Abstract
A novel series of pyranopyrazolopyridone derivatives was synthesized via multicomponent reaction involving 4-hydroxy-6-methylpyridin-2-ones, aromatic aldehydes, and pyrazolone. To this end, a new high-efficiency bimetallic cobalt-cadmium heterogeneous magnetic catalyst was designed, synthesized, characterized, and used in this reaction. Instrumental techniques such as elemental analysis (CHN), EDX, SEM, TEM, FT-IR, XRD, VSM, and TGA were used to characterize the catalyst. To maximize efficiency, reaction conditions were optimized across all parameters, including temperature, time, and the amount of catalyst used. In the optimized reaction conditions, 78 °C and 10 h in the presence of 25 mol% of the catalyst, 73–94% isolated yields of pyranopyrazolopyridone derivatives were obtained in an ethanolic solution. Key advantages of this synthesis include operational simplicity, relatively short reaction time, use of an environmentally friendly heterogeneous bimetallic catalyst, and easy magnetic separation.
Similar content being viewed by others
Introduction
It is essential to point out that the first step in the complex drug discovery process is the design and synthesis of a molecule that must be tested. Chemists use their expertise to devise a safe, practical, and economical route to afford the large quantities of active pharmaceutical ingredients required for clinical development and the manufacture of an approved drug1. According to the production process of drugs and their sources, drugs are divided into three groups, (1) Synthetic drugs using, (2) Materials of natural origin, (3) Semi-synthetic products2. Therefore, using biomass as a precursor in modern synthetic methods, including multicomponent reactions by heterogeneous or nano-catalysts, while considering biocompatibility issues, can be an important key for modifying processes to produce drugs or bioactive compounds.
4-Hydroxy-2-pyridones (HPs) are natural alkaloid products with a wide range of biological activities (Fig. 1)3. Many of these compounds have been isolated from fungi4 and plants5. In recent decades, with the increase in knowledge about the properties of 2-pyridone alkaloids3their isolation or synthesis6 has been on the agenda of many scientists active in this regard. So, these biomass compounds can be used as raw materials for synthesizing potential bioactive compounds7. In many studies, due to this feature, new reactions have been performed based on two-component8 and multi-component reactions9,10,11,12,13 and new structures obtained, and this trend continues.
Pyranopyrazoles are the fused heterocycles between a pyran and a pyrazole ring that have many agricultural activities14 pharmaceutical, and biological properties such as antimicrobial15 anticancer16 and antiviral properties17. They exist as 4 types of isomers, pyrano[2,3-c]pyrazole, pyrano[3,2-c]pyrazole, pyrano[3,4-c]pyrazole, and pyrano[4,3-c]pyrazole (Fig. 1; Ganta et al., 2021). Several methods for the synthesis of pyranopyrazoles have been presented in the literature, in single or multistep, and two-component19 or multicomponent reactions (three or more compounds)14.
Regarding biocompatibility, heterogeneous catalysts play an important role. These catalysts are easily separated from the reaction mixture and can be reused20. But they are less efficient than homogeneous catalysts21. Nano-catalysts bridge the gap between homogeneous and heterogeneous systems, and like heterogeneous catalysts, they exist in a distinct phase from reactants/products, yet their efficiency rivals homogeneous catalysts22,23. As a result, they have superior advantages over both homogeneous and heterogeneous catalysts. The use one of heterogenous nano-catalysts “magnetic nano-catalyst” is rapidly growing for the development of sustainable and green processes22. Magnetic separation avoids the need for catalyst filtration or centrifugation after completion of the reaction. Also, provides a practical technique for recovering these catalysts21. Magnetic nano-catalysts were used in various organic reactions such as multicomponent reactions, hydrogenation, reduction, oxidation, carbon-carbon coupling, Click chemistry, etc23,24,25,26,27. Core-shell-structured catalysts are a relatively new class of nanomaterials that allow a controlled integration of the functions of complementary materials with optimized compositions and morphologies28. In these systems, apart from the separate functions of core and shell, their synergy can create new features28. Also, by using different types of catalytic group (usually metals) simultaneously in a catalyst (multifunctional catalyst), their performance can be increased29. The synthesis of this type of catalyst has recently attracted the attention of many researchers and has been used in various reactions30,31. Organic chemistry research seeks to address limitations of prior work through innovative methodologies. Considering these cases and based on our previous experiences7,9,10,11,12,32we tried to green synthesize new pyranopyrazolopyridone polycyclic compounds (Scheme 3) in a multicomponent reaction, using a new bimetallic cobalt-cadmium magnetic nano-catalyst (Scheme 2). This research is important from several different aspects, including the fact that the new compounds synthesized from bio-based raw materials, and potentially have biological and medicinal activity. On the other hand, most catalysts are toxic and expensive, difficult to separate from the reaction environment, and wasted, by performing multicomponent reactions using, we have increased efficiency, and reduced time and cost.
