Abstract
Parthenium hysterophorus, identified as Congress weed, presents a considerable risk to agricultural ecosystems and human health owing to its invasive characteristics and allelopathic properties. In this study, we explore the potential of biosynthesized silver nanoparticles (AgNPs) using actinobacteria as a sustainable and effective product for inhibiting the growth of P. hysterophorus. Actinobacteria, known for their diverse metabolic capabilities, were isolated and utilized for the eco-friendly synthesis of AgNPs. The reaction mixture consisted of an aqueous silver nitrate solution (1 mM) and the cell-free culture supernatant of Actinobacteria, which served as a source of reducing and stabilizing metabolites for the biosynthesis of silver nanoparticles. The resultant AgNPs were characterised employing several analytical techniques, such as UV-Vis spectroscopy and transmission electron microscopy. The synthesized AgNPs exhibited distinct physicochemical properties such as a surface plasmon resonance peak at ~ 420 nm, spherical shape, nanoscale size of 20.2 nm, confirming their successful formation. Subsequently, the inhibitory activity of actinobacteria-synthesized AgNPs against P. hysterophorus was evaluated through comprehensive bioassays. The nanoparticles demonstrated remarkable inhibition of seed germination, seedling growth, and overall plant development. Moreover, the AgNPs displayed selective toxicity towards Parthenium weed while maintaining minimal impact on non-target plants, emphasizing their potential as a targeted and environmentally friendly solution. The study highlights the promising role of actinobacteria-synthesized AgNPs in managing P. hysterophorus, providing valuable insights into the development of sustainable strategies for weed control. This research contributes to the growing field of nanobiotechnology, showcasing the potential of bioengineered nanoparticles as an effective means to address invasive plant species and promote sustainable agricultural practices.
Similar content being viewed by others
Introduction
“Weed” refers to any unwanted or undesired plant, often characterized by its ability to grow rapidly and compete with desirable plants for water, sunlight, and nutrients. They can be invasive and disruptive to the ecosystem, detrimentally impacting the growth and productivity of crops and indigenous plant species1. Weeds are opportunistic organisms that quickly adapt their behaviour with significant phenological flexibility to better fit new prevailing conditions. Consequently, apprehension is increasing among agriculturists and weed experts over recent advancements in weed management technologies. So, for the inhibition of weeds, there is a need for weed controls that are cheap and non-toxic to other plants. The biological control of weeds involves the intentional utilisation of natural antagonists to inhibit the growth or diminish the population of problematic weed species2.
Actinobacteria constitute a category of bacteria recognised for their varied metabolic functions and the synthesis of secondary metabolites, such as antibiotics and other bioactive substances. Actinobacteria can play a significant role in inhibiting the growth and spread of weeds. Some actinobacteria have been found to possess herbicidal properties, making them effective in controlling weed populations3. These biologically derived herbicides, often referred to as bioherbicides, offer several advantages over conventional chemical herbicides4. In recent years, researchers have harnessed the potential of actinobacteria to synthesize nanoparticles (NPs), a process known as “biogenic nanoparticle synthesis“5,6. Biogenic NPs synthesized with actinobacteria often exhibit biocompatible properties, making them suitable for use in drug delivery, imaging, therapeutic applications, and have the potential to enhance precision agriculture, minimize environmental impact, and promote sustainable weed management practices, aligning with the principles of eco-friendly and effective weed control7. This process typically involves using actinobacterial strains as bioreductant to reduce and assemble metallic ions into nanoparticles. The importance of this approach using Actinobacteria for nanoparticle synthesis reduces the need for harmful chemicals and high-energy processes, making it an environmentally sustainable method.
