Abstract
Light-related visual disturbances are common symptoms among patients with dry eye disease (DED) but difficult to be evaluated in the clinical practice. This cross-sectional, multicenter, controlled study aimed at comparing light discomfort thresholds in healthy individuals and patients with DED, and to further correlate thresholds with ocular surface parameters. The Lumiz 100 device (Essilor International, Paris, France) was employed to measure light discomfort thresholds in 25 patients with DED and 25 sex- and age-matched healthy subjects under different lighting conditions: continuous warm, continuous cold and flashing warm. Participants were instructed to press a button twice to signal discomfort levels as “just perceptible” and “really disturbing”. The mean of the two values was calculated for each lighting condition. DED patients exhibited lower discomfort thresholds for all measurements compared to controls (continuous warm: 2.81 ± 0.56 log10[lux] vs. 3.47 ± 0.51 log10[lux], continuous cold: 2.78 ± 0.54 log10[lux] vs. 3.48 ± 0.50 log10[lux], flashing warm: 2.54 ± 0.53 log10[lux] vs. 3.12 ± 0.69 log10[lux], all p ≤ 0.004). A significant negative correlation was found between total light sensitivity threshold and ocular discomfort symptoms in patients with DED (r=-0.499, p = 0.011). Conversely, no significant correlation was found between light thresholds and the other ocular surface parameters. These findings show that DED patients have a higher light sensitivity than healthy subjects.
Similar content being viewed by others
Introduction
Dry eye disease (DED) is a multifactorial disorder of the ocular surface that affects millions of people globally and results in different degrees of ocular discomfort and visual disturbance. It is characterized by an imbalance in the tear film’s stability and hyperosmolarity, and is generally classified into evaporative, aqueous deficient and mixed forms1. The diagnosis and follow-up of DED can be carried out using a variety of validated methods designed to gather information about patient’s symptoms and clinical signs2. Among these, the Ocular Surface Disease Index (OSDI) questionnaire is a widely utilized tool for evaluating ocular discomfort. It is considered a reliable measure for determining the severity of DED and is favored for its efficiency in assessing the disease’s impact on patients’ quality of life3. Recently, non-invasive all-in-one devices, such as the Keratograph 5 M (Oculus Optikgeräte GmbH, Wetzlar, Germany), have been introduced in the clinical practice to assess a variety of ocular surface parameters. The repeatability and reliability of this device have been already reported4.
Beyond commonly reported symptoms such as dryness, burning, foreign body sensation, patients with DED may complain light-related visual disturbances including photophobia and glare5,6. The former does not have a universally accepted definition, and it is generally considered as an enhanced sensitivity to normal lighting conditions7,8. The latter is considered as a visual disturbance caused by stray light entering the eye that does not contribute to vision7.
The reliable quantification and precise treatment of light-related symptoms remain challenging5,8. Although various strategies including questionnaires9, scales10, and other tools11, have been employed in recent years to evaluate light sensitivity, these approaches are predominantly qualitative and rely heavily on subjective reporting. Importantly, there is currently no standardized, objective, and clinically validated tool available for quantifying photophobia in DED patients, and the relationship between light sensitivity and ocular surface parameters remains poorly understood. Lumiz 100 (Essilor International, Paris, France) is an innovative device designed to assess light discomfort thresholds through a rapid, consistent, and safe evaluation. Its feasibility and accuracy in detecting light sensitivity thresholds on healthy individuals have been recently demonstrated, offering insights into how these thresholds can be influenced by different physiological factors12,13.
The aim of the present study is to provide standardized, quantitative data on light sensitivity by comparing light discomfort thresholds under different lighting conditions between patients with DED and healthy subjects, and to further correlate them with ocular surface parameters.
Methods
In this cross-sectional, controlled study, participants were consecutively screened and recruited at two University Eye Clinics between February and March 2025. Patients with DED were compared with age- and sex-matched healthy subjects attending the general office for routine eye checkups. Exclusion criteria for both patients and controls included history of relevant ocular diseases, presence of active ocular inflammation, recent ocular surgery (within 3 months), contact lens wearing, migraine and epilepsy.