Experimental
Materials
All reactions were carried out on an efficient fume hood. All starting materials were purchased from Merck and Sigma-Aldrich and used without further purification.
Instrumentation
Melting points were measured using a Branstead Electrothermal 9200 apparatus and are reported uncorrected1. H and13C NMR spectra were recorded on a Bruker Avance 300 in 300 and 125 MHz, respectively. IR spectra were recorded on a Perkin Elmer RX 1 Fourier transform infrared spectrometer. Elemental analyses were carried out by a Perkin Elmer 2400 series II CHN/O analyzer. To evaluate the textural characteristics of the nano-catalyst, transmission electron microscopy (TEM) was conducted with a Philips EM 208 instrument at an accelerating voltage of 100 kV. The morphological characteristics of the nano-catalyst were investigated by scanning electron microscope (SEM) with a SEM MIRA III model from TESCAN, using an accelerating voltage of 15 kV for the electron beam to image the nanocatalyst surfaces with higher resolution and obtain images with magnifications of 50,000 and 150,000. Also, to identify and measure the elements present in the catalyst structure, energy dispersive X-ray (EDX) analysis was performed using a SEM FEI Quanta 200 device. XRD patterns of the synthesized magnetic nanoparticles (MNPs) were obtained on a Shimadzu XRD-6100 X-ray diffractometer instrument with Cu Kα radiation (λ = 1.542 Å) at 45 kV and 40 mA in the Bragg-Brentano configuration. XRD samples were prepared either by distributing the MNPs powder on a quartz substrate (used MNPs) or by depositing the MNP from the solution (pristine MNPs), allowing for solvent evaporation in vacuo. XRD data were collected in the range 2θ from 10° to 90° with a step of 0.1°. The approximate amount of ligand on the MNPs surfaces was estimated by TGA with a Perkin-Elmer 8000 TGA instrument. TG-DTA was performed by heating the sample to 873 K at a rate of 5 K/min and a flow rate of 50 mL/min of dry air. A vibrating sample magnetometer was used to evaluate the magnetic properties of the nano-catalyst by applying a magnetic field of 15 kOe.
Synthesis of nano-catalyst.
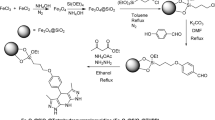
The nano-catalyst was synthesized in multiple steps. In the first, the organic ligand and the magnetic core were synthesized separately. Then from the reaction between the ligand and the synthesized magnetic core, the nano-catalyst was produced. Below are the details of these steps.
Synthesis of ligand TTC: N,N’-(thiobis(thiazole-5,2-diyl))bis(2-chloroacetamide) (TTC) as the ligand was synthesized base on our previous work33 (Scheme 1).
Magnetic core synthesis: First the bare magnetic core Fe3O4 and the core-shell of Fe3O4@SiO2 were produced based on the reported method34. Then 1 g of Fe3O4@SiO2 in 35 mL of dry toluene was placed in an ultrasonic bath for 30 min and 1 mL of N-[3-(trimethoxysilyl)propyl]ethylenediamine (TMPE) precursor added to it and was refluxed under N2 atmosphere for 24 h. The precipitate (Fe3O4@SiO2@PrEDA) was washed with toluene and ethanol and dried at room temperature. In the following, 1 g of Fe3O4@SiO2@PrEDA in 20 mL of dioxane was exposed to ultrasonic irradiation for 30 min. Then, 0.2 g of potassium carbonate and 1.2 g of ligand (TTC) were added to it and refluxed for 24 h. The resulting gray precipitate was washed with dioxane, ethanol, and water, and dried at room temperature. In last, 0.30 g of CdCl2.H2O and 0.36 g of CoCl2.6H2O were added to a solution of 1 g of Fe3O4@SiO2@PrEDA@TTC in 30 mL of dioxane and 5 mL of water and was refluxed for 24 h. The precipitate was filtered and washed with dioxane, ethanol, and water and dried. The precipitate, Fe3O4@SiO2@PrEDA@TTC@Co-Cd, was washed with dioxane, ethanol, and water and dried at room temperature. (Scheme 2)
General procedure of Preparing pyranopyrazolopyridone derivatives using Fe3O4@SiO2@PrEDA@TTC@Co-Cd nano-catalyst
In a test tube containing 1 mL of ethanol, 5 mmol of a 2-pyridone derivative and 5 mmol of phenylpyrazolone were poured, and stirred for 5 min. After the complete mixing of 2-pyridone and pyrazolone, 5 mmol of an aromatic aldehyde and 0.2 g of Fe3O4@SiO2@PrEDA@TTC@Co-Cd nano-catalyst were added to the solution. The mixture was stirred at a temperature of 75 °C for 10 h. With the completion of the reaction, the nano-catalyst was separated from the solution using a simple magnet, and was washed with 5 mL ethanol and 5 mL water, and used for the reusability test. The ethanolic solution obtained from washing the catalyst was added to the reaction solution. Then the solution was poured into 20 mL of cold water. The precipitate was filtered, and the derivatives were purified by recrystallization from DMF/H2O (1 mL/2 mL). (Scheme 3) The structures of the synthesized derivatives (Table 1) were confirmed by1HNMR and13CNMR spectral data (Refer to Supplementary Information).
