Abstract
Stroke is a leading cause of death and disability worldwide, especially in the aging population. Psychological vulnerability serves as a multidimensional mental health vulnerability state, but there is a lack of large sample studies on its association with stroke. The aim of this study was to investigate the relationship between psychological vulnerability, as quantified by the Psychological Frailty Index, and stroke. This study was based on baseline data from the China Health and Retirement Longitudinal Study (CHARLS), which included 15,284 participants ≥ 45 years of age. PFI was used as a composite measure of psychological vulnerability by quartiles. Stroke was analyzed using Cox logistic regression models to analyze the relationship between PFI and stroke risk, and the dose-response relationship was tested using restricted cubic spline (RCS). Subgroup analyses and interaction tests were performed to assess differences in different populations. In the fully adjusted Cox model, each one-IQR increase in PFI was associated with an 87% higher risk of incident stroke (HR = 1.87, 95% CI 1.54–2.27; P < 0.001). Compared with participants in the lowest PFI quartile (Q1), those in the highest quartile (Q4) exhibited a more than three‐fold increased stroke risk (HR = 3.12, 95% CI 1.99–4.91; P < 0.001). Restricted cubic spline analysis demonstrated a linear dose–response relationship between PFI and stroke risk (P for nonlinearity > 0.05). In subgroup analyses, the association between PFI and stroke was significantly modified by age and marital status (P for interaction < 0.05). In this study, we found that psychological vulnerability was significantly positively and linearly associated with stroke risk. Assessment of psychological vulnerability may be an important component of a comprehensive stroke prevention and control strategy, with particular attention to the elderly and those with social support deficits. Future longitudinal studies should be conducted to clarify the causal relationship.
Similar content being viewed by others
Background
Stroke is one of the leading causes of death and disability globally, especially in aging populations1. According to the Global Burden of Disease study, about 12.2 million incident strokes and 6.6 million stroke-related deaths were reported worldwide in 20192. In China, the rising prevalence of stroke, with high recurrence and mortality rates, poses a major challenge to the healthcare system3,4. The aging population and the increased prevalence of cardiometabolic risk factors, such as hypertension, diabetes, and hyperlipidemia, continue to drive an upward trend in the incidence of stroke5,6,7. Recently emerging evidence suggests that psychosocial and psychological factors may also play a crucial role in the development and prognosis of stroke8,9. Psychological vulnerability is often associated with a chronic low-grade systemic inflammatory state, and increased inflammatory factors (e.g., IL-6, CRP) promote platelet adhesion and aggregation, exacerbating hemodynamic disturbances10,11. Neuroimmune interactions can also damage neurovascular units and weaken brain tissue tolerance to ischemia-reperfusion, thereby increasing stroke incidence and brain damage12. Behaviorally, psychologically vulnerable individuals are more likely to experience depression, anxiety, and social withdrawal, which are closely related to unhealthy lifestyles (e.g., sedentary lifestyle, eating disorders, smoking, and drinking) and poor chronic disease management (e.g., insufficient adherence to hypertension and diabetes mellitus)13,14,15.
Originally conceptualized as a geriatric syndrome characterized by decreased physiological reserves and increased vulnerability to stressors, frailty has increasingly been recognized as a multidimensional disease16,17. Psychological vulnerability has emerged as an important but underdeveloped area18. Unlike physical frailty, mental frailty focuses on mental and emotional vulnerability, which may independently affect health outcomes19,20. The Psychological Fragility Index (PFI) was developed as a composite measure to capture the psychological vulnerability of older adults, providing a new way to quantify psychological vulnerability21. The PFI comprises four equally-weighted subdimensions: (1) depressive symptoms, assessed via eight items from the simplified CES-D scale (score range 0–15); (2) subjective cognitive complaints, covering self-rated memory and attention (total score 0–10); (3) coping ability, measured by a four-item coping questionnaire (total score 0–8); and (4) emotional instability, evaluated through a self-reported mood fluctuation scale (total score 0–6). Each subscale score is first converted to a Z-score and then summed to yield the raw PFI. Previous studies have preliminarily revealed significant correlations between PFI and a variety of health outcomes. For example, a validation study based on the CHARLS data found that the risk of all-cause mortality nearly doubled with each increase in the level of PFI per IQR, and the number of outpatient visits and hospitalization rates also increased significantly, respectively22. Another cohort study noted that higher psychological vulnerability scores were strongly associated with an increased risk of heart failure recurrence and postoperative complications23. However, there is a lack of research on the relationship between psychological frailty and stroke, especially in large population groups in low- and middle-income countries such as China.
