Abstract
Spexin (SPX), a recently identified adipokine, shows potential in enhancing insulin sensitivity, offering promising implications for metabolic health. This study aimed to investigate the effect of 8 weeks of high intensity interval training (HIIT) on insulin resistance through liver gluconeogenesis, lipolysis, inflammation, oxidative stress, and lipogenesis in Type 2 diabetic rats with a special focus on the role of SPX. In this study, 28 male Wistar rats were divided into four groups: Healthy Control (CON), Diabetes Control (T2D), HIIT, and Diabetes+HIIT (T2D+HIIT). After diabetes induction (high-fat diet+streptozotocin), the exercise groups underwent an 8-week HIIT protocol. HOMA-β, HOMA-IR, QUICKI, fasting blood sugar, insulin, and SPX were measured in liver and serum. The expression of Galanin/2, FOXO-1, PGC-1α, G6Pase, PEPCK, CPT1A, AMPK, PPARα, ACC, FAS, SREBP-1c, SIRT-1, and inflammatory/antioxidant markers were analyzed in the liver. Our results showed that HOMA-β, QUICKI, insulin, and serum level of SPX levels were higher in T2D+HIIT than T2D group. Similarly, SPX, GALR2, FOXO-1, PGC-1α, CPT1A, PPARα, AMPK, and SIRT-1 levels were higher in the livers of rats in the T2D+HIIT than T2D group. However, HOMA-IR, PEPCK, G6Pase, ACC, FAS, and SREBP-1c levels were lower in T2D+HIIT than T2D group. Also, HIIT+T2D reduced inflammation and increased antioxidants, indicating an improved overall health status of the liver. HIIT benefits diabetic liver with reducing gluconeogenesis, inflammation, oxidative stress, and lipogenesis while increasing lipolysis. These improvements coincided with elevated hepatic SPX signaling and are associated with reduced insulin resistance. These findings suggest a potential association between HIIT induced SPX signaling modulation in liver and improving insulin resistance, though further mechanistic studies are required to confirm the causality.
Similar content being viewed by others
Introduction
Diabetes is a widespread metabolic disease defined by abnormal digestion of proteins, lipids, and carbohydrates as well as persistently elevated blood sugar levels1,2. By 2030, itis estimated that nearly 366 million people will be living with diabetes. Of these individuals, approximately 90% to 95% are expected to have type 2 diabetes (T2D), which is the most common form of the disease3,4.
Two key features of T2D are insulin resistance (IR) and elevated gluconeogenesis5,6.Studies have shown that adipokines, bioactive molecules (such as hormones and cytokines) secreted by adipose tissue, could affect these pathological process5,6. A 14-amino acid peptide, spexin (SPX) is the most recently discovered adipokine considered to be involved in a number of homeostatic processes, including metabolism, energy balance, and reproduction6,7,8. Its expression has been reported in the liver, adipose tissue, pancreas, kidneys, and skeletal muscle9,10. Previous clinical studies demonstrated that IR, obesity, and T2D are associated with low blood concentrations of SPX11,12. In addition, serum SPX concentrations negatively correlated with body mass index (BMI)11,12. Furthermore, SPX reduced rate of dyslipidemia, gluconeogenesis, lipogenesis, and hepatic fat accumulation but increased lipolysisin vitro6,13. Through its receptor (i.e. Galanin 2/3 (GAL2/3)), SPX stimulates upregulation of fatty acid translocase (FAT/CD36), carnitine palmitoyl transferase 1A (CPT1A), peroxisome proliferator-activated receptor alpha (PPAR-α), and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) in rat cardiomyocytes7,14.
Regular physical exercise was shown to increase SPX blood concentration14,15. Both resistance and aerobic training can stimulate SPX expression, reduce the levels of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα), and improve glucose uptake and insulin sensitivity in skeletal muscles14,15. Leciejewska et al.16 reported that exercise can increase not only SPX secretion but also GALR2 and GALR3 expression in peripheral tissues. In addition, it has been found that high intensity interval training (HIIT) is more effective in increasing the levels of SPX than moderate intensity aerobic training17. Therefore, the purpose of this study was to investigate the effect of HIIT on IR in T2D rats with special focus on the role of SPX in enhancing hepatic lipolysis and reducing gluconeogenesis and lipogenesis.
Materials and methods
Animal models
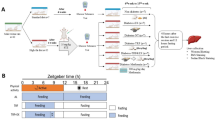
28 male Wistar rats weighing 130–160 g, with an average age of 7–8 weeks, were purchased from the animal farm of Kerman University of Medical Sciences. During the experiment, the animals were housed in a 12-h light/dark cycle at a constant temperature (22 ± 1/4 °C), and humidity (50 ± 4%), with access to food and water ad libitum. The animals were randomly divided into four groups (n = 7 in each group) following the adaptation period of one week: control (CON), type 2 diabetes (T2D), high intensity interval training (HIIT), and T2D + HIIT. Kerman University of Medical Sciences Experimental Animals Ethics Committee approved all procedures (Ethical code: IR.KMU.AEC.1404.005).
