Abstract
This study examines the feeding ecology and trophic dynamics of the critically endangered large yellow croaker (Larimichthys crocea) in the differing habitats of Hong Kong and Taiwan using 18S gut content metabarcoding and stable isotope analysis. As a top predator, the large yellow croaker plays a crucial role in regulating fish populations and maintaining balance in the marine ecosystem. We found isotopic niche differences between juveniles and adults, with juveniles consuming more planktonic prey and adults more benthic species. In the more disrupted waters of Hong Kong, disrupted ontogenetic trophic transitions were observed, as adults exhibited unexpectedly low stable nitrogen isotope values, indicating prolonged consumption of prey at low trophic levels. In contrast, the relatively less impacted waters of Taiwan showed normal ontogenetic trophic transition. These findings highlight the impact of habitat degradation on large yellow croaker and underscore the urgent need for conservation measures, including stricter fishing regulations and habitat protection, to preserve this essential species and its ecological role.
Similar content being viewed by others
Introduction
Anthropogenic pressures such as overfishing, habitat destruction, and pollution have significantly altered trophic dynamics across ecosystems, leading to profound dietary shifts in various species. These pressures also complicate ontogenetic trophic transition, where fish typically occupy higher trophic positions as they mature1. Ontogenetic shifts in diet preferences are essential for the growth, survival, and reproductive success of fish, influencing population dynamics and ecosystem health. Juvenile fish generally consume smaller, lower trophic-level prey, while adults transition to larger, higher trophic-level prey2. For example, juvenile Atlantic cod (Gadus morhua) primarily feed on zooplankton and small invertebrates, whereas adults shift to a diet dominated by fish and larger invertebrates3. However, disruptions in habitats can have profound implications for ontogenetic trophic transitions. For instance, in the case of the Nassau grouper (Epinephelus striatus), overfishing has led to a significant reduction in adult populations, causing juveniles to remain at lower trophic levels due to a lack of adult guidance and competition4.
The South China Sea, a major component of the western Pacific Ocean, encompasses the coastlines of multiple countries, including China, the Philippines, Brunei, Indonesia, Singapore, Malaysia, and Vietnam, covering an area of approximately 3,500,000 km2. This semi-enclosed marginal sea is renowned for its rich biodiversity, hosting over 22,629 marine species, including a vast array of crustaceans, molluscs, and fish5,6,7,8,9,10. Within this vast and diverse marine region lie Hong Kong and Taiwan, which boast their unique marine environments.
Hong Kong’s waters, covering an area of 1640.4 km211 and home to over 1260 fish species12, are divided into a western estuarine zone influenced by the Pearl River Estuary and an eastern oceanic zone shaped by ocean currents13. Seasonal currents bring diverse species to these waters. However, this environment is threatened by coastal development, pollution, overfishing, and biological invasions, leading to habitat loss and fragmentation14. Overfishing has caused the decline of many fish species, with some, such as the Chinese bahaba (Bahaba taipingensis), now critically endangered15.
Taiwan, comprising one main island and multiple offshore islands, boasts over 3200 documented fish species due to its unique geographic location on the edge of the continental shelf and at the intersection of different ocean currents, which contribute to its rich fishery resources8,16,17,18. With the Taiwan Strait to its west and the vast Pacific Ocean to its east, Taiwan’s waters are influenced by the East China Sea coastal current and the Kuroshio current, which transport diverse marine species from both the north and south19.
The large yellow croaker (Larimichthys crocea), a species of considerable economic importance within the family Sciaenidae, has historically been a key fishery resource in East Asia, particularly along the coasts of the South China Sea, East China Sea, and Yellow Sea20. This species plays a significant role in local fisheries and marine ecosystems21. Despite its importance, the large yellow croaker has experienced dramatic population declines due to overfishing, habitat degradation, and environmental pollution22, leading to its classification as critically endangered on the International Union for Conservation of Nature (IUCN) Red List23.
This study aims to investigate the trophic dynamics of juvenile and adult large yellow croaker populations in two distinct regions: the polluted and degraded waters of Hong Kong and the comparatively less impacted waters of Taiwan. By employing both gut content DNA metabarcoding and stable isotope analysis, we aim to provide a comprehensive understanding of the dietary habits and trophic positions of the large yellow croaker across different life stages and geographic regions. Additionally, potential prey items, including fish and marine invertebrates, were collected and analyzed for stable carbon and nitrogen isotope signatures to elucidate the trophic interactions within Hong Kong’s marine food web.
Understanding the ecological consequences of anthropogenic pressures on marine ecosystems is crucial for developing effective conservation and management strategies. The large yellow croaker populations in Hong Kong and Taiwan provide a unique opportunity to study the impacts of environmental degradation on marine food webs. This research will explore potential shifts in trophic dynamics and their broader implications for marine biodiversity. The findings will highlight the urgent need for measures to protect and restore vulnerable marine species and their habitats, providing critical insights essential for predicting and mitigating the impacts of global environmental change on marine biodiversity.
Results
Morphometric data of large yellow croaker
In this study, 73 large yellow croaker specimens were collected from six different sites in Hong Kong. The mean total length (TL), standard length (SL), and weight ± S.D. of the 53 juvenile specimens from sites SKC, HLC, and SKI were 17.22 ± 1.85 cm (range: 14.7–23.3 cm), 13.61 ± 1.75 cm (range: 11.0–19.0 cm), and 45.90 ± 16.83 g (range: 24.8–103.6 g), respectively. For the 20 adult specimens from sites TYI, MWI, and TLC, these measurements were 43.27 ± 3.96 cm (range: 38.3–55.1 cm), 36.13 ± 3.69 cm (range: 32.0–47.5 cm), and 704.56 ± 226.90 g (range: 429.0–1491.3 g).
In Taiwan, 24 large yellow croaker specimens were collected from two sites. For the 19 juvenile specimens, the mean total length (TL), standard length (SL), and weight ± S.D. were 29.68 ± 4.75 cm (range: 20.1–35.3 cm), 24.50 ± 4.08 cm (range: 15.8–29.0 cm), and 275.05 ± 110.79 g (range: 76.0–440.0 g), respectively. For the five adult specimens, these measurements were 43.22 ± 2.91 cm (range: 39.1–46.4 cm) for TL, 36.81 ± 2.93 cm (range: 33.3–40.5 cm) for SL, and 752.80 ± 141.86 g (range: 564.0–912.0 g) for weight.