1HNMR and13CNMR spectral data of 4a as a sample: Pink solid in 90% yield: m.p.: 220–222 °C; IR (KBr): C-N (1108), NO2 (1350, 1510), 1438 (C = C Aromatic), C = N (1638), N-C = O (1596) cm−11. HNMR (300 MHz, DMSO-d6) δ: 1.98 (s, 3 H, CH3-Pyrazolone), 2.27 (s, 3 H, CH3-Pyridone), 4.72 (wide peak, 2 H, CH2-Benzyle), 5.29 (s, 1 H, CH), 5.82 (s, 1 H, CH-Pyridone), 7.01–7.16 (m, 5 H, ArH), 7.30–7.45 (m, 3 H, ArH), 7.49–7.52 (d, J = 8.4 Hz, 2 H, Ar-H), 7.69–7.73 (dd, J = 2.9 Hz، 5.8 Hz, 2 H, Ar-H), 8.14–8.17 (d, J = 8.4 Hz, 2 H, Ar-H) ppm13. C NMR (75 MHz, DMSO-d6) δ: 12.4 (CH3−Pyrazolone), 19.8 (CH3-Pyridone), 38.7 (CH-bridge), 46.8 (CH2-Benzyl), 99.3 (CH-Pyridone), 102.4 (C-Pyridone), 120.6 (C-Pyrazolone), 123.3 (CH-Ar), 124.3 (CH-Ar), 126 (CH-Ar), 127.8 (CH-Ar), 128.6 (CH-Ar), 128.7 (CH-Ar), 128.9 (CH-Ar), 130.6 (CH-Ar), 137.1 (C-Ar), 139 (C-Ar), 145.7 (C-Ar), 145.8 (C-Pyrazolone), 146.3 (C-Ar), 147.4 (C-Pyridone), 150.3 (C-O Pyran), 151.8.1 (C-O Pyran), 156 (C = O of Pyridone) ppm.
Results and discussion
In this research, magnetic nanoparticles were prepared by co-precipitation method35 from iron II and iron III salts. In the next step, the surface of the magnetic nanoparticles is covered with a silica shell, which prevents the oxidation of the magnetic core. Also, the number of surface hydroxyl groups increases, which facilitates the connection of organic groups in the next step. Then, organic-inorganic precursor and TTC ligand were connected onto the magnetic core-shell structure in order. Finally, in the last step, metal-coordination sites of the TTC ligand (oxygen, nitrogen and sulfur atoms), enabling ligand to coordinate with cobalt and cadmium metals. (Scheme 2)
Identification and determination of nano-catalyst structure
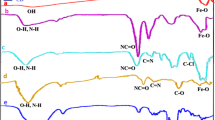
FT-IR spectra of nano-catalyst layers and ligand precursor are shown in Fig. 2 for comparison. In the IR spectrum of Fe3O4 MNPs, the absorption frequency corresponding to Fe-O is observed in 572 cm−1, and stretching and bending vibrations related to O-H have appeared in 3396 cm−1 and 1621 cm−1, respectively (Fig. 2a)33. In the spectrum of the Fe3O4@SiO2 core-shell MNPs, in addition to the aforementioned bands, the asymmetric Si-O-Si stretching vibration is observed in the region of 1098 cm−1 (Fig. 2b)33. The Fe3O4@SiO2@PrEDA composition shows N-H stretching is placed below the OH stretching vibration band (3421 cm−1). In addition, the absorption frequency appearing at 1584 cm−1 can be related to C-N stretching (Fig. 2c)29. Also, the presence of absorption peaks appearing at 2935 and 2883 cm−1 are related to asymmetric and symmetric stretching vibrations of the aliphatic C-H bonds of anchored alkyl groups, respectively33. The final form of the catalyst without of cation (Fe3O4@SiO2@PrEDA@TTC; Fig. 2e), in addition to having the mentioned peaks in the IR spectrum of Fe3O4@SiO2@PrEDA (Fig. 2c), has C = O absorption bands (1692 cm−1) related to the TTC ligand and iminic of thiazole ring at 1556 cm−1 (Fig. 2d) comparable with amidic C = O frequency 1692 cm−1 and 1553 cm−1 in the TTC ligand (Fig. 2e)34. The spectrum of the final catalyst (Fig. 2f) shows similar absorption frequencies to the spectrum of Fe3O4@SiO2@PrEDA@TTC at the absorption frequencies corresponding to C = N, C = O, and OH (with a slight shift in frequencies). Therefore, no structural degradation occurred in the final catalyst.
