Abstract
Home ranges reflect a trade-off between the costs and benefits associated with acquiring resources and are influenced by complex interactions among intrinsic and extrinsic factors. These factors can lead to different spatial and temporal patterns to acquire the necessary resources that meet energetic and reproductive needs. Identifying the drivers of these strategies concurrently across spatiotemporal scales remains rare but is essential for identifying landscape constraints on populations in rapidly changing systems. We examined spatiotemporal drivers of home range size of the federally endangered ocelot (Leopardus pardalis; [22 Males, 12 Females]) in the two remaining populations in the USA. Males increased home range size during reproductive periods while females constrained their home range, but increased in size to match the demands of reproduction. Habitat complexity and the associated prey diversity and abundance were related to smaller home range size. Our results suggest that home range variation is a response to environmental conditions and annual changes in life history. Sex-specific drivers of home range size across space and time—in the context of habitat loss, shifting climate patterns, and changing resource productivity—can help identify management and habitat restoration targets for small and declining populations.
Similar content being viewed by others
Introduction
Animals must make fundamental trade-offs between maximizing energy acquisition and minimizing energy expenditure1. Foraging theory predicts that individuals’ behavioral decisions should maximize fitness by optimizing energetic trade-offs in the exploitation of resources2. The restriction of movement and space use while pursuing resources facilitates the formation of home ranges, which are defined as the area used by animals to meet life history requirements3. Consequently, this restriction of space use can impact many ecological processes, including the distribution and abundance of animals4 predator-prey dynamics5 and community dynamics6. Predicting spatial patterns of home ranges and space use that mediate the distribution and abundance of animals is a principal concern for species conservation in the face of environmental change7.
Similar to energetic trade-offs animals face while foraging, the size of a home range reflects a trade-off between the costs and benefits associated with acquiring resources, such as food and mates, over a given period of time8. Whereas metabolic requirements for a given body size commonly drives interspecific variation in home range size9,10 intraspecific variation can be influenced by multiple intrinsic (e.g., age or sex) and extrinsic (e.g., forage and climate) factors that maximize fitness and resource acquisition11. Home ranges are shaped by the distribution, predictability, and accessibility of resources, and are often smaller as resource quality and abundance increases12,13,14. Home ranges then should be spatially (e.g., patches of high habitat quality) and temporally (e.g., reproductive cycles, seasonality) dynamic, influencing population-level effects as well as displaying ecosystem-level variation in time scale-specific responses4,15.
Mammalian carnivores can have a disproportionate effect on ecosystems relative to their abundance16,17 yet declines in their distribution and abundance have been widespread. These declines are often consequences of large spatial requirements related to diet specializations and life histories18. Many carnivores are territorial and exclude conspecifics from part of their home range. Thus, the size of the home range is not only constrained by environmental factors but also by social and competitive interactions that can be sex-specific19,20. For species that are long-lived and have low reproductive rates, an area-minimizing approach can be effective to meet energetic and reproductive needs21. However, the ranging behavior of polygynous male carnivores may benefit most from a resource-maximizing strategy to overlap with multiple females while also selecting for high-resource habitat patches22. Sex-specific fitness requirements, coupled with extrinsic factors that dictate the abundance and distribution of food, should strongly influence carnivore home range size23,24,25. For carnivores, their home range size often decreases as prey availability (e.g., productivity) and landscape connectivity increases13,26,27. However, variation in this response is common28 but few studies have concurrently examined variation in sex-specific home range strategies across spatiotemporal scales and environmental variation.
Ocelots (Leopardus pardalis), a medium-sized solitary and territorial carnivore that are endangered in the United States, exhibit sex-specific differences in space use and strategies for maximizing reproductive success29,30,31. Generally, throughout their distribution from southern Texas, USA to southern Brazil, males have larger home ranges than females, and both sexes have been observed to change their space use in response to changes in resources32,33,34. Although the space use of ocelots has been widely studied across their broad geographic distribution19 approaches to estimate home ranges have varied, and past studies have been limited by sample size, duration, and tracking methodologies19,35 challenging the quantification of sex-specific patterns and responses to environmental variation. In the United States, population declines have been largely due to the loss of native woodlands that have been converted to agriculture and urban development in the 1900s36,37. Ocelot recovery is currently limited by available habitat and extensive fragmentation, making a better understanding of how space use and landscape conditions may influence sex-specific density paramount to help inform habitat management actions.
We estimated home ranges across multiple spatiotemporal scales and evaluated the effects of intrinsic and extrinsic factors on home range size in the two extant ocelot populations in the United States. We considered the intrinsic factor of sex and extrinsic factors that would influence foraging, competitive interactions, and physiological constraints, including vegetation connectivity, productivity, habitat structure, drought conditions, and temperature. We expected that both sexes would have smaller home ranges with better foraging conditions, and as productivity increased. However, we predicted that males would have larger home ranges than females, but female core ranges would have less variability and be constrained to balance the competing demands of reproduction. Consequently, we expected that males would show greater variability in their home range to maximize overlap with females and to meet energetic demands for covering greater distances. Ultimately, understanding the intrinsic and extrinsic effects on spatiotemporal variation in home range strategies offers opportunities to understand landscape constraints on populations in rapidly changing systems.