Induction of diabetes
To induce T2D, we adapted the method introduced by Magalhaes et al.18, with modifications to made it more feasible in our lab. We used this method in our previous publications19,20,21. Animals in T2D and T2D+HIIT groups were fed a high-fat diet (HFD) for 2 months (60% fat, 20% carbohydrates, and 20% protein). Then, the animals were injected with streptozotocin (STZ) at a low dose (30 mg/kg). Blood glucose levels were measured with a glucometer 3 days after the injection, after 12 h of fasting. Animals with fasting blood glucose higher than 300 mg/dl were considered diabetic. Animals in CON and HIIT groups consumed a normal diet (20% fat, 60% carbohydrates, and 20% protein).
Treadmill running protocol
After the induction of T2D, animals in HIIT and T2D+HIIT groups were acclimated to treadmill for 5 days (with speed of 8 m/min for 10 min). Then, we used stepwise testing to assess each rat’s maximum speed (Vmax). Starting with 6 m/min, the treadmill speed increased every 2 min until the rat reached exhaustion. Each rat’s final effort was taken as Vmax. Vmax was measured every two weeks. We have described the main training protocol in detail in previous publications19,20,22,23,24,25,26,27,28,29,30,31. Briefly, the protocol involved 4–10 intervals (2 min at high intensity and 1 min at low intensity) with 80–100% of Vmax (Table 1).
Serum and tissue sampling
A combination of ketamine (80 mg/kg) and xylazine (10 mg/kg) was prepared fresh on the day of the procedure and administered via intraperitoneal injection in a total volume of 1 mL/kg body weight to euthanasia of the animals. Then, blood samples were taken from the animals’ heart. The whole liver tissue was then carefully removed, immediately frozen in liquid nitrogen, and stored at 80 °C for further analysis.
ELISA
Inflammatory and anti-inflammatory cytokines, namely TNF-α and interleukin-10 (IL-10) (Karmania Pars Gene Company, Iran), insulin serum concentration (The ALPCO Company, United States), Spexin in serum and liver (PHOENIX PHARMACEUTICALS, INC, United States), ALT (Karmania Pars Gene Company, Iran), and AST (Karmania Pars Gene Company, Iran) were all measured according to the instructions of their respective ELISA kits32,33.
Measurement of oxidative stress indices
The measurement of glutathione peroxidase (GPX) was based on the ability of glutathione peroxidase to oxidize glutathione (GSH) to oxidized glutathione (GSSH). Glutathione reductase converts GSSH to GSH using nicotinamide dinucleotide phosphate (NADPH). The decrease in NADPH levels, measured at 340 nm, indicated GPX activity, which was measured using a kit (Behboud Tahghigh Kerman Company, Iran). The activity of SOD and CAT was indirectly measured using a colorimetric method inhibiting pyrogallol oxidation, according to the kits instruction(Behboud Tahghigh Kerman Company, Iran).
Western blotting
All antibodies used in the study were purchased from Santa Cruz Biotechnology, Inc, U.S.. The expression of galanin (GAL) (sc-16219), forkhead box protein O1 (FOXO-1) (sc-374427), peroxisome coactivator 1 alpha (PGC-1α) (sc-544812), carnitine palmitoyl transferase 1A (CPT1A) (sc-514555), Peroxisome proliferator-activated receptor α (PPARα) (sc-398394), Phosphoenolpyruvate carboxykinase (PEPCK) (sc-271029), Sirtuin 1 (SIRT-1) (sc-523698), Glucose 6-phosphatase (G6Pase) (sc-25840), Acetyl-CoA carboxylase (ACC) (sc-140258), Fas Fatty acid synthase (FAS) (sc-263879), AMP-activated protein kinase (AMPK) (sc-104369), and Sterol regulatory element-binding protein 1 (SREBP-1c) (sc-323698) were assessed using the western blotting technique. Western blot protocol has been described in detail elsewhere18,34. Firstly, liver samples were lysed using lysis buffer (150 mM sodium chloride, 1% Triton X-100, 0.2% deoxycholate sodium, 0.1% SDS, 50 mM Tris, pH 8.0). Using an Eppendorf 5415R centrifuge, the samples were centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatant was collected. A Bradford assay determined the protein concentrations in samples stored at 80 °C. After separation by SDS-PAGE, the proteins were transferred to a PVDF membrane at 120 mV for 1.5 h. Incubation with specific primary antibodies was conducted for 18 h after blocking the membranes with 5% skim milk for 1 h at room temperature. After washing with TBS-T, the membranes were incubated with secondary antibodies conjugated with horseradish peroxidase. Using ECL reagents, the proteins of interest were visualized. Finally, ImageJ software converted qualitative measurements to quantitative information.
Calculation of insulin resistance/sensitivity indices
The Homeostasis Model Assessment (HOMA) was utilized to evaluate insulin resistance (HOMA-IR) and β-cell function (HOMA-β). HOMA-IR and HOMA-β scores were computed using the following formulas: HOMA-IR = [(fasting glucose (mmol/l) × fasting insulin (μU/ml))/22.5]. HOMA β-cell = [(20 × fasting insulin (μU/ml))/(fasting glucose (mmol/l) − 3.5)35. The Quantitative Assessment of Insulin Sensitivity Index (QUICKI) was also employed due to its strong correlation with glucose clamp determination of insulin sensitivity. QUICKI was calculated using the formula of Katz and colleagues: (i.e., 1/[log (fasting insulin in μU/ml) + log (fasting glucose in mg/dl)])36.