Ethical approval for the study was obtained from the Ethics Committee “Comitato Etico Territoriale Lazio Area 2” (protocol number 316.24, approval date 13-02-2025). Informed consent was obtained from all participants, and the study was conducted in accordance with the tenets of the Declaration of Helsinki.
Data acquisition
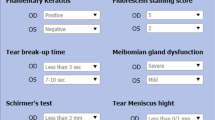
Diagnosis of DED was obtained using the following Tear Film & Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) II criteria: positive symptom score (OSDI score ≥ 13) and non-invasive break-up time (NIBUT) < 10 s (s)2. Briefly, the OSDI questionnaire is a 12-item questionnaire composed of 3 parts related to vision function, ocular symptoms, and environmental triggers. Patients rate the frequency of their symptoms from 0 to 4 with 0 indicating “none of the time” and 4 corresponding to “all of the time”. Each response is given score from 0 to 4. The sum of all values will give the OSDI score that ranges from 0 to 100. A score of 13 or higher is indicative of DED. The severity was further calculated as follows: mild = 13–22, moderate = 23–32, severe > 323. After the eligibility screening, participants fulfilling study criteria were analyzed for the collection of the following data: age, sex, history sunglasses use, self-reported light sensitivity, best-corrected visual acuity (BCVA), iris color analysis (dark vs. light) and objective light discomfort thresholds measured by means of Lumiz 100 device (Essilor International, Paris, France). Briefly, a validated questionnaire was used to rate the self-perception of light sensitivity by asking participants to reply to the question: “Do you feel sensitive to light?” with one of the following options: (1) Yes, a lot; (2) Yes, a bit; (3) No, not really; (4) No, not at all13. BCVA was converted to logMAR for analysis, and the mean BCVA, considered as the average of the values of both eyes, was calculated. Light discomfort thresholds were measured using the Lumiz 100, a portable device that provides uniform diffuse illumination across the user’s visual field. Since photosensitivity has a logarithmic relationship with light intensity, the lux thresholds were log-transformed, and all statistical analyses were conducted using the log10 (lux) thresholds. A tablet application controls light intensity ranging from 10 lx (log10[lux] = 1) to 10,211 lx (log10[lux] = 4.01) at eye level. If a threshold was not reached at 10,211 lx, the subsequent level (12,253 lx) was arbitrarily assigned. Thresholds were determined for two levels of discomfort under three lighting conditions simulating different everyday environments: two with continuous light increases and one with discontinuous increases. For continuous increase, light starts at 25 lx for 5 s and increases every second using a 20% increase step, using either warm light (4000 K color temperature) mimicking natural light or cold light (6500 K color temperature) mimicking artificial light. For discontinuous increases, light starts at 10 lx for 5 s followed by increases to 25 lx for half a second and then decreases back to 10 lx for 2 s, before a 44% increase from the previous flash using warm light (4000 K). This flashing increase does not provide enough time for the visual system to adapt and has been chosen to reflect the most bothering situations in everyday life. Participants were instructed to press a button twice to signal discomfort levels. The first level, called the “just perceptible” discomfort threshold, occurs when the discomfort is initially noticed, and participants feel mild symptoms such as “tension in the eyelids or tingling.” The second level, the “really disturbing” discomfort threshold, is reported when the discomfort becomes bothersome requiring effort to keep their eyes open. The mean of these two levels represented the mean threshold for each of the three lighting conditions. Total light sensitivity threshold was calculated as the mean of six logarithmic illumination thresholds. The protocol and measurement reliability have been detailed in previous studies12,13.