The ¹HNMR spectrum of TTC ligand (Fig. 3) has shown three single signs with integrals of 4, 2, and 2 correlated with CH2-CO (4.37 ppm), CH = N (7.74 ppm) and NH (12.74 ppm), respectively. In the13C NMR spectrum of ligand (refer to Sporting Information) has seen five signals in 42.1, 122.3, 143.3, 160.3, and 165.4 ppm concern CH2, S-C-S, C = N, =C-N, respectively.
Energy-dispersive X-ray spectroscopy (EDX) is used to identify catalyst as shown in Fig. 4 This analysis clearly proves that the desired elements C, N, O, Co, Cd, S, Si, and Fe are present in the synthesized nano-catalyst and the synthesis process was completely successful.
X-ray diffraction spectroscopy (XRD)
When the X-ray impacts crystals in which atoms are arranged in a specific order, it results in the diffraction phenomenon, and it is scattered when impacting amorphous materials. For a pure substance, the X-ray diffraction pattern is like a fingerprint for that substance36. These patterns are collected as a database for inorganic and organic compounds. So, using this database and with the help of searching and matching, the composition of unknown crystal materials can be determined. The relative intensity and position of the lines are identification factors37. The X-ray diffraction pattern of the Fe3O4@SiO2@PrEDA@TTC@Co-Cd magnetic nano-catalyst is shown in Fig. 5. The results obtained are consistent with the crystalline values reported for Fe3O4 (2θ: 30.28°, 35.58°, 43.17°, 57.07°, 62.75°, 70.96°, 76.45°)38.
This means that the cubic crystal structure of Fe3O4 remained constant throughout the Fe3O4@SiO2@PrEDA@TTC@Co-Cd magnetic nano-catalyst synthesis process.
Scanning electron microscopy (SEM)
The electrons interact with the atoms of the sample producing different signals that contain information about the surface topography and the composition of the sample39 Field Emission SEM is mostly used when the characteristics of a specific sample are due to its higher resolution, it does not provide a clear or good morphology39 Therefore, SEM images of the Fe3O4@SiO2@PrEDA@TTC@Co-Cd magnetic nano-catalyst were taken and shown in Fig. 6. The SEM images reveal a nearly uniform distribution of spherical particles with an average size of about 60 nm (evaluated using ImagJ software). Moreover, the images indicate the existence of particle agglomerations, which can be ascribed to the magnetic interactions among the particles.
Transmission electron microscopy (TEM)
Evidence from the TEM images of the Fe3O4@SiO2@PrEDA@TTC@Co-Cd magnetic nano-catalyst (Fig. 7) show that the nanoparticles are spherical and have a core-shell shape and the average particles size were about 20 to 30 nanometers (analyzed by ImagJ software).
Vibrating sample magnetometer (VSM)
Creating a change in the magnetic field of a particle causes the formation of an induced electric field, and by measuring this new electric field, the change in its magnetic field can be detected40. When the external magnetic field is removed for the superparamagnetic particles, they do not show demagnetization and magnetization completely40 The magnetic property of the Fe3O4@SiO2@PrEDA@TTC@Co-Cd magnetic nano-catalyst was measured using a vibrating magnetometer (Fig. 8). The saturation magnetization value for the Fe3O4@SiO2@PrEDA@TTC@Co-Cd magnetic nano-catalyst is approximately equal to 7.12 emu/g. This value is less compared to the Fe3O4 bare core (54 emu/g) because of the presence of different layers on the magnetic nano-catalyst41 However, the magnetic property has been preserved to an acceptable extent and the magnetic nano-catalyst can be easily separated from the reaction medium with a simple magnet.
Thermal gravimetric analysis (TGA)
This analysis provides information about physical phenomena, such as phase transition, and physical adsorption, as well as chemical phenomena, including chemical adsorption, thermal decomposition, and gas-solid reactions (such as oxidation or reduction)42. The thermal stability of the Fe3O4@SiO2@PrEDA@TTC@Co-Cd magnetic nano-catalyst using TGA analysis has been evaluated (Fig. 9) The results from the TGA curve show four weight losses. The weight loss at low temperatures (below 100 ℃) is due to the hydroxy groups and the solvent molecules that were previously adsorbed on the surface43 The weight loss (between about of 100–270 ℃) is due to the loss of physically adsorbed water, solvent and surface hydroxyl groups44 and above 270 ℃ up to 410 ℃ (27% weight loss) is due to the decomposition of organic group24 The reduction in final weight can also be due to the oxidation of metals.