Among the adult specimens collected in Hong Kong, two males and 18 females were identified, with females being predominant across all sampling months. The mean gonad length and weight ± S.D. for males were 13.95 ± 0.07 cm (range: 13.9–14.0 cm) and 3.24 ± 0.32 g (range: 3.01–3.46 g), respectively, while for females, these values were 15.50 ± 3.16 cm (range: 11.0–23.5 cm) and 61.12 ± 40.92 g (range: 26.00–197.84 g). In Taiwan, the mean gonad length and weight ± S.D. of the two female specimens were 17.85 ± 0.49 cm (range: 17.5–18.2 cm) and 86.00 ± 19.80 g (range: 72.00–100.00 g).
Gonadal examinations revealed that in Hong Kong, the male testes were in stage IV (milky white), while the female ovaries were in stages IV and V (large, yellow-pigmented eggs), indicating readiness for spawning in late November and December. Similar developmental stages were observed in Taiwan. The gonadosomatic index (GSI) values in Hong Kong were 0.48 and 0.5 for males in October and November, respectively, while for females, the values were 7.79 (September), 7.25 (October), 10.53 (November), and 13.27 (December), indicating an increasing trend towards the breeding season. In Taiwan, the GSI value for females in October was 10.86, slightly higher than that of females in Hong Kong during the same month.
Stable isotope analysis, isotopic variation, trophic niche metrics, and trophic positions of large yellow croaker in Hong Kong and Taiwan
For juvenile large yellow croakers in Hong Kong, the stable carbon isotope values ranged from − 20.33 to − 17.22‰, with a mean value of − 18.36‰ (Table 1; Supplementary Table 1). The stable nitrogen isotope values ranged from 11.60 to 13.40‰, with a mean value of 12.40‰. In contrast, adult large yellow croakers in Hong Kong exhibited stable carbon isotope values ranging from − 17.3 to − 16.52‰, with a mean value of − 16.91‰, and stable nitrogen isotope values ranging from 11.26 to 12.70‰, with a mean value of 11.72‰. The stable carbon isotope values were slightly more enriched in the adult fish, while the stable nitrogen isotope values unexpectedly fell within the range observed in the juvenile fish and even exhibited lower values. The mean values of C:N ratios for juvenile and adult fish were 3.71 and 3.61, respectively.
For juvenile large yellow croakers in Taiwan, the stable carbon isotope values ranged from − 16.35 to − 15.30‰, with a mean value of − 15.72‰. The stable nitrogen isotope values ranged from 11.88 to 14.60‰, with a mean value of 12.98‰. Adult large yellow croakers in Taiwan showed stable carbon isotope values ranging from − 16.00 to − 15.23‰, with a mean value of − 15.60‰, and stable nitrogen isotope values ranging from 11.90 to 14.71‰, with a mean value of 13.63‰. The C:N ratios for both life stages of fish in Taiwan were lower than those in Hong Kong, with mean values of 3.10 for juveniles and 3.17 for adults.
Based on the bivariate isotope plots, fish in Hong Kong show a leftward offset towards lower stable carbon isotope values, whereas fish in Taiwan exhibit a rightward offset towards higher stable carbon isotope values (Fig. 1a,b). Adult fish in Taiwan represent the highest stable nitrogen isotope values among all the fish specimens collected from both locations.
Bivariate isotope plots for large yellow croaker specimens illustrating (a) δ13C and δ15N values, and (b) the mean and standard deviation (± SD) of these values. (c) A biplot of the corrected standard ellipse area (SEAc), which represents the core isotopic niche sizes adjusted for small sample sizes, and (d) a density box plot of the Bayesian standard ellipse area (SEAb), illustrating the distribution of core isotopic niche sizes estimated using Bayesian inference, depict the isotopic niche sizes for different life stages of large yellow croaker specimens from Hong Kong and Taiwan. Solid lines show standard ellipse area (40% of the data) and dashed lines show total convex hull area (100% of the data). Coloured boxes represent 50%, 75%, and 95% credible intervals, while central black dots and grey ‘X’s represent modes and the SEAc point, respectively.
The trophic niche metrics, including the total area (TA), Bayesian standard ellipse area (SEAb), and corrected standard ellipse area (SEAc), for juvenile fish in Hong Kong (3.28‰2, 0.71‰2, and 0.73‰2, respectively) were notably higher than those for adult fish (0.78‰2, 0.21‰2, and 0.23‰2, respectively), with 0% isotopic niche overlap, suggesting a more varied diet and a wider dietary niche in juveniles (Fig. 1c,d). The trophic niche metrics for juvenile fish in Taiwan (2.12‰2, 0.84‰2, and 0.89‰2, respectively) were also higher than those for adult fish (0.42‰2, 0.42‰2, and 0.55‰2, respectively), with 29.7% isotopic niche overlap, indicating a highly varied diet and a wider dietary niche in juveniles.
A significant positive relationship between fish body size and stable carbon isotope value was observed in fish from Hong Kong (R2 = 0.532, p < 0.001), suggesting a decreasing reliance on pelagic sources and an increasing reliance on benthic sources as the fish grow, whereas a weak positive relationship was found in fish from Taiwan (R2 = 0.109, p = 0.064), indicating a limited dietary shift from pelagic to benthic sources with growth (Fig. 2a,b). Regarding stable nitrogen isotope values, a significant negative relationship with fish body size was detected in Hong Kong specimens (R2 = 0.314, p < 0.001), indicating a dietary transition toward prey at lower trophic levels as the fish grow (Fig. 2c). In contrast, a positive relationship was observed in fish from Taiwan (R2 = 0.171, p = 0.026), reflecting an increasing consumption of prey at higher trophic levels during growth (Fig. 2d).