Patients underwent non-invasive ocular surface work-up by means of Keratograph 5 M (Oculus, Wetzlar, Germany). All the measurements were taken between 9:00 a.m. and 12:00 p.m. in a dimly lit room with controlled temperature (21–24 °C) and humidity (30–60%). The following parameters were measured: NIBUT-first, NIBUT-average, tear meniscus height (TMH), meibomian gland loss (MGL), bulbar redness. For each parameter, the mean of the values obtained from both eyes was calculated and used for analysis. The NIBUT was measured as the time in s between the last complete blink to the start of interruption in the placid rings reflected on the surface of the cornea, which is automatically recognized by the tool. The instrument generated two measures: the time until the tear film’s initial disruption (NIBUT-first) and the average duration of all instances of rupture (NIBUT-average). Values < 10 s are considered pathological by the instrument for both parameters14. Photographs of the lower TMH were acquired and evaluated for each participant. The measurements were obtained by placing a ruler perpendicular to the edge of the lid at the central position with respect to the center of the pupil. The cut-off provided by the instrument (0.20 mm) was used to distinguish between normal (≥) or pathological values (<)14,15. Infrared meibography of upper and lower eyelids was performed to assess the extent of MGL. The process included utilizing a grading system known as meiboscore, which classifies deficiencies on a scale ranging from 0 to 3: grade 0 means no gland loss, grade 1 indicates up to 33% gland loss, grade 2 represents 33–66% gland loss, and grade 3 suggests 67% or more gland loss15. Measurements of bulbar redness were automatically acquired, and the R-Scan module automatically categorizes redness levels in the bulbar and limbal areas, identifies blood vessels in the conjunctiva, and scores redness levels using a grading system15.
Statistical analysis
Statistical analysis was conducted using SPSS for Macintosh software (version 30.0.0.0, SPSS, Inc.) Means ± standard deviations (SDs) were calculated for numerical continuous variables, while percent distributions were presented for categorical data. The distribution of variables was evaluated using the Shapiro-Wilk test. Fisher’s exact test and Chi-squared test were employed to compare categorical variables. Non-parametric (Mann–Whitney U test and Kruskal–Wallis test) tests were used to compare variables among groups. The relationships between the total light sensitivity and other numerical data were evaluated using Spearman’s correlation test. A post hoc power analysis was conducted by using G*Power software (version 3.1.9.6) to assess the statistical power of the study. Based on the difference in the primary outcome (total light sensitivity threshold 3.35 ± 0.53 log10[lux] and 2.71 ± 0.52 log10[lux]) respectively between the two groups (n = 25), the effect size (Cohen’s d) was calculated as approximately 1.22. Using a two-sided test with an alpha level of 0.05, the calculated power was approximately 0.99 (99%), indicating a high likelihood of detecting a true difference between the groups. This indicates that our sample size was adequate to detect the observed difference. A p-value of less than 0.05 was considered statistically significant.
Results
Demographic data
Overall, 100 eyes of 50 Caucasians patients (22 men, 28 women; mean age 55.68 ± 16.46 years) were included in the study analysis. Of these, 25 patients were affected by DED (group 1) and 25 were matched healthy subjects (group 2). There were no statistically significant differences between the two groups in terms of age (56.44 ± 17.69 vs. 54.92 ± 5.45 years; p = 0.48, Mann–Whitney U test), gender [8 males (32%) and 17 females (68%) vs. 14 males (56%) and 11 females (44%); p = 0.15, Fisher’s exact test], iris colors [20 (80%) patients had dark irises and 5 (20%) had light irises in both groups; p = 1, Fisher’s exact test], sunglasses users [22 (88%) participants were sunglasses users and 3 (12%) reported to not use sunglasses vs. 15 (60%) participants were sunglasses users and 10 (40%) reported to not use sunglasses (p = 0.051, Fisher’s exact test)]. In group 1, 16 (64%) patients rated their self-perception of light sensitivity as 1, 8 (32%) as 2, 1 (4%) as 3; in group 2, 5 (20%) patients rated their self-perception of light sensitivity as 1, 4 (16%) as 2, 6 (24%) as 3 and 10 (40%) as 4 (p < 0.001, Chi-squared test). Mean values for each ocular parameter investigated in DED and healthy patients are shown in Table 1.