The China Health and Retirement Longitudinal Study (CHARLS) provides nationally representative data on the health and psychosocial status of Chinese adults aged 45 and older24. Understanding the relationship between PFI and stroke may help in the early identification of high-risk individuals. Therefore, we hypothesized an association between PFI and stroke. The purpose of this study was to assess the association between PFI and stroke using data from CHARLS to inform more comprehensive prevention strategies that incorporate mental health into stroke risk assessment and management.
Materials and methods
Study design and population
This study utilized data from the China Health and Retirement Longitudinal Study (CHARLS), a nationally representative longitudinal survey conducted biennially since 2011. CHARLS employs a multi-stage stratified probability sampling method to ensure broad coverage across 150 counties or districts and 450 urban communities or villages spanning 28 provinces in China25.
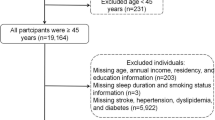
Our analysis participants were surveyed in Wave 4 (2018), which served as the baseline for psychological vulnerability assessment. At this wave, a total of 17,617 individuals participated. We excluded participants younger than 45 years or with missing age information (n = 219), those lacking stroke outcome data in the 2018 survey (n = 1,262), and those with incomplete PFI and covariates information (n = 852). Consequently, 15,284 participants remained for final analysis. Incident stroke events were identified based on self-reports collected during Wave 5 (2020) follow-up surveys, representing new cases occurring after the baseline PFI measurement. This design established a clear temporal sequence with psychological vulnerability assessed prior to stroke onset, supporting the cohort study framework.
Figure 1 illustrates the detailed inclusion and exclusion process. The overall follow-up period between baseline assessment and outcome ascertainment was approximately two years. Loss to follow-up and missing data were carefully addressed to minimize bias, ensuring the validity of the temporal relationship between PFI and incident stroke.
Definition of PFI
The PFI consists of 26 items from the CHARLS data covering four sub-dimensions. Depressive symptoms (8 items, based on a simplified version of the CES-D, 4-point Likert scale ranging from 0 to 3), subjective cognitive complaints (6 items, self-assessment of memory and attention, 5-point Likert scale ranging from 1 to 5), coping (6 items, Coping Styles Questionnaire, 5-point Likert scale ranging from 1 to 5) and emotional instability (6 items, self-assessment of mood swings, 4-point Likert scale ranging from 1 to 5), and mood instability (6 items, self-assessment of mood swings, level 4 Likert scale, scores 0–3)26. All reverse entries were reverse coded to ensure that higher scores represented greater psychological vulnerability. First, the scores of the entries within each subdimension were summed to obtain the raw subscale total scores. Subsequently, each of the four subscale total scores was Z-score normalized to eliminate scale differences across scales. Finally, the standardized scores of the four subscales were summed by equal weights to generate the original PFI values. To facilitate the interpretation of the regression model effects, we further standardized the value in terms of the interquartile range (IQR) of the PFI, so that the model regression coefficients reflected the effect of each additional IQR unit of psychological vulnerability on the risk of stroke27. The construction of the PFI is based on established literature and previous studies, and its multidimensional composite properties help to fully quantify psychological vulnerability21.
Assessment of stroke
The outcome variable in this study was the participant’s first stroke event, with stroke onset defined as the first reported stroke between the baseline survey (CHARLS wave 4, 2018) and the follow-up period (wave 5, 2020). Stroke onset was captured by medically trained investigators based on a standardized questionnaire and relied primarily on participants’ self-reported history of physician diagnosis28. The data were verified and validated by the study team to maximize the accuracy of the information. However, stroke diagnosis was not combined with clinical history or imaging data, and there was some risk of information bias.
