDM.
Researchers, such as Izadi et al.9, emphasize plant growth rate (PGR) as a crucial parameter directly linked to crop yield. A negative PGR indicates reduced plant dry weight due to the shedding of photosynthetic tissues40. Becker et al.41 noted that salinity stress can accelerate the completion of the growth cycle, reducing plant dry matter production and yield. In the present study, enhanced PGR was observed under 10 dS.m−1 salinity due to maintained GA and photosynthesis, though increased salinity induced water stress and reduced growth. Severe stress significantly impacts plant growth, limiting cell expansion and reducing both photosynthesis and TDM accumulation. This is consistent with findings for other Salicornia species, such as S. rubra42, S. dolichostachya Moss43, and S. perennans44. Research on these plants shows that increased salt concentration causes wilting and other effects that reduce green area and the overall growth rate.
Plant height and forage yield
The Urmia ecotype showed the greatest PH under 10 dS.m−1, suggesting that low saline conditions can promote its vegetative growth. However, increasing salinity resulted in a reduction in PH, highlighting the negative impact of high salinity on plant growth. The different ways these ecotypes respond to salinity suggest they have unique adaptation strategies. For instance, the Central Plateau and Urmia ecotypes showed a quadratic response in PH, which first increased up to 10 dS.m−1 before declining. Meanwhile, the Bushehr ecotype’s height showed a consistent, linear decrease as salinity levels rose. Numerous investigations have demonstrated that growth inhibition, manifested in the reduced elongation of both stems and roots, alongside a concomitant decrease in dry matter accumulation, are commonly observed indicators of oxidative stress45. A pivotal mechanism underlying this growth reduction under saline conditions may reside in the compromised allocation of metabolic energy. Specifically, the energetic demands associated with ion homeostasis and osmotic adjustments escalate, consequently diverting metabolic resources away from growth processes46. Moreover, it has been proposed that reduced growth can be considered an adaptive mechanism for halophytes under severe salt stress, thereby enhancing survival rates47. Therefore, it is reasonable to posit that osmotic stress, ion toxicity, and altered nutrient homeostasis represent crucial determinants contributing to decreased plant height in saline environments. Further, it has been suggested that physiological drought induced in the root zone by competitive interactions among chloride, sulfate, and nitrate ions, is a contributing factor to this reduced stature. In particular, high salinity at the root zone, especially during early developmental stages, instigates osmotic stress, leading to diminished cellular water content, reduced cell elongation, and a sluggish restoration of cell turgor and subsequent expansion even after osmotic balance has been re-established46. In contrast, the findings of Araus et al.14, focusing on S. europaea ecotypes, revealed that salinity levels can, paradoxically, promote plant height. This observation is consistent with the work of Beyrami et al.48, who reported increased plant height and various morphological traits in both S. persica and S. bigelovii under a salinity of 8 dS.m−1. However, they also found that increasing the severity of salt stress up to 25 dS.m−1 resulted in a decline in these same characteristics, an observation that closely aligns with the present study’s findings.
The Central Plateau ecotype under 10 dS.m−1 showed the highest Fy, indicating its potential for forage production under moderate saline conditions. The quadratic polynomial models for Fy across ecotypes suggested an optimum salinity level for Fy in the Central Plateau and Urmia ecotypes, with reduction in Fy under higher salinity conditions. Interestingly, even though the Bushehr ecotype comes from the southern coasts, it showed lower PH and less Fy than the other two ecotypes, especially when the salt levels were high (20 and 40 dS.m−1). This suggests that the Bushehr ecotype is more suited to lower salt environments. It naturally grows along rivers that are less salty before they empty into the Persian Gulf. In contrast, the Central Plateau ecotype is adapted to the very salty, dry soils of central Iran, and the Urmia ecotype is from the extremely salty Urmia Lake. The findings of this study are consistent with other research on Salicornia species. Studies by De Souza et al.49, and Ranjbar et al.6,7 have all shown that forage yield peaks under moderate salinity conditions. These peak forage yields were consistently higher than those observed in control environments that had no salt. Similarly, investigations into other halophytes, such as Salsola and Suaeda, have revealed a comparable trend50: forage production exhibited an increase in response to moderate salinity levels. However, higher salinity concentrations were unequivocally correlated with a reduction in dry matter accumulation. Consequently, and in line with their deductions, it can be posited that a salinity tolerance mechanism, which facilitates enhanced growth at elevated salinity levels, is inherent in halophytes such as S. persica ecotypes. Nonetheless, it appears that a more protracted exposure time may be required to induce this mechanism and effectively mitigate the adverse effects of high salinity stress. The Pearson’s correlation matrix revealed significant positive relationships between PH, Fy, Pn, and SC, suggesting that efficient gas exchange and carbon assimilation are key traits for forage production. Conversely, a strong negative correlation between the Na+/K+ ratio and many of the quality and growth traits suggested that high salinity-induced ionic imbalance is detrimental to the overall forage performance of S. persica ecotypes.