Statistical analysis
Results are shown as the mean ± standard deviation (SD). Data normality was assessed with the Kolmogorov–Smirnov test. To compare variables between groups, we employed a Two-Way ANOVA followed by a Tukey post-hoc test. All statistical analyses were conducted using GraphPad Prism version 9.0. A p-value less than 0.05 was considered statistically significant.
Results
Body weight and blood glucose concentrations
The data indicated a significant increase in body weight in both T2D and T2D+HIIT compared to CON and HIIT groups after diabetes induction (month 2) (P < 0.0001). However, month 4 body weight was reduced in the T2D and T2D+HIIT compared to CON and HIIT groups (P < 0.01), with a greater decrease in the T2D group (P < 0.0001) (Fig. 1A). Fasting blood glucose (FBG) was significantly increased after diabetes induction in both T2D and T2D+HIIT groups compared to the baseline (P < 0.0001). HIIT led to reduce FBG values in T2D+HIITcompared to the T2D group (P < 0.0001) (Fig. 1B).
Body weight (A) and fasting blood glucose (FBG) (B) before starting the intervention (month 0), after diabetes induction (month 2), and 48 h after the last training session (month 4) in all groups. The homeostatic model assessment (HOMA)-β (C), HOMA of insulin resistance (HOMA-IR) (D), Quantitative insulin sensitivity check index (QUICKI) (E), serum insulin (F) (Mean ± SD, n = 7 in each group). Abbreviations: CON: control, T2D: type 2 diabetic , HIIT: high intensity interval training. #(P < 0.0001) significant difference between T2D and T2D + HIIT with other groups, *(P < 0.01) significant difference between T2D and T2D + HIIT. ****(P < 0.0001), ***(P < 0.001), **(P < 0.05).
Insulin resistance/sensitivity indexes, and serum insulin concentrations
HOMA-β index was higher in the HIIT group (P < 0.0001, 6.74% higher) and lower in the T2D one (P < 0.0001, 82.47% lower) compared to the CON group. Additionally, the interaction between T2D and HIIT was significant (P < 0.0001, 55.68% higher in T2D+HIIT compared with T2D) (Fig. 1C).
HOMA-IR index was significantly higher in T2D compared to the CON group (P < 0.0001, 43.46% higher). There were no significant changes in HOMA-IR levels between CON and HIIT groups. However, HOMA-IR index was lower in the T2D+HIIT group compared to the T2D group (interaction effect) (P < 0.001, 19.39% lower) (Fig. 1D).
QUICKI index were significantly lower in the T2D group compared to the CON one, (P < 0.0001, 118.46% lower). Furthermore, significant interaction between T2D and HIIT groups was found (P < 0.0001, 97.18% higher in T2D+HIIT compared to T2D group) (Fig. 1E).
Serum insulin concentrations were significantly lower in the T2D group compared to the CON (P < 0.0001, 213.79% lower). and the T2D+HIIT (P < 0.0001, 64.66% lower) groups (Fig. 1F).
GAL-R2 expression levels in the liver and SPX expression levels in liver and serum concentrations
HIIT significantly increased GAL-R2 expression levels in the liver (P < 0.0001, 70% increase). Conversely, T2D was associated with a significant decrease in GAL-R2 liver expression (P < 0.0001, 43.11% decrease). GAL-R2 levels were significantly higher in the T2D+HIIT group compared to the T2D group (P < 0.001, 32.48% higher) (Fig. 2) (All western blot images have been provided in Supplementary material).
GAL-R2 (A), liver SPX (B), serum SPX (C) FOXO-1 (D), PGC-1α (E), G6Pase (F), and PEPCK (G) expression in the liver (mean ± SD, n = 7 in each group). GAL-R2 Galanin receptor 2, SPX spexin, FOXO-1 Forkhead box protein O, PGC-1α peroxisome proliferator-activated receptor gamma coactivator (PGC)-1alpha, G6Pase glucose 6-phosphatase, PEPCK phosphoenolpyruvate carboxykinase, CON control, T2D Type 2 diabetes, HIIT high intensity interval training. ****(P < 0.0001), ***(P < 0.001), **(P < 0.01), and *(P < 0.05).
Both SPX liver expression levels (P < 0.0001, 60.23% lower) and serum concentrations (P < 0.0001, 59.41% lower) were significantly lower in the T2D group compared to the CON one. However, the T2D+HIIT (interaction effect) group showed higher SPX values in both serum (P < 0.001, 98.02% higher) and liver (P < 0.001, 76.42 higher) compared to the T2D group (Fig. 2B,C).
FOXO-1, PGC-1α, G6Pase, and PEPCK expression levels in the liver
HIIT significantly increasedFOXO-1 expression levels compared to CON group in the liver (P < 0.0001, 97.32% decrease). In contrast, the T2D group exhibited a substantial decrease in expression levels ofFOXO-1 compared to CON group in the liver (P < 0.0001, 48.62% decrease). Moreover, this group presented lower levels of FOXO-1 compared to the T2D+HIIT group (P < 0.0001, 35.21% lower) (Fig. 2D).
HIIT significantly increased PGC-1α expression levels compared to CON group in the liver (P < 0.0001, 96.71 increase). Conversely, T2D was associated with a remarkable decrease in PGC-1α levels (P < 0.0001, 72.14% decrease). Furthermore, PGC-1α levels were significantly higher in the T2D+HIIT group compared to the T2D one (P < 0.05, 79.23% higher) (Fig. 2E).