Synthesis of pyrazolo-pyrano-pyridones using Fe3O4@SiO2@PrEDA@TTC@Co-Cd magnetic nano-catalyst
Considering the importance of pyranopyrazolopyridone compounds in pharmaceutical and biological fields, we tried to prepare these compounds by using efficient method. For this aim, a blend of phenylpyrazolone, p-nitrobenzaldehyde and 1-benzyl-4-hydroxy-6-methyl-2-pyridone was stirred in ethanol solution with 50 mol% of tin chloride catalyst (base on our previous work13 and under reflux conditions for 6 h, as a result 4a was formed with 72% isolated yield (Table 1, Entry 1). According to the result of the reaction and the fact that the reaction in the presence of Lewis acid gave a good yield, some other Lewis acids such as cadmium chloride, nitrate and cobalt nitrate were used to perform the reaction (Table 1, Entries 2–4). As a result of which cadmium chloride and cobalt nitrate obtained better results. Because these two metal salts are considered environmental pollutants, to reduce the pollution of cadmium and cobalt in catalyzing this reaction, it was decided to make a heterogeneous magnetic nano-catalyst from them and then optimization of the reaction condition in the presence of different amount of the magnetic nano-catalyst were performed (Table 1, Entries 6–8). The best value was obtained for 25% w/w of pyridine (Table 1, Entry 7). The reaction was also studied in the absence of the catalyst to clearly determine the effect of the catalyst on the reaction (Table Entry 5).
Also, the temperature and reaction time conditions in the presence of the magnetic nano-catalyst were optimized (Table 1, Entries 9–13). The hot filtration test was also performed. For this purpose, after one hour of the reaction, the catalyst was separated, and it was observed that after the catalyst separation, there was no significant increase in yield of 4a, at the end of the reaction. After obtaining the best reaction conditions, ethanol as solvent, 0.2 gr (for 5 mmol of other substrates) of Fe3O4@SiO2@PrEDA@TTC@Co-Cd magnetic nano-catalyst, 75 °C and 10 h, the synthesis of other derivatives was carried out (Table 2).
The structure and yield of the synthesized pyranopyrazolopyridone derivatives using Fe3O4@SiO2@PrEDA@TTC@Co-Cd magnetic nano-catalyst, were shown in Table 2.
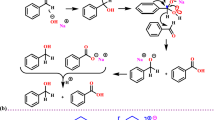
As shown in Table 2, NH-containing pyridones exhibited lower reactivity, likely due to tautomerism of enol-keto-2-pyridone (Scheme 4). Pyridones with an R group on nitrogen have a lower tendency to form an aromatic ring, so carbon number 3 is a stronger nucleophile and can attack the aldehyde directly. In fact, NH-containing pyridones have a tendency to form an aromatic ring7. Also, NH derivative pyridine (4 h), the ring is not closed, but in pyridone derivatives with ethyl and benzyl groups, a pyran ring is formed. Aromatic aldehydes with electron-withdrawing groups, have better reactivity (4a).
Notably, NH-containing pyridones reacted poorly with electron-deficient benzaldehydes (e.g., p-Cl, p-OMe), yielding only trace of the corresponding 4,4’-(arylmethylene)bis(1 H-pyrazol-5-ols)45,46 products. A possible mechanistic route based on some reports in the literature47,48 is shown in scheme 5. The formation of these products suggests that pyrazolone probably reacts with the aldehyde first to produce the Knoevenagel intermediate, then pyridone derivatives (depending on their nucleophilicity) or pyrazolone react with the Knoevenagel product via a Michael addition reaction (Scheme 5). Finally, after performing dehydration and cyclization reactions, the desired pyranopyrazolopyridone will be obtained (Scheme 5).