In general, scatterplots reveal that as age and body size increase, fish in Hong Kong exhibit enrichment in stable carbon isotope values but depletion in stable nitrogen isotope values. In contrast, fish in Taiwan exhibit enrichment in both stable carbon and nitrogen isotope values. The variation in stable carbon isotope values indicates an ontogenetic dietary transition from pelagic to benthic sources. Higher stable nitrogen isotope values, resulting from consuming prey at higher trophic levels, are observed in adult fish in Taiwan, but surprisingly not in those in Hong Kong.
Composition of trawl survey catch, stable isotope analysis of potential prey items, and trophic interactions in the Hong Kong marine food web
In the trawling survey, Decapoda and fish were the major catch groups, accounting for 41.93% and 35.53% of the total catch, respectively. The Decapoda catch included at least 85 species from 46 genera and 23 families. The fish catch comprised 109 species from 68 genera and 39 families, none of which were migratory. Other catches included barnacles (0.09%), bivalves (1.59%), brittle stars (0.65%), cephalopods (0.53%), feather stars (0.01%), gastropods (6.36%), polychaetes (0.14%), sea anemones (0.34%), sea cucumbers (1.06%), sea pens (7.73%), sea urchins (0.08%), corals (0.19%), starfish (0.01%), stomatopods (3.74%), and tunicates (0.03%).
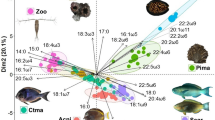
47 selected invertebrate and 43 fish samples from trawling were analyzed for stable isotope analysis (Supplementary Fig. 1; Supplementary Table 2). These samples were classified into various groups, including at least two species of Cnidarians (one coral species and at least one sea anemone species), 22 species of Crustaceans (one barnacle species, 19 Decapoda species, and three Stomatopoda species), one species of echinoderm (at least one sea cucumber species), 43 fish species, 13 species of Mollusks (six Bivalvia species, two Cephalopoda species, and five Gastropoda species), and at least three species of sea pens (Table 2; Supplementary Table 3).
Among the potential food items analyzed, echinoderms exhibited the lowest mean stable carbon isotope value (δ13C) at − 18.48‰, whereas sea pens had the highest mean stable carbon isotope value at − 13.80‰ (Table 2). In terms of stable nitrogen isotope values (δ15N), sea pens recorded the lowest mean value at 6.09‰, while fish showed the highest mean value at 11.47‰ (Table 2).
The bivariate isotope plot indicates that large yellow croakers of both life stages occupy the highest positions due to their enrichment in stable nitrogen isotope values (Fig. 3). Furthermore, marine invertebrates such as Cnidarians, Crustaceans, Echinoderms, Mollusks, and sea pens exhibit relatively lower stable nitrogen isotope values (below 11.00‰) compared to fish. These invertebrate groups share similar mean stable carbon isotope values, ranging from − 18.48 to − 13.80‰.
Diet analysis of large yellow croaker
The 18S rRNA V9 barcoding diet analysis revealed that juvenile large yellow croakers in Hong Kong have a diverse diet, with 20 prey groups identified (Fig. 4a). The most common prey groups were Polychaeta (20.00%), fish (18.67%), and Decapoda (17.33%). Other significant prey included Copepoda (13.33%) and Isopoda (4.00%). Adults have a less diverse diet, comprising 10 prey groups, with an increased reliance on fish (38.10%) and Decapoda (19.05%), as well as consuming Isopoda, Amphipoda, comb jellies, jellyfish, and Mysida. In Taiwan, juvenile large yellow croakers have a diet with 10 prey groups, primarily fish (40.00%) and Decapoda (20.00%), followed by Bivalvia (10.00%), Cephalopoda (6.67%), and Gastropoda (6.67%). Adults have a less diverse diet with four prey groups, predominantly fish (66.67%) and Decapoda (16.67%).
Juvenile large yellow croakers in Hong Kong have the highest number of reads, identifying at least 75 prey species. Fish dominate their diet (74.07%), followed by Decapoda (18.92%) and Mysida (4.29%) (Fig. 4b). Adult fish have at least 21 prey species, with fish making up 98.93% of their diet. In Taiwan, juveniles have at least 29 prey species, with fish (54.93%) and Cephalopoda (21.28%) being the most abundant. Adults have at least 11 prey species, primarily fish (91.97%).
A shift in diet from planktonic to benthic prey species was observed in both locations, corresponding to distinct differences in diet composition between juveniles and adults (Fig. 5). Juvenile fish in Hong Kong consumed a mixture of planktonic and benthic species but fed exclusively on benthic species during the adult stage. During the juvenile stage, they relied not only on pelagic fish Engraulis sp. and planktonic mysid Rhopalophthalmus sp., but also on demersal fish Pagrus major and benthic shrimp Sicyonia sp. In the adult stage, their diet was dominated by the demersal fish Pagrus major, with some benthic shrimp Sicyonia sp. and planktonic mysid Rhopalophthalmus sp. Predation on planktonic organisms such as copepods and jellyfish were largely reduced. Juvenile and adult fish in Taiwan consumed small proportions of planktonic species and large proportions of benthic species, gradually increasing their reliance on benthic species. Juvenile fish relied on demersal fish of the families Derichthyidae and Cynoglossidae, and benthic cephalopod Euprymna sp. They also showed reliance on planktonic species such as Anostraca (branchiopod) and mysid Gastrosaccus sp. Adult fish greatly increased reliance on demersal fish in the family Derichthyidae, and slightly increased reliance on the demersal fish in the family Cynoglossidae, with some benthic gastropods in the order Neogastropoda.
The NMDS plots were supplemented by SIMPER analysis, which identified an overall dietary dissimilarity of 73.43% between juvenile and adult fish in Hong Kong, primarily driven by fish, which contributed 67.99% to this difference (Fig. 6a; Table 3). In Taiwan, the dietary dissimilarity was 50.04%, primarily due to fish, which accounted for 53.04% (Fig. 6b; Table 3). Although ANOSIM results indicated non-significant dietary differences between juvenile and adult fish in Hong Kong (Global R = 0.025, p = 0.305) and Taiwan (Global R = -0.31, p = 0.895), these findings are likely influenced by the extremely small and uneven sample sizes rather than a true absence of dietary differences. The sample sizes were notably imbalanced, with 53 juvenile fish compared to 20 adult fish in Hong Kong, and six juvenile fish versus two adult fish in Taiwan. Such small and unequal sample sizes reduce the statistical power of ANOSIM, making it difficult to detect significant dietary differences even if they exist. Additionally, the negative R-value in Taiwan (− 0.31) suggests that within-group dissimilarity was more significant than between-group dissimilarity, further highlighting the limitations posed by the small sample size. Therefore, despite the non-significant ANOSIM results, the substantial dissimilarity percentages from SIMPER suggest that there are meaningful dietary differences between juvenile and adult fish in Hong Kong and Taiwan.