Results
We estimated 224 home ranges from 30 ocelots (19 M, 11 F) across different temporal scales – monthly (n = 167), seasonal (n = 42), and half-year (n = 15) (Table 1). The monthly core area of male home ranges (2.57 km2; 95% CI [1.77, 3.38] km2 was approximately 3x times larger than that of females (0.76 km2; 95% CI [0.38, 1.14] km2, and the full home range was 2.7x larger (males: 10.55 km2; 95% CI [7.48, 13.61] km2 and females: 3.92 km2; 95% CI [2.22, 5.61] km2. Home range size was similar across temporal scales (month, seasonal, and half-year), but both sexes showed an increase (females: 12%; males: 20%) for home ranges estimated during the half-year period compared to monthly. Spatially, the full home range showed greater variability compared to core home ranges among individuals (Fig. 1). However, female home range size was less variable compared to males across temporal scales (month, seasonal, and half-year) and spatial scales (full and core home range) (Fig. 1; Table 1). For individuals with successive monthly home ranges (30 individuals), we found that location of the core home range centroid moved an average of 298 m for females and 480 m for males, while the core seasonal home ranges (11 individuals) shifted 240 m for females and 979 m for males. We found support for intra-annual variation in home range size (AICc weight = 1) with male home ranges at a minimum and female home ranges at a maximum during the summer months (Fig. 2).
Average individual home range size (km2 for male and female ocelots in Texas, USA between 2011 and 2024. Home ranges were estimated using an autocorrelated kernel density estimator at the core (50%) and full (95%) after fitting a continuous-time movement model to monthly (calendar months), seasonal (3-month periods), and half-year (April–September) GPS location data for each individual.
Intra-annual variation in the full (95%) home range size for male and female ocelots (Leopardus pardalis) in Texas, USA between 2011 and 2024. Shaded areas show the 95% confidence intervals and points represent individual home range size. Home ranges were estimated using an autocorrelated kernel density estimator after fitting a continuous-time movement model to monthly GPS locations.
We found sex-specific differences in home range size relative to multiple landscape and climate variables, and in some cases, variation between spatial scales (core vs. full). In general, males responded more strongly to landscape and climate conditions compared to females, especially at the core spatial scale (Figs. 3 and 4). For males, we found that home range size was smaller as woody cover, vegetation heterogeneity, and mean temperature increased (Fig. 3; Supplementary Information Table S1). In contrast, greater canopy cover was associated with larger home ranges for males (Fig. 3; Supplementary Information Table S1). Most landscape and climate conditions had a minimal effect on female home range size across spatial scales (Figs. 3 and 4). However, the proportion of canopy cover was likely to have a negative effect on female home range size at the 50% (pd = 97%) and 95% (pd = 89%) home range size, and the proportion of woody land cover at the 95% female home range size (pd = 100%; Figs. 3 and 4; Supplementary Information Table S1). The negative response to canopy cover of females contrasted the positive response in males across both spatial scales (Fig. 4). We found positive effects between home range size and both drought and the connectivity of woody cover for males and females (Figs. 3 and 4; Supplementary Information Table S1).
Beta coefficient estimates of landscape effects on ocelot home range size in Texas, USA between 2011 and 2024. Generalized linear mixed effects models with a gamma distribution and log link were fitted to test the effect of landscape variables on the monthly core (50%) and full (95%) home range size for males and females. Home range size was estimated using an autocorrelated kernel density estimator after fitting a continuous-time movement model from GPS location data for each individual.
Conditional effects of covariates on the monthly core (50%) and full (95%) home range size of male and female ocelots in Texas, USA between 2011 and 2024. Separate generalized linear mixed effects models with a gamma distribution and log link were fit for males and females for 50% and 95% home range size. Home range size was estimated using an autocorrelated kernel density estimator after fitting a continuous-time movement model from GPS location data for each individual. The y-axis was scaled logarithmically to improve visualization.
Discussion
Our study examined the effect of intrinsic and extrinsic factors on home range size across multiple spatial and temporal scales using GPS data from the only extant populations of an endangered carnivore in the United States. Concurrent analyses across spatiotemporal scales remain uncommon, but partitioning these scale-dependent processes is important for identifying mechanisms that affect variation in home range size. We found that interactions between intrinsic and extrinsic factors influenced home range size, but that relationships varied across spatial scales. In general, males responded more strongly than females to changes in environmental conditions across spatial scales (core and full home range), while female home range size generally showed a minimal response across predictors, especially in their core range. This variation in sex-specific responses may be due in part to differences in philopatry and dispersal behaviors that differentially expose males to broader landscape variation. Female carnivores often display higher philopatry and can lead to fitness benefits due to familiarity with resources within their natal home range38. On the other hand, males generally disperse from natal home ranges and maintain larger home ranges, likely leading to the development of behavioral plasticity to modify home ranges according to environmental conditions39.