Reveals that actinobacteria are not very good sources of bioherbicides, which suggests that there is still an opportunity to explore their metabolites and secondary products for weedicidal purposes. This is where Actinobacteria-mediated silver nanoparticle (AgNPs) manufacturing comes in handy for managing invasive weeds like the extremely troublesome Parthenium hysterophorus L. Because of their special qualities, particularly as an enhanced surface area and bioavailability, these NPs may make weedicides more effective. This might result in more focused and ecologically friendly weed control solutions.8
Reported study discusses the herbicidal effects of Streptomyces metabolites and the processes for isolating and optimizing their bioherbicidal activity. In light of these considerations, the current study aims to screen, characterise, and identify Actinobacteria, as well as evaluate their weedicidal activity against Parthenium weed. This approach presents a sustainable and environmentally friendly method for targeted weed suppression, thereby decreasing dependence on conventional chemical herbicides. As research progresses, these environmentally friendly alternatives could play a vital role in integrated weed management strategies and contribute to more sustainable agricultural practices.9
Results
Isolation of actinobacteria
Initially, eleven pure isolates were identified based on the morphology of their colonies, microscopic, and biochemical properties. The isolated isolates were filamentous, Gram-positive, nonmotile, and aerobic, exhibiting catalase and oxidase activity.
Production of actinobacteria metabolite
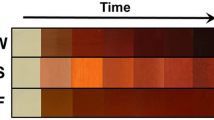
After inoculating the ISP-2 medium with a new and active culture of Actinobacteria, it was placed in an incubator for around 25 days while continuously shaken at 180 rpm. The color of the yeast-malt extract broth changed from dark brown to yellowish brown during the fermentation process (Fig. 1). After fermentation, the medium was centrifuged to proceed for the liquid-liquid extraction procedure.
Screening for antimicrobial activity of the metabolite
The results showed that 9 of the isolates were effective against the pathogenic microorganisms. The maximum antimicrobial activity was observed against all bacterial and fungal species compare to other species.
Molecular characterization of the selected isolates using 16 S rRNA sequence amplification
Molecular characterization of selected isolates was performed using 16 S rRNA gene sequencing for taxonomic identification. Initially six bacterial isolates were selected based on their strong antibacterial activity. Later, these isolates were subjected to secondary screening which were further selected for molecular characterization. BLAST analysis using the NCBI GenBank database identified two isolates as members of the phylum Actinobacteria. Phylogenetic analysis further resolved their classification within the genus Microbacterium. Specifically, isolates SKI-(H) and SKI-(X) were identified as Microbacterium testaceum with 100% sequence similarity. Isolate LA2(O) was identified as Streptomyces rochei with 100% similarity and Microbacterium trichothecenolyticum with 98% similarity. Partial 16 S rRNA gene sequences of isolates SKI-(H), SKI-(X), and LA2(O) have been submitted to the NCBI GenBank database under accession numbers PP107951, OP071245 and PV460900 respectively. The phylogenetic tree of the isolate SKI-(H) and SKI-(X) is depicted in Fig. 2 (a, b, and c).
The sequences were aligned using MEGA X software and the Phylogenetic tree of Microbacterium trichothecenolyticum SKI‒[H], SKI‒[X], and LA2(O) sp. was constructed using Neighbor-Joining” or “Maximum Likelihood” approach the UPGMA tree (constructed using MEGA X software, 6.0) depicts the evolutionary distances between Actinobacteria and other similar genera based on available M. testaceum SKI‒[H], SKI‒[X] sp. and Streptomyces rochei LA2(O) sequences from soil sample, as indicated by yellow highlight (Accession number: PP107951, OP071245 and PV460900). The nodes provide bootstrap values, such as 1000 replicates, to illustrate how reliable the branching patterns are. The scale bar represents 0.3% estimated phylogenetic divergence.
Characterization of secondary metabolite extract of SKI-[H], SKI-[X] and LA2(O)
The compounds were identified by analyzing the peak area, molecular weight, formula, and similarity index. The GC-MS analysis of the ethyl acetate extract of Microbacterium testaceum SKI-[H], Microbacterium trichothecenolyticum SKI-[X], and Streptomyces rochei LA2(O) are shown in Fig. 3a–c, respectively.
The chemicals obtained from the GC-MS analysis are displayed in the Table 1. The primary chemical found in all extracts was n-hexadecanoic acid, with a similarity index of 92% for each extract.