Light discomfort thresholds between groups
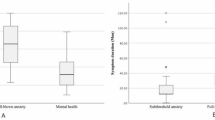
Patients belonging to group 1 exhibited lower discomfort thresholds for all measurements compared to those of group 2, as presented in Table 2. Figure 1 shows the different resistance to continuous warm, continuous cold and flashing warm lights reported by a representative healthy subject (A-C) and by a representative patient with DED (D-F).
Clinical test comparisons
Differences in the total light sensitivity threshold across the categorical variables were analyzed in Table 3.
There was no effect of iris color, sunglasses use and self-perception of light sensitivity on the total light sensitivity threshold in both groups. The gender significantly influenced the total light sensitivity threshold in group 1 with females showing significantly lower threshold than males (2.55 ± 0.51 log10[lux] vs. 3.06 ± 0.35 log10[lux], p = 0.007, Mann–Whitney U test).
Correlation analyses
A possible correlation between total light sensitivity threshold and age and BCVA was explored. A significant positive correlation was found between total light sensitivity and age in group 2 (r = 0.439, p = 0.028, Spearman’s correlation test), but not in group 1 (r=−0.260, p = 0.209, Spearman’s correlation test). No significant correlation was found in group 1 between the total light sensitivity threshold and BCVA (r=−0.154, p = 0.462, Spearman’s correlation test).
The total light sensitivity threshold was evaluated for a possible correlation with OSDI score and the other continuous variables analyzed with Keratograph 5 M (Table 4).
A significant negative correlation was found between total light sensitivity threshold and OSDI score (r=−0.499, p = 0.011, Spearman’s correlation test) in group 1, but not in group 2. No significant correlation was observed among total light sensitivity thresholds and the continues variables analyzed with Keratograph 5 M in both groups.
Discussion
Light-related visual disturbances are common DED symptoms difficult to be evaluated in the routine clinical practice. The present study highlighted the utility of employing Lumiz 100 for assessing light discomfort thresholds in patients with DED. Significant differences in light sensitivity were found between patients with DED and controls. Light discomfort thresholds correlated with ocular discomfort symptoms but not with ocular parameters analyzed with Keratograph 5 M.
DED represents a common etiology of light-related visual disturbances. Consistently, Buchanan et al. reported in a chart review on 111 adults that DED was the second most common cause of photophobia (36.1%) after migraine headache (53.7%)6. Furthermore, on a large cohort of DED patients, 299 (79.9%) reported photophobia for more than half of the time16; similarly, on a population of 236 veterans with mild or greater DED symptoms, 75% reported photophobia17. In our study, significant lower thresholds were found for all the light conditions in DED patients compared to healthy individuals. Different mechanisms have been proposed to explain how DED may lead to enhanced light sensitivity. Direct irritation of the trigeminal afferents that innervate the eye and subsequently altered neuronal pathways may be responsible of photophobia in DED patients8. Moreover, tear film instability is responsible for increased ocular forward light scattering and veiling luminance on the retina leading to glare which is also associated to a greater neuronal response5. Hence, it is widely accepted that central sensitization and neurons changes, along with tear film changes, contributes to photophobia in DED patients18.
None of the variables analyzed appeared to influence light discomfort thresholds in both groups except for gender. Among DED patients, females exhibited lower total discomfort threshold compared to males. This gender difference was not observed among healthy participants. This discordance aligns with previous studies that reported contrasting results. Some authors have identified lower light discomfort thresholds in women12,13, while others have found no meaningful differences between genders19,20,21. Iris color did not seem to affect total light sensitivity threshold, which is consistent with previous studies reporting minimal or no influence of iris pigmentation on light discomfort13,21. In our study, we found no significant difference in light sensitivity thresholds between individuals who regularly used sunglasses and those who did not. This finding contrasts with the results reported by Marié et al.13. Variations in the types of sunglasses used or in the criteria applied to define regular users could explain such discrepancies.