Discussion
This study is the first to reveal disrupted ontogenetic trophic transition in marine fish, specifically in the large yellow croaker populations of Hong Kong. By uncovering these unexpected patterns in stable nitrogen isotope values, our research provides novel insights into the complexities of marine food webs and the impacts of environmental pressures on fish dietary habits.
Dietary niche divergence is a common strategy among marine organisms to minimize predation risk during growth24. In this study, significant variations in stable carbon isotope values and distinct differences in diet composition indicate a clear dietary niche divergence and a shift in habitat utilization among different life stages of large yellow croakers in both Hong Kong and Taiwan. The findings revealed that as the croakers grow, their foraging behavior changes from consuming planktonic prey species to benthic prey species, leading to distinct dietary differences between juvenile and adult stages. The gut content analysis showed that juveniles primarily consumed Decapoda and a mix of pelagic and demersal fish species, indicating near-surface and bottom foraging in shallow waters with a mobile foraging strategy. In contrast, adults primarily consumed demersal fish species, reflecting a stationary foraging strategy near the substrate in deeper waters. This shift in diet and habitat utilization underscores the importance of habitat connectivity for the species’ lifecycle. Additionally, the large yellow croaker transitions from a carnivorous mesopredator in its juvenile stage to a piscivorous predator in adulthood, underscoring its role in regulating fish populations and establishing its significance as a key predator within its ecosystem.
Based on DNA metabarcoding results, it was expected that increased consumption of Pagrus major, a predatory carnivorous fish with high stable nitrogen isotope values due to its high trophic level, would lead to higher stable nitrogen isotope values in adult croakers compared to juveniles. Notably, the stable nitrogen isotope value exhibited a significant decreasing trend with age. This discrepancy may be attributed to methodological differences: DNA metabarcoding reflects short-term dietary intake (days to weeks), while stable nitrogen isotope values from muscle tissue represent longer-term dietary patterns (weeks to months or years) and trophic level estimations25,26. Furthermore, DNA metabarcoding cannot differentiate the developmental stages (and hence, variations in nitrogen sources) of prey through molecular approaches27. It is plausible that this limitation affects our understanding of the dietary contributions of prey to predators’ diets and their trophic positions within the food web. Adult croakers captured between September and December may consume P. major eggs or newly hatched juveniles instead of larger, mature individuals, resulting in lower stable nitrogen isotope values. An increase in the gonado-somatic index of mature P. major was observed during this period, indicating the onset of spawning and the provisioning of eggs and juveniles28.
Our study provides evidence of disrupted ontogenetic trophic transition in the local marine food web, highlighted by the unexpectedly low stable nitrogen isotope values in adult large yellow croakers (averaging 11.72‰) compared to juveniles (averaging 12.40‰) in Hong Kong. In contrast, this unusual pattern was not observed in the same fish population in Taiwan, where stable nitrogen isotope values increased from an average of 12.98‰ in juveniles to 13.63‰ in adults. Factors such as overfishing, environmental degradation from coastal construction, and inadequate marine management have contributed to the decline of apex predators in the region. Typically, a positive relationship is expected between stable nitrogen isotope values and fish body size due to increased energy requirements29. Notably, for fish in the family Sciaenidae, stable nitrogen isotope values generally increase with age and body size, as observed in species like Sciaenops ocellatus and Pennahia argentata30,31. However, our study found a weak and even declining shift in stable nitrogen isotope values with growth, indicating disrupted ontogenetic trophic transition. Such prey-switching behavior could reduce nutritional quality and energy intake, potentially impacting the overall nutritional status of the large yellow croaker32. More importantly, disrupted ontogenetic trophic transition reflects a shortening of the food chain, ultimately simplifying the food web structure and weakening the ecosystem33.
Unusual depletions in stable nitrogen isotope values with age have been reported in freshwater ecosystems due to invasive species or changes in water management practices, such as pond emptying. For instance, archaeological roach fish (Rutilus rutilus) from Belgian sites with a standard length of 30 cm showed enriched stable carbon isotope values but depleted stable nitrogen isotope values compared to those under 20 cm34. Typically, stable nitrogen isotope values increase with age due to dietary shifts from planktonic crustaceans and plants in juveniles to mollusks in adults. The authors suggested that regular pond emptying inhibited mollusk growth, altering adult diets to plant feeders. Similarly, lake whitefish (Coregonus clupeaformis) in the Great Lakes had depleted stable nitrogen isotope values after dreissenid mussel invasions35. Comparing historical isotopic signatures (1947–1997) with modern ones (2001–2005) showed a significant drop in stable nitrogen isotope values. This was attributed to the mussels’ impact on the abundance of the lake whitefish’s prey, Diporeia sp., causing a dietary shift from the profundal to the pelagic zone, thus decreasing their stable nitrogen isotope values over time.
Using trawling to collect potential prey items for the large yellow croaker proved to be limited, as it did not align with the prey species identified through DNA metabarcoding, except for the Decapoda Charybdis (Charybdis) japonica. This method was ineffective for capturing planktonic or gelatinous prey, such as Amphipoda, Copepoda, and comb jellies, due to the net’s inappropriate size for small or surface-dwelling organisms.