Silver nanoparticle synthesis of actinobacteria
The AgNPs were produced by incubating 1 mM AgNO3 with 333 µg/ml of secondary metabolites LA2(O) at 40 °C for a duration of 48 h. The secondary metabolites collectively exhibit a synergistic capacity to produce reducing potential, which, in an aqueous solution, can efficiently transform AgNO3 (aq) into Ag (aq) nanoparticles. The effectiveness of a reaction in the absence of salt has been confirmed. Secondary metabolites involved in the production of AgNps. Similarly, incubating secondary metabolites alone in ddw resulted in the absence of any AgNP-specific assimilation peak.
Color change
The early evidence of nanoparticle formation is noticed through a change in colouration. The aggregation of AgNPs to a greater size is shown by a colour shift in the solution from yellow to brown.
UV spectrometry
AgNPs exhibit a distinct absorption peak in UV absorption spectra, attributed to the excitation of their surface plasmon. Bio-AgNPs were confirmed by UV–visible (UV–Vis) spectrophotometer (Shimadzu No-UV 1800) with a resolution of 1 nm in the range of 300–800 nm. Absorption maximum at 413 nm is the characteristic one of the dye (Fig. 4) whereas for strain LA2(O) (OAgNPs) the peak is accredited to the surface plasmon resonance band of the AgNPs.
Fourier transform infrared (FTIR) spectroscopy
The FTIR analysis indicates the presence of various functional groups in the sample LA2(O), including amino, alkynes, amide, ether, and alcohol groups. The observed peak positions and transmittance values provide insights into the chemical composition and molecular structure of the sample (Fig. 5). Based on FTIR analysis of sample (LA2 O): The amide (–C = O) bond is 1635.94 cm−1 with transmittance (24.41%), the ether (C–O) bond is (1383.90 cm−1) with transmittance (39.45%), the alcohol (C–O) bond (1051.42 cm−1) with transmittance (35.67%) and the amino (–NH) bond (3411.05 cm−1) with transmittance (7.3%).
Transmission electron microscopy
The nanoparticles have a consistent size distribution and given that the sizes of the particles ranged from 11 to 42 nm and spherical in shape (Fig. 6). Using Image J software, the average size of the AgNPs was calculated to be 20.2 nm, according to the examination of TEM data. The transmission electron micrographs showed that the AgNPs particles were distinct and separated from one another.
Weedicidal effect of actinobacteria on plant growth
The mature seeds and tubers of the aforementioned weeds were collected, processed, and subsequently utilised to investigate their weedicidal action. Simultaneous investigation of effect on Pisum sativum, Cicer arietinum (White), V. radiata, Cicer arietinum (black), and Lens culinaris was also done and observations showed that the secondary metabolite extract were harmless and did not inhibit the growth of these crop plants (Fig. 7 Table 2).
The weedicidal analysis of LA2(O), LA2(O)*, SKI-[H], SKI-[X], and paper towel assays along with untreated control and media flask assays along with untreated control (Fig. 8).
LA2(O)*-Nano formulation
LA2(O), LA2(O)*, SKI-[H], and SKI-[X] weedicidal analysis is shown in Fig. 9 together with a control employing seed coating, and Fig. 10 illustrates the corresponding extract and direct injection of extract in soil in a pot setting.
Weedicidal analyses of LA2(O), LA2(O)*, SKI-[H], and SKI-[X] are presented in Tables 3 and 4, respectively, together with controls for seed coating with the corresponding extract and spraying the extract on plants in a container.
The impact of spraying SKI-[Z], SKI-[H], and SKI-[X] on the number of leaves, growth inhibition count, and stem length of Parthenium is shown graphically in Fig. 11 (a, b, c).
The growth analysis of Parthenium seeds coated in SKI-[Z], SKI-[H], and SKI-[X] is shown graphically in Fig. 12a–c and is broken down by the number of leaves, growth inhibition count, and stem length of the Parthenium.