Total discomfort threshold and light self-perception were not significantly associated. In the literature, conflicting results are available, and a significant association was found in some studies13,22, but not in others21,23. These conflicting findings highlight the subjective nature of questionnaire-based assessments and emphasize the need for novel measurement tools, such as the Lumiz 100, able obtain more reliable and accurate data regarding patients’ light-related complaints.
Controversial data are available about the impact of age on light sensitivity. In our study, a significant positive correlation was found between light discomfort threshold and age in healthy patients but not in DED patients. While some studies reported that age does not significantly influence light sensitivity thresholds13,21, others showed that younger individuals are more sensitive to light than older ones who present lower contrast sensitivity and a different modulation of high cortical processes24,25. Furthermore, although disturbances in light adaptation are known to impair visual function and reduce visual acuity, BCVA did not correlate with light sensitivity thresholds in DED patients in our cohort.
A significant negative correlation was found between total light sensitivity threshold and ocular discomfort symptoms in DED patients. Galor et al. reported that patients with DED and photophobia have a more chronic disease course and more severe symptoms than their counterparts without photophobia26. Similarly, a recent study showed that photophobic patients have higher OSDI score compared to non-photophobic patients16.
Conversely, no significant correlation was observed between total light sensitivity threshold and ocular surface parameters analyzed with Keratograph 5 M. Singh and colleagues reported that tear film parameters assessed with the same device did not predict the presence or absence of photophobia in DED16. This discrepancy is not unexpected, given the well-established observation that DED symptoms often show poor correlation with clinical signs of ocular surface damage18,26. Therefore, interventions aimed solely at tear film stabilization may be insufficient in alleviating photophobia. This could explain the limited therapeutic response of photophobic DED patients to tear substitutes, further substantiating the neurogenic etiology underlying this symptom18,27.
Although this study offers novel insights into the assessment of light sensitivity thresholds in patients with DED, the limitation related to the cross-sectional design that precludes the assessment of causal relationships should be mentioned.
In conclusion, this study demonstrated that patients with DED exhibit significantly lower discomfort thresholds for all light conditions compared to healthy individuals. These thresholds were associated with ocular discomfort symptoms but not with the other ocular surface parameters measured with Keratograph 5 M. Future studies with larger sample size and longitudinal design are needed to further explore the characteristics of light sensitivity in different DED profiles and to evaluate potential therapeutic approaches able to modify light thresholds.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DED:
-
Dry eye disease
- OSDI:
-
Ocular Surface Disease Index
- TFOS:
-
Tear Film & Ocular Surface Society
- DEWS:
-
Dry Eye Workshop
- NIBUT:
-
Non-invasive break-up time
- BCVA:
-
Best-corrected visual acuity
- TMH:
-
Tear meniscus height
- MGL:
-
Meibomian gland loss
- SD:
-
Standard deviation
References
Craig, J. P. et al. Ocul Surf. ;15(3):276–283. doi: https://doi.org/10.1016/j.jtos.2017.05.008. (2017). Epub 2017 Jul 20. PMID: 28736335.
Wolffsohn, J. S. et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 15 (3), 539–574. https://doi.org/10.1016/j.jtos.2017.05.001 (2017). Epub 2017 Jul 20. PMID: 28736342.
Schiffman, R. M., Christianson, M. D., Jacobsen, G., Hirsch, J. D. & Reis, B. L. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. ;118(5):615 – 21. (2000). https://doi.org/10.1001/archopht.118.5.615. PMID: 10815152.
Vigo, L. et al. Diagnostic performance of a novel noninvasive workup in the setting of dry eye disease. J. Ophthalmol. 2020, 5804123. https://doi.org/10.1155/2020/5804123 (2020). PMID: 33489333; PMCID: PMC7803097.