G6Pase expression levels were lower in the HIIT group (P < 0.0001, 55.36% lower) and higher in the T2D group (P < 0.0001, 23.63% higher) compared to the CON one. Furthermore, significant interaction effect was observed for G6Pase (P < 0.0001, 32.21% lower in T2D+HIIT compared with T2D).Furthermore, PEPCK showed higher levels in the T2D group compared to the CON (P < 0.0001, 17.32% higher) and the T2D+HIIT (P < 0.0001, 60.23% change) ones. HIIT also decreased PEPCK compared with CON group (P < 0.0001, 78.23% decrease) (Fig. 2F,G) (All western blot images have been provided in Supplementary material).
CPT1A, PPARα and SIRT1 expression levels in the liver
Both CPT1A and PPARα expression levels were significantly lower in the T2D group compared to the CON one (P < 0.05, 20.04% lower and P < 0.05, 27.87% lower, respectively). The T2D + HIIT group exhibited significantly higher levels of both CPT1A (P < 0.001, 26.14% higher) and PPARα (P < 0.01, 25.25% higher) compared to the T2D group (Fig. 3A,B) (All western blot images have been provided in Supplementary material).
CPT1A (A), PPARα (B), and SIRT-1 (C) AMPK (D), SREBP-1c (E), ACC (F) and FAS (G) expression in the liver (Mean ± SD, n = 7 in each group). CPTIA carnitine palmitoyl transferase IA, PPARα peroxisome proliferator-activated receptor α, AMPK AMP-activated protein kinase, SREBP-1c sterol regulatory element-binding protein 1, ACC Acetyl-CoA carboxylase, FAS fatty acid synthase, CON control, T2D Type 2 diabetes, HIIT high intensity interval training. ****(P < 0.0001), **(P < 0.01).
Additionally, SIRT-1 expression levels were significantly lower in the T2D group compared to the CON (P < 0.05, 39.32% lower) and the T2D+HIIT (P < 0.0001, 32.62% lower) ones (Fig. 3C).
AMPK, SREBP-1c, ACC, and FAS expression levels in liver
HIIT led to a significant increase in AMPK expression levels compared to CON group (P < 0.0001, 156.57% increase). Conversely, the T2D group was associated with a significant decrease in AMPK levels in the liver (P < 0.0001, 60.79% decrease). Furthermore, AMPK levels were significantly higher in the T2D+HIIT group than in the T2D one (P < 0.0001, 60.56% higher). (Fig. 3D).
Rats ACC levels were significantly higher in the T2D group compared to the CON (P < 0.0001, 97.36% change). and T2D+HIIT ones (P < 0.0001, − 387.36% higher) (Fig. 3E). Rats with T2D exhibited lower SREBP-1c levels compared to both the CON group (P < 0.01, 49.58% lower) and the T2D+HIIT group (P < 0. 01, 39.86% lower) (Fig. 3F).
In addition, FAS levels were higher in the T2D group (P < 0.0020, 112.69% higher) compared to the CON and the T2D+HIIT ones (P < 0.0001, 125.98% higher) (Fig. 3G) (All western blot images have been provided in Supplementary material).
TNFα and IL-10 expression levels in the liver
TNFα expression levels in the liver were significantly lower in the HIIT group (P < 0.0001, − 59.39% lower) and higher in the T2D group (P < 0.0001, − 34.26% higher) compared to the CON one. Furthermore, TNFα levels were significantly lower in the T2D+HIIT group than the T2D one (P < 0.01, − 18.22% lower) (Fig. 4A).
TNFα (A) and IL-10 (B), SOD (C), GPX (D) and CAT (E) levels in the liver, and serum levels of ATL (F) and AST (G) (Mean ± SD, n = 7 in each group). TNFα tumor necrosis factor alpha, IL-10 interleukin-10, SOD superoxide dismutase, GPX glutathione peroxidase, CAT catalase, ALT alanine aminotransferase, AST aspartate aminotransferase, CON control, T2D Type 2 diabetes, HIIT high intensity interval control. ****(P < 0.0001), ***(P < 0.001), **(P < 0.01), *(P < 0.05).
HIIT was associated with increased IL-10 levels (P < 0.0001, 33.75% increase) andT2D was associated with decreased IL-10 levels compared to CON group (P < 0.0001, 59.62% change). Furthermore, IL-10 levels were significantly higher in the T2D+HIIT group compared to T2D one (P < 0.01, 32.31% change) (Fig. 4B).
SOD, GPX, and CAT expression levels in in the liver
SOD expression levels in the liver were significantly lower in the T2D group compared to the CON (P < 0.0001, 46.36% lower) and the T2D+HIIT (P > 0.0001, 40.74% lower) groups (Fig. 4C).
Also, GPX levels were significantly different between CON and T2D groups (P < 0.0001, 38.10% lower in T2D).Furthermore, the HIIT group showed higher levels of GPX than the CON group (P < 0.0001, 31.03% higher). Moreover, there was a significant difference between T2D and T2D+HIIT groups (P < 0.01, 61.90% lower in T2D compared to HIIT+T2D) (Fig. 4D).
