Abstract
To construct a precise and personalized nomogram model and assess the risk factors associated with deep vein thrombosis (DVT) in patients undergoing (traumatic brain injury) TBI. Clinical data from TBI patients between January 2015 and January 2020 were retrospectively gathered. Divided into the model training set and the model validation set in chronological order. The risk factors for DVT were analyzed using LASSO regression and multifactor logistic regression. Post-modeling assessments were conducted for differentiation, consistency, and clinical efficacy. LASSO regression results showed that Age, BMI, smoking history, balance of intake and output, interval between operation and injury, preoperative D-dimer, preoperative FIB, and preoperative PT were the risk factors of DVT in patients with TBI after surgery (P < 0.05). The nomograph model was constructed using the above 8 risk factors. The AUC of the training set and validation set models were 0.833 (0.790–0.876) and 0.815 (0.748–0.882) respectively, and the Brier values of the training set and verification set were 0.157 and 0.165 respectively, indicating that the calibration of the model was good. Clinical decision curves for both sets confirmed the model’s high net benefit, indicating its effectiveness. Age, BMI, smoking history, balance of intake and output, interval between operation and injury, preoperative D-dimer, preoperative FIB, and preoperative PT are identified as significant risk factors for DVT development in TBI patients. The risk prediction model exhibits robust consistency and prediction efficiency, offering valuable insights for medical practitioners in early identification and targeted invervention for high-risk TBI patients prone to DVT.
Similar content being viewed by others
Introduction
Postoperative individuals with traumatic brain injuries (TBI) commonly experience lower limb muscle weakness, paralysis, severe trauma, prolonged operation duration, mannitol usage, and the contraindication of routine anticoagulant administration with the first 3 days after surgery. Consequently, this demographic represents a high-risk category for developing deep venous thrombosis (DVT)1,2,3. The presence of one or more risk factors can predispose postoperative TBI patients to DVT, with an incidence ranging from 13.0 to 80%, markedly surpassing rates observed in patients undergoing other neurosurgical procedures4. DVT, once manifest, can lead to life-threatening pulmonary embolism and sudden death. Historical perspectives held that DVT predominantly occurs within 7 days following TBI5. In clinical TBI scenarios, one of the primary contributors to mortality is DVT, particularly acute pulmonary embolism resulting from lower extremity deep venous thrombosis (LEDVT). Simultaneously, hemorrhagic brain injury exhibit an elevated mortality risk6. Coagulation abnormalities secondary to TBI tend to normalize within approximately one week. Studies also indicate enhanced coagulation function during anesthesia induction, skin incision, and the entire operative process. However, around one week post-craniotomy, all indicators gradually revert to normal levels7.
Hence, it becomes imperative to investigate the risk factors associated with early postoperative DVT in TBI patients and construct a predictive model. Currently, there is a paucity of risk prediction models in this domain. Through a retrospective analysis of clinical data from TBI patients, this study establishes a pertinent risk prediction model by exploring postoperative DVT risk factors. The model’s predictive efficacy is internally validated, aiming to facilitate early identification of high-risk DVT patients and the formulation of targeted interventions to enhance the recovery of TBI patients.
Methods
General information
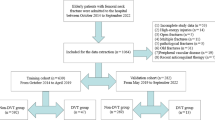
A retrospective collection of diverse medical records was conducted, encompassing patients with traumatic traumatic brain injury (TBI) admitted to the neurosurgery departments of the Tianjin Medical University General Hospital and the Ordos Central Hospital from January 2016 to January 2021. Traumatic TBI diagnosis adhered to the criteria proposed by Wang8considering medical history, disease assessment, physical examination and auxiliary examination. DVT diagnosis aligned with the Guidelines for the Diagnosis and Treatment of Deep Vein Thrombosis (Third Edition), relying on color Doppler ultrasound results showing definitive signs of deep vein lumen obstruction or filling defect. Inclusion criteria were age ≥ 18 years, traumatic TBI diagnosed by brain CT, complete medical records, while exclusion criteria involved a history of previous DVT, heparin or warfarin anticoagulation therapy, multiple injuries of the trunk and lower limbs, cerebrovascular disease, severe coagulation dysfunction, blood system-related diseases, and hospitalization time < 72 h. The specific process of the entire sample filtering can be seen in Fig. 1. The clinical data of this study were sourced from the clinical electronic medical record system and were approved by the Medical Ethics Committee of the Tianjin Medical University General Hospital (Ethics Approval No.: IRB2022-YX-006-01) and Ordos Central Hospital Hospital (Ethics Approval No.: 2022-081). Informed consent was obtained from all subjects and/or their legal guardian(s). I confirm that all methods were carried out in accordance with relevant guidelines and regulations. All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Treatment method
Upon admission, all patients underwent surgical interventions such as decompressive craniectomy and evacuation of hematoma, followed by comprehensive symptomatic treatment including nutritional rehydration, anti-infection measures and osmotic dehydration. The DVT group comprised 184 patients with DVT occurring within 2 weeks post-surgery, while the control group included 333 patients without DVT. Patients in DVT group received 2500 IU/d subcutaneous injection of low molecular weight heparin calcium once a day for three consecutive days after DVT diagnosis. By time segment, all enrolled cases will be included in the training set for model development with July 1, 2019 as the boundary, and the patients after July 1, 2019 will be included in the validation set for model verification.