Discussion
The findings of this study demonstrate that secondary metabolites derived from Streptomyces rochei LA2(O), SKI-[H], and SKI-[X], along with their synthesized silver nanoparticles (AgNPs), exhibit strong herbicidal activity against Parthenium hysterophorus, an invasive and ecologically destructive weed. The lowest germination percentage was recorded for LA2(O)* (6.6%) compared to the control (96.5%), and resulting in complete seedling mortality under post-emergence treatment. This is consistent with recent reports indicating that AgNPs synthesized using biological agents can significantly suppress weed growth. For example, demonstrated the herbicidal potential of AgNPs synthesized from Zanthoxylum nitidum against Bidens pilosa22achieving notable suppression of seed germination and seedling growth10. Similarly, reported enhanced phytotoxicity of AgNPs synthesized using Azadirachta indica extract on Echinochloa crus-galli, confirming the broad-spectrum herbicidal action of bio-fabricated nanoparticles11.
GC-MS analysis of actinobacterial extracts identified n-hexadecanoic acid as the major compounds with a similarity of 92%. This compound is well known for its allelopathic, herbicidal and antimicrobial properties12. For instance13, identified n-hexadecanoic acid in rice root exudates as a phytotoxic agent inhibiting weed seedling growth in paddy fields13. Furthermore, in studies involving marine actinobacteria, the same compound has been linked to both antifungal and herbicidal activities, reinforcing its contribution to the bioactivity observed in our results14. The size and morphology of AgNPs in our study ranging from 11 to 42 nm and confirmed as spherical via TEM are consistent with nanoparticle characteristics that enhance plant bioactivity. Reported that smaller, uniformly shaped AgNPs synthesized by actinomycetes exhibited increased penetration into plant tissues leading to greater biocidal activity15. The absorption peak at 413 nm in our UV–Vis analysis further aligns with known surface plasmon resonance (SPR) values for biologically synthesized AgNPs, indicating successful synthesis16.
This research used three different experimental settings for analyzing the herbicidal activity of the three actinobacterial strains along with actinobacteria-synthesized AgNPs against P. hysterophorus. Treatment of selected weeds with the secondary metabolite of LA2(O), SKI-[H], and SKI-[X] in a moist chamber revealed the potency of these in inhibiting the growth of P. hysterophorous L. Similar results were obtained when seeds of P. hysterophorous L. coated in 10 ml of cell-free filtrate of LA2(O), SKI-[H], SKI-[X], and LA2(O)* were grown in pots and germination percentage (G%) was calculated to be 13.6%, 52.8%, 66.3%, and 6.6% respectively. Whereas the G% for the control pot with 30 wild-type Parthenium seeds was found to be 96.5%. In a different experiment, freshly germinated Parthenium seeds were sprayed with cell-free filtrate of LA2(O), SKI-[H], and SKI-[X] cultures. The survival percentage of Pathenium seeds coated with LA2(O)* was the lowest, at 0.00%, followed by LA2(O) (13.3%), SKI-[X] (36.0%), and SKI-[H] (40.0%). As shown in this study, the effectiveness of actinobacteria-synthesized AgNPs against parthenium weed highlights their potential as a viable alternative to conventional herbicides17. Have also reported that the use of AgNPs production was demonstrated to increase the activity of Actinobacteria. AgNPs have demonstrated promise in improving soil health and crop productivity in addition to controlling weeds.
Importantly, our findings also reflect the growing interest in dual-function bioformulations that not only suppress weeds but also improve soil and crop health. Recently, reported that actinobacterial strains producing indole-3-acetic acid (IAA) and siderophores improved crop vigor while suppressing weed emergence, suggesting a multifunctional role in agroecosystems18. This synergistic potential supports the sustainable integration of actinobacteria-based biocontrol agents in modern agriculture.
In comparison with previous studies, our research contributes novel insights by evaluating both pre-emergence and post-emergence effects of microbial AgNPs on Parthenium weed. While most existing studies focus solely on seed germination inhibition, our findings on seedling mortality post-application represent an advancement in the practical applicability of these biocontrol agents. However, future investigations should address environmental safety, non-target effects, and long-term field efficacy to ensure scalable implementation.