Regardless of spatiotemporal scale, male ocelots had larger and more variable home ranges than females. Beyond body size differences, the larger home ranges of males are common among mammalian taxa and are often attributed to a behavioral response to overlap with multiple females during reproductive periods40. Ocelots can reproduce year-round, and in this system, parturition is thought to occur more frequently with wetter conditions in the spring and fall41. We found some support for males reducing their home ranges during summer months when females were more likely to be with offspring and anestrus, therefore reducing the value of larger home ranges that would contact multiple females. Concomitant with the decrease in male home ranges, females showed an increase in home range size during summer months, potentially due to behavioral and energetic requirements that change with dependent offspring. Two strategies have been hypothesized to influence home range size: resource maximization, which would promote acquiring more resources that lead to larger home range, and area minimization, which meets a minimum resource threshold for survival and reproduction in the smallest possible unit area42. Males appeared to employ a resource maximization strategy during reproductive periods while females constrained their home range in an area-minimizing approach, only increasing in size to match the demands of reproduction24.
Increasing vegetation heterogeneity, our proxy for prey diversity, was related to smaller home range sizes. Mechanistic and empirical models have found that resource aggregation (i.e., increasing patchiness of resources across space) dictates the shape and spatial distribution of home ranges and tends to lead to greater movement and larger individual home ranges12,42. However, in some cases, greater habitat heterogeneity can increase availability of resources within a smaller area, thereby reducing the need for extensive movements43. In this system, vegetation heterogeneity may be associated with a more uniform distribution of prey and foraging opportunities, particularly for dietary generalists and opportunistic foragers like ocelots44,45,46. Similarly, the home range size was smaller with greater amounts of woody cover. The response to woody cover in the region is consistent with previous work that found resource selection and even spatial partitioning of sympatric carnivores were affected by structural complexity of the vegetation47. Selection for woody cover likely provided access to mates, protection from interspecific competitors, and greater prey availability48,49,50 that reduces the space needed to meet energetic demands.
Contrary to our hypothesis, we observed a minimal effect of productivity on home range size for both sexes. Productivity (e.g., NDVI) as a measure of prey availability has been commonly used to test expectations on home range size, with responses often varying by individuals, populations, and species12,28,51. The lack of seasonal changes (e.g., wet and dry seasons) in ocelot home range size has been observed in other portions of their range33 as well as other felids and carnivores28,52,53. However, the degree of seasonality in a system may interact with productivity to shape home ranges of carnivores28. Most of our home ranges were estimated between April and September, representing both wet and dry periods, but relatively few samples came from the coldest months. In this system, plant flowering and fruiting can occur year-round, even in colder months54 which may have dampened any response of home range size to productivity. Further, carnivores such as ocelots that are dietary generalists may be able to maintain stable home ranges despite seasonal changes in productivity due, in part, to wide diet variation28.
The home range size of males and females responded differently to horizontal and vertical habitat structure. Male home range size was greater as canopy cover increased, as more structural complexity in vegetation within a home range may provide the necessary cover to access additional hunting opportunities or explore new areas for food or mates. On the other hand, female home range size decreased as canopy cover increased. This contradictory effect may be tied to differences in behavioral strategies to balance the energetic costs of reproduction and vulnerability of young (e.g55.,. Many female carnivores with young reduce their movements to account for greater energetic demands and select for structural complexity that provides stable thermal properties and protection from weather and predators40,56,57. Consequently, female space use and ranging behavior can have impacts on juvenile survival and reproductive success that influence population dynamics58,59.
Considering multiple spatial and temporal scales when defining a home range is critical to evaluate mechanisms that might directly or indirectly affect ranging behavior60 (Fieberg and Börger 2012). Despite our coarse resolution of weather predictors, we captured variation in weather conditions across 12 years of ocelot home ranges; however, the effects of temperature and drought had a minimal effect on home range size. This suggested that indirect predictors, like weather conditions, were less important than direct predictors like habitat composition and configuration at monthly timescales to influence home range size. Weather conditions have been shown to influence short temporal scales (e.g., days and weeks11, and although we examined environmental conditions across multiple spatial and temporal scales, their impact may be more pronounced over shorter time frames or finer movement behaviors, such as daily distance traveled or resource selection within a home range. For example, drought may reduce the density of prey, but not the distribution within the home range, leading to decreased encounters that require greater daily movements within the home range to acquire enough prey. Alternatively, we may not have captured changes that would influence prey abundance and distribution using the short-term drought metric (3-month SPEI), even though it captures changes in vegetation productivity. In addition, weather conditions interact with vegetation composition and structure and this interaction may mediate space use during periods of high temperature and drought61.
Understanding sex-specific variation and multiscale drivers of home range variation has important implications for species management and conservation. Recovery of ocelots in the United States has been slow and thought to be constrained by available habitat, thereby requiring action to improve connectivity and maximize available space through habitat restoration. We found that interactions between intrinsic and extrinsic factors can influence home range size and that efforts to promote woody vegetation and structural complexity could potentially (1) increase density by providing habitat to minimize female home range size and (2) provide landscape connectivity through restoration of habitat structure that males can utilize to maximize resources. Consequently, understanding the drivers of sex-specific home range size across space and time in the presence of habitat loss, shifting climate patterns, and resource productivity can help provide the necessary management and habitat restoration targets for the conservation of small and declining populations.