Physiological responses and photosynthetic characteristics
SC (Stomatal conductance) showed a complex response pattern, with the highest value observed in the Urmia ecotype under 10 dS.m−1 and the lowest in the Central Plateau ecotype under 40 dS.m−1. This highlights the important role of stomata in regulating gas exchange under stress conditions. Prior investigations have unequivocally demonstrated that salinity stress exerts a considerable influence on the physiological responses of Salicornia plants51,52. Researchers have posited that salinity, by diminishing the osmotic potential of the soil solution, impairs water absorption by roots, subsequently inducing stomatal closure and consequently, reduced SC43. Supporting this notion, Ayala and O’Leary35 confirmed a decline in SC in S. bigelovii with increasing salinity levels. Conversely, Pérez-Romero et al.53 reported that the highest SC in S. ramosissima was achieved at its optimal salinity concentration. These findings underscore the fact that SC responses in Salicornia vary markedly across different species and even at differing salinity intensities, thereby highlighting the role of inherent genetic variations in determining species-specific salinity tolerance.
Pn (Net photosynthesis rate) was also impacted by both ecotype and salinity. The Urmia ecotype under control conditions demonstrated the highest Pn, but Pn decreased with increasing salinity, indicating a salinity-induced limitation of carbon assimilation. The quadratic polynomial model accurately described the Pn response in the ecotypes, suggesting an optimal salinity level for Pn, beyond which productivity declines. Researchers have identified a reduction in photosynthetic activity as a primary indicator of osmotic stress damage in Salicornia plants54. Elevated salinity levels in halophytes curtail the capacity of the photosynthetic system to generate ATP and NADPH, and also diminish Pn during the dark reactions, resulting in decreased carbohydrate production. This, in turn, contributes to a decline in dry weight accumulation at higher salinity concentrations55. Aside from impaired CO2 uptake, investigators attribute the reduction in Pn to phytochemical factors such as alterations in photosynthetic pigments (chlorophylls and carotenoids) and the inactivation of the Rubisco enzyme, often linked to damage to PSII54. The findings of this study indicate that the more stress-tolerant ecotypes are capable of enhancing Pn through improved translocation of carbohydrates from photosynthetic organs to reproductive sinks. However, the protective mechanisms used by the more resistant ecotypes (Central Plateau and Urmia) differ from those of the sensitive Bushehr ecotype. These differences relate to their defense against the harmful effects of high salinity, like that found in seawater. This finding supports previous research by De Souza et al.49.
The T (transpiration rate) was initially enhanced at 10 dS.m−1 in Central Plateau and Urmia ecotypes, likely due to increased water uptake to compensate for salt stress, but was reduced in all ecotypes under higher salinity. Conversely, the Bushehr ecotype showed a linear decrease in T with increased salinity. The observed reduction in T within the studied S. persica ecotypes, when subjected to elevated salinity stress, can plausibly be attributed to diminished water uptake. This, in turn, triggers stomatal closure as a response to the osmotic shock induced by high salt concentrations in the root zone. Consequently, the reduction in tissue water content leads to a decline in T by the plant. Notably, the salt-tolerant S. persica ecotypes exhibited the capacity to maintain a more stable T by protecting their stomata from degradation. This observation aligns with findings from other researchers concerning S. fruticosa, further reinforcing the role of stomatal regulation in halophytic stress responses56.
The Ci (intercellular CO2 concentration) decreased with increasing salinity across all ecotypes, potentially reflecting an increase in stomatal closure in response to salt stress. The Central Plateau ecotype, when exposed to 10 dS.m−1 salinity, exhibited a lower Ci concentration. This finding suggests a more effective photosynthetic process and a higher conversion rate of absorbed CO2 into carbohydrates relative to the other studied ecotypes. Furthermore, the sustained rate of CO2 uptake under severe salinity stress in all three studied ecotypes is a result of their ability to counteract the deleterious effects of high salt concentrations on the photosynthetic machinery. This action prevents the accumulation of CO2 within the substomatal chamber. As previously reported by Gulzar et al.57, a decrease in Ci concentration due to increased salinity restricts the supply of CO2 for photosynthesis, thereby lowering the photosynthetic rate. This observation aligns with the results of the present study.
Interestingly, the ecotypes showed different responses in carboxylation efficiency (CE) to salinity. The Central Plateau and Urmia ecotypes increased their CE up to 20 dS.m−1 and then decreased it, whereas the Bushehr ecotype’s CE continuously and linearly increased with rising salinity. A pivotal factor limiting the rate of Pn under salinity stress is mesophyll conductance, often referred to as CE58. Reduced Pn rates coupled with CO2 accumulation within photosynthetic tissues indicate an impaired ability of mesophyll cells to effectively utilize CO2 and a diminished CE of the plant. The build-up of CO2 in photosynthetic organs under stress conditions renders the plant incapable of using it, despite its passage through stomatal cells. In essence, environmental stressors, by increasing stomatal limitations, induce alterations in Ci concentration, Pn, T, and CE59.