Despite mariculture and restocking efforts since 1987, these initiatives have failed to restore large-scale spawning and have reduced the genetic diversity of large yellow croaker36,37. Such inadequate practices have highlighted the disconnect between mariculture and fishery management, which fails to address overfishing20. To protect the remaining wild population, we recommend shifting focus to management and enforcement to reduce fishing pressure. Establishing marine parks or reserves in the southern waters of Hong Kong, such as Shek Kwu Chau and Hei Ling Chau, is crucial for protecting nursery grounds and spawning groups. These areas are vital habitats for both juvenile and adult fish, and effective management will enhance their growth, survival, and reproductive success. Marine protected areas have proven effective in conserving species and mitigating ecological impacts38.
Regulating minimum catch and mesh sizes is necessary to prevent overexploitation. Implementing a 55 mm square-mesh cod-end can help avoid capturing undersized individuals, thereby promoting more sustainable fishing practices, as opposed to the current 255 mm minimum catch size adopted in Sansha Bay39. Continuous management and evaluation are crucial to ensure compliance with these regulations. Additionally, implementing a fishing moratorium from September to February could protect the spawning large yellow croaker and other species like seabream and grunts during their active months40. Although a moratorium currently exists in the South China Sea from June to August, extending it to cover the autumn spawning peak is strongly recommended.
Conclusions
Our study highlights that a disrupted ontogenetic trophic transition is a significant indicator of the large yellow croaker’s critically endangered status, revealing ecological stressors and environmental changes affecting the species. These alterations, observed in the Hong Kong population but not in the less urbanized and disturbed Taiwan population, underscore the specific threats like habitat degradation, pollution, and food scarcity faced by different life stages of the croaker. To further investigate these patterns, we recommend stable isotope analysis on both juvenile and adult fish from various geographic stocks. This will help identify if similar disruptions occur elsewhere and provide deeper insights into the species’ feeding ecology and environmental challenges. Understanding these shifts is crucial for developing targeted conservation strategies, such as habitat restoration, implementing fishing moratoriums, regulating minimum catch and mesh sizes, and establishing marine protected areas to aid in the recovery of this increasingly rare species.
Materials and methods
Specimen collection for large yellow croaker
Large yellow croaker (Larimichthys crocea) specimens were sourced from local fishermen in Hong Kong (HK) and Taiwan (TW) (Supplementary Fig. 2). In Hong Kong, specimens were collected between June and December 2021 (Table 4). During June and July 2021, 53 juvenile croakers were caught nearshore (6.4 m to 17.3 m depth) using gill nets and hand lines at northwest Shek Kwu Chau (SKC), east Hei Ling Chau (HLC), and southwest Soko Island (SKI) (Supplementary Fig. 2). From September to December 2021, 20 adult croakers were caught in deeper waters (32 m to 42 m depth) near west Tsing Yi Island (TYI), south Ma Wan Island (MWI), and south Tang Lung Chau (TLC) .
In October 2021, two adult croakers were purchased from Long Fong Fishing Port (LFP), Taiwan, and caught using set nets. In August 2024, six juvenile croakers were caught near Kinmen Island (KMI), Taiwan, using hand lines. All specimens were labeled and frozen at -20 °C upon arrival at the laboratory. Additionally, three adults and 13 juveniles collected from LFP between February and October 2020 were included for isotope analysis. These specimens were not included in the gut content analysis.
Specimen collection and trawling survey for potential prey items
The potential food items, including fish and marine invertebrates, were sampled in Hong Kong’s southern and southwestern waters between April and May 2022 using the trawling method with a scientific trawling permit (R1710058). The sampling area encompassed Hong Kong sites with latitudes ranging from 22° 18ʹ 96ʺ to 22° 16ʹ 66ʺ and longitudes from 114° 08ʹ 16ʺ to 114° 18ʹ 41ʺ.
A commercial shrimp trawler with a 15 m outrigger was used to tow 4 to 8 nets (net width: 2 m; stretched mesh size: 2 cm) along the sea floor. For each site, two transects were trawled during daylight at a speed of 6 km/h for 10 min, creating a total swept area of 0.22 km2 (ranging from 0.008 to 0.016 km2). Catches from each transect were roughly sorted and stored on ice on board and then frozen at − 20 °C upon transport to the laboratory. Identification and measurement of all organisms were completed within 48 h to avoid altering stable isotope signatures41.
Morphometric measurements for large yellow croaker and potential prey items
Potential prey items were identified to the lowest possible taxonomic level based on morphological characteristics. Each specimen was measured to the nearest 0.1 cm and weighed to the nearest 0.1 g with an electronic balance. Fish specimens were measured for total length (TL) and standard length (SL). For marine invertebrates, including coral, sea anemones, barnacles, stomatopods, sea cucumbers, bivalves, gastropods, and sea pens, total length (TL) was recorded, while decapods were measured for carapace length (CL) and cephalopods were measured for mantle length (ML). Any observable wounds, such as tail or claw loss, were recorded.
The life stage and sex of large yellow croaker specimens were determined by examining their gonads. These gonads were classified into stages, with immature juveniles exhibiting thin, glassy, and colourless gonads42,50. Mature males have milk‐white gonads, while mature females have yellow gonads with visible eggs. Gonads were extracted, measured for length to the nearest 0.1 cm, and weighed to the nearest 0.1 g. The gonadosomatic index (GSI) was calculated using:
Stable isotope analysis
Stable carbon and nitrogen isotopes are commonly used to estimate predators’ long‐term dietary patterns43. Stable carbon isotopes identify major food sources, while stable nitrogen isotopes indicate the trophic level of consumers within the food web. These isotopes are expressed in delta notation (‰) relative to international standards. Approximately 0.5 g of dorsal muscle tissue above the lateral line from fish, abdominal muscle tissue from crustaceans, and foot muscle tissues from gastropods and bivalves were extracted and stored at − 20 °C. Fish (except large yellow croaker) and marine invertebrates were grouped into broad taxonomic categories at the order level.