Methods
Study area
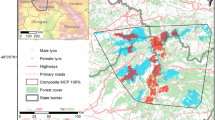
This study was conducted at two sites within coastal southern Texas, USA: one on private working ranch lands and the other in and around the Laguna Atascosa National Wildlife Refuge (Fig. 5; < 35 km apart). These areas are home to the last two breeding populations of ocelots in the United States and have limited connectivity between them (Lehnen et al. 2021, Veals et al. 2022). Ranchlands included inland dunes, coastal prairie, emergent herbaceous and woody wetlands, and large extensive mixed to dense live oak (Quercus virginiana)-thornscrub and mesquite (Proposis glandulosa)-thornscrub communities (Lombardi et al. 2021). Laguna Atascosa National Wildlife Refuge (LANWR) and adjacent private lands were characterized by a mosaic of protected and private patches of mixed to dense thornscrub and coastal prairie within a mosaic with row-crop agriculture, urbanization and extensive road networks62,63. The regional climate was semi-arid subtropical with annual temperatures ranging from 10 to 36 °C, with summer peaks of > 40 °C during the summer. Annual precipitation was highly variable (313–529 mm) with more during summer62. Episodic moderate and severe droughts were common62. Generally, these precipitation patterns lead to a growing season from April to through September and a dry winter season from October through March.
Study areas of ocelot (Leopardus pardalis) GPS collar data in Texas, USA between 2011 and 2024 and land cover from the 2021 National Land Cover Database generated in R (v4.4.3; R Core Team 2024; https://www.R-project.org/). Black polygons represent the general area of ocelot distribution in the region including private working ranch lands in the north and the other in the south around the Laguna Atascosa National Wildlife Refuge.
GPS collar data
We collected GPS collar data from 34 individuals (22 M, 12 F) over 12 years across both study sites (17 individuals per site). Ocelots were captured using Tomahawk box-traps (10 × 51 × 38 cm; Tomahawk Trap, Hazelhurst, WI, USA) from January and May from 2011 to 2017 and November and April from 2019 to 2024. We immobilized ocelots using intramuscular injection of a ketamine-medetomidine mixture and medetomidine was reversed with atipamezole. We fit captured ocelots with GPS collars (G5-PC, Advanced Telemetry Systems, Isanti, MN, USA or TGW-4177-4, Telonics, Mesa, AZ, USA) and programmed collars to attempt a fix every 1 h and automatically release after 6 (N = 7) or 12 months (N = 5). In addition, we incorporated GPS collar data from previously captured ocelots (N = 22; 2012 to 2021) that included location fix rates from every 30 min to 5 per day, and were programmed to automatically release after 4–12 months31,47,64,65. We filtered data for outliers and erroneous locations by removing points with high dilution of precision (DOP; 3D fixes > 10 or 2D fixes > 5) and improbable velocities (≥ 5 km/h) between successive fixes (Bjørneraas et al., 2009). All experimental protocols were approved by the United States Fish and Wildlife Service, Texas Parks and Wildlife Department, and Texas A&M University - Kingsville’s Institute for Animal Care and Use Committee (USFWS: ES822908; TPWD: SPR-1123-136; TAMUK: 2023-10-20). We carried out all methods in accordance with relevant guidelines and regulations including ARRIVE guidelines for reporting animal research66.
Home range Estimation
We fit continuous-time movement models to individual ocelot GPS relocations at monthly, seasonal (3-month period), and half-year (6-month period) timeframes using the R (R Core Team 2024) package ctmm67,68. Our temporal periods reflected differences between the summer growing season and dry winter season. We visually assessed variograms of time-series movement patterns to identify individuals that did show range-residency (i.e., no clear asymptote) and subsequently removed them from further analyses (n = 4). We calculated autocorrelated kernel density estimators (aKDE), which account for autocorrelation, small effective sample sizes, and irregular sampling in time69,70,71. We calculated a home range as the minimum area in which an animal has some probability of being located72. We considered the 95% home range as an area of active use and the 50% area as an area of core use, hereafter core (50%) and full (95%) home range67. We compared the 50% and 95% aKDE isopleth to identify differences in spatial scale.
We used a cosinor model to examine how monthly home range size changes within the year (sensu24. We had an average of 4.25 (range: 1–10) and 9.7 (range: 1–19) observations per month for females and males, respectively. This model fits a cosine curve to periodic data within a regression model and can be used to identify seasonal patterns. We implemented a single component cosinor analysis using the R (R Core Team 2024) package GLMMcosinor68,73. The cosinor model was fit with a Gamma distribution with a log link and included sex as a fixed effect and a single cosinor component grouped by sex over 12 months. We assessed the effect of an annual cycle on home range size by comparing our cosinor model with an intercept-only model without temporal change within the year using Akaike information criterion (AICc).
Home range size analysis
We identified a set of landscape variables a priori that could influence the size of an ocelot home range by impacting foraging, competitive interactions, and physiological constraints (Table 2). Across their geographic range, ocelots select for a high proportion of woody cover and closed canopy that provides foraging opportunities, denning locations, and protection from competitors45,49,74. We characterized horizontal habitat structure through composition and configuration of woody vegetation. We created a woody cover land cover category by combining National Land Cover Dataset (NLCD) cover classes (forest, shrubland, and woody wetlands; Fig. 575;. We then calculated the proportion and connectivity (i.e., the average distance an organism can move within a patch) of woody cover within each home range using the R (R Core Team 2024) package landscapemetrics68,76. To estimate land cover metrics, we matched the time of the individual home ranges to the closest available NLCD year (2011, 2013, 2016, 2019, 2021). We represented vertical habitat structure by estimating the percentage of canopy cover across our study area using a GEDI-fusion approach (e.g74.,. We acquired canopy cover estimates from GEDI level 2b product footprints77 and filtered footprints by degrade flag (0), quality flag (1), and beam sensitivity (≥ 0.95) through Google Earth Engine. To predict continuously across our study area, we fitted a random forest regression model of canopy cover to continuous remote sensing products, including Landsat, synthetic aperture radar, and spectral indices (see Supplementary Information for additional details; Table S2; Fig. S1). We calculated the average proportion of canopy cover within individual home ranges from annual rasterized maps.