Covariates
Covariates were identified via structured CHARLS questionnaires and onsite measurements. In addition to socio-demographic factors (age, sex, place of residence, marital status, education), we included lifestyle variables and key comorbidities. Smoking status was classified as never smoker (never smoked ≥ 100 cigarettes in lifetime), current smoker (smoked ≥ 1 cigarette in the past month), or former smoker (smoked ≥ 100 cigarettes lifetime but abstinent ≥ 1 year; those abstinent < 1 year were classified as current). Alcohol consumption was categorized as frequent (> 1 time/month in the past year), occasional (< 1 time/month but ≥ 1 time in the past year), or none (no lifetime drinking), with one drinking episode defined as ≥ 50 ml white spirit, ≥ 500 ml beer, or ≥ 150 ml wine. Diabetes mellitus was defined by any of: self-reported physician diagnosis; current use of oral hypoglycemic agents or insulin; fasting plasma glucose ≥ 7.0 mmol/L; or HbA1c ≥ 6.5%29. Hypertension was defined by any of: self-reported diagnosis; current antihypertensive medication; or mean measured systolic blood pressure ≥ 140 mmHg or diastolic ≥ 90 mmHg30. Dyslipidemia was determined by self-report, lipid-lowering medication use, or laboratory criteria (total cholesterol ≥ 6.2 mmol/L, LDL-C ≥ 4.1 mmol/L, HDL-C < 1.0 mmol/L, or triglycerides ≥ 2.3 mmol/L)31. Heart disease was ascertained by self-reported physician diagnosis or follow-up interview confirmation32.
Statistical analyses
All variables were described according to the occurrence of stroke at baseline in this study. Continuous variables were first tested for normality, and for variables that were approximately normally distributed, they were expressed as mean ± standard deviation (SD) and compared between groups using analysis of variance (ANOVA). For non-normally distributed variables, medians and quartiles were described and compared using non-parametric tests. Categorical variables were expressed as frequencies and percentages, and differences between groups were analyzed using the chi-square test. PFI was treated as a continuous variable and standardized by its interquartile range (IQR), and the lowest quartile group was used as the reference group when dividing based on quartiles. Cox proportional risk regression modeling was used to assess the association between PFI and the risk of stroke occurrence. Potential non-dose-response relationships between PFI and stroke risk were explored using restricted cubic spline (RCS). The vertical axis of the RCS model reflects the predicted probability of disease from the cox model. Heterogeneity of the association between PFI and stroke in different demographic and clinical subgroups was assessed by subgroup analysis and interaction tests. Because multiple comparisons increase the risk of type I error, we used the Bonferroni correction method in the subgroup analysis. A total of 22 comparisons were made, resulting in an adjusted significance threshold of α’ = 0.05/28 ≈ 0.00227. Correlations were only considered statistically significant if the p-value was below this threshold. It should be noted that Bonferroni correction is a conservative method that may increase the risk of type II error; therefore, the findings should be interpreted with caution and further studies are needed to validate these subgroup effects. Statistical analysis was performed using R4.4.2 (R Foundation, http://www.R-project.org). A two-sided P value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics of participants by incident stroke status during follow-up
A total of 15,284 adults without stroke at baseline and 323 (2.11%) with first stroke during follow-up were included in this study. Age and PFI were significantly higher in the stroke group than in the non-stroke group (P < 0.001). The proportion of unmarried persons was 18.27% in the stroke group, which was significantly higher than that of 13.70% in the non-stroke group (P = 0.020). The proportion of those with elementary school education or less was 77.09%, which was higher than 65.76% in the non-stroke group (P < 0.001), and the proportion of rural residents was 70.28%, which was also significantly higher than 62.23% in the non-stroke group (P = 0.010). In terms of comorbidities, the prevalence of hypertension was 57.28% in the stroke group, which was significantly higher than that of 32.64% in the non-stroke group (P < 0.001). The prevalence of diabetes mellitus was 17.03%, which was higher than that of 11.30% in the non-stroke group (P = 0.010). The prevalence of dyslipidemia was 33.44%, higher than 19.43% in the non-stroke group (P < 0.001). The prevalence of heart disease was 34.37%, which was significantly higher than 17.18% in the non-stroke group (P < 0.001). In addition, the proportion of frequent drinkers in the stroke group was 19.81%, which was significantly lower than the 27.24% in the non-stroke group (P = 0.010). Gender distribution (P = 0.420) and smoking status (P = 0.160) were not significantly different between the two groups (Table 1).
Continuous variables with approximately normal distribution are presented as mean ± SD and compared using analysis of variance (ANOVA). Non-normally distributed variables are presented as median [interquartile range] and compared using non-parametric tests. Categorical variables are presented as number (percentage) and compared using the chi-square test. Abbreviations: DM, diabetes mellitus.