Furthermore, based on its formula, Pwue (photosynthetic water use efficiency) is an index that expresses the rate of photosynthesis per unit of transpiration36. The elevated Pwue observed in the Bushehr ecotype under salinity stress, compared to the control conditions, is likely attributable to a higher instantaneous photosynthetic rate or carbon assimilation rate in this treatment. Conversely, the lower Pwue under control conditions without stress can be ascribed to a higher SC leading to increased T rates in conjunction with the instantaneous Pn rate. This combination results in reduced instantaneous photosynthetic efficiency under favorable environmental conditions. Researchers have reported that a decrease in Pwue with increasing stress levels may result from a reduced Ci concentration, a phenomenon influenced more by non-stomatal factors than stomatal ones60. In line with the results of our study, other investigators have observed an increase in Pwue under elevated salinity stress in the halophyte quinoa61. In this experiment, as salinity levels increased, the reduction in Pn rate occurred at a slower pace than the decrease in T intensity. This resulted in the maintenance of Pwue at the highest possible level under salinity stress. Despite a significant decrease in intrinsic Pwue at the highest salinity level (40 dS.m−1), the Central Plateau and Urmia ecotypes are still recommended for cultivation in areas with moderate root-zone salinity (10 to 20 dS.m−1) due to the lower risk involved.
Forage quality, nutritional value, and soil electrical conductivity
Our study revealed a positive relationship between salinity levels and forage N (nitrogen) and CP (crude protein) percentage up to 10 dS.m−1, particularly for the Central Plateau ecotype, which demonstrated the highest average N and CP under 10 dS.m−1. The quadratic polynomial models for the Central Plateau and Urmia ecotypes showed an optimal salinity level (10 and 20 dS.m−1, respectively), after which a reduction was observed. In contrast, the Bushehr ecotype showed a linear decline in N and CP percentages, highlighting the varied nutritional quality response to salinity. In this vein, researchers have articulated that when stress intensity surpasses a plant’s tolerance threshold, akin to kochia (Bassia prostrate) in similar studies, a reduction in CP content of the forage occurs. They attribute this outcome to a diminished rate of protein synthesis within photosynthetic tissues, concomitant with an increase in protease activity, leading to rapid protein degradation62.
It appears that the increase in Ash (ash content) with escalating salinity levels in this study arises from heightened uptake of elements such as Na+, resulting from the increased salt concentrations in plant tissues under elevated salinity. In this context, researchers have posited that the percentage of Ash reflects the mineral content of forage7. When a halophyte is under saline stress, it temporarily increases the concentration of ions like Na+, Cl−, and K+ in the apoplast, the space outside the cell membrane. To tolerate this, the plant increases the water content within its mesophyll cells, which are the main photosynthetic cells in the leaf. This influx of water effectively dilutes the salts and enhances the plant’s ability to absorb these ions from the63. In line with this, the increased ash content in S. sinus persica ecotype resulting from elevated salinity levels has also been corroborated by Ranjbar and Pirasteh-Anosheh64., which is consistent with the results of this study.
The nutritional value of forage plants is assessed based on crude protein and fiber content, organic matter, NDF (neutral detergent fiber) percentage, ADF (acid detergent fiber) percentage, and true dry matter digestibility. The NDF content provides an estimate of the cell wall minus pectin, while the ADF content represents the cell wall minus pectin and hemicellulose. In this context, a decrease in NDF percentage in halophytes such as Lotus ulginosus and Agropyron elongatum has been reported with increasing salinity up to 25 dS.m−165. Moreover, regarding ADF, researchers in another study confirmed an increase in ADF content in the halophyte Lolium multifloru with increasing salinity levels in irrigation water66. However, in agreement with the results of this study, some researchers have reported that salt stress did not significantly affect CF, NDF and ADF content in the forage of different ecotypes of the halophytes S. persica67 and quinoa68. Based on this information, it can be inferred that alterations in crude fiber due to salinity are likely more associated with changes in lignin content, while NDF and ADF are less influenced by salinity stress and are more determined by genetics and plant species.