In addition, as a proxy for prey abundance, we estimated vegetation productivity using MODIS vegetation index products78,79. We calculated the average monthly normalized difference vegetation index (NDVI) from both Terra and Aqua satellite datasets to represent vegetation productivity. NDVI has been associated with changes in small mammal abundance in many systems80,81,82 and has a broader spatial and temporal coverage than many field studies, making it an important proxy to describe prey abundance83. As a proxy for prey diversity, we calculated a metric of vegetation heterogeneity (i.e., 2nd order entropy) within individual home ranges from a gray-level co-occurrence matrix (GLCM) derived from composite MODIS images of vegetation productivity (i.e., NDVI84,85;. Vegetation heterogeneity is often associated with greater species richness and diversity86.
We selected climate variables of temperature and drought as they could influence the availability of prey and impose physiological constraints on ocelot movement and space use61,87. We calculated average monthly temperature and drought from gridMET datasets88. Drought was estimated using the standardized precipitation-evaporation index (SPEI) that accounts for atmospheric evaporative demand and precipitation at a scale of three months89. SPEI values indicate the number of standard deviations by which the observed water balance deviates from the long-term mean. A SPEI time scale of threemonths is a measure of short-term drought that has been associated with changes in vegetation productivity in arid systems90. For all extrinsic variables, we accounted for temporal variation throughout by estimating landscape and climate variables for the specific month or year for which each individual’s home range was estimated.
We used Bayesian generalized linear mixed models to test the effect of sex, landscape, and climate variables on home range size. We fit a Gamma distribution with a log link because of non-negative and right-skewed home range sizes in separate models for each sex and aKDE isopleth (50% and 95%) with individual identification as a random effect. Models were implemented in the R (R Core Team 2024) packages brms91 with four chains of 4,000 iterations and 2,000 iterations as burn-in. To evaluate model convergence, we required R-hat values < 1.01 and visually inspected traceplots92. We assessed model fit through posterior predictive checks (Fig. S2)93. All variables were scaled and centered prior to model fitting and had Pearson’s correlation coefficients |<0.7|. To describe the posterior effects of parameter estimates, we calculated the 95% credible intervals and probability of direction using the R (R Core Team 2024) package bayestestR68,94. The probability of direction (pd) is the percentage of the posterior distribution of the parameter that is greater than or less than zero.
Data availability
The data and code that support the findings of this study are available on Dryad at https://doi.org/10.5061/dryad.1ns1rn95b
References
Schoener, T. W. Theory of feeding strategies. Annu. Rev. Ecol. Syst. 2, 369–404 (1971).
Pyke, G. H., Pulliam, H. R. & Charnov, E. L. Optimal foraging: a selected review of theory and tests. Annual Rev. Ecol. Systematics 52, 137–154 (1977).
Burt, W. H. Territoriality and home range concepts as applied to mammals. J. Mammal. 24, 346–352 (1943).
Wang, M. & Grimm, V. Home range dynamics and population regulation: An individual-based model of the common shrew Sorex araneus. Ecol. Model. 397–409. https://doi.org/10.1016/j.ecolmodel.2007.03.003 (2007).
Dulude-de Broin, F. et al. Predator home range size mediates indirect interactions between prey species in an Arctic vertebrate community. J. Anim. Ecol. 92, 2373–2385 (2023).
Costa-Pereira, R., Moll, R. J., Jesmer, B. R. & Jetz, W. Animal tracking moves community ecology: opportunities and challenges. J. Anim. Ecol. 91, 1334–1344 (2022).
Tucker, M. A. et al. Moving in the anthropocene: global reductions in terrestrial mammalian movements. Science 359, 466–469 (2018).
Mcnab, B. K. Bioenergetics and the determination of home range size. Am. Nat. 97, 133–140 (1963).
Gittleman, J. L. & Harvey, P. H. Behavioral ecology and sociobiology carnivore Home-Range size, metabolic needs and ecology. Behav. Ecol. Sociobiol. 10, 57–63 (1982).
Lindstedt, S. L., Miller, B. J. & Buskirk, S. W. Home range, time, and body size in mammals. Ecology 67, 413–418 (1986).
Van Beest, F. M., Rivrud, I. M., Loe, L. E., Milner, J. M. & Mysterud, A. What determines variation in home range size across Spatiotemporal scales in a large browsing herbivore? J. Anim. Ecol. 80, 771–785 (2011).
Brown, M. B. et al. Ranging behaviours across ecological and anthropogenic disturbance gradients: A pan-African perspective of giraffe (Giraffa spp.) space use. Proceedings of the Royal Society B: Biological Sciences 290, (2023).
Dickie, M. et al. Resource exploitation efficiency collapses the home range of an apex predator. Ecology 103, e3642 (2022).