Observations
Relevant data of each case were collected through the hospital network information system, covering disease-related data and preoperative laboratory indicators. Disease-related data included gender, age, body mass index (BMI), smoking history (defined as smoking for 6 months or more of continuous or cumulative smoking in life), drinking history (defined as drinking at least once a month or drinking for more than half a year), diabetes, hypertension, hyperlipidemia, Glasgow Coma Scale (GCS) score at admission, trauma causes, brain injury types, interval between surgery and injury, hospital stay, open TBI, blood transfusion, pulmonary infection, and balance of intake and output. Preoperative laboratory indicators encompassed prothrombin time (PT), fibrinogen (FIB), D-dimer, platelet count (PLT), white blood cell count (WBC), albumin (Alb), hemoglobin.
The calculation of the incoming and outgoing volume 24 h as a time unit, with the observation window starting at 8:00 on the second day of each patient’s hospitalization. Total input and output were assessed for each time unit, classifying a balance of ≥ 300 ml as positive and < 300 ml as negative9,10.
Statistical methods
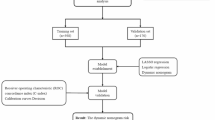
In this study, the R language (Version R 4.0.3) was employed for data processing, and statistical significance was set at a two-tailed P value of < 0.05. Categorical variables were described by frequency and percentage, with group-wise comparisons conducted using the chi-squared test. Due to the non-normal distribution of continuous variables, and the median and quartile were used for statistical description, while group-wise comparisons utilized the Mann-Whitney U test. Subsequently, in model construction, the LASSO model, incorporating ten-fold cross-validation, was implemented to facilitate the selection of more efficacious variables and mitigate the risk of model overfitting within the training set. The variables identified through LASSO regression, coupled with the incidence of deep vein thrombosis (DVT), were designated as independent and dependent variables, respectively. Multivariate logistic regression analysis established through back stepwise selection method ensued, resulting in the formulation of a predictive model for the risk of DVT following traumatic brain injury (TBI). The “rms” package in the R language was employed to visualize the model and generate a nomogram. Model performance metrics were extracted from the receiver operating characteristic curve (ROC), with the area under the curve (AUC) quantifying the model’s discriminative ability in distinguishing patients with and without postoperative DVT. Calibration curves, generated with 1000 bootstrap resamples, assessed the consistency between predicted and actual DVT occurrences. The model’s clinical utility was further evaluated through a clinical decision curve. And the model’s validation was conducted using an independent validation set.
Results
A total of 1513 cases of TBI underwent screening. After applying the inclusion and exclusion criteria, 517 TBI patients were included in the study. Of these, 184 patients (35.60%) developed DVT postoperatively, with 87.50% of cases occurring within 7 days. The remaining 333 patients did not experience DVT, and they were divided into a training set (361 patients) and a validation set (156 patients).