The Na+ concentration increased with increasing salinity, while K+ decreased. The higher Na+ accumulation and K+ reduction at higher salinity (40 dS.m−1) resulted in the highest Na+/K+ ratio, indicating the detrimental effect of high salinity on ionic balance in S. persica ecotypes. This can reduce plant palatability and nutritive value as forage. The ecotypes showed specific responses to salinity, with Na+ accumulation best described by quadratic polynomial models in Urmia and Bushehr and a linear model for Central Plateau. K+ content decreased with salinity in all ecotypes, and the Na+/K+ ratio increased in a linear pattern. The findings from our study also showed that differences in how the ecotypes absorbed nutrients such as Na+ and K+, as well as the quality of their forage, likely depend on the ecological origins of which they came. The accumulation of Na+ in the plant tissues is a common mechanism for halophytes to tolerate salt stress, while the reduction in K+ can be a consequence of the competition between Na+ and K+ transport channels. In this context, researchers have reported that increasing salinity levels lead to a reduction in K+ and an increase in Na+ within S. rubra plants42 and other halophytes such as Purslane (Portulaca oleracea L.)69. They posited that salinity likely impairs the K+ uptake mechanism by the roots, resulting in a decreased K+ concentration within plant tissues. Furthermore, under saline conditions, the abundance of Na+ ions at the root surface impedes K+ uptake, competing with this element for binding sites within the cells, and causing passive K+ absorption70. Other investigators have also stated in their studies that elevated levels of sodium chloride in the root zone diminish the absorption of nutrients, particularly K+, a finding that aligns with the results of the present research71,72.
Lastly, the EC (electrical conductivity) of the soil extracts significantly increased with increasing salinity, reflecting the experimental design and the impact of applied saline water. As anticipated, the application of increasing saline water concentrations resulted in a linear increase in soil EC across all ecotypes, a direct consequence of the accumulation of soluble ions in the soil solution. However, the variation in the slopes of this relationship, with the Urmia and Bushehr ecotypes showing a greater increase in EC per unit of applied salinity compared to the Central Plateau ecotype, is particularly insightful. This finding suggests that the Urmia and Bushehr ecotypes may have a lower capacity for active salt exclusion from the rhizosphere, or conversely, a higher rate of salt uptake and accumulation in their tissues to maintain osmotic balance. Ecotypes with a robust ability to sequester salts in their vacuoles can reduce the ionic concentration in the surrounding soil, a mechanism critical for survival in hypersaline environments6,15. Therefore, the comparatively steeper increase in soil EC in the Urmia and Bushehr ecotypes could indicate a different, and perhaps less effective, salt management strategy compared to the Central Plateau ecotype.
The results of this study underscore the critical role of genetic and ecological origins in shaping a plant’s specific physiological mechanisms for coping with salinity. This inherent diversity holds significant implications for practical application in saline agriculture. By identifying and utilizing high-performing ecotypes, future breeding programs can be strategically designed to select for elite ecotypes that not only exhibit robust salt tolerance but also possess desirable agronomic traits, such as high forage yield and superior nutritional quality for livestock fodder. This approach is particularly relevant for cultivating Salicornia in coastal, arid, and semi-arid areas, where the use of abundant seawater for irrigation can transform unproductive lands into a viable and profitable agricultural system.
Conclusion
In conclusion, this study underscores the significant variations in salinity tolerance and performance among S. persica ecotypes. The Central Plateau and Urmia ecotypes demonstrated superior forage yield and nutritional quality under moderate salinity (10–20 dS.m−1), highlighting their potential for cultivation in saline environments. These ecotypes exhibited efficient gas exchange and carbon assimilation, coupled with relatively stable water use efficiency, making them suitable candidates for forage production in salt-affected regions. Conversely, while the Bushehr ecotype showed different adaptive strategies under salinity, its lower forage yield and nutritional value under stress conditions suggest its limitations for saline agriculture. Therefore, the findings of this research recommend the selection of Central Plateau and Urmia ecotypes for sustainable forage production in salt-affected areas, particularly where soil salinity ranges from 10 to 20 dS.m−1. These results offer valuable insights into the potential of utilizing halophytes like S. persica for both forage production and soil reclamation, thereby providing a dual benefit for agriculture in arid and semi-arid regions. Therefore, future research should focus on scaling up the cultivation of these superior ecotypes and evaluating their performance under field conditions for practical application.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author (Yazdan Izadi, Email: izady.nike70@gmail.com) on reasonable request.
References
Dos Santos, T. B., Ribas, A. F., de Souza, S. G. H., Budzinski, I. G. F. & Domingues, D. S. Physiological responses to drought, salinity, and heat stress in plants: a review. Stresses 2 (1), 113–135. https://doi.org/10.3390/stresses2010009 (2022).
Balasubramaniam, T., Shen, G., Esmaeili, N. & Zhang, H. Plants’ response mechanisms to salinity stress. Plants 12 (12), 2253. https://doi.org/10.3390/plants12122253 (2023).
Hussain, S. et al. Salinity stress in arid and semi-arid climates: effects and management in field crops. Clim. Change Agric. (CC&A). 13, 201–226. https://doi.org/10.5772/intechopen.87982 (2019).
Rahman, M. M. et al. Adaptive mechanisms of halophytes and their potential in improving salinity tolerance in plants. Int. J. Mol. Sci. 22 (19), 10733. https://doi.org/10.3390/ijms221910733 (2021).