Valeix, M., Hemson, G., Loveridge, A. J., Mills, G. & Macdonald, D. W. Behavioural adjustments of a large carnivore to access secondary prey in a human-dominated landscape. J. Appl. Ecol. 49, 73–81 (2012).
Börger, L., Dalziel, B. D. & Fryxell, J. M. Are there general mechanisms of animal home range behaviour? A review and prospects for future research. Ecol. Lett. 11, 637–650 (2008).
Estes, J. A. et al. Trophic downgrading of planet Earth. Science 333, 301–306 (2011).
Ritchie, E. G. et al. Ecosystem restoration with teeth: what role for predators? Trends Ecol. Evol. 27, 265–271 (2012).
Ripple, W. J. et al. Status and ecological effects of the world’s largest carnivores. Science 343, 1241484 (2014).
Gonzalez-Borrajo, N., López-Bao, J. V. & Palomares, F. Spatial ecology of jaguars, pumas, and ocelots: a review of the state of knowledge. Mammal Rev. 1–14, https://doi.org/10.1111/mam.12081 (2017).
Potts, J. R. & Börger, L. How to scale up from animal movement decisions to Spatiotemporal patterns: an approach via step selection. J. Anim. Ecol. 92, 16–29 (2023).
Mitchell, M. S. & Powell, R. A. Optimal use of resources structures home ranges and Spatial distribution of black bears. Anim. Behav. 74, 219–230 (2007).
Le Roex, N., Mann, G. K. H., Hunter, L. T. B. & Balme, G. A. Relaxed territoriality amid female trickery in a solitary carnivore. Anim. Behav. 194, 225–231 (2022).
Aronsson, M. et al. Intensity of space use reveals conditional sex-specific effects of prey and conspecific density on home range size. Ecol. Evol. 6, 2957–2967 (2016).
Johansson, Ö. et al. Sex-specific seasonal variation in puma and snow leopard home range utilization. Ecosphere 9, e02371 (2018).
Loveridge, A. J. et al. Changes in home range size of African lions in relation to pride size and prey biomass in a semi-arid savanna. Ecography 32, 953–962 (2009).
Morato, R. G. et al. Space use and movement of a neotropical top predator: The endangered jaguar. PLoS ONE 11, e0168176 (2016).
Rodríguez-Recio, M. et al. Estimating global determinants of Leopard home range size in a changing world. Anim. Conserv. 25, 748–758 (2022).
Nilsen, E. B., Herfindal, I. & Linnell, J. D. C. Can intra-specific variation in carnivore home-range size be explained using remote-sensing estimates of environmental productivity?. Ecoscience 12, 68–75 (2005).
Di Bitetti, M. S., Paviolo, A. & De Angelo, C. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic forest of misiones, Argentina. J. Zool. 270, 153–163 (2006).
Satter, C. B. et al. Long-term monitoring of ocelot densities in Belize. J. Wildl. Manage. 83, 283–294 (2019).
Veals, A. M. et al. Multiscale habitat relationships of a habitat specialist over time: the case of ocelots in Texas from 1982 to 2017. Ecosphere 13, e4204 (2022).
Azevedo, F. C. C. et al. Spatial organization and activity patterns of ocelots (Leopardus pardalis) in a protected subtropical forest of Brazil. Mammal Res. 64, 503–510 (2019).
Dillon, A. & Kelly, M. J. Ocelot home range, overlap and density: comparing radio telemetry with camera trapping. J. Zool. 275, 391–398 (2008).
Goulart, F. et al. Ecology of the ocelot (Leopardus pardalis) in the Atlantic forest of Southern Brazil. Neotropical Biology Conserv. 4, 137–143 (2009).
Fleming, C. H. et al. Estimating where and how animals travel: an optimal framework for path reconstruction from autocorrelated tracking data. Ecology 97, 576–582 (2016).
Jahrsdoerfer, S. E. & Leslie, D. M. Tamaulipan Brushland of the Lower Rio Grande Valley of South Texas: Description, Human Impacts, and Management Options. (1988).
Tremblay, T. A., White, W. A. & Raney, J. A. Native woodland loss during the mid 1900s in Cameron county, Texas. The Southwest. Naturalist 50, 479–482 (2005).
de Oliveira, M. E., Saranholi, B. H., Dirzo, R. & Galetti, P. M. A review of philopatry and dispersal in felids living in an anthropised world. Mammal Rev. 52, 208–220 (2022).
Gantchoff, M., Conlee, L. & Belant, J. Conservation implications of sex-specific landscape suitability for a large generalist carnivore. Divers. Distrib. 25, 1488–1496 (2019).
Elbroch, L. M., Lendrum, P. E., Alexander, P. & Quigley, H. Cougar Den site selection in the Southern Yellowstone ecosystem. Mammal Res. 60, 89–96 (2015).
Laack, L. L., Tewes, M. E., Haines, A. M. & Rappole, J. H. Reproductive life history of ocelots Leopardus pardalis in Southern Texas. Acta Theriol. 50, 505–514 (2005).
Mitchell, M. S. & Powell, R. A. A mechanistic home range model for optimal use of spatially distributed resources. Ecol. Model. 177, 209–232 (2004).
Mangipane, L. S. et al. Influences of landscape heterogeneity on home-range sizes of brown bears. Mammalian Biology. 88, 1–7 (2018).