Patient baseline characteristics
Baseline characteristics of all participants in both the training and validation cohorts are presented in Table 1. No statistically significant differences were observed in characteristics between the training and validation cohorts. Subsequently, the characteristics of all participants in the training cohort were compared between the control group and DVT groups (Table 2). Comparing baseline data, including age, gender ratio, and BMI, between the two groups revealed significant difference only in age and BMI, with the DVT group having a higher mean age and BMI (P < 0.05). In the DVT group, the proportion of patients smoking and receiving blood transfusion, the incidence of brain contusion with traumatic subarachnoid hemorrhage and intracranial hematoma, negative balance of inflow and outflow, interval between operation and injury more than 6 h, fibrinogen (FIB), D-dimer, and platelet count (PLT) were significantly higher than those in the control group (P < 0.05). Moreover, prothrombin time (PT) were lower than those in the control group (P < 0.05). Out of 184 DVT cases, 44 (24.46%) occurred within 3 days, 116 (63.04%) within 4–7 days, and 24 (12.50%) after 7 days, with a median DVT onset time of 7 (4,7) days.
Model construction
To enhance the selection of effective variables and prevent model overfitting, the LASSO model with ten-fold cross-validation was employed to screen variables in the training set, as illustrated in Figs. 2 and 3. LASSO regression analysis identified 8 non-zero coefficient predictive variables (age, BMI, smoking history, balance of intake and output, interval between operation and injury, preoperative D-dimer, preoperative FIB, and preoperative PT). These variables, along with DVT occurrence, were used as independent and dependent variables, respectively, in multivariate logistic regression analysis. Results indicated that age, BMI, smoking history, negative balance of inflow and outflow, interval between operation and injury more than 6 h, higher fibrinogen (FIB), higher D-dimer and lower PT were the primary risk factors for post-TBI DVT (P < 0.05). Refer to Table 3 for details. Visualization of the model and patient risk assessment were conducted using the “rms” package in R, generating a nomogram (Fig. 4).
Model validation
Model discrimination was assessed through the receiver operating characteristic curve (ROC) and its area under the curve (AUC). The training set model demonstrated an AUC of 0.833 (0.790–0.876). Verification using the validation set yielded an AUC of 0.815 (0.748–0.882), indicating high discriminatory ability (Fig. 5A and B). Calibration curves for both training and validation sets demonstrated close alignment between predicted and actual values. Brier values for the training and validation sets were 0.157 and 0.165, respectively, suggesting good model calibration (Fig. 6A and B).
Clinical decision curve analysis of the model
The efficacy of the nomogram model was evaluated through clinical decision curves for both the training and validation sets. Results revealed a high net benefit value, indicating the effectiveness of the constructed model (Fig. 7A and B).
Clinical decision curve of nomogram model. A: Clinical decision curve of training set (n = 361); B: Clinical decision curve for validation set (n = 156). The horizontal axis is the threshold probability, and the vertical axis is the net benefit value; None indicates that all patients have no DVT after surgery, and the net benefit is 0; All represented that all patients had DVT after surgery, and the net benefit was the inverse slope of the slope; When the model prediction probability of patients after TBI is n, the higher the corresponding net benefit value is, the better. That is, the farther the model curve is from the horizontal axis and vertical axis, the better the effectiveness of patients.
Discussion
The genesis of deep vein thrombosis (DVT) redominantly results from vein wall injury, hypercoagulability of blood, and sluggish blood flow11. In the clinical management of severe brain injury, intravenous administration of drugs such as mannitol, hemostatic agents, and hormones is common to mitigate intracranial pressure and cerebral hemorrhage. However, these medications may induce vascular endothelial damage, a critical factor in DVT development. Furthermore deep vein catheterization, frequently employed for treatment facilitation, can contribute to vein wall damage, initiating the endogenous coagulation system. In addition, patients with impaired hypothalamus function, common in brain trauma, may experience stress-induced states such as high fever and diabetes insipidus, elevating blood viscosity, and promoting a hypercoagulable state. In addition, patients with severe brain trauma are often accompanied by severe consciousness disorder. The braking time in bed is long, and the function of lower limb muscle pump is weakened, resulting in slow blood flow speed, which can cause leukocyte adhesion, which is easy to induce thrombosis12. DVT not only hampers the treatment and rehabilitation of brain trauma patients but also extends hospital stays, elevates medical costs, and even causes unnecessary doctor-patient disputes. Identifying DVT risk factors is crucial for preventing postoperative DVT and mitigating complications. This study revealed a DVT incidence of 35.60% in TBI patients after surgery, within 87.50% occurring within 7 days, aligning with prior research13. Therefore, it is necessary to evaluate the risk factors of DVT in the early stage, including ultrasonic examination of lower limbs on the 3rd and 7th days after operation14. This study aims to assess postoperative DVT risk in TBI patients through a nomogram model, intending to provide a reliable basis for prevention and treatment strategies.