Cox proportional hazards analysis of PFI and incident stroke risk
To assess the dynamic risk between PFI and first-ever stroke, we constructed an analytic model using the Cox proportional risk regression model (Table 2). In the crude model, the risk of stroke increased 2.57-fold (95% CI: 2.18–3.04, P < 0.001) for each IQR increase in the PFI continuous variable. In Model 1, the HR was 2.20 (95% CI: 1.82–2.67, P < 0.001). In Model 2, the HR was 1.87 (95% CI: 1.54–2.27, P < 0.001). After grouping PFIs by quartiles, there was a dose-dependent increase in the risk of stroke in Q2-Q4, using the Q1 group as a reference (P for trend < 0.001).
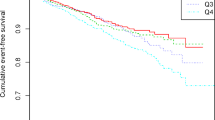
Nonlinear analysis of the association between PFI and stroke risk
The relationship between PFI and stroke risk was assessed using the RCS model (Fig. 2). We built RCS curves based on 3 previously established models. All three RCS curves were statistically different (P for overall all < 0.001). In the crude model, there was a linear correlation between PFI and stroke risk (P for nonlinear = 0.985). In particular, in the fully adjusted model, PFI and stroke risk continued to show a linear association (P for nonlinear = 0.789).
Subgroup analyses and interaction tests
Figure 3 presents the results of subgroup analyses and interaction tests examining the association between PFI and stroke risk. To account for multiple comparisons, Bonferroni correction was applied. After correction (adjusted significance level α′ = 0.05/22 ≈ 0.00227), significant associations remained in the subgroups of age (45–60 and ≥ 60 years), sex (female and male), marital status (married), location (rural and urban), smoking status (never and former smokers), drinking status (none and frequent drinkers), hypertension (yes and no), diabetes mellitus (no), dyslipidemia (yes and no), and heart disease (yes and no) (all P < 0.00227). Associations in other subgroups did not reach statistical significance after correction. Interaction tests indicated that age and marital status significantly modified the relationship between PFI and stroke risk (P for interaction < 0.05).
Investigation of the association between PFI and stroke by subgroup. Note 1 The above model adjusted for age, gender, location, marital status, smoking status, drinking status, hypertension, diabetes, dyslipidemia, and heart disease. Note 2 In each case, the model is not adjusted for the stratification variable. Note 3 Given the multiple subgroup analyses, we acknowledge the potential for Type I errors. After Bonferroni correction, significant associations remained in age (45–60 and ≥ 60 years), sex (female and male), married status, location (rural and urban), never smokers, former smokers, none drinkers, frequent drinkers, hypertension (yes and no), no diabetes, dyslipidemia (yes and no), and heart disease (yes and no). Associations in other subgroups did not reach statistical significance after correction and should be interpreted with caution.
Discussion
In this large, nationally representative cohort study based on the CHARLS database, we observed a robust positive association between the PFI and incident stroke risk. Higher PFI was independently associated with increased stroke risk even after adjustment for demographic factors, comorbidities, and lifestyle variables. Subgroup analyses further revealed that this association was particularly pronounced in middle-aged adults (45–60 years) and among married individuals, highlighting important sociodemographic modifiers. These findings underscore the potential of psychological vulnerability as a valuable target for stroke risk stratification and prevention.
We found that those who had a stroke during the follow-up period were older, had a higher proportion of unmarried individuals, had lower levels of education, and tended to live in rural areas compared with those who did not have a stroke. In addition, the prevalence of major comorbidities such as hypertension, diabetes mellitus, dyslipidemia, and heart disease was significantly higher in the stroke group than in the nonstroke group. These descriptive results are consistent with previous epidemiologic studies, suggesting that stroke occurs as a result of a combination of demographic, socioeconomic, and clinical multifactors33,34,35. It is important to note that the present comparison of baseline characteristics reflects only between-group differences, and we subsequently used Cox regression modeling to adjust for potential confounders to further explore the independent effect of psychological vulnerability on stroke risk36,37,38. Demographic social factors and metabolic comorbidities may work in conjunction with psychological vulnerability to further exacerbate stroke susceptibility39,40. Therefore, future adjustment for these confounders is necessary to identify independent effects of psychological vulnerability on stroke risk. In addition, RCS analysis suggested a linear association between PFI and stroke risk. This linear characterization suggests that even moderate psychological vulnerability may adversely affect the occurrence of cerebrovascular events. Subgroup analyses showed that the effect of psychological vulnerability on stroke risk was more pronounced in the middle-aged group aged 45–60 years. The middle-aged population may be more prone to behavioral risk factors (e.g., unhealthy lifestyle) and metabolic abnormalities in the face of psychological vulnerability compared with the older age group, thereby amplifying stroke risk41. Furthermore, despite the prevalence of decreased neurovascular regulation and immune function in the elderly population, making them physiologically more susceptible to psychological factors, the relatively low HR suggests that their psychological vulnerability has a smaller marginal impact on stroke risk42,43. With regard to marital status, the positive association between PFI and stroke risk was significant in the married population, possibly due to the emotional pressure or responsibility in the spousal relationship that makes psychological vulnerability more directly affect health behaviors and physiological responses44. In contrast, unmarried individuals, although lacking spousal support, may be somewhat buffered by other social relationships (family, friends, community support, etc.), resulting in a non-significant association between psychological vulnerability and stroke risk45. In addition, the small number of stroke events and limited statistical efficacy in the unmarried group may also have affected the results of the significance test46. The complexity and diversity of social support warrant further in-depth study to clarify how different forms of support modulate the effect of psychological vulnerability on stroke risk.