Koh, S. Mechanisms of Visual Disturbance in Dry Eye. Cornea. ;35 Suppl 1:S83-S88. (2016). https://doi.org/10.1097/ICO.0000000000000998. PMID: 27583799.
Buchanan, T. M., Digre, K. B., Warner, J. E. A. & Katz, B. J. The unmet challenge of diagnosing and treating photophobia. J. Neuroophthalmol. 42 (3), 372–377. https://doi.org/10.1097/WNO.0000000000001556 (2022). Epub 2022 Mar 25. PMID: 35427241.
Mainster, M. A. & Turner, P. L. Glare’s causes, consequences, and clinical challenges after a century of ophthalmic study. Am J Ophthalmol. ;153(4):587 – 93. (2012). https://doi.org/10.1016/j.ajo.2012.01.008. PMID: 22445628.
Digre, K. B. & Brennan, K. C. Shedding light on photophobia. J. Neuroophthalmol. 32 (1), 68–81. https://doi.org/10.1097/WNO.0b013e3182474548 (2012). PMID: 22330853; PMCID: PMC3485070.
Choi, J. Y. et al. Usefulness of a photophobia questionnaire in patients with migraine. Cephalalgia 29 (9), 953–959. https://doi.org/10.1111/j.1468-2982.2008.01822.x (2009). Epub 2009 Feb 27. PMID: 19298545.
Cortez, M. M. et al. Validation of a photophobia symptom impact scale. Cephalalgia. ;39(11):1445–1454. doi: 10.1177/0333102419845641. Epub 2019 May 22. PMID: 31116567; PMCID: PMC6999072. (2019).
Durgam, S. S. et al. Visual photosensitivity threshold and objective photosensitivity luminance in healthy human eyes assessed using an automated ocular photosensitivity analyser: a step towards translation of a clinical tool for assessing photophobia. Ophthalmic Physiol. Opt. 42 (2), 311–318. https://doi.org/10.1111/opo.12923 (2022). Epub 2021 Nov 30. PMID: 34846070; PMCID: PMC9739816.
Montés-Micó, R., Cerviño, A., Martínez-Albert, N., García-Marqués, J. V. & Marie, S. Performance of a new device for the clinical determination of light discomfort. Expert Rev. Med. Devices. 17 (11), 1221–1230 (2020). Epub 2020 Oct 27. PMID: 33054420.
Marié, S., Montés-Micó, R., Martínez-Albert, N., García-Marqués, J. V. & Cerviño, A. Evaluation of physiological parameters on discomfort glare thresholds using LUMIZ 100 tool. Transl Vis. Sci. Technol. 10 (8), 28. https://doi.org/10.1167/tvst.10.8.28 (2021). PMID: 34319385; PMCID: PMC8322719.
Tian, L., Qu, J. H., Zhang, X. Y. & Sun, X. G. Repeatability and reproducibility of noninvasive keratograph 5 M measurements in patients with dry eye disease. J. Ophthalmol. 2016, 8013621. https://doi.org/10.1155/2016/8013621 (2016). Epub 2016 Apr 12. PMID: 27190639; PMCID: PMC4844888.
Vaccaro, S. et al. A Novel Noninvasive Screening Tool for Dry Eye Disease. Diagnostics (Basel) ;14(12):1209. doi: https://doi.org/10.3390/diagnostics14121209. (2024). PMID: 38928625; PMCID: PMC11202972.
Singh, S., Maity, M., Ali, M. H. & Basu, S. Tear film and dry eye characteristics as predictors of photophobia in 374 patients with dry eye disease. Semin Ophthalmol. 40 (3), 235–238 (2025). Epub 2024 Nov 19. PMID: 39563106.
Galor, A., Levitt, R. C., Felix, E. R. & Sarantopoulos, C. D. What can photophobia tell Us about dry eye? Expert Rev. Ophthalmol. 11 (5), 321–324. https://doi.org/10.1080/17469899.2016.1222905 (2016). Epub 2016 Aug 23. PMID: 28845186; PMCID: PMC5571866.