Grigore, M. N. & Vicente, O. Wild halophytes: tools for Understanding salt tolerance mechanisms of plants and for adapting agriculture to climate change. Plants 12 (2), 221. https://doi.org/10.3390/plants12020221 (2023).
Ranjbar, G., Pirasteh-Anosheh, H., Dehghanie, F., Keshtkar, S. & Race, M. Feasibility of growing salicornia species in a coastal environment through planting date and density management in a direct seawater irrigation system. Environ. Sci. Pollut Res. 29 (31), 47800–47809. https://doi.org/10.1007/s11356-022-19089-y (2022).
Ranjbar, G., Khademi, R., Dehghanie, F., Keshtkar, S. & Islam, K. R. Salicornia sinus-persica: a high-yielding species for fodder production with seawater. Arid Land. Res. Manage. 38 (1), 97–108. https://doi.org/10.1080/15324982.2023.2238258 (2024).
Chaturvedi, T., Christiansen, A. H. C., Goł ˛ebiewska, I. & Thomsen, M. H. Salicornia species: current status and future potential. In Future of Sustainable Agriculture in Saline Environments 461–482 (CRC, 2021).
Izadi, Y., Nabipour, M. & Ranjbar, G. H. Growth, development, and flowering responses of salicornia genotypes to photoperiod. Int. J. Veg. Sci. 28 (1), 40–58. https://doi.org/10.1080/19315260.2020.1825149 (2022).
Lin, Z. & Khodayari, H. Genetic characterization of Salicornia persica Akhani (Chenopodiaceae) assessed using random amplified polymorphic DNA. Caryologia. 75 (2), 59–69. https://doi.org/10.36253/caryologia-1493 (2022).
Chatrenoor, T. & Akhani, H. An integrated morpho-molecular study of salicornia (Amaranthaceae‐Chenopodiaceae) in Iran proves Irano‐Turanian region the major center of diversity of annual glasswort species. Taxon 70 (5), 989–1019. https://doi.org/10.1002/tax.12538 (2021).
Akhani, H. Salicornia persica Akhani (Chenopodiaceae), a remarkable new species from central Iran. Linzer Biologische Beitr. 35, 607–612 (2003).
Salehi, M., Dehghany, F. & Ebrahimi, N. G. Successful salicornia seed production using saline water. J. Water Sustainable Dev. (JWSD). 4 (1), 37–46. https://doi.org/10.22067/jwsd.v4i1.56175 (2017).
Araus, J. L. et al. Effect of irrigation salinity and ecotype on the growth, physiological indicators and seed yield and quality of Salicornia Europaea. Plant. Sci. 304, 110819. https://doi.org/10.1016/j.plantsci.2021.110819 (2021).
[Izadi, Y., Nabipour, M. & Ranjbar, G. Investigation on germination and seedling growth of three salicornia species in response to different levels of salinity stress originated from sodium chloride using Gompertz function. Environ. Stress Crop Sci. 15 (1), 231–246. https://doi.org/10.22077/escs.2020.3569.1880 (2022).
Izadi, Y., Nabipour, M. & Ranjbar, G. Photoperiod affects growth, seed production, and biochemical composition in salicornia species under salinity. Plant. Species Biol. 38 (3), 109–130. https://doi.org/10.1111/1442-1984.12403 (2023).
Ventura, Y. et al. Effect of seawater concentration on the productivity and nutritional value of annual salicornia and perennial sarcocornia halophytes as leafy vegetable crops. Sci. Hort. 128 (3), 189–196. https://doi.org/10.1016/j.scienta.2011.02.001 (2011).
Glenn, E. P., O’Leary, J. W., Watson, M. C., Thompson, T. L. & Kuehl, R. O. Salicornia bigelovii torr.: an oilseed halophyte for seawater irrigation. Science 251 (4997), 1065–1067. https://doi.org/10.1126/science.251.4997.1065 (1991).
Amiri, B. et al. Effect of salinity on growth, ion content and water status of glasswort (Salicornia herbacea L). Casp. J. Environ. Sci. 8 (1), 79–87 (2010).
Rathore, A. P., Kumari, A., Chaudhary, D. R. & Rathore, M. S. Phenological and physio-biochemical variations in Salicornia brachiata roxb. Under different soil and water treatments (salinity). Aquat. Bot. 174, 103429. https://doi.org/10.1016/j.aquabot.2021.103429 (2021).
Ranjbar, G., Pirasteh-Anosheh, H., Tafti, S., Nikkhah, M. & M., & Effect of water salinity on biomass, Ash content and some ions concentration in pickleweed (Salicornia bigelovii Torr). Environ. Stress Crop Sci. 14 (4), 1069–1079. https://doi.org/10.22077/escs.2020.3670.1889 (2021).
Alfheeaid, H. A. et al. Salicornia bigelovii, S. brachiata and S. herbacea: their nutritional characteristics and an evaluation of their potential as salt substitutes. Foods 11 (21), 3402. https://doi.org/10.3390/foods11213402 (2022).