Materials and methods
Collection of weeds
The ICAR-Directorate of Weed Research, Maharajpur, Jabalpur-482,004 (M.P.), India (23.90° North Latitude, 79.58° East Longitude) is where the weed seeds were purchased. After being taken to the lab for further identification and growth inhibition tests, the samples were kept for later use at room temperature.
Soil sample collection
Collecting soil samples for the isolation of Actinobacteria requires careful planning and attention to sterile techniques to avoid contamination. Actinobacteria are commonly located in the upper soil layers, particularly within the rhizosphere of plants. In this study, soil samples were gathered from agricultural fields, (Dumariya Ganj, 27.2078° N, 82.6526° E and Rajesh Nursey, Basti 26.8140° N, 82.7630° E and Integral University Lucknow N 26° 57’ 30.4344”, E 80° 59’ 53.1924) at different depths (e.g., surface soil and subsoil) to capture a representative range of Actinobacteria.
Isolation of actinobacteria
The soil samples were diluted with sterile saline solution to create a series of dilutions. At 10− 6 dilution, 1 ml sample was spread on Actinomycetes isolation agar (AIA) and starch casein agar to isolate Actinobacteria. Then, the plates were incubated at an appropriate temperature (usually around 28–30 °C) for 4 to 7 days, as Actinobacteria typically have a slow growth rate. After incubation, we examined the plates for the growth of colonies with typical Actinobacteria morphology, e.g. powdery appearance, and chalky texture19. Thereafter, individual colonies were isolated by streaking them onto fresh nutrient agar plates to obtain pure cultures. Pure colonies of Actinobacteria were characterized with gram staining to screen gram-positive bacteria, and conducted some biochemical tests to identify its characteristics such as catalase and oxidase activity20.
Preliminary antimicrobial screening of actinobacteria
A single streak of an Actinobacteria isolate that tested positive against pathogenic and multi-drug resistant (MDR) pathogens at 90 °C on Mueller Hinton agar was used for the initial screening21. Mueller Hinton agar was streaked with the actinobacterial isolates in a parallel pattern, and they were incubated at 28˚C for four to six days22. Actinobacterial strains were cultivated on media, followed by inoculation of specific pathogenic bacteria strains, Staphylococcus aureus (ATCC-6538), Pseudomonas aeruginos a (NCIM-5029), Salmonella abony (ATCC6017), Klebsiella pneumoniae (NCIM-2957), Bacillus subtilis (MTCC-441), Escherichia coli (MDR)(ATCC-25923), human pathogenic fungi- Aspergillus niger (ITCC 545), Aspergillus flavus (MTCC 277) and Aspergillus parasiticus (MTCC-2796). These plates were streaked perpendicular to the previous streak of actinobacteria and incubated at 30˚C and used in calculating zone of inhibition after 72 h and 24 h for fungi and bacteria, respectively. Out of 9 bacterial strain tested, actinobacterial isolates were positive in the preliminary screening and processed for molecular characterization19.
Molecular characterization using 16 S rRNA amplification of isolated actinobacterial strains
The molecular characterization i.e., PCR amplification, sequencing and restriction analysis, 16S rRNA sequence amplification was conducted at Bio-kart India Pvt. Ltd, located in Bangalore. The amplification of the 16S ribosomal sequence was performed by using F243 (5’ GGATGAGCCCGCGGCCTA 3’) and 1378R (5’ CGGTGTGTACAAGGCCCGG 3’) primers23. The method of amplifying 16 S rRNA sequences was utilised for molecular characterisation, facilitating the secondary screening of the isolates selected after the initial screening phase. The BLAST tool in the GenBank database was employed to achieve a comprehensive categorisation of the strains. The NCBI GenBank now holds the partial 16 S rRNA gene sequence of the SKI-(H), SKI-(X), and LA2(O) isolates32.