Diel, R. J. et al. Photophobia: shared pathophysiology underlying dry eye disease, migraine and traumatic brain injury leading to central neuroplasticity of the trigeminothalamic pathway. Br. J. Ophthalmol. 105 (6), 751–760. https://doi.org/10.1136/bjophthalmol-2020-316417 (2021). Epub 2020 Jul 23. PMID: 32703784; PMCID: PMC8022288.
Verriotto, J. D. et al. New methods for quantification of visual photosensitivity threshold and symptoms. Transl Vis. Sci. Technol. 6 (4), 18. https://doi.org/10.1167/tvst.6.4.18 (2017). PMID: 28845363; PMCID: PMC5566267.
Vanagaite, J. et al. Light-induced discomfort and pain in migraine. Cephalalgia. ;17(7):733 – 41. (1997). https://doi.org/10.1046/j.1468-2982.1997.1707733.x. PMID: 9399002.
Pierson, C., Wienold, J. & Bodart, M. Review of factors influencing discomfort glare perception from daylight. LEUKOS 14 (3), 111–148. https://doi.org/10.1080/15502724.2018.1428617 (2018).
Rodriquez, R. & Pattini, A. Tolerance of discomfort glare from a large area source for work on a visual display. Lighting Res. Technol. 46 (2), 157–170. https://doi.org/10.1177/1477153512470386 (2014).
Rodriguez, R., Yamín Garretón, J. & Pattini, A. Glare and cognitive performance in screen work in the presence of sunlight. Lighting Res. Technol. 48 (2), 221–238. https://doi.org/10.1177/1477153515577851 (2016).
Wolska, A. & Sawicki, D. Evaluation of discomfort glare in the 50 + elderly: experimental study. Int. J. Occup. Med. Environ. Health. 27 (3), 444–459. https://doi.org/10.2478/s13382-014-0257-9 (2014). Epub 2014 Jun 21. PMID: 24952143.
Facchin, A., Daini, R. & Zavagno, D. The glare effect test and the impact of age on luminosity thresholds. Front. Psychol. 8, 1132. https://doi.org/10.3389/fpsyg.2017.01132 (2017). PMID: 28713326; PMCID: PMC5492864.
Galor, A. et al. Dry eye symptom severity and persistence are associated with symptoms of neuropathic pain. Br. J. Ophthalmol. 99 (5), 665–668. https://doi.org/10.1136/bjophthalmol-2014-306057 (2015). Epub ird14 Oct 21. PMID: 25336572.
Galor, A. et al. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br. J. Ophthalmol. 100 (6), 745–749. https://doi.org/10.1136/bjophthalmol-2015-307094 (2016). Epub 2015 Sep 16. PMID: 26377416.
Acknowledgements
None.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization, F.L., G.C., E.V. and G.G.; Data curation, F.L., C.C., and G.G.; Formal analysis, F.L., C.C., A.C. and C.S.; Investigation, F.L., C.C., A.C. and C.S.; Methodology, F.L., G.C., C.C., E.V. and G.G.; Project administration, F.L., G.C., E.V. and G.G.; Resources, G.C., E.V. and G.G.; Software, F.L.; Supervision, G.G., G.C. and E.V.; Validation, F.L., G.C., E.V. and G.G.; Visualization, G.C., E.V. and G.G.; Writing—original draft, F.L., G.C. and G.G.; Writing—review and editing, F.L., G.C. and G.G. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of “Comitato Etico Territoriale Lazio Area 2” (approval date 13-02-2025). Written informed consent was obtained from all participants.
Consent for publication
All authors gave final approval of the version to be published.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lixi, F., Coco, G., Corda, C. et al. Light discomfort thresholds under different lighting conditions in healthy subjects and dry eye patients. Sci Rep 15, 29213 (2025). https://doi.org/10.1038/s41598-025-15633-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15633-1