Parks, G. E., Dietrich, M. A. & Schumaker, K. S. Increased vacuolar Na+/H+ exchange activity In Salicornia bigelovii torr. In response to NaCl. J. Exp. Bot. 53 (371), 1055–1065. https://doi.org/10.1093/jexbot/53.371.1055 (2002).
Bozorgmehr, A., Asgary, H. R., Farzam, M. & Ranjbar, G. Investigating the effect of using salty water on the accumulation of elements in the aerial organs and the quality of fodder in several salicornia species. Environ. Stress Crop Sci. 17 (2), 319–335. https://doi.org/10.22077/escs.2023.5771.2175 (2024).
Beyrami, H., Rahimian, M. H., Dehghani, F., Ranjbar, G. & Hasheminejhad, Y. Effect of irrigation water salinity on the yield of two salicornia species (Salicornia bigelovii and S. persica). J. Agric. Sci. Sust Prod. 29 (3), 95–111 (2019).
Ventura, Y. & Sagi, M. Halophyte crop cultivation: the case for salicornia and sarcocornia. Environ. Exp. Bot. 92, 144–153. https://doi.org/10.1016/j.envexpbot.2012.07.010 (2013).
Gunning, D. & Cultivating Salicornia europaea (marsh samphire). Dublin, Ireland: Irish Sea Fisheries Board, 4, 1–95. (2016).
Maas, E. V. & Hoffman, G. J. Crop salt tolerance—current assessment. J. Irrig. Drain. Div. 103 (2), 115–134. https://doi.org/10.1061/JRCEA4.0001137 (1977).
Maas, E. V., Hoffman, G. J., Chaba, G. D., Poss, J. A. & Shannon, M. C. Salt sensitivity of corn at various growth stages. Irrig. Sci. 4, 45–57. https://doi.org/10.1007/BF00285556 (1983).
Saeedinia, M., Broomand Nasab, S., Hooshmand, A. R., Mohammadi, S., Andarzian, B. & A., & Applicability of CWSI index for irrigation scheduling of maize using saline water in Ahvaz. Water Soil. Sci. 26 (1–1), 173–185 (2016).
Martínez, J. & Reca, J. Water use efficiency of surface drip irrigation versus an alternative subsurface drip irrigation method. J. Irrig. Drain. Eng. 140 (10), 04014030. https://doi.org/10.1061/(ASCE)IR.1943-4774.000074 (2014).
Khan, M. A. & Weber, D. J. Factors influencing seed germination in Salicornia Pacifica var. Utahensis. Am. J. Bot. 73 (8), 1163–1167. https://doi.org/10.1002/j.1537-2197.1986.tb08562.x (1986).
Abdulrahman, F. S. & Williams, G. J. Temperature and salinity regulation of growth and gas exchange of Salicornia fruticosa (L.) L. Oecologia 48, 346–352. https://doi.org/10.1007/BF00346493 (1981).
Lv, S. et al. Sodium plays a more important role than potassium and chloride in growth of Salicornia Europaea. Acta Physiol. Plant. 34, 503–513. https://doi.org/10.1007/s11738-011-0847-0 (2012).
Ayala, F. & O’Leary, J. W. Growth and physiology of Salicornia bigelovii torr. At suboptimal salinity. Int. J. Plant. Sci. 156 (2), 197–205. https://doi.org/10.1086/297241 (1995).
Reis, A. M., Mendes, A. N. G., Abrahão, J. C. D. R., Santos, M. D. O. & Silva, V. A. Early selection of drought-tolerant Coffea Arabica genotypes at the seedling stage using functional divergence. Pesquisa Agropecuária Trop. 52, e72412. https://doi.org/10.1590/1983-40632022v5272412 (2022).
Lotfi, S., Rouzbehan, Y., Fazaeli, H., Feyzbakhsh, M. T. & Rezaei, J. The nutritional value and yields of Amaranth (Amaranthus hypochondriacus) cultivar silages compared to silage from corn (Zea mays) harvested at the milk stage grown in a hot-humid climate. Anim. Feed Sci. Technol. 289, 115336. https://doi.org/10.1016/j.anifeedsci.2022.115336 (2022).
Hanušovský, O. et al. The nutritional potential of grape by-products from the area of Slovakia and Austria. Emirates J. Food Agric. 32, 1–10. https://doi.org/10.9755/ejfa.2020.v32.i1.2051 (2020).
Asch, J., Johnson, K., Mondal, S. & Asch, F. Comprehensive assessment of extraction methods for plant tissue samples for determining sodium and potassium via flame photometer and chloride via automated flow analysis#. J. Plant. Nutr. Soil. Sci. 185 (2), 308–316. https://doi.org/10.1002/jpln.202100344 (2022).
