Abstract
This study provides the first evidence related to the identification of microbial strains closely associated with muscle strength enhancement, independent of the host’s genetic background. Fecal transplants from humans into mice revealed a significant impact of gut bacteria on muscle strength, with some mice experiencing increases, while others showed no change or decreases. Interestingly, analysis of the fecal and gastrointestinal tract bacteria from each mouse classified by the degree of muscle strength revealed significant differences based on muscle strength. Furthermore, a more diverse microbial community was observed in the gastrointestinal tract compared to the feces. Further investigation identified two bacterial species, Lactobacillus johnsonii (L. johnsonii) and Limosilactobacillus reuteri (L. reuteri), that are related to improved muscle strength. Indeed, we confirmed that the supplementation with these bacteria in aged mice significantly enhanced their muscle strength by increasing the mRNA expression levels of follistatin (FST) and insulin-like growth factor-1 (IGF1) in muscle tissue. Overall, this study provides the first evidence that specific gut bacteria can directly improve muscle strength and introduces a novel approach to studying the gut microbiome’s influence on complex traits.
Similar content being viewed by others
Introduction
In the process of evolution, species have adapted to their environments to survive and thrive, resulting in an astonishing diversity of life on earth, ranging from the smallest microbes to the largest mammals1. Humans, in particular, have formed symbiotic relationships with various environmental microorganisms, which play crucial roles in survival and overall health1. Among these, the microbes residing in the human body are especially fascinating, as they have evolved to create highly specialized ecosystems specific to each environment, such as the gastrointestinal (GI) tract, skin, and oral cavity2. These microbial communities contribute significantly to human well-being by supporting immune function, aiding in nutrient metabolism, and protecting against pathogens. Advancements in next-generation sequencing have revolutionized our understanding of these human-associated microbes, providing insights into their diversity and biochemical capabilities3. These discoveries have opened new avenues for improving healthcare and developing microbiome-based therapeutic strategies.
The microbial system associated with humans is vital for maintaining health and homeostasis, while an imbalance in the microbiota, often referred to as dysbiosis, is associated with various health problems. Maintaining a healthy microbial balance is crucial for preventing diseases and promoting overall well-being4. Dysbiosis has been implicated in gastrointestinal diseases such as Crohn’s disease, colitis, and colorectal cancer, as well as systemic conditions including obesity, diabetes, depression, neurodegenerative diseases, and skin disorders5,6,7,8,9,10. These findings highlight the importance of understanding how to maintain or restore beneficial microbial communities and their relationship with overall health.
Physical exercise is widely recognized as a key factor in enhancing immune function and reducing the incidence of inflammatory and metabolic diseases11. Regular, moderate exercise is known to prevent various gastrointestinal disorders, although the mechanisms underlying this protective effect remain unclear. It is increasingly believed that the gut microbiome plays a pivotal role in mediating the beneficial effects of exercise12,13. Recent studies suggest that the gut microbiota not only influences disease prevention but also affects exercise performance and capacity14. Skeletal muscles, which constitute approximately 40% of total human body mass, are essential for mobility, thermoregulation, and maintaining metabolic homeostasis15. Signals originating from the gut microbiota, including microbial metabolites, peptides, oligosaccharides, and interleukins, form a communication network between the gut and skeletal muscles, potentially regulating inflammation and metabolic activity16. However, most research on skeletal muscle and the gut microbiota has been limited to muscle wasting or metabolic dysfunctions17. Therefore, a more detailed exploration of the relationship between skeletal muscle and the microbiome is warranted.
In this study, we address key limitations of traditional microbiome research, such as variability in host genetic backgrounds and the over-reliance on fecal microbiota as a proxy for the entire gut microbiome. To minimize rapid shifts in the microbial community caused by genetic influences within animal models, we utilized human fecal microbiota transplantation (FMT) into mice as a robust experimental approach to evaluate the microbiome’s functional contributions. By comparing compositional changes in the GI microbiota before and after FMT, we identified specific bacterial strains associated with improved muscle strength and locomotor performance. Through proof-of-concept experiments adhering to Koch’s postulates, we isolated Lactobacillus johnsonii (L. johnsonii) and Limosilactobacillus reuteri (L. reuteri), two bacterial species enriched in the group with greater muscle strength. Furthermore, supplementation of these strains in aged mice significantly improved their locomotor abilities by upregulating follistatin (FST) and insulin-like growth factor-1 (IGF1) expression in muscle tissue. This study not only highlights the potential of gut microbial interventions in enhancing muscle performance but also provides a foundational framework for understanding the gut-muscle axis and its broader implications for health.
Methods
Identification of specific microbial strains related muscle strength
Fifty C57BL/6J female mice (9 months old) were obtained from the Animal Facility of Aging Science at the Korea Basic Science Institute (Gwangju, Republic of Korea). The mice were housed individually in a specific-pathogen-free (SPF) facility with sterilized food and water provided ad libitum. Environmental conditions were maintained at a temperature of 22 ± 1 °C, with a relative humidity of 40–50%, and a 12-h light/dark cycle. To standardize the intestinal environment and eliminate variability caused by individual microbial communities, intestinal microbiota were depleted using a combination of antibiotics and antifungals. A mixture containing azithromycin (15 mg/kg), neomycin (25 mg/kg), ciprofloxacin (20 mg/kg), and miconazole (30 mg/kg) was administered once daily for two consecutive days.
Following the microbiota depletion, fecal microbiota transplantation (FMT) was performed to colonize the mice with human gut microbiota and investigate the microorganisms closely associated with muscle strength. Briefly, fecal samples were obtained from healthy adult volunteers who met the following criteria: no chronic illness or gastrointestinal disorders, no antibiotic or probiotic use within the last six months, and maintenance of a regular diet including daily meat intake. These donors provided informed consent under institutional review board (IRB) approval (IRB No. CHU_KOREAN_FOOD_2-2_2010). All procedures involving human participants were conducted in accordance with institutional guidelines and regulations, and adhered to the principles outlined in the Declaration of Helsinki and its subsequent amendments. Equal volumes of stool from each donor were pooled to prepare a composite fecal suspension.
The preparation of the fecal mixture for FMT was adapted from a previously established method18. Fecal samples were collected from 10 healthy human volunteers and mixed in JM medium, which included 19 edible plants, yeast extract (Duchefa Biochemie), glucose (Duchefa Biochemie), mineral solution, amino acids, and pig blood (Alpha Biochemicals Co., Jeonju, Republic of Korea) to create a composite fecal suspension containing a diverse microbial community. The fecal mixture was administered orally to the mice at a dosage of 20 μL twice daily for 3 months19.
To evaluate the effects of FMT on muscle strength, we conducted two functional tests: the Rotarod test and the Wire suspension test. Muscle strength was assessed for each mouse before the FMT (baseline, 0 months) and again after 3 months of treatment. In addition to measuring muscle strength, we recorded the body weight of individual mice and collected fecal and blood samples for subsequent analyses. For the follow-up validation experiment using the strains identified from the screening analysis, we used a separate group of 12-month-old female C57BL/6J mice to better represent an aging mode. At the end of the study, all mice underwent cardiac blood collection while inhaling isoflurane to minimize distress. Immediately after blood collection, the mice were euthanized by cervical dislocation. All procedures were conducted in accordance with ethical guidelines and were approved by the Institutional Animal Care and Use Committee of the Korea Basic Science Institute (KBSI-IACUC-23–12).