Reusability of the Fe3O4@SiO2@PrEDA@TTC@Co-Cd magnetic nano-catalyst
Among the very important features of the magnetic nano-catalyst is easy separation and reuse potential. During the synthesis of pyranopyrazolopyridone derivatives, after cooling the reaction container, the nanoparticles were separated from the reaction medium by a simple magnet and washed several times with ethanol and then placed in an oven at 60 °C for 5 h. The desired catalyst can be recovered up to 5 times and its performance will not change much (Fig. 10). TEM and SEM analysis of catalyst used was performed after the reusability test (Fig. 11a and b, respectively). These images indicated that the structure of catalyst was retained but minor aggregation of the nanoparticles of the catalyst compared with the fresh one was observed. Moreover, the leaching rate of cadmium and cobalt active sites from the Fe3O4@SiO2@PrEDA@TTC@Co-Cd magnetic nano-catalyst surface was proved by ICP analysis according to Table 3. Hot filtration at the reaction temperature (75 °C) was performed to separate the Fe3O4@SiO2@PrEDA@TTC@Co-Cd magnetic nano-catalyst from the reaction medium using a simple magnet. The reaction mixture before and after separation of the nano-catalyst was analyzed by ICP to investigates the leaching of cadmium and cobalt active sites, and the results are presented in Table 3. No significant reaction progress was observed after nano-catalyst separation, confirming the results of ICP analysis and the absence of leached cadmium and cobalt active sites. In other words, we have taken measures to immobilize the metal ions using a highly stable polydentate ligand system incorporating N, O, and S donor atoms. This strong chelation significantly minimizes the possibility of metal leaching. ICP analysis of the reaction filtrate after each catalytic cycle and hot filtration test confirmed negligible leaching (below detection limits), and the catalyst was successfully recycled five times without significant activity loss, suggesting stable retention of Cd and Co within the catalyst matrix.
Conclusion
In this research we have synthesized a novel magnetic and bimetallic catalytic system, nominated as Fe3O4@SiO2@PrEDA@TTC@Co-Cd. The properties of the synthesized magnetic nano-catalyst were verified through several instrumental analyses such as FT-IR, XRD, SEM, EDX, TEM, TGA, and ICP-OES. We produced pyranopyrazolopyridone derivatives (70–94% yield at 78 °C) under optimized reaction conditions, employing the magnetic nano-catalyst Fe3O4@SiO2@PrEDA@TTC@Co-Cd with ethanol as a green solvent. This high efficiency can be attributed to the characteristics of the catalyst such as the bimetallic nature of that, the lack of leaching of the Co-Cd metallic active centers due to the use of a ligand with very strong chelators, and the significant solubility of it and other reactants in ethanol solvent. Furthermore, the magnetic properties of the Fe3O4@SiO2@PrEDA@TTC@Co-Cd nano-catalyst remove the requirement for centrifugation or filtration of the reaction mixture, allowing for the separation of the nano-catalyst from the reaction medium with the use of a simple magnet. In conclusion, the magnetic nano-catalyst demonstrated reusability for at least five cycles with minimal loss of activity.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Rotella, D. P. The critical role of organic chemistry in drug discovery. ACS Chem. Neurosci. 7, 1315–1316 (2016).
Alamgir, A. N. M. & Drugs Their natural, synthetic, and biosynthetic sources. in 105–123 (2017). https://doi.org/10.1007/978-3-319-63862-1_4
Jessen, H. J. & Gademann, K. 4-Hydroxy-2-pyridone alkaloids: structures and synthetic approaches. Nat. Prod. Rep. 27, 1168 (2010).
Breinholt, J. et al. A novel, antifungal N -Methyl-4-hydroxy-2-pyridone from Fusarium oxysporum. J. Nat. Prod. 60, 33–35 (1997). Oxysporidinone.
Wachira, S. et al. Toxicity of six plant extracts and two pyridone alkaloids from ricinus communis against the malaria vector Anopheles Gambiae. Parasit. Vectors. 7, 312 (2014).
Torres, M., Gil, S. & Parra, M. New synthetic methods to 2-Pyridone rings. Curr. Org. Chem. 9, 1757–1779 (2005).
Kamali, M. & Samadikhah, M. Design-Expert a Powerful Tool for Optimizing Organic Synthesis: A Green and Facile Multicomponent Synthesis of New 2‐Pyridone‐pyrimidine Derivatives by p‐Toluene Sulfonic Acid in Water. ChemistrySelect 8, (2023).
Katsina, T., Anagnostaki, E. E., Mitsa, F., Sarli, V. & Zografos, A. L. Palladium-catalyzed direct alkenylation of 4-hydroxy-2-pyridones. RSC Adv. 6, 6978–6982 (2016).
Kamali, M. & Shahi, S. & Mashhadi Akbar Bujar, M. Temperature-Dependent Green Synthesis of New Series of Mannich Bases from 4‐Hydroxy‐pyridine‐2‐one and Their Antioxidant Activity Evaluation. ChemistrySelect 5, 1709–1712 (2020).
Kamali, M. & Shahi, S. Catalytic switching in the Multi-component synthesis of novel thioethers based on 4-Hydroxy-2-pyridones. Org. Prep Proced. Int. 54, 167–177 (2022).
Kamali, M. & Ebrahimi, A. One-pot multicomponent green synthesis of novel series of 4-hydroxy-2-pyridone-fused spiropyrans catalyzed by acetic acid. Synth. Commun. 53, 1439–1450 (2023).