Production of secondary metabolites
Secondary metabolites were extracted using Actinobacteria that were evaluated largely for antibacterial activity. Submerged state fermentation (SmF) was used to produce secondary metabolites from Actinobacteria. In a 500 ml flask with 100 ml of ISP-2 medium (g/L): yeast extract, 4.0; malt extract, 10.0; dextrose, 4.0; Agar, 20.0; double distilled water (ddw), 1000 mL, and pH 7.3, the isolates SKI-(H), SKI-(X), and LA2(O) were inoculated. For five to seven days, the flasks containing the inoculated strains were incubated at 28 °C at 200 rpm in a rotary shaker. Following growth, the cultures were centrifuged at 10,000 rpm, and the supernatant was employed in further studies24.
Screening of actinobacteria for herbicidal activity
The germination and growth inhibitory assay was done using P. hysterophorus. Sterile cotton was laid on the petri dishes, with a cotton being soaked with the cell-free filtrate from the selected actinobacterial cultures. Seeds of weeds were placed on absorbent cotton and were kept in the growth cabinet at 28 °C for 5 days after which herbicidal effect was noted25.
Synthesis of silver nanoparticle
A 1 mM aqueous solution of 50 µl AgNO3 was used for the in-vitro synthesis of AgNPs. This solution was first combined with 50 ml Actinobacteria supernatants at a pH of 8.5.16. incubated for five days at 37 °C in the dark in a rotary shaker set to 200 rpm. The control experiments used an uninoculated medium and AgNO3 solution to perform the procedure in order to assess if bacteria are involved in the formation of nanoparticles. A UV-Vis spectrophotometer, sample collection at predefined intervals, and UV-Vis spectrum observation were used to study silver ion reduction26.
Characterization of synthesized silver nanoparticle
The initial indication of extracellular AgNPs formation is a change in colour. The color of the synthesized AgNPs was turned to dark brown. UV–visible spectroscopy and transmission electron microscopy were employed to analyse the synthesised AgNPs26. The general production of AgNPs was confirmed by a UV–Visible spectrophotometer (Shimadzu dual beam-model UV1601 PC) with the wavelength of 1 nm. The size of the inorganic core A drop of AgNPs (suspension) was dried on carbon-coated TEM with copper grids at 80 kV by TEM Tecnai TM G2 Spirit Bio-TWIN (FEI, Hillsboro, OR, USA) to determine the percentage composition of the inorganic core27.
UV–Vis spectrometry
UV–Vis spectroscopy is a highly effective and easy method for verifying nanoparticle synthesis. Absorption measurements were conducted utilising a UV–visible spectrometer (Eppendorf, Germany) to quantify the decrease of metal ions. The characterisation of nanoparticles encompasses light wavelengths ranging from 200 to 800 nm. The absorbance spectrum of the colloidal sample was recorded between 200 and 800 nm using a UV–Vis spectrophotometer (sterile water was used as a reference). UV–vis absorption measurements were carried out in a quartz cuvette using a Shimadzu dual-beam UV-1601 PC spectrophotometer (model) with a 1 nm resolution. All samples were measured for their optical densities (O.D.) and were visualized using the following counter units28.
FTIR spectroscopy of biogenic AgNPs
The formation of numerous functional groups on the surface of AgNps was confirmed using surface Perkin–Elmer Spectrum 2 FT-IR (Perkin Elmer Version 10.03.06 instrument; Waltham, MA, USA). This approach used global attenuated reflectance sampling (scaled to the full surface) and the recorded spectra were analyzed using the transmission technique (at 4 cm1 resolution) over the entire wavenumber range (4,000–4,500 cm1).The objective of FTIR is to quantify the absorption of light at each wavelength by the materials29.
Transmission electron microscopy (TEM)
To perform TEM, a drop of GNP (Graphene nanoplatelets)solution was dried on carbon-coated TEM copper grids. The grid is put inside the TEM apparatus once it has dried. The nanoparticles are exposed to a concentrated electron beam, which creates high-resolution photographs that show their size, shape, and distribution at the nanoscale. Using a TEM Tecnai TM G2 Spirit Bio-TWIN (FEI, Hillsboro, OR, USA) to dry a drop of generated AgNPs (suspension) at 80 kV on a carbon-coated TEM copper grid allowed the proportion of inorganic core to be calculated10,30.