Culture of Lactobacillus johnsonii and Limosilactobacillus reuteri
The two strains of L. johnsonii (GMB 1195) and L. reuteri (GMB 2061) were obtained from Gut Microbe Bank (https://www.gmbank.org), and cell culture was performed the same as those used in previous studies18,20.
Evaluation of muscle strength in mice administered identified strains
After identifying specific microbial strains associated with muscle strength, we administered these strains to mice to evaluate their effects on motor performance. For this experiment, 12-month-old C57BL/6J female mice (n = 40) were obtained from the Animal Facility of Aging Science at the Korea Basic Science Institute (Gwangju, Republic of Korea). The mice were randomly divided into four experimental groups (n = 10 per group): CTRL (control), LJ (Lactobacillus johnsonii), LR (Lactobacillus reuteri), and LJ + LR (L. johnsonii and L. reuteri mixture).
To standardize the intestinal environment and ensure consistent colonization, gut microbiota were depleted in all mice by administering a combination of antibiotics once daily for two consecutive days, following the same protocol described earlier. Subsequently, L. johnsonii and/or L. reuteri were orally administered to the respective experimental groups daily for 3 months to establish a microbial community in the mouse intestine. Body weight measurements and behavioral tests were conducted monthly to monitor changes in motor performance and evaluate the effects of the administered strains.
Rotarod
Motor coordination, muscle strength, and balance were assessed using the Rotarod apparatus (B.S Technolab INC, Seoul, South Korea). The Rotarod, which measures the time each animal can maintain balance while walking on a rotating drum, was conducted following the same methods as in previous studies20. For the test, the Rotarod was set to accelerate to 40 rpm over a period of 5 min. Each mouse was given three trials with a maximum duration of 5 min each. The latency to fall was used as a measure of muscle strength. The mice were tested at 30-day intervals. The average latency to fall from the Rotarod was recorded and used for subsequent analysis.
Wire suspension
This test was used to evaluate forelimb strength18. The apparatus consisted of two vertical supports spaced 60 cm apart and a stainless-steel wire (50 cm in length, 2 mm in diameter) horizontally fixed between them. The mice were required to grasp the center of the wire with their front paws, and the latency to fall from the wire to the bedding was measured. The experiment was conducted in triplicate for each mouse, and the average value was used for evaluation. A rest pause of 3 min was allowed between consecutive attempts.
Analyses of biochemical parameters
The serum levels of TCHO, TG, and HDL-C and blood glucose level were determined using commercial assay kits (Asan Pharmaceutical, Seoul, South Korea) following the manufacturer’s instructions, as previously described21,22.
DNA extraction and 16S rRNA gene sequencing
The DNA from each fecal or gastrointestinal whole bacterial genome was extracted using the phenol–chloroform-isoamyl alcohol extraction method, following the same procedure as previously described23,24,25. Briefly, the samples were subjected to bead beating in a buffer solution (200 mM NaCl, 200 mM Tris-HCl (pH 8.0), 20 mM EDTA). Genomic DNA was extracted from the phenol:chloroform:isoamyl alcohol phase. DNA precipitation was achieved by adding 3 M sodium acetate and isopropanol. After washing with 70% ethanol and air-drying, the DNA pellet was dissolved in TE buffer (10 mM Tris–HCl (pH 8.0), 1 mM EDTA). The concentration and purity of the extracted DNA were measured using a BioSpec-nano spectrophotometer (Shimadzu Biotech, Kyoto, Japan), and its integrity was assessed on a 1% (w/v) agarose gel.
The metagenomic sequencing analysis of the intestinal microbial DNA samples was conducted by ebiogen, Inc. (South Korea). In brief, each sequenced sample was prepared according to the Illumina 16S Metagenomic Sequencing Library protocol, and genes were amplified using the 16S V3-V4 primers. The forward primer for 16S Amplicon PCR included the sequence 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′, while the reverse primer included the sequence 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′. The genomic DNA input was amplified with 16S V3-V4 primers. Subsequent amplification steps were performed to add the multiplexing index and Illumina sequencing adapters. The final products were normalized and pooled using PicoGreen, and the size of the library was validated using the Agilent TapeStation DNA ScreenTape D1000 system (Agilent Technologies, Santa Clara, CA, USA). Lastly, the pooled library was sequenced (2 × 300) using the MiSeq platform (Illumina, San Diego, CA, USA). The amplicon error was modeled from merged fastq using DADA2 (ver.1.10.1); noise sequence was filtered out, errors in marginal sequences were corrected, chimeric sequences and singletons were removed, and sequences were de-replicated. The 16S rRNA sequencing data generated during this study have been deposited in the NCBI Sequence Read Archive (SRA) under the BioProject accession number PRJNA1214556 (ID 1214556—BioProject—NCBI).
Data and statistical analyses
All data and statistical analyses were conducted as previously described18,23,25. In brief, the Q2-Feature Classifier is a Naive Bayes classifier trained on the SILVA reference (V3-V4 region) database (https://www.arb-silva.de/) to classify bacterial species. After setting the default parameters with Denoise single function, the dataset was classified using the program. Diversity calculations and statistical tests utilized the q2 diversity of the “sampling depth” option. We used a minimum sequencing quality score threshold of 20 and a rarefaction depth of 11,510. After confirming the quality of the sequencing results, the sequencing output in the “table.qzv” file was filtered using thresholds in QIIME 2. The metagenomic data OTU and taxonomic classification tables were imported into R version with the phyloseq (1.28.0) package. Statistical analysis was performed using Kruskal–Wallis rank-sum test for α-diversity and permutation multivariate analysis of variance (PERMANOVA) from the vegan package in R to detect differences in β-diversity measures between groups. ADONIS, with 999 permutations from the vegan package in R, was used to quantify the effect size of variables explaining Bray–Curtis distances. All p-values were adjusted using the Benjamini and Hochberg method, and significance was declared at P < 0.05.
The α-diversity analysis for relative abundance evaluation of material and microbiome
Α-diversity analysis for relative abundance was conducted as previously described18,23,24. We identified core taxa present in each group using the phyloseq (version 1.28.0) and MetagenomeSeq (version 1.16.0) packages. Metadata, OTU, and taxonomic classification tables were imported into the phyloseq package, and the data were processed according to guidelines24. Phyloseq class objects were converted to MetagenomeSeq objects, and the data were normalized using Cumulative Sum Scaling (CSS) specifically designed for metagenomic data in the Bioconductor package metgenomeSeq (version 1.16.0). Normalized data were then converted to phyloseq class objects for further analysis and visualization in R.
The normalized OTU data were used for abundance calculations, and each taxonomic level was grouped for plotting. To clearly visualize abundance data, classification groups were collected as ‘Others’ if their relative abundance was less than 5%, excluding the kingdom and class levels.
The β-analysis for relative abundance evaluation of material and microbiome
The β-diversity was calculated using Bray–Curtis dissimilarity on log-transformed OTU data, as previously described18,23,24, and non-metric multidimensional scaling (NMDS) was performed using the ‘MetaMDS’ function from the ‘vegan’ package. NMDS aims to reduce dimensionality while retaining as much information as possible about relationships between samples. It was conducted on the Bray–Curtis dissimilarity matrix to assess relationships between samples.