Abreu, K. C. et al. Feeding habits of ocelot (Leopardus pardalis) in Southern Brazil. Mammalian Biology. 73, 407–411 (2008).
Cruz, P. et al. Cats under cover: habitat models indicate a high dependency on woodlands by Atlantic forest felids. Biotropica 51, 266–278 (2019).
Emmons, L. H. Comparative feeding ecology of felids in a Neotropical rainforest. Behav. Ecol. Sociobiol. 20, 271–283 (1987).
Sergeyev, M. et al. Selection in the third dimension: Using LiDAR derived canopy metrics to assess individual and population-level habitat partitioning of ocelots, bobcats, and coyotes. Remote Sens. Ecol. Conserv. rse2.369, https://doi.org/10.1002/rse2.369 (2023).
Booth-Binczik, S. D. et al. FOOD HABITS OF OCELOTS AND POTENTIAL FOR COMPETITION WITH BOBCATS IN SOUTHERN TEXAS. Source: Southwest. Naturalist. 58, 403–410 (2013). http://www.fws.gov/southwest/
Wang, B. et al. Habitat use of the ocelot (Leopardus pardalis) in Brazilian Amazon. Ecol. Evol. 9, 5049–5062 (2019).
Horne, J. S., Haines, A. M., Tewes, M. E. & Laack, L. L. Habitat partitioning by sympatric ocelots and bobcats: implications for recovery of ocelots in Southern Texas. The Southwest. Naturalist 54, 119–126 (2009).
Herfindal, I., Linnell, J. D. C., Odden, J., Nilsen, E. B. & Andersen, R. Prey density, environmental productivity and home-range size in the Eurasian Lynx (Lynx Lynx). J. Zool. 265, 63–71 (2005).
Kubala, J. et al. Factors shaping home ranges of Eurasian Lynx (Lynx Lynx) in the Western Carpathians. Sci. Rep. 14, 21600 (2024).
Phillips, D. M., Harrison, D. J. & Payer, D. C. Seasonal changes in Home-Range area and fidelity of Martens. J. Mammal. 79, 180–190 (1998).
Lonard, R. I. & Judd, F. W. Phenology of native angiosperm of South Padre Island, Texas. in Proceedings of the North American Prairie Conferences 217–222 (1989).
Serieys, L. E. K., Leighton, G. R. M., Merondun, J. & Bishop, J. M. Denning and maternal behavior of caracals (Caracal caracal). Mammalian Biology. 104, 615–621 (2024).
Matthews, S. M. et al. Reproductive Den selection and its consequences for fisher neonates, a cavity-obligate Mustelid. J. Mammal. 100, 1305–1316 (2019).
Morrison, E. E., Lehman, C. P., Neiles, B. Y. & Rota, C. T. Resource selection of Den sites for bobcats (Lynx rufus) in the Northern great plains, united States. J. Mammal. https://doi.org/10.1093/jmammal/gyae105 (2024).
Broekhuis, F. Natural and anthropogenic drivers of Cub recruitment in a large carnivore. Ecol. Evol. 8, 6748–6755 (2018).
Davies, A. B., Marneweck, D. G., Druce, D. J. & Asner, G. P. Den site selection, pack composition, and reproductive success in endangered African wild dogs. Behav. Ecol. 27, 1869–1879 (2016).
Fieberg, J. & Börger, L. Could you please phrase home range as a question? J. Mammal. 93, 890–902 (2012).
Sergeyev, M. et al. Influence of abiotic factors on habitat selection of sympatric ocelots and bobcats: testing the interactive range-limit theory. Front. Ecol. Evol. 11, 1166184 (2023).
Blackburn, A. M., Heffelfinger, L. J., Veals, A. M., Tewes, M. E. & Young, J. H. Cats, cars, and crossings: The consequences of road networks for the conservation of an endangered felid. Global Ecol. Conservation 27, e01582 (2021).
Veals, A. M. et al. Landscape connectivity for an endangered carnivore: habitat conservation and road mitigation for ocelots in the US. Landsc. Ecol. 38, 363–381 (2023).
Lehnen, S. E., Sternberg, M. A., Swarts, H. M. & Sesnie, S. E. Evaluating population connectivity and targeting conservation action for an endangered Cat. Ecosphere 12, e03367 (2021).
Lombardi, J. V., Tewes, M. E., Perotto-Baldivieso, H. L., Mata, J. M. & Campbell, T. A. Spatial structure of Woody cover affects habitat use patterns of ocelots in Texas. Mammal Res. 65, 555–563 (2020).
Percie, D. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMC Vet. Res 16, 242 (2020).
Calabrese, J. M., Fleming, C. H. & Gurarie, E. Ctmm: an r package for analyzing animal relocation data as a continuous-time stochastic process. Methods Ecol. Evol. 7, 1124–1132 (2016).
R Core Team. A language and environment for statistical. (2024).
Fleming, C. H. et al. Correcting for missing and irregular data in home-range Estimation. Ecol. Appl. 28, 1003–1010 (2018).
Fleming, C. H. & Calabrese, J. M. A new kernel density estimator for accurate home-range and species-range area Estimation. Methods Ecol. Evol. 8, 571–579 (2017).
Silva, I. et al. Autocorrelation-informed home range estimation: A review and practical guide. Methods Ecol. Evol. 13, 534–544 (2022).