By leveraging routinely collected clinical laboratory parameters, our model offers several unique advantages for predicting DVT risk. First, it avoids additional testing and associated costs, thereby reducing the financial burden on patients. Second, these standard variables have well-defined clinical meanings and reference ranges with which physicians are already familiar, making the model easier to interpret and validate. Third, since these measures are automatically captured in electronic health records as part of routine care, the model can be seamlessly integrated into existing electronic medical record systems or clinical decision support platforms to provide real‐time alerts and automated risk assessments without extra workflow steps. Notably, numerous established examples exist of risk‐prediction tools built on routine laboratory data in other disease contexts, underscoring the feasibility and value of this approach. For example, Wang et al. utilized routine blood count and biochemical testing data (including potassium (K), total protein (TP), albumin (ALB), and indirect bilirubin (NBIL)) to construct machine learning diagnostic models for cardiovascular pan-disease, achieving excellent performance with an AUC of 0.992115. Similarly, Harris et al. developed a prediction model using routine clinical parameters to stratify survival in malignant pleural mesothelioma patients undergoing cytoreductive surgery, demonstrating fair-to-good performance with a Harrell’s concordance statistic of 0.6216. These examples collectively demonstrate that routine clinical parameter-based models represent a practical, cost-effective approach to advancing precision medicine while seamlessly integrating into existing healthcare infrastructure.
The findings identified smoking history as a DVT risk factor in patients after TBI. Smoking, coupled with trauma and intravenous drug administration, damages vascular endothelial cells, promoting the release of vasoactive substances and inducing blood circulation disorders and thrombosis17,18. In our risk factor analysis, we found that GCS scores, which reflect the severity of TBI, showed no significant association with the development of DVT. Our findings are consistent with previous research findings19,20.
At present, there is no literature report on the relationship between DVT and inflow and outflow of patients after TBI. This study discovered a correlation between negative balance of inflow and outflow and DVT occurrence in acute TBI patients. Negative fluid balance, often resulting from dehydration or excessive fluid loss, can lead to hemoconcentration and increased blood viscosity, which slows venous blood flow and promotes venous stasis—one of the key components of Virchow’s triad for thrombosis formation21. Negative balance, indicative of reduced inflow compared to outflow, may render patients hypercoagulable, increasing DVT susceptibility22. This state not only facilitates the accumulation of clotting factors but also increases the risk of thrombus formation. Additionally, hypovolemia can cause microcirculatory disturbances and endothelial dysfunction, further contributing to a hypercoagulable state23,24. Experimental and clinical studies have shown that dehydration and hemoconcentration are associated with increased secretion of prothrombotic factors such as von Willebrand factor, enhanced platelet aggregation, and elevated D-dimer levels, all of which promote thrombosis25. Therefore, careful monitoring and management of fluid balance are essential in TBI patients to avoid excessive negative fluid balance and reduce the risk of DVT.
The incidence of surgical DVT was lowest within 24 h after the patient’s injury, and the longer the time of injury, the higher its incidence, which was related to the impaired consciousness, reduced limb movement, slow venous blood flow, hypercoagulation, and vascular wall damage after TBI26. Elevated BMI emerged as a risk factor, consistent with prior research associating obesity with a 2–3 times increased risk of venous thromboembolism27. Mechanisms linking BMI and venous thrombosis involve increased blood viscosity, clot formation, venous stasis, and pre-thrombotic conditions28. Higher than normal BMI is significantly associated with impaired fibrinolytic activity of the coagulation system and elevated plasma coagulation factor concentrations29. Other identified risk factors included preoperative FIB, PT and D-dimer, likely tied to early occurrence of fibrinolysis, vascular endothelial damage, platelet dysfunction and other conditions in patients after TBI30. Therefore, while controlling acute brain injury bleeding, we should also be alert to blood hypercoagulability, closely monitor the blood biochemistry and coagulation function of patients, which is extremely important for the prognosis of patients.