Construction of heatmap and phylogenetic tree
Heatmaps and cluster analyses were generated using the Heatplus package (version 2.30.0) from Bioconductor and the ‘vegan’ package in R, as previously described18,23. These analyses utilized relative abundance values of all OTU or core abundant OTU across samples. For cluster analysis and heatmap generation, average linkage hierarchical clustering and Bray–Curtis distance measurement were employed, respectively. To generate the heatmap, unsupervised prevalence filtering with a 5% threshold across all samples was performed to collect the most abundant classification groups.
To directly visualize sample abundance with respect to taxonomic classification, phylogenetic trees were constructed for each group from raw sequences without filtering, as previously described18,23. In brief, classification groups that could not be classified at the species level were reclassified based on NCBI accession numbers using the Taxonomizr package (version 0.5.3) in R. Subsequently, the 16S rRNA sequences of each group were aligned using ClustalW with default parameters. The resulting alignments were used to construct maximum likelihood phylogenetic trees with 500 bootstrap replicates in MEGAX. All phylogenetic trees were visualized using iTOL.
Co-occurrence network construction
To observe the concurrent occurrence relationships in the mouse muscle strength records, we constructed a co-abundance network using the permutation-reordering-bootstrap strategy as previously described18,23,24. In essence, the unnormalized abundance data were uploaded to CoNet, a Java Cytoscape plugin. All networks were independently constructed by partitioning the OTU abundance matrix into SMS, MMS, and WMS groups. Microbial networks and links or edges were derived from OTU occurrence data. Using CoNet’s multiple ensemble correlation approach, significant co-occurrence across the entire sample was identified, while OTUs occurring in fewer than three samples were removed (‘row_minocc’ = 3). For ensemble network generation, five similarity metrics including Spearman and Pearson correlation coefficients, Mutual Information Score, Bray–Curtis, and Kullback–Leibler Dissimilarity were computed in CoNet, and p-values were merged using the Brown method. P-values were adjusted using the Benjamini–Hochberg correction (adjusted P < 0.05). If two or more out of the five metrics indicated significant co-occurrence between two OTUs, the relationship was retained as an edge in the final network. The final co-abundance network model was represented using the Louvain algorithm implementation in the igraph package in R, identifying communities within each network to maximize the modularity score of each OTU within the given network.
Differential abundance
Differential abundance analysis was conducted as previously described18,23. To estimate changes in taxonomic composition of gut microbial clusters according to changes in muscle strength groups, we used DESeq2 (version 1.24.0)26,27. Classification groups not observed in more than 1% of the samples were excluded from the DESeq2 analysis.
Enzyme-linked immunosorbent assay
The concentrations of IL-6 in serum were measured using Mouse IL-6 PicoKine ELISA Kit (MBS175960, MyBioSource). The ELISA kits were performed according to the manufacturer’s protocols and measured using SpectraMax Plus 384 Plate Reader (MD, USA).
Histological analysis
Tissue histological analysis was conducted following the same procedures as described previously18,23,24. The Sol, GA, and EDL muscles were collected and fixed in 10% neutral buffered formalin embedded in paraffin. After a series of 6 μm thick sections, tissue sections were deparaffinized using hot water and stained with hematoxylin–eosin (H&E) and Masson’s trichrome following standard procedures. Images of stained tissue sections were captured using a slide scanner (KFBIO Magscanner KF-FL-005, Guangzhou, China). Fibre cross-sectional area in each image was quantified using ImageJ software following the manufacturer’s instructions for H&E images. At least 6 images of muscle sections captured from different fields of each mouse sample were examined.
Reverse transcription quantitative polymerase chain reaction
After euthanizing the experimental group, each mouse was dissected to isolate the leg muscles. The muscle tissues were homogenized, and then centrifuged at 3000 rpm for 10 min at 4 °C. After centrifugation, the supernatant was collected and used to extract genomic DNA following the manufacturer’s instructions of the Genomic DNA Extraction kit (iNtRON, 17231, South Korea) to prepare samples for RT-qPCR. Subsequently, RT-qPCR was performed according to the manufacturer’s manual of the PrimerScript RT Reagent kit (TaKaRa, Japan). The primers used for RT-qPCR were as follows: FST forward: 5'-CCAGGCAGCTCCACTTGTGT-3' and reverse: 5′-AGTCACTCCATCATTTCCACAAAG-3′, IGF1 forward: 5′-GGACCAGAGACCCTTTGCGGGG-3′ and reverse: 5′-GGCTGCTTTTGTAGGCTTCAGTGG-3′. The RT-qPCR reaction was carried out using the iQ SYBR Green SuperMix Kit (BioRad, USA) under the following conditions: DNA denaturation at 95 °C for 3 min, followed by 40 cycles of DNA amplification at 95 °C for 15 s and 60 °C for 30 s. The real-time PCR standard curve was generated by serial dilution of recombinant plasmid and used for quantification as a standard. The Ct values of each sample were used for quantification using the standard curve.
Statistical analysis
All statistical analyses are reported as the mean ± S.E.M., and the differences in the relative abundance of bacterial populations containing feces and GI were analyzed using the Mann–Whitney sum rank tests in R software. Comparisons between two groups were carried out using two-tailed, paired t-tests, and the p-values were calculated. Statistical differences were considered significant when p < 0.05. Asterisks indicate *p < 0.05; **p < 0.01; and ***p < 0.001.
Results
The FMT of human feces in C57BL/6 mice has variable effects on muscle strength
To examine the effects of human fecal microbiota transplantation (FMT) on muscle strength in C57BL/6 mice, we established an experimental protocol incorporating standardized behavioral assessments. A schematic of the procedure is presented in Fig. 1a. To assess strength, we utilized two commonly used tests, the Rotarod and Wire suspension tests, which measure motor coordination, balance, and endurance in mice7. First, we compared changes in Rotarod performance in the same mice before and after FMT to assess the effect of gut microbiota on strength while controlling for genetic factors. Interestingly, we found that replacing the native gut microbiota via FMT had variable effects on strength (Fig. 1b,c). During the 3-month experimental period, changes in Rotarod performance were categorized into three groups: the strengthened group (Rota_SMS; n = 7; Δ latency to fall = 18.6 ± 6.2 s), the intermediate group (Rota_MMS; n = 7; Δ latency to fall = − 0.3 ± 1.4 s), and the weakened group (Rota_WMS; n = 7; Δ latency to fall = − 42.6 ± 5.6 s) (Fig. 1b,c).
FMT of a human fecal mixture into C57BL/6 mice leads to a modification in the muscle strength of the mice. (a) Schematic representation of the experimental strategy. (b) Comparison of Rotarod records from individual mice in the Rotarod experimental group before and 3 months after FMT. (c) Comparison of changes in Rotarod records of each mouse group before and after FMT. (d) Comparison of Wire suspension records from individual mice in the Wire suspension experimental group before and 3 months after FMT. (e) Comparison of changes in Wire suspension records of each mouse group before and after FMT. The values represent mean ± S.E.M. Rota, mouse groups classified based on the Rotarod records; Wire, mouse groups classified based on the Wire suspension records; SMS, mouse group with stronger muscle strength after FMT; MMS, mouse group with unchanged in muscle strength after FMT; WMS, mouse group with weakened muscle strength after FMT. *p < 0.05; ***p < 0.001.