Multiple potential mechanisms may explain the observed association between psychological vulnerability and increased risk of stroke. First, psychological vulnerability is usually accompanied by chronic psychological stress47, systemic inflammation48, and dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis49. And chronic stress leads to prolonged activation of the HPA axis, resulting in elevated cortisol levels, endothelial dysfunction, and elevated blood pressure50. Previous studies have demonstrated that these factors may contribute to cerebrovascular pathology51. Second, people with high psychological vulnerability may be more prone to depressive symptoms, anxiety, cognitive decline, and social withdrawal52. These conditions are independently associated with poor cardiovascular outcomes and may increase the risk of stroke53. Third, From a behavioral perspective, individuals who are psychologically weak may be less likely to engage in health-promoting behaviors54. Instead, they usually have bad habits such as smoking, which is consistent with our findings. They may also exhibit lower adherence to prescribed medications and medical advice, which may affect the management of cardiovascular risk factors such as hypertension or diabetes55. Finally, psychological vulnerability may be associated with reduced access to or utilization of health-care services. This may stem from a lack of health awareness, cognitive impairment, or a lack of social support, which delays the diagnosis and possible treatment of stroke. These interrelated biological and behavioral pathways may collectively explain the high vulnerability of psychologically vulnerable individuals to stroke. However, given the limitations of our dataset, we recognize that these proposed mechanisms remain speculative in the context of this study, and future studies will need to validate these pathways in conjunction with relevant biomarker and mediator analyses.
The present study found that the risk of stroke increased by approximately 87% for each IQR increase in PFI. This effect remained significant after adjusting for classical stroke risk factors, indicating the importance of psychological vulnerability as an independent risk factor. It is noteworthy that the magnitude of the effect of PFI on stroke risk was comparable to that of some classical risk factors (e.g., hypertension, diabetes), suggesting that mental health status has a non-negligible place in stroke risk assessment56. In addition, the PFI, as a multidimensional psychological composite indicator, provides an important addition to stroke risk identification by capturing individual psychological and behavioral risk characteristics that are difficult to be reflected by traditional physiological indicators. Clinically, our findings support the incorporation of psychological vulnerability assessment tools, such as the PFI, into routine stroke risk screening systems. Through early identification of high-risk individuals with higher psychological vulnerability, individualized interventions such as targeted psychological counseling, behavioral interventions, and social support enhancement can be implemented. This multidimensional and comprehensive management strategy is expected to mitigate the direct and indirect effects of psychological vulnerability on stroke occurrence and promote comprehensive stroke prevention and health management.
Strengths and limitations
This study has several significant strengths. First, we used a large-scale, nationally representative sample from the CHARLS to ensure the broad applicability and representativeness of the findings. Second, this study uses a validated psychological vulnerability index that integrates multiple dimensions such as depression, cognition, and coping ability to more comprehensively reflect an individual’s mental health status. Third, we utilized the RCS method to explore the relationship between psychological vulnerability and stroke risk in depth, which enhanced the granularity of the analysis. Fourth, interaction and stratification analyses were conducted for different subgroups, which effectively revealed the differential effects of psychological vulnerability in different populations and provided a basis for personalized risk assessment. The present study also suffers from the following limitations. First, due to the cross-sectional design, the causal relationship between psychological vulnerability and stroke could not be clarified. Second, both PFI and stroke events relied on self-reporting, which may have information bias and affect the accuracy of the results. Third, although we adjusted for multiple known stroke risk factors, we were unable to completely exclude potential residual confounding effects. Finally, this study lacked validation from an external independent dataset, limiting the generalizability and generalization of the results. Given that the study population consisted of Chinese adults aged 45 years and older, the applicability of the psychological vulnerability index and its association with stroke risk across different cultural backgrounds and healthcare systems needs to be further explored. Future validation studies should be conducted in diverse populations and in different regions to enhance the robustness of the findings and their value for widespread application.