In addition, the HIIT (P < 0.0001, 169.23 higher) and the T2D (P < 0.0001, 65.12% lower) groups showed higher and lower CAT levels compared to the CON group, respectively. Finally, CAT levels were significantly higher in the T2D+HIIT group than in the T2D one (P < 0.0001, 61.76% higher) (Fig. 4E).
ALT and AST concentrations in serum
ALT concentrations in serum were significantly higher in the T2D group compared to the CON (P < 0.0001, 21.04% higher) and the T2D+HIIT groups (P < 0.01, 14.83% higher) (Fig. 4F).
Furthermore, HIIT (P < 0.001, 15.41% lower) and the T2D (P < 0.0001, 17.70% higher) groups showed lower and higher serum concentrations of AST compared to the CON group, respectively. Moreover, serum AST concentrations were significantly lower in the T2D+HIIT group compared to the T2D one (P < 0.0001, 22.72% lower) (Fig. 4G).
Discussion
The aim of the study was to investigate the effect of HIIT on IR through hepatic lipolysis, gluconeogenesis, and lipogenesis with a particular focus on adipokine SPX in a T2D rat model.
Our findings disclosed that 8-week HIIT protocol upgraded significantly the expression levels of SPX and GAL-R2 in liver of diabetic rats. Furthermore, an increase in FOXO1 and PGC1 as well as a decrease in G6Pase, suggested a decline in gluconeogenesis. Promotion of lipolysis confirmed by high expression of CPT1A, PPARY, and SIRT-1 in T2D+HIIT compared to T2D group. Also, elevation of AMPK and SREBP and reduction of ACC and FAS verified reduced lipogenesis. Exercise training was found to raise SPX serum concentrations and its expression levels in the adipose tissue of obese mice14 and humans37. Moreover, moderate and constant intensity exercise has demonstrated the ability to enhance adipose tissue SPX expression and secretion, resulting in reduced obesity-related glucose intolerance and IR14. A 12-week combined training can effectively improve SPX levels, lipid accumulation products, visceral adiposity index, and body composition in adults with T2D15. Likewise, chronic treatment with SPX improved glucose tolerance and decreased IR in obese and T2D rats38,39. Our results showed that SPX serum concentrations and liver expression levels decreased in T2D, which was associated with higher HOMA-IR and lower HOMA-β and QUICKI values. These data showed higher IR values in T2D group which can also affect the lower weight of this group compared to the CON one in month 4. In the absence of insulin, the body’s inability to effectively use glucose for energy leads to the breakdown of fat and muscle tissue, while excess glucose is excreted in urine (glycosuria) causing calorie deficit, and the metabolic shift to alternative energy sources further increases energy demand—all of which contribute to significant weight loss in uncontrolled diabetes40,41. However, 8 weeks of HIIT treatment could increase both the serum and liver level of SPX, as well GAL-R2 liver expression.
Likewise, these changes were associated with HOMA-β and QUICKI values improvement. These data suggested a possible role for SPX in the improvement of IR. However, mechanistic studies should examine this hypothesis. Recent evidence showed that SPX treatment diminished glucose-induced insulin release in isolated pig islets42. Also, glucose tolerance improvement and reduction of IR and hepatic lipids was observed after chronic treatment with SPX in both obese and T2D rats38,39. Our study highlights the potential role of SPX in improving IR in rodents. This modification was possibly mediated by binding of SPX to GALR2 and, finally, by the promotion of FOXO1/PGC-1α and suppression of G6Pase and PEPCK. However, while we did not directly investigate SPX binding to GALR2, previous studies reported the interaction of SPX with GALR243 and its modulatory role on metabolic pathways associated with gluconeogenesis regulation, including insulin signaling and oxidative stress18. The regulation of both PEPCK and G6Pase genes at the transcriptional level involves the crosstalk between a network of transcription factors44. Indeed, transcription factors, such as FOXO1 and PGC-1α, stimulated the PEPCK promoter9. Previous studies confirmed the key role of PGC-1α in regulating glucose and lipid metabolism45. Thus, reduction of PGC-1α function is involved in impaired insulin signaling, reduced glucose uptake, and increased hepatic glucose production. These effects were followed by higher blood glucose concentrations and contributed to diabetes development46.
Moreover, PGC-1α signaling reduction is engaged in disrupted lipid metabolism, which is characterized by elevated lipogenesis and reduced fatty acid oxidation. Recent evidence showed that liver adenovirus-mediated overexpression of PGC-1α leads to significantly enhancement of glucose production in rat47. Notably, PGC-1α regulates transcription genes, such as PEPCK, G6Pase and FOXO1, where FOXO1 is a transcription factor modulating the gluconeogenic enzyme expression48.
Our data demonstrated that exercise raised SPX, FOXO1 and PGC-1α expression levels in the liver. These findings suggest that a partnership between PGC-1α and FOXO1 could possibly represent a mechanism monitoring gluconeogenesis. Given that SPX knockdown leads to upregulation of PEPCK and G6Pase, causing gluconeogenesis in HepG2 cells6, SPX maight be involved in the inhibition of G6Pase and PEPCK. Consequently, the binding of SPX to GALR2 likely could possibly promoted the FOXO1/PGC-1α expression. Previous studies showed that SPX knockdown results in increased levels of PEPCK and G6Pase, and, eventually, induction of gluconeogenesis in HepG2 cells6. SPX could also promotes lipolysis by enhancing the expression levels of CPT1A, PPARα, and PGC-1α49. Wang et al. reported that the stimulation of fatty acids metabolism and the breakdown of lipoproteins induced by SPX administration They explained that SPX increased FAT/CD36, CPT1A, PPARα, SIRT1, and PGC-1α expression10.