In our study, we found that D-dimer was a key factor in the predictive model. D-dimer, a fibrin degradation product indicating active coagulation and secondary fibrinolysis, demonstrates significant predictive value for deep vein thrombosis risk31. Previous studies have proven that patients with preoperative D-dimer > 1.0 µg/mL had a significantly higher incidence of DVT than those with < 0.5 µg/mL (14.8% vs. 8.98%), demonstrating its utility for early identification of high-risk individuals and optimization of prophylaxis timing32,33. Early TBI patients often present with subtle clinical manifestations that may be obscured by altered mental status. However, brain injury triggers the release of tissue factor-rich microvesicles into circulation, causing rapid D-dimer elevation that reflects both coagulation activation and fibrinolytic activity34,35. As elevated D-dimer is a critical predictor of DVT risk, it provides an objective biomarker for early identification of high-risk patients when clinical signs may be unreliable, enabling timely implementation of targeted prophylactic strategies.
The nomogram, employed as a statistical model, offers accuracy, repeatability, visualization, and ease of use without requiring computer software intervention. It aids clinicians in standardized decision-making and is increasingly employed as a clinical aid. Many scholars at home and abroad have used nomograms to establish prediction models for survival probability and prognosis of different diseases. For example, Fang et al.36 used nomograms to predict early seizures in patients with cerebral vein thrombosis; Pan et al.37 used nomograms to predict the possibility of DVT occurring within 14 days in patients with acute stroke. However, there is no report on the nomogram prediction model related to TBI at home and abroad. The nomogram in this paper is mainly divided into three parts: risk indicators, scoring and prediction probability of DVT. Users can quickly obtain the prediction probability of DVT according to the indicators of patients with brain trauma. It can be seen that compared with the binary variable logistic regression formula model reported by Yang et al.38 and others, the nomogram has the advantages of simple calculation, convenience and intuition, and has higher clinical practical value. Cheng and Xu39 found that the AUC of the two assessment models were 0.738 and 0.633 respectively when verifying the predictive value of Caprini risk assessment model and Autar thrombus risk assessment scale for DVT formation risk of inpatients. The AUC for the nomogram was 0.822 in the training set, and 0.803 in the validation set, outperforming other prediction tools. Calibration curves and Brier values demonstrated good model calibration, affirming its accuracy in predicting postoperative DVT in TBI patients.
In this investigation, the predictive capacity of the risk assessment model was validated through the construction of ROC curves. The outcomes indicate that the risk prediction model, founded on independent risk factors for deep vein thrombosis (DVT) in traumatic brain injury patients, exhibits robust predictive efficacy. Both its discriminatory and calibration abilities were found to be notably high. The proposed prediction model holds clinical utility for evaluating DVT risk in traumatic brain injury patients, with the nomograms developed in this study serving as valuable tools for post-traumatic patient assessment. Identification of high-risk patients using these nomograms allows for timely intervention, enhancing early clinical screening and optimizing treatment plans. Given its foundation on independent DVT risk factors, as well as the integration of diverse clinical data and biochemical indices specific to traumatic brain injury patients, this predictive model offers valuable insights into DVT occurrence in this patient population.
This study still has some limitations. Although the survey samples of this study come from two different centers, due to the limitation of the number of samples, there may be some bias and the possibility of over fitting the model. Future research endeavors should prioritize expanding the sample size to address these limitations, improve model robustness, and undertake external validation for comprehensive. Currently, the model does not account for the temporal probability of DVT onset. To enhance its predictive performance, future work should include the collection of time-to-event data for DVT, and subsequent model refinement based on these temporal parameters.
In conclusion, the risk prediction model exhibits robust consistency and prediction efficiency, offering valuable insights for medical practitioners in early identification and targeted invervention for high-risk TBI patients prone to DVT.
Data availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- DVT:
-
Deep vein thrombosis
- TBI:
-
Traumatic brain injury
- LEDVT:
-
Lower extremity deep venous thrombosis
- BMI:
-
Body mass index
- GCS:
-
Glasgow Coma Scale
- PT:
-
Prothrombin time
- FIB:
-
Fibrinogen
- PLT:
-
PLT D-dimer, platelet count
- WBC:
-
White blood cell count
- Alb:
-
Albumin
- ROC:
-
Receiver operating characteristic curve.
- AUC:
-
Area under the curve.
References
Ryan, C. G. et al. Acute traumatic subdural hematoma: current mortality and functional outcomes in adult patients at a level I trauma center. J. Trauma. Acute Care Surg. 73, 1348–1354 (2012).
Davanzo, J. R., Sieg, E. P. & Timmons, S. D. Management of traumatic brain injury. Surg. Clin. North. Am. 97, 1237–1253 (2017).