Conclusion
In this study of 15,284 Chinese middle-aged and older adults, there was a significant and linear positive association between PFI and stroke risk. Age and marital status had a significant modifying effect on the association, suggesting that specific populations may have higher susceptibility. Therefore, psychological assessment should be incorporated into routine stroke risk screening and targeted interventions should be developed to reduce psychological vulnerability in high-risk populations and thereby prevent stroke.
Data availability
The data used in this study are publicly available from the CHARLS database (http://charls.pku.edu.cn/).
Abbreviations
- ANOVA:
-
Analysis of variance
- CHARLS:
-
China Health and Retirement Longitudinal Study
- PFI:
-
Psychological Fragility Index
- DM:
-
Diabetes mellitus
- RCS:
-
Restricted cubic spline
- IQR:
-
Interquartile range
References
He, M. et al. Global, regional, and National epidemiology of ischemic stroke in young adults, 1990–2021. J. Neurol. 272 (5), 354 (2025).
eClinicalMedicine. The rising global burden of stroke. EClinicalMedicine 59, 102028 (2023).
Ji, C., Ge, X., Zhang, J. & Tong, H. The stroke burden in China and its Long-Term trends: insights from the global burden of disease (GBD) study 1990–2021. Nutrition Metabolism Cardiovasc. Diseases :103848. (2025).
Zhao, Y. et al. Increasing burden of stroke in china: A systematic review and meta-analysis of prevalence, incidence, mortality, and case fatality. Int. J. Stroke: Official J. Int. Stroke Soc. 18 (3), 259–267 (2023).
Bourassa, K. J. et al. Accelerated epigenetic aging and prospective morbidity and mortality among U.S. Veterans. The journals of gerontology series A, biological sciences and medical sciences 2025.
Aalami Harandi, S. et al. Do cardiometabolic risk factors relative risks differ for the occurrence of ischemic heart disease and stroke?? Res. Cardiovasc. Med. 5 (1), e30619 (2016).
Kamabu, E. M. et al. Impact of diabetes mellitus on 30-day mortality among acute stroke patients in Northern Tanzania. PloS One. 20 (4), e0321988 (2025).
Thomas, K. et al. Associations of psychosocial factors with multiple health behaviors: A Population-Based study of Middle-Aged men and women. International J. Environ. Res. Public. Health 17(4). (2020).
Zhou, X. et al. Analysis of the incidence and influencing factors of depression in the acute stage of ischemic stroke: A retrospective clinical study. Brain Behav. 15 (4), e70483 (2025).
Kim, J. et al. A double-hit of stress and low-grade inflammation on functional brain network mediates posttraumatic stress symptoms. Nat. Commun. 11 (1), 1898 (2020).
Maydych, V. The Interplay Between Stress, Inflammation, and Emotional Attention: Relevance for Depression. Volume 13–2019. (2019).
Enzmann, G., Kargaran, S. & Engelhardt, B. Ischemia-reperfusion injury in stroke: Impact of the brain barriers and brain immune privilege on neutrophil function. Ther. Adv. Neurol. Disord. 11, 1756286418794184 (2018).
Wong, V. W., Yiu, E. K., Ng, C. H., Sarris, J. & Ho, F. Y. Unraveling the associations between unhealthy lifestyle behaviors and mental health in the general adult Chinese population: A cross-sectional study. J. Affect. Disord. 349, 583–595 (2024).
Huang, Y., Loux, T., Huang, X. & Feng, X. The relationship between chronic diseases and mental health: A cross-sectional study. Mental Health Prev. 32, 200307 (2023).
Ridley, M., Rao, G., Schilbach, F. & Patel, V. Poverty, depression, and anxiety: Causal evidence and mechanisms. Science (New York, NY) 370(6522). (2020).