Lambers, H., Oliveira, R. S., Lambers, H. & Oliveira, R. S. Growth and allocation. Plant Physiol. Ecol, Springer, Cham. 385–449. (2019). https://doi.org/10.1007/978-3-030-29639-1_10
Becker, V. I. et al. Combined effects of soil salinity and high temperature on photosynthesis and growth of Quinoa plants (Chenopodium Quinoa). Funct. Plant. Biol. 44 (7), 665–678. https://doi.org/10.1071/FP16370 (2017).
Khan, M. A., Gul, B. & Weber, D. J. Effect of salinity on the growth and ion content of Salicornia rubra. Commun. Soil. Sci. Plant. Anal. 32 (17–18), 2965–2977. https://doi.org/10.1081/CSS-120000975 (2001).
Katschnig, D., Broekman, R. & Rozema, J. Salt tolerance in the halophyte Salicornia dolichostachya moss: growth, morphology and physiology. Environ. Exp. Bot. 92, 32–42. https://doi.org/10.1016/j.envexpbot.2012.04.002 (2013).
Akcin, A. & Yalçın, E. The effect of salinity on anatomical characteristics of two halophyte species from Turkey. Bot. Lett. 170 (4), 581–590. https://doi.org/10.1080/23818107.2023.2189936 (2023).
Bose, J., Rodrigo-Moreno, A. & Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 65 (5), 1241–1257. https://doi.org/10.1093/jxb/ert430 (2014).
Jamali, S. & Sharifan, H. Investigation the effect of different salinity levels on yield and yield components of Quinoa (Cv. Titicaca). J. Water Soil. Conserv. 25 (2), 251–266. https://doi.org/10.22069/JWSC.2018.13721.2841 (2018).
Riadh, K., Wided, M., Hans-Werner, K. & Chedly, A. Responses of halophytes to environmental stresses with special emphasis to salinity. Adv. Bot. Res. 53, 117–145. https://doi.org/10.1016/S0065-2296(10)53004-0 (2010).
Beyrami, H., Rahimian, M. H. & Dehghany, F. Estimation of water requirement and crop coefficients of two salicornia species in Yazd. J. Water Res. Agric. 34 (3), 401–414. https://doi.org/10.22092/JWRA.2020.342669.784 (2020).
De Souza, M. M., Mendes, C. R., Doncato, K. B., Badiale-Furlong, E. & Costa, C. S. Growth, phenolics, photosynthetic pigments, and antioxidant response of two new genotypes of sea asparagus (Salicornia Neei Lag.) to salinity under greenhouse and field conditions. Agriculture 8 (7), 115. https://doi.org/10.3390/agriculture8070115 (2018).
Altay, V. & Ozturk, M. The genera Salsola and Suaeda (Amaranthaceae) and their value as fodder. Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture, Springer, Cham. 1–12. (2020). https://doi.org/10.1007/978-3-030-17854-3_97-1
Algharib, A. M., Örçen, N. & Nazarian, G. R. Effect of salt stress on plant growth and physiological parameters of common glasswort (Salicornia europaea). Int. J. Biosci. 8 (2), 218–227. https://doi.org/10.12692/ijb/8.2.218-227 (2016).
Homayouni, H. et al. Temporal changes in biochemical responses to salt stress in three salicornia species. Plants 13 (7), 979. https://doi.org/10.3390/plants13070979 (2024).
Pérez-Romero, J. A., Redondo-Gómez, S. & Mateos-Naranjo, E. Growth and photosynthetic limitation analysis of the Cd-accumulator Salicornia Ramosissima under excessive cadmium concentrations and optimum salinity conditions. Plant. Physiol. Biochem. 109, 103–113. https://doi.org/10.1016/j.plaphy.2016.09.011 (2016).
Siddiqui, S. A., Khatri, K., Patel, D. & Rathore, M. S. Photosynthetic gas exchange and chlorophyll a fluorescence in Salicornia brachiata (Roxb.) under osmotic stress. J. Plant. Growth Regul. 41, 429–444. https://doi.org/10.1007/s00344-021-10311-8 (2022).
Lovelock, C. E. & Ball, M. C. Influence of salinity on photosynthesis of halophytes. In Salinity: environment-plants-molecules 315–339 (Springer Netherlands, 2002). https://doi.org/10.1007/0-306-48155-3.
Marco, P. & Carvajal, M. Del Carmen Martinez-Ballesta, M. Efficient leaf solute partioning in Salicornia fruticosa allows growth under salinity. Environ. Exp. Bot. 157, 177–186. https://doi.org/10.1016/j.envexpbot.2018.10.001 (2019).
Gulzar, S., Hussain, T., Gul, B. & Hameed, A. Photosynthetic adaptations and oxidative stress tolerance in halophytes from warm subtropical region. In Handbook of Halophytes (ed. Grigore, M. N.) (Springer, 2020). https://doi.org/10.1007/978-3-030-17854-3_52-1.