Agarwal, N. et al. Risk-to-benefit ratio of venous thromboembolism prophylaxis for neurosurgical procedures at a quaternary referral center. Neurosurgery 84, 355–361 (2019).
Zhang, Z. L. et al. Clinical efficacy of Rivaroxaban in prevention and treatment of postoperative deep vein thrombosis for severe traumatic brain injury. Zhonghua Yi Xue Za Zhi. 97, 3558–3561 (2017).
Gunning, A. C., Maier, R. V., de Rooij, D., Leenen, L. P. H. & Hietbrink, F. Venous thromboembolism (VTE) prophylaxis in severely injured patients: an international comparative assessment. Eur. J. Trauma. Emerg. Surg. 47, 137–143 (2021).
Chipman, A. M. et al. Therapeutic anticoagulation in patients with traumatic brain injuries and pulmonary emboli. J. Trauma. Acute Care Surg. 89, 529–535 (2020).
Laroche, M., Kutcher, M. E., Huang, M. C., Cohen, M. J. & Manley, G. T. Coagulopathy after traumatic brain injury. Neurosurgery 70, 1334–1345 (2012).
Wang, Z. Zhongcheng Neurosurgery (Hubei Science and Technology, 2005).
Besen, B. A. & Taniguchi, L. U. Negative fluid balance in sepsis: when and how? Shock 47, 35–40 (2017).
Alsous, F., Khamiees, M., DeGirolamo, A., Amoateng-Adjepong, Y. & Manthous, C. A. Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. Chest 117, 1749–1754 (2000).
Broderick, C., Watson, L. & Armon, M. P. Thrombolytic strategies versus standard anticoagulation for acute deep vein thrombosis of the lower limb. Cochrane Database Syst. Rev. 1, CD002783 (2021).
Li, Q., Yu, Z., Chen, X., Wang, J. & Jiang, G. Risk factors for deep venous thrombosis of lower limbs in postoperative neurosurgical patients. Pak J. Med. Sci. 32, 1107–1110 (2016).
Rodier, S. G. et al. Early anti-xa assay-guided low molecular weight heparin chemoprophylaxis is safe in adult patients with acute traumatic brain injury. Am. Surg. 86, 369–376 (2020).
Kimmell, K. T. & Jahromi, B. S. Clinical factors associated with venous thromboembolism risk in patients undergoing craniotomy. J. Neurosurg. 122, 1004–1011 (2015).
Wang, Z. et al. Construction of machine learning diagnostic models for cardiovascular pan-disease based on blood routine and biochemical detection data. Cardiovasc. Diabetol. 23 (1), 351 (2024).
Harris, E. J. A. et al. Prediction modelling using routine clinical parameters to stratify survival in malignant pleural mesothelioma patients undergoing cytoreductive surgery. J. Thorac. Oncol. 14 (2), 288–293 (2019).
Bratseth, V., Pettersen, A., Opstad, T. B., Arnesen, H. & Seljeflot, I. Markers of hypercoagulability in CAD patients. Effects of single aspirin and clopidogrel treatment. Thromb. J. 10, 12 (2012).
Incalza, M. A. et al. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 100, 1–19 (2018).
Chen, D. et al. Venous thrombus embolism in polytrauma: special attention to patients with traumatic brain injury. J. Clin. Med. 12(5) (2023).
Cole, K. L. et al. Factors associated with venous thromboembolism development in patients with traumatic brain injury. Neurocrit Care. 40 (2), 568–576 (2024).
Umar, H. et al. Triple emergencies: hyperosmolar hyperglycemic state, venous thromboembolism, and huge free-floating right heart thrombus successfully managed with anticoagulation. Clin. Case Rep. 9 (12), e04710 (2021).
McCullough, M., Kholdani, C. & Zamanian, R. T. Prevention of deep vein thrombosis and pulmonary embolism in high-risk medical patients. Clin. Chest Med. 39, 483–492 (2018).
Ding, J. et al. Dehydration in cerebral venous sinus thrombosis. CNS Neurosci. Ther. 30 (5), e14760 (2024).
Dmitrieva, N. I. & Burg, M. B. Secretion of von Willebrand factor by endothelial cells links sodium to hypercoagulability and thrombosis. Proc. Natl. Acad. Sci. U S A. 111 (17), 6485–6490 (2014).
Shi, Z. et al. Contribution of dehydration to END in acute ischemic stroke not mediated via coagulation activation. Brain Behav. 9 (6), e01301 (2019).