Second, we analyzed changes in Wire suspension performance of the same mice before and after FMT to further evaluate grip strength and endurance (Fig. 1d,e). Similarly, changes in Wire suspension performance over the 3-month period were grouped as follows: the strengthened group (Wire_SMS; n = 8; Δ hanging time = 24.5 ± 4.7 s), the intermediate group (Wire_MMS; n = 8; Δ hanging time = − 7.2 ± 2.6 s), and the weakened group (Wire_WMS; n = 8; Δ hanging time = − 28.8 ± 2.9 s) (Fig. 1d,e).
In addition, body weight, blood glucose, and blood lipid levels of the two experimental groups were measured before and 3 months after FMT. Both body weight and blood glucose levels increased after 3 months, likely due to the aging process of the mice (Supplementary Fig. 1a,b,g,h). Interestingly, high-density lipoprotein (HDL) cholesterol levels were found to have increased in the muscle-strengthened groups (Rota_SMS and Wire_SMS).
Taken together, these results suggest that the gut microbiome may play a role in the complex interactions between physical performance and age-related metabolic health.
FMT altered the composition of the gut microbiome of each mouse
The phenotypic differences observed in individual mice following FMT led us to examine whether corresponding changes were also reflected in the fecal microbiota composition. To this end, we analyzed the gut microbiome before and after FMT using 16S rRNA amplicon sequencing. The V3-V4 regions of the 16S rRNA genes, extracted from the genomic DNA of the gut microbiome and filtered for sequencing errors, were represented as operational taxonomic units (OTUs). These OTUs were matched against the SILVA reference database (region V3-V4) to identify individual microbial species (https://www.arb-silva.de/) (Supplementary Table S1). All identified 16S rRNA sequences were classified down to the species level.
In the Rotarod experimental group, species richness, as measured by Fisher and ACE indices, showed a significant increase after FMT, indicating an expansion in the number of microbial species in the gut microbiome (Fig. 2a, Supplementary Fig. S2a, Supplementary Table S2). However, despite this increase in species richness, the species evenness—assessed using Simpson, Evenness, InvSimpson, and Shannon indices—did not change significantly, suggesting that the distribution patterns of microbial species remained relatively stable (Fig. 2a, Supplementary Fig. S2a, Supplementary Table S2).
FMT induces significant compositional changes in the gut microbiome of C57BL/6 mice. (a–e) Analysis of fecal gut microbiome before and 3 months after FMT in the Rotarod experimental group and (f,j) Wire suspension experimental group are shown. (a,f) Comparisons of α-diversity indices of the fecal gut microbiome, measured using Fisher (left) and Simpson (right) methods, between before and 3 months after FMT are shown in box plots. (b,g) Comparisons of phylogenetic compositions of the fecal gut microbiome at the phylum level between before and 3 months after FMT. (c,h) The β-diversity plots of the fecal gut microbiome, measured using multidimensional scaling plot before and 3 months after FMT. (d,i) Maximum-likelihood phylogenetic tree comprising taxa of the fecal gut microbiome before FMT (left) and 3 months after FMT (right). The circular dendrogram rings represent phylum levels, with corresponding taxa depicted in the inner layer. See Supplemental Fig. 4 for the circular ring (phylum) and inner layer (family) color legends. (e,j) Color-coded network graphs of the fecal gut microbiome before FMT (left) and 3 months after FMT (right), illustrating co-occurrence and mutual exclusion interactions among operational taxonomic units (OTUs). The network was developed using class-level OTUs. Additional network information is provided in Supplemental Table 3. Rota, mouse groups classified based on the Rotarod records; Wire, mouse groups classified based on the Wire suspension records; 0 m, before FMT; 3 m, 3 months after FMT; Feces, fecal gut microbiome.
Consistent with the increase in species richness, phylogenetic classification revealed substantial changes and diversification in microbial composition after FMT. Before FMT, the gut microbiome was primarily composed of Bacteroidetes (57.120%) and Firmicutes (39.869%) (Fig. 2b). After FMT, the relative abundance of Verrucomicrobia increased (9.029%), while the abundance of Bacteroidetes (50.352%) and Firmicutes (36.649%) decreased (Fig. 2b). Microbial distributions at lower taxonomic levels are detailed in Supplementary Fig. S2b–d. Additionally, analyses of β-diversity (Fig. 2c, Supplementary Fig. S2e–g), phylogenetic trees (Fig. 2d), and co-occurrence networks (Fig. 2e, Supplementary Table S3) further confirmed the distinct shifts in gut microbiome composition following FMT in the Rotarod experimental group.
A similar pattern was observed in the Wire suspension experimental group. Species richness, as measured by Fisher and ACE indices, also showed a dramatic increase after FMT, while species evenness, assessed using Simpson, Evenness, InvSimpson, and Shannon indices, remained relatively unchanged (Fig. 2f, Supplementary Fig. S3a, Supplementary Table S2). Prior to FMT, the gut microbiome in this group was predominantly composed of Bacteroidetes (54.994%) and Firmicutes (41.866%) (Fig. 2g). After FMT, the abundance of Verrucomicrobia increased (9.060%), while the relative abundance of Bacteroidetes (49.785%) and Firmicutes (37.415%) decreased (Fig. 2g). Microbial distributions at lower taxonomic levels are provided in Supplementary Fig. S3b-d. Furthermore, analyses of β-diversity (Fig. 2h, Supplementary Fig. S3e–g), phylogenetic trees (Fig. 2i), and co-occurrence networks (Fig. 2j, Supplementary Table S3) confirmed significant shifts in gut microbiome composition after FMT in the Wire suspension experimental group.
Taken together, these results demonstrate that FMT reproducibly altered the gut microbiota in both experimental groups, leading to increased species richness and taxonomic diversification.
Potential contributions of fecal microbiome to muscle strength in enhanced groups
To investigate the relationship between gut microbiome composition and muscle strength, fecal samples from mice were analyzed before and after FMT using 16S rRNA sequencing. In the Rotarod experimental group, species richness significantly increased across all groups after FMT, as measured by Fisher and ACE indices, although the Rota_SMS group, which showed improved muscle strength, exhibited slightly lower richness indices compared to the Rota_MMS and Rota_WMS groups. Despite this, species evenness remained stable across all groups, as measured by Simpson, Evenness, Inverse Simpson, and Shannon indices (Fig. 3a, Supplementary Fig. S5a, Supplementary Table S2). Phylogenetic classification revealed that while the overall microbial compositions were altered in all groups after FMT, the Rota_SMS group displayed distinct patterns, including higher Verrucomicrobia abundance and reduced Bacteroidetes and Firmicutes compared to the other groups (Fig. 3b, Supplementary Table S4). These differences were further supported by heatmap and β-diversity analyses, which highlighted unique clustering patterns in the Rota_SMS group (Fig. 3c,d, Supplementary Fig. S5b–d, Supplementary Tables S5–S9). Similarly, in the Wire suspension experimental group, species richness also improved in all groups after FMT, with no significant changes in species evenness (Fig. 3e, Supplementary Fig. S8a, Supplementary Table S2). However, the Wire_SMS group, which exhibited enhanced grip strength, showed distinct microbial compositions, including increased abundance of certain microbial groups compared to the other groups (Fig. 3f, Supplementary Table S10). Heatmap and β-diversity analyses further confirmed that the microbial community in the Wire_SMS group was distinct from the Wire_MMS and Wire_WMS groups (Fig. 3g,h, Supplementary Fig. S8b–d, Supplementary Tables S11–S15). While α-diversity indices showed no consistent differences across groups, the distinct microbial signatures identified in strength-enhanced groups (Rota_SMS and Wire_SMS) suggest a potential link between specific gut microbes and muscle strength, highlighting the need for further functional validation.