All tissue samples were freeze‐dried at ‐80 °C for at least 48 h, then ground into homogeneous powder using a mortar and pestle before analysis. Samples were weighed to approximately one mg and placed in tin capsules for stable carbon (δ13C) and nitrogen (δ15N) isotope analysis using a Eurovector Elemental Analyser (Isomass Scientific Inc., Alberta, Canada) coupled with a Nu Perspective Isotope Ratio Mass Spectrometer (Nu Instruments Ltd., Wrexham, U.K.). A mathematical normalization technique was applied to avoid errors introduced by more negative stable carbon isotope values caused by lipid content accumulation in muscle tissue when the C:N ratio was equal to or larger than 3.5: δ13Cnormalized = δ13Cuntreated – 3.32 + 0.99 × C:N44. To estimate the isotopic niche sizes and overlaps of large yellow croaker between life stages, corrected standard ellipse areas (SEAc) and Bayesian standard ellipse areas (SEAb) were calculated using the package ‘SIBER’ in R via RStudio45.
Gut content analysis
Gut content samples were sent to the Beijing Genomics Institute (BGI) for meta-rDNA extraction and 18S amplicon PE300 sequencing on the HiSeq 2500 platform to observe species-specific genomic sequences. Previous studies on the diets of large yellow croakers revealed them to be carnivores, primarily preying on decapods and fish46,47. Customized primers 1391F (5′‐GTACACACCGCCCGTC‐3′) and EukB (5′‐TGATCCTTCTGCAGGTTCACCTAC‐3′) that amplify the V9 region of 18S rRNA were selected due to their short target sequence (approximately 200 bp), broad taxonomic coverage, and frequent use in metabarcoding studies of marine invertebrate48,49. Polymerase chain reaction (PCR) was performed for DNA amplification by adding 30 ng of qualified DNA template with the customized primers. Reactions included an initial 5-min incubation at 95 °C, followed by 30 cycles of 94 °C for 30 s, 57 °C for 45 s, and 72 °C for 60 s, with a final 2-min incubation at 72 °C. PCR products were purified using Agencourt AMPure XP beads and dissolved in Elution Buffer for library construction. The Agilent 2100 Bioanalyzer was used to determine library size and concentration. Qualified libraries were sequenced on the HiSeq platform, generating at least 50 k tags with a minimum 15 bp overlapping length from each sample.
Raw reads were demultiplexed and trimmed of specific primers using Cutadapt v4.9, generating clean reads. These were further trimmed using the q2-dada2 plugin in QIIME2 ver. Amplicon 2024–05 for quality filtering and removal of low-quality or chimeric sequences. The remaining pair-end reads were joined, denoised, and grouped into amplicon sequence variants (ASVs) using q2-dada2 for taxonomical classification. ASVs classified as class Actinopterygii were further annotated to higher taxonomic levels using BLAST + . The CRABS software package was used to construct primer-specific reference databases. The 18S rRNA sequences for eukaryotic groups and taxonomic information were downloaded from the NCBI GenBank database. Primer-specific sequences were extracted using in silico PCR analysis and pairwise global alignment in CRABS. Extracted reads were dereplicated, assigned taxonomy, and used to train the Qiime2 naïve-Bayes classifier. Non-dietary taxa and those with low taxonomic resolution were discarded from the dataset. Sequences matching the DNA of the large yellow croaker were also discarded. The geographic distribution of taxa was checked using FishBase (https://www.fishbase.se), SeaLifeBase (https://www.sealifebase.ca/), the Hong Kong Biodiversity Information Hub (https://bih.gov.hk/), the Hong Kong Register of Marine Species (https://www.marinespecies.org/hkrms/), and the Taiwan Biodiversity Network (https://www.tbn.org.tw/). Scientific names were verified using WoRMS (https://www.marinespecies.org/). Conservation status was determined according to the IUCN Red List (https://www.iucnredlist.org/).
Multivariate analysis
A bubble plot was used to summarize the relative abundance of each prey species found in the gut contents of large yellow croaker specimens across different life stages. A two-dimensional non-metric multi-dimensional scaling (NMDS) ordination plot visualized the Bray–Curtis distances among prey items in the gut contents of large yellow croaker specimens between life stages. This technique is robust and insensitive to outliers. One-way analysis of similarity (ANOSIM) was used to test the null hypothesis of no differences in diet composition between life stages. Global R and p values statistically determined the similarity of prey assemblages between life stages. Similarity percentage (SIMPER) analysis determined the percentage contribution of each food item to dietary dissimilarity between life stages. Overall average dissimilarity, average abundance, average dissimilarity, and cumulative contribution were calculated to identify typifying and discriminating prey among life stages. NMDS, ANOSIM, and SIMPER analyses were performed using the community ecology package ‘VEGAN’ in R via RStudio. All analyses were run with 9999 permutations based on the Bray–Curtis dissimilarity matrix. However, the unbalanced sample sizes between life stages constrain statistical power, which might obscure dietary shifts and inflate the dissimilarity contributions of rare prey, particularly in groups with smaller sample sizes. Additionally, a non-significant result might indicate limited statistical power rather than a true biological homogeneity.
Data availability
The data that support the findings of this study are available at Mendeley Data https://data.mendeley.com/datasets/s4nhh57jdr/1.
References
Romanuk, T. N., Hayward, A. & Hutchings, J. A. Trophic level scales positively with body size in fishes. Glob. Ecol. Biogeogr. 20(2), 231–240. https://doi.org/10.1111/j.1466-8238.2010.00579.x (2011).
Werner, E. E. & Gilliam, J. F. The ontogenetic niche and species interactions in size-structured populations. Annu. Rev. Ecol. Syst. 15, 393–425. https://doi.org/10.1146/annurev.es.15.110184.002141 (1984).
Link, J. S. & Garrison, L. P. Trophic ecology of Atlantic cod Gadus morhua on the northeast US continental shelf. Mar. Ecol. Prog. Ser. 227, 109–123. https://doi.org/10.3354/meps227109 (2002).
Sadovy, Y., Eklund, A. M. Synopsis of biological data on the Nassau grouper, Epinephelus striatus (Bloch, 1792), and the jewfish, E. itajara (Lichtenstein, 1822). NOAA Technical Report NMFS 146 (1999).
Liu, J. Y. Status of marine biodiversity of the China seas. PLoS ONE 8(1), e50719. https://doi.org/10.1371/journal.pone.0050719 (2013).
Liu, R. Progress of marine biodiversity studies in China seas. Biodivers. Sci. 19(6), 614 (2011).