Praeger, A. J. et al. Deep vein thrombosis and pulmonary embolus in patients with traumatic brain injury: a prospective observational study. Crit. Care Resusc. 14, 10–13 (2012).
Ntinopoulou, P. et al. Obesity as a risk factor for venous thromboembolism recurrence: a systematic review. Med. (Kaunas). 58, 1290 (2022).
Klarin, D., Emdin, C. A., Natarajan, P., Conrad, M. F. & Kathiresan, S. Genetic analysis of venous thromboembolism in Uk biobank identifies the ZFPM2 locus and implicates obesity as a causal risk factor. Circ. Cardiovasc. Genet. 10, e001643 (2017).
Stein, P. D., Beemath, A. & Olson, R. E. Obesity as a risk factor in venous thromboembolism. Am. J. Med. 118, 978–980 (2005).
Vella, M. A., Crandall, M. L. & Patel, M. B. Acute management of traumatic brain injury. Surg. Clin. North. Am. 97, 1015–1030 (2017).
Luo, C. Y. & Roan, J. N. Re-visiting D-dimers and fibrin degradation products for the diagnosis of acute aortic dissection. J. Thorac. Dis. 9 (7), 1744–1747 (2017).
Imamura, H. et al. Preoperative D-dimer value and lower limb venous ultrasound for deep venous thrombosis prevents postoperative symptomatic venous thromboembolism in patients undergoing colorectal surgery: A retrospective study. J. Anus Rectum Colon. 7 (3), 159–167 (2023).
Tatarano, S. et al. Significance of preoperative screening of deep vein thrombosis and its indications for patients undergoing urological surgery. Investig Clin. Urol. 62 (2), 166–171 (2021).
Chen, X. et al. Plasma D-dimer levels are a biomarker for in-hospital complications and long-term mortality in patients with traumatic brain injury. Front. Mol. Neurosci. 16, 1276726 (2023).
Zhang, J., Zhang, F. & Dong, J. F. Coagulopathy induced by traumatic brain injury: systemic manifestation of a localized injury. Blood 131 (18), 2001–2006 (2018).
Fang, Y., Song, G., Lin, J., Ye, X. & Huang, S. Predicting the occurrence of early seizures after cerebral venous thrombosis using a comprehensive nomogram. Epilepsy Res. 178, 106820 (2021).
Pan, X., Wang, Z., Chen, Q., Xu, L. & Fang, Q. Development and validation of a nomogram for lower extremity deep venous thrombosis in patients after acute stroke. J. Stroke Cerebrovasc. Dis. 30, 105683 (2021).
Yang, T., Wei, G., Zhu, C. & Pan, A. Incidence and risk factor analysis of deep venous thrombosis in patients with severe traumatic brain injury. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 31, 182–186 (2019).
Cheng, L. & Xu, Q. The screening effect of two thrombus risk assessment models on deep venous thrombosis in hospitalized patients. Chin. J. Clin. Healthc. 23, 247–251 (2020).
Funding
None.
Author information
Authors and Affiliations
Contributions
Wei Hao, Jiancheng Feng, Hongliang Luo and Yong Liu conceived and designed the study and analyzed the data, Ruifang Ma, Xuan Lu and Dongsheng Xiong developed the methodology. Wei Hao, Jiancheng Feng and Hongliang Luo carried out the experiments. Wei Hao, Jiancheng Feng, Hongliang Luo and Ruifang Ma analyzed the data and interpreted the results. Wei Hao, Jiancheng Feng and Hongliang Luo wrote the manuscript. Ruifang Ma , Xuan Lu and Dongsheng Xiong provided technical support. Yong Liu reviewed and revised the manuscript and supervised the study. All authors have read and approved the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
This study was approved by the Medical Ethics Committee of the Tianjin Medical University General Hospital (Ethics Approval No.: IRB2022-YX-006-01) and Ordos Central Hospital Hospital (Ethics Approval No.: 2022-081). Informed consent was obtained from all subjects and/or their legal guardian(s). I confirm that all methods were carried out in accordance with relevant guidelines and regulations. All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent for publication
Not Applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hao, W., Feng, J., Luo, H. et al. Assessment of risk factors related to early occurrence of deep vein thrombosis after TBI using nomogram model. Sci Rep 15, 29313 (2025). https://doi.org/10.1038/s41598-025-15287-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15287-z