Chen, X., Mao, G. & Leng, S. X. Frailty syndrome: An overview. Clin. Interv. Aging. 9, 433–441 (2014).
Kojima, G., Liljas, A. E. M. & Iliffe, S. Frailty syndrome: implications and challenges for health care policy. Risk Manage. Healthc. Policy. 12, 23–30 (2019).
Nobre, J. et al. Psychological Vulnerability Indices and the Adolescent’s Good Mental Health Factors: A Correlational Study in a Sample of Portuguese Adolescents. Children (Basel Switzerland) 9(12). (2022).
Cozza, M. & Boccardi, V. Cognitive frailty: A comprehensive clinical paradigm beyond cognitive decline. Ageing Res. Rev. 108, 102738 (2025).
Jiang, R. et al. Associations of physical frailty with health outcomes and brain structure in 483 033 middle-aged and older adults: a population-based study from the UK biobank. Lancet Digit. Health. 5 (6), e350–e359 (2023).
Zhao, J., Liu, J. Y. W., Fernández, D. & Tyrovolas, S. Development of a psychological frailty index: Results from the China health and retirement longitudinal study. Front. Psychol. 16, 1495733 (2025).
Zhao, J., Liu, J. Y. W., Fernández, D. & Tyrovolas, S. Development of a psychological frailty index: results from the China health and retirement longitudinal study. Volume 16–2025. (2025).
Chung, K., Wilkinson, C., Veerasamy, M. & Kunadian, V. Frailty scores and their utility in older patients with cardiovascular disease. Interventional Cardiol. (London England). 16, e05 (2021).
Liu, P., Zhang, Z. & Luo, M. Relationship between air pollution exposure and insulin resistance in Chinese middle-aged and older populations: Evidence from Chinese cohort. Front. Public. Health. 13, 1551851 (2025).
Chen, X., Wang, Y., Strauss, J. & Zhao, Y. China Health and Retirement Longitudinal Study (CHARLS). In: Encyclopedia of Gerontology and Population Aging. edn. Edited by Gu D, Dupre ME. Cham: Springer International Publishing; : 948–956. (2021).
Lajoie, C., Poleksic, J., Bracken-Roche, D., MacDonald, M. E. & Racine, E. The concept of vulnerability in mental health research: A mixed methods study on researcher perspectives. J. Empir. Res. Hum. Res. Ethics: JERHRE. 15 (3), 128–142 (2020).
Shang, Q. et al. Sleep duration and the risk of new-onset arthritis in middle-aged and older adult population: results from prospective cohort study in China. Front. Public. Health. 12, 1321860 (2024).
Wang, B., Li, L., Tang, Y. & Ran, X. Joint association of triglyceride glucose index (TyG) and body roundness index (BRI) with stroke incidence: A National cohort study. Cardiovasc. Diabetol. 24 (1), 164 (2025).
Qu, L. et al. Association between atherogenic index of plasma and new-onset stroke in individuals with different glucose metabolism status: insights from CHARLS. Cardiovasc. Diabetol. 23 (1), 215 (2024).
Yan, J., Zhang, M. Z. & He, Q. Q. Association of changes and cumulative measures of triglyceride-glucose index-body mass index with hypertension risk: A prospective cohort study. BMC Public. Health. 24 (1), 2652 (2024).
Mo, D., Zhang, P., Zhang, M., Dai, H. & Wang, G. Association between the atherogenic index of plasma and incident hypertension across different blood pressure states: A National cohort study. Cardiovasc. Diabetol. 24 (1), 219 (2025).
Tang, X., Zhang, K. & He, R. The association of triglyceride-glucose and triglyceride-glucose related indices with the risk of heart disease in a National. Cardiovasc. Diabetol. 24 (1), 54 (2025).
Avan, A. et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: An ecological analysis from the global burden of disease study 2017. BMC Med. 17 (1), 191 (2019).
Tian, D. S. et al. Prevalence and risk factors of stroke in china: a National serial cross-sectional study from 2003 to 2018. Stroke Vascular Neurol. 8 (3), 238–248 (2023).
Wang, H., Wu, M., Tu, Q. & Li, M. Risk factors for stroke in a population of central china: A cross-sectional study. Medicine 101 (46), e31946 (2022).
Gan, Y. et al. Prevalence and risk factors associated with stroke in middle-aged and older chinese: A community-based cross-sectional study. Sci. Rep. 7 (1), 9501 (2017).