Ng, P. K. L. & Tan, K. S. The state of marine biodiversity in the South China Sea. Raffles Bull. Zool. 8, 3–7 (2000).
Ng, S. L. et al. An updated checklist of fishes of Dongsha Island, Taiwan, northern South China Sea. ZooKeys 1220, 175. https://doi.org/10.3897/zookeys.1220.131100 (2024).
Randall, J. E. & Lim, K. K. P. A checklist of the fishes of the South China Sea. Raffles Bull. Zool. 8, 569–667 (2000).
Teh, L., Cashion, T., Alava, J. J., Cheung, W. W. & Sumaila, U. R. Status, trends, and the future of fisheries in the East and South China Seas. Fisheries Centre Res. Rep. 27(1), 101 (2019).
Lands Department – Hong Kong Geographic Data 2025. https://www.landsd.gov.hk/en/resources/mapping-information/hk-geographic-data.html (Accessed 26 June 2025).
Astudillo, J.C., Williams, G.A., Leung, K.M.Y., Cannicci, S., Yasuhara, M., Yau, C., Qiu, J.W., Ang, P.O., To, A.W.L., Shea, S.K.H. (Eds.), 2025. Hong Kong Register of Marine Species. https://www.marinespecies.org/hkrms/. (Accessed 12 March 2025).
Morton, B. Protecting Hong Kong’s marine biodiversity: present proposals, future challenges. Environ. Conserv. 23(1), 55–65. https://doi.org/10.1017/s037689290003825x (1996).
Reeves, R. J., Au, C. L., Cook, R. S. Hong Kong port in the 21st century. Present in the International Conference on Port Development for the Next Millennium (p. 117–124), Hong Kong: Hong Kong Institution of Engineers (1992).
Liu, M. Bahaba taipingensis. The IUCN Red List of Threatened Species 2020: e.T61334A130105307. https://doi.org/10.2305/IUCN.UK.2020-2.RLTS.T61334A130105307.en. (Accessed 12 March 2025) (2020).
Koeda, K., Ho, H.C. Fishes of Southern Taiwan. K. Koeda and H. C. Ho (Eds). National Marine Museum of Biology and Aquarium, Pingtung, Taiwan, 1353 pp (2019).
Shao, K. T. The fish database of Taiwan. Available at: https://fishdb.sinica.edu.tw/ (Accessed 12 March 2025) (2017).
Shao, K. T. et al. A checklist of the fishes of southern Taiwan, northern South China Sea. Raffles Bull. Zool. 19, 233–271 (2008).
Chang, C. H. et al. DNA barcodes of the native ray-finned fishes in Taiwan. Mol. Ecol. Resour. 17(4), 796–805. https://doi.org/10.1111/1755-0998.12601 (2017).
Liu, M. & Sadovy de Mitcheson, Y. Profile of a fishery collapse: why mariculture failed to save the large yellow croaker. Fish Fish. 9(3), 219–242. https://doi.org/10.1111/j.1467-2979.2008.00278.x (2008).
Kon, T. et al. Whole-genome resequencing of large yellow croaker (Larimichthys crocea) reveals the population structure and signatures of environmental adaptation. Sci. Rep. 11(1), 11235. https://doi.org/10.1038/s41598-021-90645-1 (2021).
Lin, C. H. et al. Evidence of lower age, fast growth, and shrinking habitat in critically endangered croaker fish Larimichthys crocea. Estuar. Coast. Shelf Sci. 313, 109095. https://doi.org/10.1016/j.ecss.2024.109095 (2025).
Liu, M., Cheng, J. H., Nguyen Van, Q., Sasaki, K., Hoshino, K., Sakai, T., Santos, M., Yang, C. H., Chen, M. H., Yeh, H. M., Mok, H. K., Liu, S. H., Seah, Y. G. Larimichthys crocea. The IUCN Red List of Threatened Species 2020: e.T49182559A49239394. https://doi.org/10.2305/IUCN.UK.2020-1.RLTS.T49182559A49239394.en. (Accessed 12 March 2025) (2020).
Snover, M. L. Ontogenetic habitat shifts in marine organisms: influencing factors and the impact of climate variability. Bull. Mar. Sci. 83(1), 53–67 (2008).
Abend, A. G. & Smith, T. D. Differences in stable isotope ratios of carbon and nitrogen between long-finned pilot whales (Globicephala melas) and their primary prey in the western North Atlantic. ICES J. Mar. Sci. 54(3), 500–503. https://doi.org/10.1006/jmsc.1996.0192 (1997).
Sweeting, C., Jennings, S. & Polunin, N. Variance in isotopic signatures as a descriptor of tissue turnover and degree of omnivory. Funct. Ecol. 19(5), 777–784. https://doi.org/10.1111/j.1365-2435.2005.01019.x (2005).
Traugott, M., Thalinger, B., Wallinger, C. & Sint, D. Fish as predators and prey: DNA-based assessment of their role in food webs. J. Fish Biol. 98(2), 367–382. https://doi.org/10.1111/jfb.14400 (2021).
Law, C. S. W. & Sadovy de Mitcheson, Y. Reproductive biology of black seabream Acanthopagrus schlegelii, threadfin porgy Evynnis cardinalis and red pargo Pagrus major in the northern South China Sea with consideration of fishery status and management needs. J. Fish Biol. 91(1), 101–125. https://doi.org/10.1111/jfb.13331 (2017).
Newsome, S. D., Etnier, M. A., Monson, D. H. & Fogel, M. L. Retrospective characterization of ontogenetic shifts in killer whale diets via δ13C and δ15N analysis of teeth. Mar. Ecol. Prog. Ser. 374, 229–242. https://doi.org/10.3354/meps07747 (2009).
Choi, B., Kim, W. S., Ji, C. W., Kim, M. S. & Kwak, I. S. Application of combined analyses of stable isotopes and stomach contents for understanding ontogenetic niche shifts in silver croaker (Pennahia argentata). Int. J. Environ. Res. Public Health 18(8), 4073. https://doi.org/10.3390/ijerph18084073 (2021).