Analysis of the fecal gut microbiome before and after FMT shows that the fecal microbiome had little effect on changes in muscle strength in mice. (a–d) Analysis of fecal gut microbiome before and 3 months after FMT in the Rotarod experimental group and (e–h) Wire suspension experimental group are shown. (a,e) Comparisons of α-diversity indices of the fecal gut microbiome in each group, measured using Fisher and Simpson methods, between before and 3 months after FMT are shown in box plots. (b,f) Comparisons of phylogenetic compositions of the fecal gut microbiome in each group at the phylum level between before and 3 months after FMT. (c,g) The β-diversity plots of the fecal gut microbiome, measured using multidimensional scaling plot before FMT (upper) and 3 months after FMT (lower) in each group. (d,h) Heatmap of the microbial composition in the fecal gut microbiome of each group before FMT (left) and 3 months after FMT (right) based on Bray–Curtis distance matrix calculated at the species level.
Potential contributions of GI microbiome to muscle strength with greater diversity and detailed profiles compared to fecal microbiome
To further explore the relationship between the gut microbiome and muscle strength, we analyzed the microbiome composition derived from gastrointestinal (GI) contents, in contrast to fecal samples. In the Rotarod experimental group, the species richness and distribution patterns of the GI gut microbiome were markedly different from those of the fecal gut microbiome, indicating greater diversity and distinct microbial profiles (Fig. 4a, Supplementary Fig. S11a, Supplementary Table S2). The composition of gut microbes isolated from the GI contents not only differed significantly from those isolated from feces but also exhibited clear group-specific differences (Fig. 4b, Supplementary Fig. S11b-d, Supplementary Tables S4–S9). Analyses of α-diversity indices (Fig. 4a, Supplementary Fig. S11a, Supplementary Table S2), β-diversity indices (Fig. 4c,d, Supplementary Fig. S12), phylogenetic trees (Fig. 4e), and co-occurrence network analysis (Fig. 4f, Supplementary Table S3) further confirmed that the GI gut microbiome composition differed significantly across the three experimental groups.
Analysis of the GI gut microbiome elucidates that the GI gut microbiome is responsible for the alteration of muscle strength in mice in the Rotarod experimental group. (a) Comparisons of α-diversity indices of the GI gut microbiome, measured using Fisher and Simpson methods, 3 months after FMT are shown in box plots. (b) Comparison of phylogenetic compositions of the GI gut microbiome at the phylum level, 3 months after FMT. (c) The β-diversity plot of the GI gut microbiome, measured using multidimensional scaling plot, 3 months after FMT. (d) Heatmap of the microbial composition in the GI gut microbiome of each group 3 months after FMT based on Bray–Curtis distance matrix calculated at the species level. (e) Maximum-likelihood phylogenetic tree comprising taxa of the GI gut microbiome 3 months after FMT in the Rota_SMS group (left), the Rota_MMS group (middle), and the Rota_WMS (right). The circular dendrogram rings represent phylum levels, with corresponding taxa depicted in the inner layer. See Supplemental Fig. 4 for the circular ring (phylum) and inner layer (family) color legends. (f) Color-coded network graphs of the GI gut microbiome 3 months after FMT in the Rota_SMS group (left), the Rota_MMS group (middle), and the Rota_WMS group (right), illustrating co-occurrence and mutual exclusion interactions among OTUs. The network was developed using class-level OTUs. Additional network information is provided in Supplemental Table 3. Rota, mouse groups classified based on the Rotarod records; 3 m, 3 months after FMT; GI, gastrointestinal gut microbiome; SMS, mouse group with stronger muscle strength after FMT; MMS, mouse group with unchanged in muscle strength after FMT; WMS, mouse group with weakened muscle strength after FMT.
Similarly, in the Wire suspension experimental group, the GI gut microbiome exhibited greater diversity and distinct microbial compositions compared to the fecal gut microbiome. Species richness and microbial distribution patterns derived from the GI contents were significantly different across the groups (Fig. 5a, Supplementary Fig. S13a, Supplementary Table S2). The composition of the GI gut microbiome also demonstrated clear group-specific differences (Fig. 5b, Supplementary Fig. S13b–d, Supplementary Tables S10–S15), while β-diversity analyses (Fig. 5c,d, Supplementary Fig. S14), phylogenetic trees (Fig. 5e), and co-occurrence network analysis (Fig. 5f, Supplementary Table S3) confirmed that the GI microbiome varied significantly among the strength-enhanced and other groups.
Analysis of the GI gut microbiome elucidates that the GI gut microbiome is responsible for the alteration of muscle strength in mice in the Wire suspension experimental group. (a) Comparisons of α-diversity indices of the GI gut microbiome, measured using Fisher and Simpson methods, 3 months after FMT are shown in box plots. (b) Comparison of phylogenetic compositions of the GI gut microbiome at the phylum level, 3 months after FMT. (c) The β-diversity plot of the GI gut microbiome, measured using multidimensional scaling plot, 3 months after FMT. (d) Heatmap of the microbial composition in the GI gut microbiome of each group 3 months after FMT based on Bray–Curtis distance matrix calculated at the species level. (e) Maximum-likelihood phylogenetic tree comprising taxa of the GI gut microbiome 3 months after FMT in the Wire_SMS group (left), the Wire_MMS group (middle), and the Wire_WMS group (right). The circular dendrogram rings represent phylum levels, with corresponding taxa depicted in the inner layer. See Supplemental Fig. 4 for the circular ring (phylum) and inner layer (family) color legends. (f) Color-coded network graphs of the GI gut microbiome 3 months after FMT in the Wire_SMS group (left), the Wire_MMS (middle), and the Wire_WMS (right), illustrating co-occurrence and mutual exclusion interactions among OTUs. The network was developed using class-level OTUs. Additional network information is provided in Supplemental Table 3. Wire, mouse groups classified based on the Wire suspension records; 3 m, 3 months after FMT; GI, gastrointestinal gut microbiome; SMS, mouse group with stronger muscle strength after FMT; MMS, mouse group with unchanged in muscle strength after FMT; WMS, mouse group with weakened muscle strength after FMT.
Our findings emphasize that the GI microbiome is not only more diverse but also provides a more accurate representation of the overall gut microbiome compared to fecal microbiome analyses. This is consistent with recent studies demonstrating that the fecal microbiome does not fully represent the composition and functional diversity of the entire gut microbiome28,29. By analyzing the GI microbiome, we have demonstrated that assessing the GI contents allows for a more detailed and comprehensive understanding of the relationship between microbial composition and muscle strength, providing insights that may not be fully captured through fecal microbiome analysis.