Kamali, M. & Mohammadzadeh, S. One-pot Biginelli synthesis of novel series of 2-oxo/thioxo/imino-1,2,3,4-tetrahydropyrimidine based on 4-hydroxy-2-pyridone. Chem. Pap. 77, 4943–4952 (2023).
Kamali, M. & Keramat Pirolghor, F. One-pot three‐component synthesis of novel chromeno[3,2‐c ]pyridine‐1,9(2H)‐diones by using SnCl2⋅2H2O as catalyst. J. Heterocycl. Chem. 59, 655–663 (2022).
Mamaghani, M. Hossein nia, R. A review on the recent multicomponent synthesis of Pyranopyrazoles. Polycycl. Aromat. Compd. 41, 223–291 (2021).
Ahmad, A., Rao, S. & Shetty, N. S. Green multicomponent synthesis of pyrano[2,3- c ]pyrazole derivatives: current insights and future directions. RSC Adv. 13, 28798–28833 (2023).
Musa, A. et al. The anticancer and EGFR-TK/CDK-9 dual inhibitory potentials of new synthetic Pyranopyrazole and pyrazolone derivatives: X-ray crystallography, in vitro, and in Silico mechanistic investigations. J. Biomol. Struct. Dyn. 41, 12411–12425 (2023).
Malebari, A. M. et al. Exploring the dual effect of novel 1,4-diarylpyranopyrazoles as antiviral and anti-inflammatory for the management of SARS-CoV-2 and associated inflammatory symptoms. Bioorg. Chem. 130, 106255 (2023).
Ganta, R. K., Kerru, N., Maddila, S. & Jonnalagadda, S. B. Advances in Pyranopyrazole scaffolds’ syntheses using sustainable Catalysts—A review. Molecules 26, 3270 (2021).
Farooq, S. & Ngaini, Z. Recent Synthesis of Mono- & Bis‐Pyranopyrazole Derivatives. ChemistrySelect 9, (2024).
Mukhtar, A. et al. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew. Sustain. Energy Rev. 157, 112012 (2022).
Chen, T., Peng, Y., Qiu, M., Yi, C. & Xu, Z. Heterogenization of homogeneous catalysts in polymer nanoparticles: from easier recovery and reuse to more efficient catalysis. Coord. Chem. Rev. 489, 215195 (2023).
Chen, M. N., Mo, L. P., Cui, Z. S. & Zhang, Z. H. Magnetic nanocatalysts: synthesis and application in multicomponent reactions. Curr. Opin. Green. Sustain. Chem. 15, 27–37 (2019).
Rai, P. & Gupta, D. Magnetic nanoparticles as green catalysts in organic synthesis-a review. Synth. Commun. 51, 3059–3083 (2021).
Mobaraki, A., Hajibeygi, M., Moradi, H., Pirasteh, M. & Takallou, A. Design of an efficient magnetic brush solid acid and its catalytic use in organic reactions. Sci. Rep. 15, 2828 (2025).
Poursattar Marjani, A., Asadzadeh, F. & Danandeh Asl, A. Fe3O4@Glycerol-Cu as a novel heterogeneous magnetic nanocatalyst for the green synthesis of 2-amino-4H-chromenes. Sci. Rep. 12, 22173 (2022).
Payamifar, S. et al. Magnetic nickel nanoparticle catalyst on β-cyclodextrin-modified Fe3O4 for nitroarene hydrogenation. Sci. Rep. 14, 28493 (2024).
Asadzadeh, F. & Poursattar Marjani, A. Revolutionizing acridine synthesis: novel core-shell magnetic nanoparticles and Co-Zn zeolitic imidazolate framework with 1-aza-18-crown-6-ether-Ni catalysts. Sci. Rep. 14, 25739 (2024).
Das, S. et al. Core–shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem. Soc. Rev. 49, 2937–3004 (2020).
Liao, M. et al. Quinary metal oxide NiCoMnCeCaOx Nanorod as a multifunctional catalyst towards hydrogen production from ethanol steam reforming: synergistic effect of polymetallic component. Chem. Eng. J. 497, 154646 (2024).
Bikas, S., Poursattar Marjani, A., Bibak, S. & Sarreshtehdar Aslaheh, H. Synthesis of new magnetic nanocatalyst Fe3O4@CPTMO-phenylalanine-Ni and its catalytic effect in the Preparation of substituted pyrazoles. Sci. Rep. 13, 2564 (2023).
Huang, Y. B., Liang, J., Wang, X. S. & Cao, R. Multifunctional metal–organic framework catalysts: synergistic catalysis and tandem reactions. Chem. Soc. Rev. 46, 126–157 (2017).