Garcia, E.M. Red Drum (Sciaenops ocellatus) Trophic Web Reconstruction Using Stable Isotopes in Two Systems in the Northwestern Gulf of Mexico. Theses and Dissertations. 1070. (2022) https://scholarworks.utrgv.edu/etd/1070
Berumen, M. L., Pratchett, M. S. & McCormick, M. I. Within-reef differences in diet and body condition of coral-feeding butterflyfishes (Chaetodontidae). Mar. Ecol. Prog. Ser. 287, 217–227. https://doi.org/10.3354/meps287217 (2005).
Dobson, A. et al. Habitat loss, trophic collapse, and the decline of ecosystem services. Ecology 87(8), 1915–1924. https://doi.org/10.1890/0012-9658(2006)87[1915:hltcat]2.0.co;2 (2006).
Fuller, B. T., Müldner, G., Van Neer, W., Ervynck, A. & Richards, M. P. Carbon and nitrogen stable isotope ratio analysis of freshwater, brackish and marine fish from Belgian archaeological sites (1 st and 2nd millennium AD). J. Anal. At. Spectrom. 27(5), 807–820. https://doi.org/10.1039/C2JA10366D (2012).
Rennie, M. D., Sprules, W. G. & Johnson, T. B. Resource switching in fish following a major food web disruption. Oecologia 159(4), 789–802. https://doi.org/10.1007/s00442-008-1271-z (2009).
Ding, A. X. & He, Y. E. Test on release and proliferation of Pseudosciaena crocea in Daiquyang sea area. South China Fisheries Sci. 7(1), 73–77 (2011).
Zhang, Q., Hong, W., Yang, S. & Liu, M. Review and prospects in the restocking of the large yellow croaker (Larimichthys crocea). Modern Fisheries Inf. 25(12), 3–5 (2010).
Malakhoff, K. D. & Miller, R. J. After 15 years, no evidence for trophic cascades in marine protected areas. Proc. R. Soc. B 288(1945), 20203061. https://doi.org/10.1098/rspb.2020.3061 (2021).
Yu, M., Herrmann, B., Liu, C., Zhang, L. & Tang, Y. Effect of codend design and mesh size on the size selectivity and exploitation pattern of three commercial fish in stow net fishery of the Yellow Sea China. Sustainability 15(8), 6583. https://doi.org/10.3390/su15086583 (2023).
Wilson, K. D., Wong, E. C. Fisheries, mariculture and marine protected areas in Hong Kong. Coastal Infrastruct. Dev. Hong Kong: Rev., 215–231 (1996s).
Perkins, M. J. et al. Short-term tissue decomposition alters stable isotope values and C:N ratio, but does not change relationships between lipid content, C: N ratio, and Δδ13C in marine animals. PLoS ONE 13(7), e0199680. https://doi.org/10.1371/journal.pone.0199680 (2018).
Chu, C. Y. The yellow croaker fishery of Hong Kong and preliminary notes on the biology of Pseudosciaena crocea (Richardson). Hong Kong Univ. Fisheries J. 3, 111–164 (1960).
Fry, B. Stable isotope ecology (Vol. 521, p. 318). New York: Springer (2006).
Post, D. M. et al. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152(1), 179–189. https://doi.org/10.1007/s00442-006-0630-x (2007).
Jackson, A. L., Inger, R., Parnell, A. C. & Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80(3), 595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x (2011).
Xu, J. Y., Cheng, J. J., Tian, F. G. & Xu, Z. L. Food composition and feeding habits of Guanjingyang large yellow croaker in summer. J. Fishery Sci. China 19(1), 94–104 (2012).
Yang, J. M. & Zheng, Y. Feeding habit and seasonal variation of the food contents of the large yellow croaker, Pseudosciaena crocea (Richardson), off Chekiang and Kiangsu. Studia Marina Sinica 2, 14–30 (1962).
Duarte, S., Leite, B. R., Feio, M. J., Costa, F. O. & Filipe, A. F. Integration of DNA-based approaches in aquatic ecological assessment using benthic macroinvertebrates. Water 13(3), 331. https://doi.org/10.3390/w13030331 (2021).
Waraniak, J. M., Marsh, T. L. & Scribner, K. T. 18S rRNA metabarcoding diet analysis of a predatory fish community across seasonal changes in prey availability. Ecol. Evol. 9(3), 1410–1430. https://doi.org/10.1002/ece3.4857 (2019).
Lin, D., Zhan, J., Luo, J., Zheng, Z. & Shi, X. Studies on the gonadal development and the annual reproductive cycle of the cultured large yellow croaker, Pseudosciaena crocea (Richardson). J. Fujian Normal Univ. (Natural Science) 8(3), 81–87 (1992).
Acknowledgements
This research was funded by the Environmental and Conservation Fund of Hong Kong SAR (grant number 120/2022). The opinions, findings, conclusions, or recommendations presented in this publication are solely those of the authors and do not necessarily represent the views of the Hong Kong SAR Government. We extend our sincere gratitude to the Agriculture, Fisheries and Conservation Department for granting a scientific research permit (R1710058), which facilitated the trawling survey. Special thanks to Ms. Leung Kit Sum from the Stable Isotope Laboratory at the University of Hong Kong and Mr. Chan King Wai from the Department of Biology at the Hong Kong Baptist University for their invaluable technical support. We also express our appreciation to the fishermen in Hong Kong and Taiwan for capturing and supplying the rare large yellow croakers for this study. The authors also express their gratitude to the reviewers for the critical and constructive comments, which have significantly helped improve the manuscript.
Funding
Environmental and Conservation Fund of Hong Kong SAR, 120/2022
Author information
Authors and Affiliations
Contributions
HCW and JMYC reviewed the literature and wrote the manuscript. CMH, LYX, and KS supported data analysis and contributed to result interpretation through collaborative discussions. CHL and MTC improved and corrected the manuscript. JWQ and CKCW provided supervision. The above authors approved this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wong, HC., How, CM., Xi, L. et al. Comparative study of ontogenetic trophic transition in large yellow croaker from Hong Kong and Taiwan using metabarcoding and isotope analysis. Sci Rep 15, 30120 (2025). https://doi.org/10.1038/s41598-025-15014-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15014-8