Worton, B. J. Kernel Methods for Estimating the Utilization Distribution in Home-Range Studies. 70, 164–168 (1989).
Parsons, R., Jayasinghe, O. & Rawashdeh, O. GLMMcosinor: Fit a cosinor model using a generalized mixed nodeling framework. (2024).
Sergeyev, M. et al. Multiscale assessment of habitat selection and avoidance of sympatric carnivores by the endangered ocelot. Scientific Reports 13, 8882 (2023).
Jin, S. et al. Overall methodology design for the United States national land cover database 2016 products. Remote Sensing 11, 2971 (2019).
Hesselbarth, M. H. K., Sciaini, M., With, K. A., Wiegand, K. & Nowosad, J. Landscapemetrics: an open-source R tool to calculate landscape metrics. Ecography 42, 1648–1657 (2019).
Dubayah, R. et al. The global ecosystem dynamics investigation: High-resolution laser ranging of the earth’s forests and topography. Sci. Remote Sens. 1, 100002 (2020).
Didan, K. MODIS/Aqua vegetation indices 16-Day L3 global 250m SIN grid V061. NASA EOSDIS Land. Processes Distrib. Act. Archive Cent. https://doi.org/10.5067/MODIS/MYD13Q1.061 (2021).
Didan, K. MODIS/Terra vegetation indices 16-Day L3 global 250m SIN grid V061. NASA EOSDIS Land. Processes Distrib. Act. Archive Cent. https://doi.org/10.5067/MODIS/MOD13Q1.061 (2021).
Chidodo, D. J. et al. Application of normalized difference vegetation index (NDVI) to forecast rodent population abundance in smallholder agro-ecosystems in semi-arid areas in Tanzania. Mammalia 84, 136–143 (2020).
Kausrud, K. L. et al. Climatically driven synchrony of gerbil populations allows large-scale plague outbreaks. Proceedings of the Royal Society B: Biological Sciences 274, 1693–1969 (2007).
Pettorelli, N. et al. The normalized difference vegetation index (NDVI): unforeseen successes in animal ecology. Clim. Res. 46, 15–27 (2011).
Pettorelli, N. et al. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 20, 503–510 (2005).
Farwell, L. S. et al. Satellite image texture captures vegetation heterogeneity and explains patterns of bird richness. Remote Sens. Environ. 253, 112175 (2021).
Smith, M. M., Erb, J. D., Pauli, J. N. & Smith, M. M. Seasonality drives the survival landscape of a recovering forest carnivore in a changing world. Proceedings of the Royal Society B (2022).
Tews, J. et al. Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J. Biogeogr. 31, 79–92 (2004).
West, L. et al. Droughts reshape apex predator space use and intraguild overlap. J. Anim. Ecol. 93, 1785–1798 (2024).
Abatzoglou, J. T. Development of gridded surface meteorological data for ecological applications and modelling. Intl J. Climatology. 33, 121–131 (2013).
Vicente-Serrano, S. M., Beguería, S. & López-Moreno, J. I. A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. J. Clim. 23, 1696–1718 (2010).
Vicente-Serrano, S. M. et al. Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. USA 110, 52–57 (2013).
Bürkner, P. C. Brms: an R package for bayesian multilevel models using Stan. Journal Stat. Software 80, 1–28 (2017).
Gelman, A. & Rubin, D. B. Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–511 (1992).
Gabry, J., Simpson, D., Vehtari, A., Betancourt, M. & Gelman, A. Visualization in bayesian workflow. J. Royal Stat. Soc. Ser. A: Stat. Soc. 182, 389–402 (2019).
Makowski, D., Ben-Shachar, M., Lüdecke, D. & bayestestR Describing effects and their uncertainty, existence and significance within the bayesian framework. J. Open. Source Softw. 4, 1541 (2019).
Acknowledgements
We thank the East Foundation, US Fish and Wildlife Service, Caesar Kleberg Wildlife Research Institute at Texas A&M University-Kingsville, and private landowners. We are grateful for financial support from the East Foundation, US Fish and Wildlife Service (Grant #: F20AC11365), Tim and Karen Hixon Foundation, and the Brown Foundation. We thank the many undergraduate and graduate students, faculty, wildlife veterinarians, staff, biologists, research technicians, and volunteers for assisting with data collection over the course of this study. We thank Paul M. Kapfer, K. Whitney Hansen, and two anonymous reviewers for providing feedback on previous versions of this manuscript. This manuscript is number 25-108 from the Caesar Kleberg Wildlife Research Institute and #119 from the East Foundation.
Author information
Authors and Affiliations
Contributions
MMS, AMVD, and LSP conceived the ideas and designed methodology with support from JVL; AMVD, JVL, ABB, AMR, DGS, MS, ZMW, MET, and LSP collected the data; MMS analysed the data with support from AMVD; MMS and LSP led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Smith, M.M., Veals Dutt, A.M., Lombardi, J.V. et al. Sex-specific resource strategies mediate home range sizes of an endangered carnivore across multiple scales. Sci Rep 15, 34190 (2025). https://doi.org/10.1038/s41598-025-15493-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15493-9
Keywords
This article is cited by
-
Daily and monthly movement patterns shape bobcat home range size in two different landscapes
Landscape Ecology (2025)