Mobaraki, A., Sakhaee, N., Takallou, A. & Habibi, A. Triple Action of an Attractive Deep Eutectic Solvent in the Synthesis of Aryl Nitriles and Substituted Triazoles Using a Magnetically Reusable Fe3O4@SiO2@PrNCu Catalyst. ChemistrySelect 8, (2023).
Shockravi, A., Kamali, M., Halimehjani, A. Z. & Jafari, R. Synthesis and dynamic NMR studies of some new symmetrical Podands of dithiocarbamates formed from Bis(N-thiazol)chloroacetamides. J. Heterocycl. Chem. 50, 209–215 (2013).
Mobaraki, A., Movassagh, B. & Karimi, B. Hydrophobicity-enhanced magnetic solid sulfonic acid: A simple approach to improve the mass transfer of reaction partners on the surface of the heterogeneous catalyst in water-generating reactions. Appl. Catal. Gen. 472, 123–133 (2014).
Gomes, J. F. P., Puna, J. F. B., Gonçalves, L. M. & Bordado, J. C. M. Study on the use of MgAl hydrotalcites as solid heterogeneous catalysts for biodiesel production. Energy 36, 6770–6778 (2011).
Roos, Y. H. Methodology. in Phase Transitions in Foods 49–71 (Elsevier, doi:https://doi.org/10.1016/B978-012595340-5/50003-1. (1995).
Isobe, Y., Kamimura, A., Aoki, K. & Nakayasu, F. Prediction of fatigue damage in aluminum alloys by X-ray diffraction. in Non-destructive Testing ’92 562–566Elsevier, (1992). https://doi.org/10.1016/B978-0-444-89791-6.50120-7
Atta, A., Al-Lohedan, H. & Al-Hussain, S. Synthesis of stabilized Myrrh-Capped hydrocolloidal magnetite nanoparticles. Molecules 19, 11263–11278 (2014).
Sayid, S. A., Dadan-Garba, A., Enenche, D. E. & Ikyo, B. A. Scanning electron microscopy (SEM) of the bug eye and sand coral. Microsc Res. 08, 1–7 (2020).
Lopez-Dominguez, V. et al. A simple vibrating sample magnetometer for macroscopic samples. Rev Sci. Instrum 89, (2018).
Mohammadi, A., Barikani, M. & Barmar, M. Effect of surface modification of Fe3O4 nanoparticles on thermal and mechanical properties of magnetic polyurethane elastomer nanocomposites. J. Mater. Sci. 48, 7493–7502 (2013).
Coats, A. W. & Redfern, J. P. Thermogravimetric analysis. A review. Analyst 88, 906 (1963).
Karimzadeh, I., Aghazadeh, M., Ganjali, M. R., Doroudi, T. & Kolivand, P. H. Preparation and characterization of iron oxide (Fe3O4) nanoparticles coated with polyvinylpyrrolidone/polyethylenimine through a facile one-pot deposition route. J. Magn. Magn. Mater. 433, 148–154 (2017).
Bodaghifard, M. A. Organic base grafted on magnetic nanoparticles as a recoverable catalyst for the green synthesis of hydropyridine rings. J. Iran. Chem. Soc. 17, 483–492 (2020).
Zhou, Z. & Zhang, Y. An Eco-Friendly one-pot synthesis of 4,4’-(arylmethylene)Bis(1H-pyrazol-5-ols) using [Et3NH][HSO4] as a recyclable catalyst. J. Chil. Chem. Soc. 60, 2992–2996 (2015).
Cadena-Cruz, J. E. et al. Synthesis of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) and evaluation of their antioxidant and anticancer activities. BMC Chem. 15, 38 (2021).
Xu, Y. et al. A multi-functional composite nanocatalyst for the synthesis of biologically active pyrazolopyranopyrimidines: multifaceted antimicrobial, antioxidant, and anticancer activities. Adv. Compos. Hybrid. Mater. 8, 22 (2025).
Barakat, A. et al. Synthesis, antimicrobial activity, pharmacophore modeling and molecular Docking studies of new pyrazole-dimedone hybrid architectures. Chem. Cent. J. 12, 29 (2018).
Acknowledgements
The authors would like to thank Kharazmi University (Iran) for its financial support for this research.
Author information
Authors and Affiliations
Contributions
Mahmood Kamali: Conceptualization, Supervision, Writing, Fundingacquisition. Akbar Mobarki: Conceptualization, advisor, reviewZahra Shirpak: Investigation, Data curation, Formal analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kamali, M., Mobaraki, A. & Shirpak, Z. Multicomponent synthesis of pyrano-pyrazolo-pyridones by a bimetallic cobalt-cadmium magnetic catalyst. Sci Rep 15, 29462 (2025). https://doi.org/10.1038/s41598-025-15535-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15535-2