Furthermore, our findings revealed that the increased values of SPX following exercise was associated with an increase in AMPK expression. The latter downregulates the expression of SREBP-1c through the mTOR/AKT pathway inhibition. This might suggests a potential mechanism by which SPX could inhibit lipogenesis50. Also, the synthesis of malonyl-CoA from fatty acids decreased by SPX, indicates that SPX could possibly reduced the ACC function, a key enzyme responsible for the synthesis of malonyl-CoA from fatty acids, through AMPK activation51. Therefore, the synthesis of malonyl-CoA, a relevant component of lipid metabolism, decreased through the suppression of ACC by AMPK. Two key regulators of lipogenesis are FAS and ACC: the suppression of ACC induced by AMPK regulates SREBP-1c, a major transcription factor for the modulation of FAS and ACC52.
Recent evidence showed that AMPK activation can restrict inflammatory responses in different types of cells and tissues53,54. Inflammatory responses inhibition is mediated by several transcription factors, including SIRT1, PGC-1α, and FOXOs, that are downstream targets of AMPK signaling. Thus, AMPK signaling plays a role in inflammation reduction. Furthermore, the increase of antioxidant enzymes (i.e., SOD, GPX and CAT) defines the possible reason for reducing inflammatory responses in our study53.
This study presents some limitations. First, while food intake was not specifically monitored during the study, it is important to consider that variations in food consumption could influence metabolic outcomes. Moreover, body composition data was not assessed in this study. However, changes in body composition could significantly impact metabolic health and the response to HIIT. Future research should incorporate food monitoring and body composition measurements. Second, male rats were used in this study to reduce variability and control for sex-specific hormonal influences on metabolic outcomes. Previous research showed that male and female rats can respond differently to metabolic interventions due to hormonal differences. By using only males, we aimed to obtain more consistent and interpretable results. Future studies should include both sexes to explore potential sex-specific effects. Third, in future studies, GalR2 and GalR3 can be blocked by a selective antagonist, such as M871 or SNAP 37,889, to confirm the cause-and-effect relationship.
Conclusions
In summary, our findings demonstrate that an 8-week HIIT protocol in type 2 diabetic rats was associated with beneficial changes in hepatic metabolism and inflammation. Specifically, HIIT reduced markers of lipogenesis and gluconeogenesis, increased indicators of lipolysis, and improved antioxidant status, while also upregulating SPX signaling and related proteins in the liver. These results were accompanied by improvements in insulin sensitivity and liver function markers.
While our data suggest that HIIT may exert its positive effects in part through modulation of SPX and its downstream pathways, the precise mechanistic links—particularly regarding the role of GALR2 and the FOXO1/PGC-1α axis—remain to be fully elucidated. Our study did not directly establish causality or the specific molecular mechanisms underlying these associations. Therefore, further targeted studies are warranted to clarify whether SPX acts via GALR2 to influence insulin resistance and hepatic metabolism, and to explore the involvement of the FOXO1/PGC-1α pathway in these processes.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ramezani, N., Dezhan, M., Khalili, S. S., Shakeri, N. & Khoramipour, K. Effect of high-intensity interval training on omentin-1 serum levels, gene expression, and insulin resistance in type 2 diabetic rats. Med. Lab. J. 17(5), 20–22 (2023).
Herz, D. et al. Effects of different types of intermittent fasting interventions on metabolic health in healthy individuals (EDIF): A randomised trial with a controlled-run in phase. Nutrients 16(8), 1114 (2024).
Herz, D. et al. Efficacy of fasting in type 1 and type 2 diabetes mellitus: A narrative review. Nutrients 15(16), 3525 (2023).
Rajabi, A. et al. Effect of 8 weeks aerobic training and saffron supplementation on inflammation and metabolism in middle-aged obese women with type 2 diabetes mellitus. Sports. 10(11), 167 (2022).
Cheng, J.-X. & Yu, K. New discovered adipokines associated with the pathogenesis of obesity and type 2 diabetes. Diabetes, Metab. Syndr. Obes. Targets Ther. 15, 2381–2389 (2022).
Gu, L. et al. Spexin alleviates insulin resistance and inhibits hepatic gluconeogenesis via the FoxO1/PGC-1α pathway in high-fat-diet-induced rats and insulin resistant cells. Int. J. Biol. Sci. 15(13), 2815 (2019).
Yu, M. et al. Spexin ameliorates skeletal muscle insulin resistance through activation of GAL2 receptor. Eur. J. Pharmacol. 917, 174731 (2022).
Mirabeau, O. et al. Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res. 17(3), 320–327 (2007).
Sun, X., Yu, Z., Xu, Y., Pu, S. & Gao, X. J. P. The role of spexin in energy metabolism. Peptides 164, 170991 (2023).
Porzionato, A. et al. Spexin expression in normal rat tissues. J. Histochem. Cytochem. 58(9), 825–837 (2010).