Identification of gut microbial species associated with muscle strength
After establishing the association between the GI microbiome composition and muscle strength, we identified specific gut microbial species contributing to muscle strength using the DESeq2 method, which analyzed differential abundances as fold changes between groups. In the Rotarod experimental group, nine bacterial species showed significantly different abundances (adjusted P-value ≤ 0.05) between the Rota_SMS and Rota_WMS groups (Fig. 6a,b). Notably, seven species were enriched in the strength-enhanced Rota_SMS group, including Lactobacillus johnsonii (L. johnsonii), Limosilactobacillus reuteri (L. reuteri), and Turicibacter sanguinis (T. sanguinis). Similarly, in the Wire suspension experimental group, nine bacterial species showed differential abundances between the Wire_SMS and Wire_WMS groups (Fig. 6c,d), with four species enriched in the Wire_SMS group. Interestingly, when evaluating muscle strength through both experimental methods, the same three key species—L. johnsonii, L. reuteri, and T. sanguinis—were consistently identified. These three species were continuously enriched in the strength-enhanced groups and showed a linear, abundance-dependent correlation with muscle strength improvement (Fig. 6b,d), suggesting their critical role in enhancing muscle performance. Among these, L. johnsonii and L. reuteri were ultimately selected as the final candidates for additional functional validation, as they were consistently enriched in the gastrointestinal microbiota of the strength-enhanced (SMS) groups across both Rotarod and Wire suspension tests. This reproducible association across two independent behavioral assays suggested a strong link between these species and muscle strength improvement. While the strains were not directly isolated from mouse samples, they were chosen from among the enriched taxa for downstream experiments based on their well-characterized probiotic properties and established applicability in experimental settings.
The gut microbial species associated with muscle strength are identified. (a) Log2-fold change in abundance of most abundantly present species in the GI gut microbiome of the SMS and WMS groups in the Rotarod experimental group analyzed by DESeq2 differential abundance analysis. Each point represents a species comparison between two experimental groups. (b) The correlation analysis between gut microbiomes identified as a result of DESeq2 differential abundance analysis in (a) and Rotarod record changes are shown. (c) Log2-fold change in abundance of most abundantly present species in the GI gut microbiome of the SMS and WMS groups in the Wire suspension experimental group analyzed by DESeq2 differential abundance analysis. Each point represents a species comparison between two experimental groups. (d) The correlation analysis between gut microbiomes identified as a result of DESeq2 differential abundance analysis in (c) and Wire suspension record changes are shown.
Effects of L. johnsonii and L. reuteri administration on muscle strength and growth
To evaluate the potential of L. johnsonii and L. reuteri in enhancing muscle strength, we conducted two motor behavioral tests: the Rotarod and Wire suspension tests, over a 3-month period of bacterial administration (Fig. 7a). The Rotarod test demonstrated a significant improvement in motor coordination and endurance, as indicated by the increased time to fall in the bacterial administration groups compared to the control group (CTRL). The LJ + LR group showed the most notable improvement, with significant increases in time to fall starting from the first month and sustained over the entire study period (Fig. 7b). Similarly, in the Wire suspension test, the hanging time increased significantly after one month of L. reuteri administration, and this improvement was further pronounced in the LJ + LR group, highlighting the synergistic effect of the combined bacteria on grip strength and endurance (Fig. 7c).
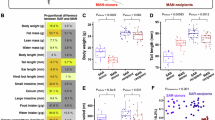
Lactobacillus johnsonii and Limosilactobacillus reuteri improve the muscle strength in mice by increasing the mRNA levels of FST and IGF1. L. johnsonii and L. reuteri increases the muscle strength. (a) Schematic representation of the efficacy test. (b) Effects of L. johnsonii and L. reuteri administration on muscle strength improvement are confirmed using Rotarod. (c) Effects of L. johnsonii and L. reuteri administration on muscle strength improvement are confirmed using Wire suspension. (d) Effects of L. johnsonii and L. reuteri administration on the body weight are shown. (e–h) Effects of L. johnsonii and L. reuteri administration on the muscle are shown. Effects of L. johnsonii and L. reuteri administration on the muscle weight (e), the skeletal muscle-related gene expression (f), the muscle fiber area (g) are shown. (h) Images of mouse muscle tissues stained with H&E and Masson’s Trichrome. Scale bars in (h), 100 μm. The values represent mean ± S.E.M. *p < 0.05; **p < 0.01; ***p < 0.001. CTRL, mouse group with administration of PBS; LJ, mouse group with administration of L. johnsonii; LR, mouse group with administration of L. reuteri; LJ + LR, mouse group with administration of L. johnsonii and L. reuteri. Sol, soleus muscle; GA, gastrocnemius muscle; EDL, Extensor digitorum longus. Values are means ± S.E.M of 10 animals per group. Significance was statistically analyzed and marked on the graphs as *p < 0.05; **p < 0.01; ***p < 0.001.
Muscle weight analysis revealed that the LJ + LR group exhibited a remarkable 157.47 ± 41.48% increase in muscle weight compared to the CTRL, highlighting the superior efficacy of the combined administration (Fig. 7e). While body weight slightly decreased in all bacterial administration groups, this effect was accompanied by significant gains in muscle mass, particularly in the LJ + LR group, suggesting that the combined administration promotes lean muscle development without adverse effects on overall body composition (Fig. 7d,e). Notably, the muscle weight in the LJ + LR group was significantly greater than in the LJ or LR groups alone, further supporting the additive or synergistic effects of co-administration.
At the molecular level, markers associated with muscle growth, Follistatin (FST) and IGF-1, were analyzed. FST, a potent inhibitor of myostatin and a key regulator of muscle growth, showed a significant 99.20 ± 3.07% increase in mRNA expression in the LJ group compared to the CTRL (Fig. 7f)30,31. IGF-1, a critical anabolic growth factor, showed the highest increase in the LJ + LR group, with a 164.00 ± 56.03% rise compared to the CTRL, indicating a substantial positive impact of the combined administration on muscle development (Fig. 7f)32,33.
The structural effects of bacterial administration on muscle fibers were assessed through histological analysis. Cross-sectional areas of muscle fibers, including the soleus (Sol), gastrocnemius (GA), and extensor digitorum longus (EDL), significantly increased across all test groups compared to the CTRL (Fig. 7g). The LJ + LR group exhibited the largest increase in muscle fiber cross-sectional area, correlating with the observed improvements in muscle strength. Masson’s trichrome staining revealed enhanced collagen accumulation in muscle fibers of the LJ + LR group, further reflecting structural adaptations induced by the bacterial administration (Fig. 7h).
Metabolic and inflammatory parameters were also evaluated to assess potential systemic effects. At the 3-month time point, the LJ + LR group showed significantly lower levels of triglycerides (TG), total cholesterol (TCHO), and low-density lipoprotein cholesterol (LDL-CHO) compared to the CTRL group, suggesting beneficial effects on metabolic and vascular health (Supplementary Fig. S15a–d). For inflammatory markers, IL-6 levels were consistently higher in the LJ group, while the LR and LJ + LR groups maintained relatively lower levels throughout the study period, indicating that co-administration may help alleviate systemic inflammation (Supplementary Fig. S15e)34.
In summary, the administration of L. johnsonii and L. reuteri, particularly in combination, significantly enhanced muscle strength and growth as demonstrated by improvements in motor performance, increased muscle weight, and structural adaptations in muscle fibers. These results highlight the potential of L. johnsonii and L. reuteri as effective probiotics for improving muscle performance, with the LJ + LR combination showing superior efficacy. Further studies are needed to explore the mechanisms underlying these effects and to address the observed inflammatory responses.