Bai, X. et al. Gender differences in risk factors for ischemic stroke: A longitudinal cohort study in East China. BMC Neurol. 24 (1), 171 (2024).
Zhou, Q. et al. Influence of lifestyle on stroke risk among adults over 65 years in Northern china: A propensity score matched study. Eur. J. Integr. Med. 58, 102224 (2023).
Deng, Y. et al. Combined lifestyle factors and metabolic syndrome risk: A systematic review and meta-analysis. Int. J. Obes. 49 (2), 226–236 (2025).
Islam, M. S. et al. The interplay of factors in metabolic syndrome: Understanding its roots and complexity. Mol. Med. (Cambridge Mass). 30 (1), 279 (2024).
Lafortune, L. et al. Behavioural risk factors in Mid-Life associated with successful ageing, disability, dementia and frailty in later life: A rapid systematic review. PloS One. 11 (2), e0144405 (2016).
Finger, C. E., Moreno-Gonzalez, I., Gutierrez, A., Moruno-Manchon, J. F. & McCullough, L. D. Age-related immune alterations and cerebrovascular inflammation. Mol. Psychiatry. 27 (2), 803–818 (2022).
Iadecola, C. & Anrather, J. The immunology of stroke and dementia. Immunity 58 (1), 18–39 (2025).
Liu, Q. et al. Association between marriage and outcomes in patients with acute ischemic stroke. J. Neurol. 265 (4), 942–948 (2018).
Chai, H. W., Ayanian, J. Z. & Almeida, D. M. Non-spousal family support, marital status, and heart problems in adulthood. Psychol. Health. 36 (8), 1003–1020 (2021).
Andersen, K. K. & Olsen, T. S. Married, unmarried, divorced, and widowed and the risk of stroke. Acta Neurol. Scand. 138 (1), 41–46 (2018).
Zhang, D. et al. Neural Mechanisms Underlying the Impact of Psychological Resilience on Psychosocial Stress Responses. Depression and anxiety 2024:5526584. (2024).
Michal, Z. S., Marquardt, C. A., Krueger, R. F., Arbisi, P. A. & Venables, N. C. Early adversity and inflammation at midlife: the moderating role of internalizing psychopathology. Psychological Medicine 2025:1–10 .
Leistner, C. & Menke, A. Hypothalamic-pituitary-adrenal axis and stress. Handb. Clin. Neurol. 175, 55–64 (2020).
Starr, L. R., Dienes, K., Li, Y. I. & Shaw, Z. A. Chronic stress exposure, diurnal cortisol slope, and implications for mood and fatigue: moderation by multilocus HPA-Axis genetic variation. Psychoneuroendocrinology 100, 156–163 (2019).
Bautista, L. E. et al. The relationship between chronic stress, hair cortisol and hypertension. Int. J. Cardiol. Hypertens. 2, 100012 (2019).
Strain, J. J. The psychobiology of stress, depression, adjustment disorders and resilience. World J. Biol. Psychiatry. 19 (sup1), S14–S20 (2018).
Zhu, Y. et al. Associations of cumulative depressive symptoms with subsequent cognitive decline and adverse health events: Two prospective cohort studies. J. Affect. Disord. 320, 91–97 (2023).
Pozzato, I. et al. The role of stress reactivity and pre-injury psychosocial vulnerability to psychological and physical health immediately after traumatic injury. Psychoneuroendocrinology 127, 105190 (2021).
Burnier, M. The role of adherence in patients with chronic diseases. Eur. J. Intern. Med. 119, 1–5 (2024).
Jackson, C. A., Sudlow, C. L. M. & Mishra, G. D. Psychological distress and risk of myocardial infarction and stroke in the 45 and up study. Circ. Cardiovasc. Qual. Outcomes. 11 (9), e004500 (2018).
Acknowledgements
We expressed our gratitude to the CHARLS staff and attendees.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
GM and ZZ designed the research. DH, LL, and ZZ collected and analyzed the data, and drafted the manuscript. LX and JJ revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The CHARLS study was approved by the Peking University Institutional Review Board (IRB00001052-11015) and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, M., Zhang, Z., Dong, H. et al. Association between the psychological frailty index and stroke: a cohort study from CHARLS. Sci Rep 15, 29756 (2025). https://doi.org/10.1038/s41598-025-15270-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15270-8