Türkel, İ, Memi, G. & Yazgan, B. Impact of spexin on metabolic diseases and inflammation: An updated minireview. Exp. Biol. Med. 247(7), 567–573 (2022).
Gu, L. et al. Spexin peptide is expressed in human endocrine and epithelial tissues and reduced after glucose load in type 2 diabetes. Peptides 71, 232–239 (2015).
Wang, M. et al. Treatment with spexin mitigates diet-induced hepatic steatosis in vivo and in vitro through activation of galanin receptor 2. Mol. Cell. Endocrinol. 552, 111688 (2022).
Fang, P. et al. Adipose tissue spexin in physical exercise and age-associated diseases. Aging Res. Rev. 73, 101509 (2022).
Mohammadi, A., Bijeh, N., Moazzami, M., Khodaei, K. & Rahimi, N. Effect of exercise training on spexin level, appetite, lipid accumulation product, visceral adiposity index, and body composition in adults with type 2 diabetes. Biol. Res. Nurs. 24(2), 152–162 (2022).
Leciejewska, N. et al. Spexin promotes the proliferation and differentiation of C2C12 cells in vitro—the effect of exercise on SPX and SPX receptor expression in skeletal muscle in vivo. Genes 13(1), 81 (2021).
Ahsan, A., Nazarali, P., Alizadeh, R. & Rezaeinezhad, N. High-intensity interval training has a greater effect on insulin resistance than moderate-intensity aerobic training by increasing spexin. Sport Physiol. 15(57), (2023).
Magalhães, D. A. D. et al. High-fat diet and streptozotocin in the induction of type 2 diabetes mellitus: a new proposal. An. Acad. Bras. Ciênc. 91(01), e20180314 (2019).
Orumiyehei, A. et al. High-intensity interval training-induced hippocampal molecular changes associated with improvement in anxiety-like behavior but not cognitive function in rats with Type 2 diabetes. Brain Sci. 12(10), 1280 (2022).
Khoramipour, K. et al. High-intensity interval training ameliorates molecular changes in the hippocampus of male rats with the diabetic brain: The role of adiponectin. Mol. Neurobiol. 60, 1–10 (2023).
Khoramipour, K. et al. Changes in the hippocampal level of tau but not beta-amyloid may mediate anxiety-like behavior improvement ensuing from exercise in diabetic female rats. Behav. Brain Funct. 20(1), 9 (2024).
Laursen P. & Martin B. Science and Application of High-Intensity Interval Training. Human kinetics, (2019).
Sadat Hosseini, N., Shirazpour, S., Sepehri, G., Dabiri, S. & Meymandi, M. S. High-intensity interval training alleviates ethanol-induced renal damage: A study on inflammation, oxidative stress, and histopathological changes in rats. Drug Alcohol Depend. Rep. 14, 100320 (2025).
Ebrahimnezhad, N., Nayebifar, S., Soltani, Z. & Khoramipour, K. High-intensity interval training reduced oxidative stress and apoptosis in the hippocampus of male rats with type 2 diabetes: The role of the PGC1α-Keap1-Nrf2 signaling pathway. Iran. J. Basic Med. Sci. 26(11), 1313 (2023).
Shirazpour, S. et al. Amelioration of chronic ethanol administration-induced learning and memory impairments by high-intensity interval training (HIIT) and ritalin. Brain Behav. 15(5), e70539 (2025).
Khoramipour, K. et al. High intensity interval training can ameliorate hypothalamic appetite regulation in male rats with type 2 diabetes: The role of Leptin. Cell. Mol. Neurobiol. 43(8), 4295–4307 (2023).
Pirani, H. et al. Lactate-induced autophagy activation: Unraveling the therapeutic impact of high-intensity interval training on insulin resistance in type 2 diabetic rats. Sci. Rep. 14(1), 1108 (2024).
Rajizadeh, M. A. et al. Lung molecular and histological changes in type 2 diabetic rats and its improvement by high-intensity interval training. BMC Pulm. Med. 24(1), 37 (2024).
Rami, M., Azimpour, M. & Khoramipour, K. The effect of 8 weeks of high intensity interval training on the levels of Wnt and NF-κB proteins in the heart tissue of male Wistar rats with type 2 diabetes. J. Sport Exerc. Physiol. 15(4), 19–30 (2022).
Soltany, A., Daryanoosh, F., Gholampour, F., Sadat Hosseini, N. & Khoramipour, K. Potential role of high-intensity interval training-induced increase in humanin levels for the management of Type 2 diabetes. J. Cell Mol. Med. 29(3), e70396 (2025).
Hosseini, N. S., Shirazpour, S., Zangiabadizadeh, M., Bashiri, H., Dabiri, S., Sepehri, G. et al. High-intensity interval training ameliorates tramadol-induced nephrotoxicity and oxidative stress in experimental rats. Cureus 16(6), (2024).
Rahmaty, S., Dehghan, P., Khoramipour, K. & Saboory, M. The effect of listening to brain waves’ relaxing and exciting music during intense endurance training on blood cortisol levels of adult men. Am. J. Sports Sci. Med. 3(4), 77–81 (2015).
Basereh, A., Ebrahim, K., Hovanloo, F., Dehghan, P. & Khoramipour, K. Effect of blood flow restriction deal during isometric exercise on growth hormone and testosterone active males. Sport Physiol. 9(33), 51–68 (2017).