Discussion
The gut microbiota is a complex of microorganisms that live in the gastrointestinal tract and consists of a variety of bacteria, fungi, protists, archaea and viruses35. The intestinal microbiome, comprised of this complex ecosystem, regulates various physiological activities that occur in the body, including digestion, metabolism, essential amino acid synthesis, detoxification, metabolism, and immune regulation35,36,37,38. According to evidence, the composition and diversity of the gut microbiota may be crucial factors in determining skeletal muscle metabolism and function39. Given that the gut microbiota is known to induce insulin resistance and obesity, at least some components of it can potentially regulate skeletal muscle mass and functionality39,40. The role of the gut microbiota in maintaining whole-body fat mass, skeletal muscle mass, and bodily function (defined as the gut-muscle axis) has been proposed by several independent research groups39,41,42. Indeed, certain probiotics, such as bifidobacteria, are widely known as the most well-known probiotics for preventing sarcopenia and muscle atrophy in rodent43. However, applying the same bacteria to humans has not achieved high reproducibility of muscle mass due to the diversity of individual populations43. In this context, detailed studies on the causal role of microbiota that promote strength or exercise performance are needed. Strength has complex characteristics greatly influenced by genetic background, nutrition, and environment. Given that genetic influences are most closely related, this study aimed to compare and analyze changes in individual mice’s fecal and overall gut compositions before and after FMT, and subsequently identify microbiota that enhance strength or exercise performance through follow-up verification, following Koch’s postulates.
In our study, human feces were administered to mice for 0 to 3 months, and the microbial diversity of feces and gastrointestinal tract were analysed through meta-analysis according to the muscle strength (Fig. 1). As a result, we found that when analysing Rotarod and Wire suspensions, microbial diversity was richer at 3 months than at 0 months, and interestingly, when we analysed the gastrointestinal tract rather than feces, we found that α, β -diversity increased and microbial richness increased (Figs. 2, 3, 4, 5). These results were consistent with previous findings29,44. And we identified two candidate strains (L. johnsonii and L. reuteri) that may help improve Rotarod and Wire suspensions using the GI tract, which is rich in microbial diversity (Fig. 6).
Finally, the two selected strains were administered to mice to evaluate their effects on Rotarod and Wire suspension performance. As shown in Fig. 7b and 7c, significant improvements in muscle strength were observed in all treatment groups—L. johnsonii, L. reuteri, and the combined administration—compared to untreated controls. In addition, elevated expression levels of follistatin (FST) and insulin-like growth factor-1 (IGF1), which are associated with muscle fiber cross-sectional area, muscle weight, and overall muscle growth, were detected in all bacterial treatment groups (Fig. 7f). Interestingly, administration of each strain, as well as their combination, resulted in a significant decrease in body weight while increasing muscle mass. Notably, the group receiving the combined treatment exhibited the greatest increase in both muscle mass and muscle fiber cross-sectional area (Fig. 7e).
Previous studies have reported that L. johnsonii and L. reuteri inhibit mitochondrial damage by effectively reducing cytochrome C45. In general, mitochondria are very closely related to motility. Mitochondrial dysfunction predisposes to calcium-mediated excitotoxicity, increases the production of reactive oxygen species, and triggers the death of motor neurons by initiating the endogenous apoptotic pathway, ultimately causing muscle strength impairment and reduced motor function46. L. johnsonii and L. reuteri are also known to contribute to the production of short-chain fatty acids, which help improve muscle synthesis and function47,48. Therefore, it is believed that the two strains may have had some effect on mitochondrial function as well as production of short-chain fatty acids in elderly mice, thereby increasing motor function. While this study provides fundamental insights into the role of gut microbiota in the regulation of muscle strength, the underlying molecular mechanisms remain to be fully clarified. Further investigation using integrated omics approaches, such as metabolomics and transcriptomics, will be essential to elucidate the relevant pathways. Moreover, as the present study was conducted using a murine model, its applicability to human physiology may be limited. Future validation studies employing human-relevant systems, including organoid cultures, ex vivo models, and population-level analyses, should be considered. These limitations are acknowledged as important aspects to be addressed in subsequent research.
Taken together, our results are the first to demonstrate the improved muscle strength of L. johnsonii and L. reuteri, suggesting potential for future use in improving various diseases related to increased motor function and muscle strength.
Conclusion
This study provides the first evidence demonstrating that L. johnsonii and L. reuteri significantly enhance muscle strength and performance, with notable effects observed in motor behavioral tests, muscle mass, and molecular markers associated with muscle growth. Through systematic FMT and detailed gastrointestinal microbiome analysis, we identified these strains as key contributors to the gut-muscle axis, highlighting their potential role in promoting skeletal muscle health.
Our findings revealed that L. johnsonii and L. reuteri improve muscle strength by potentially contributing to the production of short-chain fatty acids and other metabolic byproducts, which may activate molecular pathways associated with muscle growth and repair, including the upregulation of FST and IGF-1. These effects suggest a role for microbial metabolites in enhancing muscle synthesis and metabolic efficiency, ultimately improving physical performance.
Importantly, the combination of L. johnsonii and L. reuteri exhibited synergistic effects, resulting in the highest muscle mass, fiber cross-sectional area, and strength improvements, underscoring the potential benefits of multi-strain probiotic formulations.
These results not only shed light on the mechanisms by which specific gut microbes influence muscle strength but also provide a foundation for developing microbiota-based therapeutic strategies to combat sarcopenia, muscle atrophy, and other disorders associated with reduced muscle function.
In conclusion, L. johnsonii and L. reuteri hold significant promise as effective probiotics for enhancing muscle strength and motor function, offering potential applications in clinical and therapeutic settings to improve overall musculoskeletal health. Future studies should focus on validating these effects in human populations and further elucidating the molecular mechanisms underlying their beneficial actions.
Data availability
The 16S rRNA sequencing data generated during this study have been deposited in the NCBI Sequence Read Archive (SRA) under the BioProject accession number PRJNA1214556 (ID 1214556—BioProject—NCBI).
References
Durack, J. & Lynch, S. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 216, 20–40 (2019).
Lloyd-Price, J., Abu-Ali, G. & Huttenhower, C. The healthy human microbiome. Genome Med. 8, 1–11 (2016).
Bik, E. Composition and function of the human-associated microbiota. Nutr. Rev. 67, S164–S171 (2009).
Righetto, I., Gasparotto, M., Casalino, L., Vacca, M. & Filippini, F. Exogenous players in mitochondria-related CNS disorders: Viral pathogens and unbalanced microbiota in the gut-brain axis. Biomolecules 13, 69 (2023).
Khanna, S. & Raffals, L. The microbiome in Crohn’s disease: Role in pathogenesis and role of microbiome replacement therapies. Gastroenterol. Clin. 46, 481–492 (2017).
Pandey, K. & Umar, S. Microbiome in drug resistance to colon cancer. Curr. Opin. Physiol. 23, 100472 (2021).
Tilg, H. & Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 121, 2126–2132 (2011).
Hartstra, A., Bouter, K., Bäckhed, F. & Nieuwdorp, M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 38, 159–165 (2015).
Capuco, A. et al. Current perspectives on gut microbiome dysbiosis and depression. Adv. Ther. 37, 1328–1346 (2020).
Hirschberg, S., Gisevius, B., Duscha, A. & Haghikia, A. Implications of diet and the gut microbiome in neuroinflammatory and neurodegenerative diseases. Int. J. Mol. Sci. 20, 3109 (2019).