Gandonou, C. B., Prodjinoto, H., Wouyou, A. D., Lutts, S. & Montcho, D. H. Effects of salinity stress on growth in relation to gas exchanges parameters and water status in Amaranth (Amaranthus cruentus). Int. J. Plant. Physiol. Biochem. 10 (3), 19–27. https://doi.org/10.5897/IJPPB2018.0280 (2018).
Rasouli, F. et al. A comparative analysis of stomatal traits and photosynthetic responses in closely related halophytic and glycophytic species under saline conditions. Environ. Exp. Bot. 181, 104300. https://doi.org/10.1016/j.envexpbot.2020.104300 (2021).
Hussin, S., Geissler, N. & Koyro, H. W. Effect of NaCl salinity on Atriplex nummularia (L.) with special emphasis on carbon and nitrogen metabolism. Acta Physiol. Plant. 35, 1025–1038. https://doi.org/10.1007/s11738-012-1141-5 (2013).
Eisa, S., Hussin, S., Geissler, N. & Koyro, H. W. Effect of NaCl salinity on water relations, photosynthesis and chemical composition of Quinoa (‘Chenopodium quinoa’Willd.) as a potential cash crop halophyte. Aust. J. Crop Sci. 6 (2), 357–368. https://doi.org/10.3316/informit.054900975531667 (2012).
Waldron, B. L. et al. Salinity reduces the forage quality of forage kochia: A halophytic Chenopodiaceae shrub. Rangel. Ecol. Manage. 73 (3), 384–393. https://doi.org/10.1016/j.rama.2019.12.005 (2020).
Flowers, T. J., Munns, R. & Colmer, T. D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 115 (3), 419–431. https://doi.org/10.1093/aob/mcu217 (2015).
Ranjbar, G. H. & Pirasteh-Anosheh, H. Comparison of the accumulation of elements, Ash content and biomass of some halophytes species under irrigating with sea water. Desert Manage. 7 (14), 63–74. https://doi.org/10.22034/JDMAL.2020.38476 (2020).
Robinson, P. H. et al. Biomass accumulation and potential nutritive value of some forages irrigated with saline-sodic drainage water. Anim. Feed Sci. Technol. 111 (1–4), 175–189. https://doi.org/10.1016/S0377-8401(03)00213-X (2004).
Ben-Ghedalia, D. et al. Effect of water salinity on the composition and in vitro digestibility of winter-annual ryegrass grown in the Arava desert. Anim. Feed Sci. Technol. 91 (3–4), 139–147. https://doi.org/10.1016/S0377-8401(01)00218-8 (2001).
Moghaddam, A., Larijani, H. R., Oveysi, M., Moghaddam, H. R. T. & Nasri, M. Alleviating the adverse effects of salinity stress on salicornia persica using sodium Nitroprusside and potassium nitrate. BMC Plant. Biol. 23 (1), 166. https://doi.org/10.1186/s12870-023-04179-x (2023).
Kaya, E. & Aydemir, S. K. Determining the forage yield, quality and nutritional element contents of Quinoa cultivars and correlation analysis on these parameters. Pak J. Agric. Sci. 57 (2), 311–317. https://doi.org/10.21162/PAKJAS/20.7229 (2020).
Rahimi, Z. & Kafi, M. Effects of salinity and silicon application on biomass accumulation, sodium and potassium content of leaves and roots of purslane (Portulaca Oleracea L). J. Water Soil. 24 (2), 374–367. https://doi.org/10.22067/jsw.v0i0.3253 (2010).
Baghalian, K., Haghiry, A., Naghavi, M. R. & Mohammadi, A. Effect of saline irrigation water on agronomical and phytochemical characters of chamomile (Matricaria recutita L). Sci. Hort. 116 (4), 437–441. https://doi.org/10.1016/j.scienta.2008.02.014 (2008).
Ragel, P., Raddatz, N., Leidi, E. O., Quintero, F. J. & Pardo, J. M. Regulation of K+ nutrition in plants. Front. Plant. Sci. 10, 281. https://doi.org/10.3389/fpls.2019.00281 (2019).
Ehtaiwesh, A. F. The effect of salinity on nutrient availability and uptake in crop plants. J. Appl. Sci. 9, 55–73 (2022). https://search.emarefa.net/detail/BIM-1570585
Funding
This research has been funded by Research Vice Chancellor of Shahid Chamran University of Ahvaz, Ahvaz, Iran (Grant Number: SCU.AA98.336).
Author information
Authors and Affiliations
Contributions
Y.I. writing – original draft, visualization, methodology, investigation, formal analysis, data curation. M.N. writing, review & editing, supervision, methodology, funding acquisition, project management. G.R. writing, review & editing, visualization, methodology, investigation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Izadi, Y., Nabipour, M. & Ranjbar, G. Variations in growth, physiology and fodder quality among salicornia persica ecotypes irrigated with persian gulf seawater. Sci Rep 15, 28850 (2025). https://doi.org/10.1038/s41598-025-15008-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15008-6