Frankenberg, A. D. V., Reis, A. F. & Gerchman, F. Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: A literature review. Arch. Endocrinol. Metab. 61, 614–622 (2017).
Sims-Robinson, C., Kim, B., Rosko, A. & Feldman, E. L. How does diabetes accelerate Alzheimer disease pathology?. Nat. Rev. Neurol. 6(10), 551–559 (2010).
Katz, A. et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 85(7), 2402–2410 (2000).
Suhs, M. et al. Circulating spexin is associated with body mass index and fat mass but not with physical activity and psychological parameters in women across a broad body weight spectrum. J. Clin. Med. 11(17), 5107 (2022).
Jasmine, F. G, Walewski, J., Anglade, D. & Berk, P. (eds.). Regulation of hepatocellular fatty acid uptake in mouse models of fatty liver disease with and without functional leptin signaling: Roles of NfKB and SREBP-1C and the effects of spexin. In: Seminars in Liver Disease, Thieme Medical Publishers (2016).
Kolodziejski, P. A. et al. 30-Day spexin treatment of mice with diet-induced obesity (DIO) and type 2 diabetes (T2DM) increases insulin sensitivity, improves liver functions and metabolic status. Mol. Cell. Endocrinol. 536, 111420 (2021).
Balaji, R., Duraisamy, R. & Kumar, M. J. Complications of diabetes mellitus: A review. Drug Invent. Today 12(1), 98 (2019).
Holt, R. I. G. & Flyvbjerg, A. (eds) Textbook of Diabetes (Wiley, 2024).
Sassek, M., Kolodziejski, P. A., Szczepankiewicz, D. & Pruszynska-Oszmalek, E. J. E. Spexin in the physiology of pancreatic islets—mutual interactions with insulin. Mol. Cell. Endocrinol. 63, 513–519 (2019).
Reyes-Alcaraz, A. et al. Development of spexin-based human galanin receptor type II-specific agonists with increased stability in serum and anxiolytic effect in mice. Sci. Rep. 6(1), 21453 (2016).
Han, H.-S., Kang, G., Kim, J. S., Choi, B. H. & Koo, S.-H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 48(3), e218 (2016).
Cheng, C.-F., Ku, H.-C. & Lin, H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int. J. Mol. Sci. 19(11), 3447 (2018).
Wu, H. et al. PGC-1α, glucose metabolism and type 2 diabetes mellitus. J. Endocrinol. Investig. 229(3), R99–R115 (2016).
Chen, W., Zhao, H. & Li, Y. Mitochondrial dynamics in health and disease: mechanisms and potential targets. Stem Cell Res. Ther. 8(1), 333 (2023).
Sawada, N. et al. Endothelial PGC-1α mediates vascular dysfunction in diabetes. J. Thromb. Haemost. 19(2), 246–258 (2014).
Liu, Y. et al. Spexin protects cardiomyocytes from hypoxia-induced metabolic and mitochondrial dysfunction. Cell. Mol. Life Sci. 393, 25–33 (2020).
Li, Y. et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. J. Clin. Investig. 13(4), 376–388 (2011).
He, L. et al. Galanin ameliorates liver inflammation and fibrosis in mice by activating AMPK/ACC signaling and modifying macrophage inflammatory phenotype. Front. Immunol. 14, 1161676 (2023).
Kim, S. H. et al. Effect of isoquercitrin on free fatty acid-induced lipid accumulation in HepG2 cells. J. Ethnopharmacol. 28(3), 1476 (2023).
Salminen, A., Hyttinen, J. M. & Kaarniranta, K. J. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J. Mol. Med. 89, 667–676 (2011).
Kimura, Y. et al. Soluble uric acid promotes atherosclerosis via AMPK (AMP-activated protein kinase)-mediated inflammation. J. Thromb. Thrombolysis 40(3), 570–582 (2020).
Acknowledgements
Not applicable.
Funding
The study was supported a by grant (402000417) from Endocrinology and Metabolism Research Center, Kerman University of Medical Sciences, Kerman, Iran.
Author information
Authors and Affiliations
Contributions
Najmeh Sadat Hosseini scarify and protein assessments, wrote the main manuscript, and prepared the statistical analysis section. Kimya Khoramipour conducted animal training. Karen Khoramipour wrote the main manuscript. Kayvan Khoramipour, Mona Saheli and Jennifer W. Hill, Sergio Maroto Izquierdo and Simone Lista reviewed and revised the manuscript. Mona saheli, as the corresponding author, is responsible for the regulations, principles, and performance standards in the institute’s research. Also, all authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The ethics committee of Kerman University of Medical Sciences approved all procedures conducted in this study under the authority of the Project License (IR.KMU.AEC.1404.005). This study was conducted in accordance with the Animal Rights and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985). Also, all authors confirm that all procedures, including protocols and experiments, were conducted in full compliance with the applicable ARRIVE guidelines.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khoramipour, K., Hosseini, N.S., Hill, J.W. et al. High intensity interval training attenuate insulin resistance in diabetic rats accompanied by improvements in liver metabolism and spexin signaling. Sci Rep 15, 30682 (2025). https://doi.org/10.1038/s41598-025-15432-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15432-8