Gleeson, M. et al. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11, 607–615 (2011).
Lkhagva, E., Chung, H., Ahn, J. & Hong, S. Host factors affect the gut microbiome more significantly than diet shift. Microorganisms 9, 2520 (2021).
Bilski, J. et al. Exploiting significance of physical exercise in prevention of gastrointestinal disorders. Curr. Pharm. Des. 24, 1916–1925 (2018).
Sohail, M., Yassine, H., Sohail, A. & Thani, A. Impact of physical exercise on gut microbiome, inflammation, and the pathobiology of metabolic disorders. Rev. Diabet. Stud. 15, 35–48 (2019).
Giron, M., Thomas, M., Dardevet, D., Chassard, C. & Savary-Auzeloux, I. Gut microbes and muscle function: Can probiotics make our muscles stronger?. JCSM. 13, 1460–1476 (2022).
Wang, Y. et al. Progress of linking gut microbiota and musculoskeletal health: Casualty, mechanisms, and translational values. Gut microbes. 15, 2263207 (2023).
Wu, Y. et al. The composition of the gut microbiome in patients with sarcopenia. Turk. J. Chem. 47, 325–332 (2022).
Ahn, J., Choi, Y., Kim, H., Chung, H. & Hong, S. Identification of the intestinal microbes associated with locomotion. Int. J. Mol. Sci. 24, 11392 (2023).
Nagpal, R. et al. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci. Rep. 8, 12649 (2018).
Ahn, J. et al. Identification of muscle strength-related gut microbes through human fecal microbiome transplantation. Int. J. Mol. Sci. 25, 662 (2024).
Chung, H., Sharma, S., Kim, H., Baek, S. & Hong, S. The resveratrol-enriched rice DJ526 boosts motor coordination and physical strength. Sci. Rep. 6, 23958 (2016).
Baek, S. et al. Treatment of obesity with the resveratrol-enriched rice DJ-526. Sci. Rep. 4, 3879 (2014).
Zhai, C. et al. Comparative analysis of original and replaced gut microbiomes within same individuals identified the intestinal microbes associated with weight gaining. Microorganisms. 10, 1062 (2022).
Callahan, B. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Love, M., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Brown, M. A method for combining non-independent, one-sided tests of significance. Biometrics 31, 987–992 (1975).
Paulson, J., Stine, O., Bravo, H. & Pop, M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods. 10, 1200–1202 (2013).
Feng, L., Han, C. & Cao, S. Deficits in motor and cognitive functions in an adult mouse model of hypoxia-ischemia induced stroke. Sci. Rep. 10, 20646 (2020).
Ahn, J. et al. Fecal microbiome does not represent whole gut microbiome. Cell Microbiol. 2023, 6868417 (2023).
Sumner, C. et al. Inhibition of myostatin does not ameliorate disease features of severe spinal muscular atrophy mice. Hum. Mol Genet. 18, 3145–3152 (2009).
Lee, S. Quadrupling muscle mass in mice by targeting TGF-ß signalling pathways. PLoS ONE 2, 789 (2007).
Schiaffino, S., Reggiani, C., Akimoto, T. & Blaauw, B. Molecular mechanisms of skeletal muscle hypertrophy. J. Neuromuscul. Dis. 8, 169–183 (2021).
Barbé, C. et al. Role of IGF-I in follistatin-induced skeletal muscle hypertrophy. Am. J. Physiol. Endocrinol. Metab. 309, E557–E567 (2015).
Fiedorczuk, P. et al. Investigating the Role of Serum and Plasma IL-6, IL-8, IL-10, TNF-alpha, CRP, and S100B concentrations in obstructive sleep apnea diagnosis. Int. J. Mol. Sci. 24, 13875 (2023).
Sirisinha, S. The potential impact of gut microbiota on your health: Current status and future challenges. Asian Pac. J. Allergy Immunol. 34, 249–264 (2016).
Belizário, J., Faintuch, J. & Garay-Malpartida, M. Gut microbiome dysbiosis and immunometabolism: New frontiers for treatment of metabolic diseases. Mediat. Inflamm. 2018, 2037838 (2018).
Ahn, J. S. et al. Identification of specific gut microbes and their therapeutic potential in ameliorating systemic lupus erythematosus in a mouse model. Life Sci. 374, 123684 (2025).
Ahn, J. S., Kim, S., Han, E. J., Hong, S. T. & Chung, H. J. Increasing spatial working memory in mice with Akkermansia muciniphila. Commun. Biol. 8, 546 (2025).
Grosicki, G., Fielding, R. & Lustgarten, M. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: Biological basis for a gut-muscle axis. Calcif. Tissue Int. 102, 433–442 (2018).
Lahiri, S. et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 11, 5662 (2019).
De Sire, R. et al. Skeletal muscle-gut axis: Emerging mechanisms of sarcopenia for intestinal and extra intestinal diseases. Minerva Gastroenterol. Dietol. 64, 351–362 (2018).
Ni Lochlainn, M., Bowyer, R. & Steves, C. Dietary protein and muscle in aging people: the potential role of the gut microbiome. Nutrients 10, 929 (2018).
Prokopidis, K. et al. Impact of probiotics on muscle mass, muscle strength, and lean body mass: a systematic review and meta-analysis. JCSM. 14, 30–44 (2022).
Lkhagva, E. et al. The regional diversity of gut microbiome along the GI tract of male C57BL/6 mice. BMC microbiol. 21, 1–13 (2021).
Watthanasakphuban, N. et al. Development of high cell density Limosilactobacillus reuteri KUB-AC5 for cell factory using oxidative stress reduction approach. Microb Cell fact. 22, 86 (2023).
Ham, P. III. & Raju, R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog. Neurobiol. 157, 92–116 (2017).
Smythe, P. & Efthimiou, G. In silico genomic and metabolic atlas of Limosilactobacillus reuteri DSM 20016: An Insight into human health. Microorganisms. 10, 1341 (2022).
He, T. et al. Lactobacillus johnsonii L531 reduces pathogen load and helps maintain short-chain fatty acid levels in the intestines of pigs challenged with Salmonella enterica Infantis. Vet Microbiol. 230, 187–194 (2019).
Acknowledgements
This research was funded by the Korea Basic Science Institute (KBSI) grants C512230. This research was also supported by the Bio&Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. RS-2023-00224099).
Author information
Authors and Affiliations
Contributions
S.-T.H. and H.-J.C. were responsible for the overall content as guarantor. J.-S.A., S.-T.H., and H.-J.C. conceived the research and designed the experiments; H.-J.C. supervised the project; J.-S.A., H.-M.K. and E.-J.H. performed the experiments and data curation; J.-S.A., H.-M.K., E.-J.H. and H.-J.C. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Institutional Animal Care and Use Committee of the Korea Basic Science Institute approved the animal protocols (KBSI-IACUC-23-12), and all animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals issued by the Laboratory Animal Resources Commission of that Institute. This study is reported in accordance with the ARRIVE guidelines (https://arriveguidelines.org) to ensure the ethical and transparent reporting of animal research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ahn, JS., Kim, HM., Han, EJ. et al. Discovery of intestinal microorganisms that affect the improvement of muscle strength. Sci Rep 15, 30179 (2025). https://doi.org/10.1038/s41598-025-15222-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15222-2