Weedicidal effect of actinobacteria on plant growth
The roll paper towel assay was used for testing the effect of spores from a Streptomyces strain coated to crop and weed seeds on seed germination. Spore suspensions of Streptomyces were prepared by adding sterile double distilled water to a 10-day-old starch casein agar plate culture. Seeds of crops and weeds, which had been surface sterilised, were soaked in spore suspension of Streptomyces for 30 min, and then were air-dried in a laminar flow surface. Seeds were placed on a wetted sterile paper towel (12 × 30 cm). Towell was wrapped, sealed in plastic and aged for 5 days at ambient temperature. Three towels were used in each treatment, and the experiment was performed in three replications31.
Media trails
Parthenium seeds underwent disinfection using a 5% sodium hypochlorite solution. Autoclaved Murashige and Skoog (MS) media was supplemented with 100 ml/L of cell-free filtrates of LA2(O), LA2(O)*, SKI-[H], SKI-[X] cultures after complete cooling. The sterilized seeds were then planted into the media after it had completely hardened. Flasks were incubated in plant tissue culture laboratory under 25 °C and 72 h in dark. The flasks were transported in the light growth chamber of the PTS and incubated for 10 days at 30°C32.
Pot trails
The secondary metabolite extract of LA2(O), LA2(O)*, SKI-[H], and SKI-[X] were used in two ways for investigating its inhibitory effects on germination, leaf count, and stem length on potted Parthenium which are discussed as follows33. Each pot had a uniform size 10 inches, and was filled with 650 g of soil. The pots were arranged sequentially in the following order: control group, treatment with secondary metabolites of Actinobacteria, and treatment with their biosynthesized nanoparticles.
Seed coated in extracted metabolites
A 10 ml of cell-free filtrate of LA2(O), SKI-[H], and SKI-[X] culture was used to soak the seeds. The seeds were then left overnight in the extract to ensure better coating. Thirty soaked seeds per pot were sowed in 3 different pots along with control pots which have 30 wild-type Parthenium seeds34. The seed germination percentage (G%) was calculated using the following formula35:
Spray treatment
Thirty wild-type Parthenium seeds were planted in a control pot, and thirty seeds were planted in each of the three pots. Cell-free filtrate of LA2(O), SKI-[H], and SKI-[X] cultures were transferred in a spray bottle and 3 to 5 sprays were applied respectively, to the plant once signs of germination were visible36,37. Spraying was done after every second day for 15 days. The survival percentage (S%) was calculated using the formula:
Statistical analysis
For the experiments, a minimum of three biological replicates were employed, and the meaning of each data point indicated in the findings was derived from these repetitions. The error bars (mean ± SE) show a mean ± standard error.
Data availability
All data supporting the findings of this study are available within the paper.
References
Hussain, A. et al. Parvaiz hassan, Q. Antituberculotic activity of actinobacteria isolated from the rare habitats. Lett. Appl. Microbiol. 65, 256–264 (2017).
Dhileepan, K., Callander, J., Shi, B. & Osunkoya, O. O. Biological control of Parthenium (Parthenium hysterophorus): the Australian experience. Biocontrol Sci. Technol. 28, 970–988 (2018).
Hazarika, S. N. & Thakur, D. Actinobacteria. In Beneficial Microbes in Agro-Ecology (Academic, 443–476. (2020).
Mehta, T., Meena, M. & Nagda, A. Bioactive compounds of Curvularia species as a source of various biological activities and biotechnological applications. Front. Microbiol. 13, 1069095 (2022).
Rajesh, K., Pitchiah, S., Kannan, K. & Suresh, V. Biosynthesis of silver nanoparticles from marine actinobacterium Micromonospora sp. and their bioactive potential. Cureus 16, 2 (2024).
Bano, N. et al. Antibacterial efficacy of synthesized silver nanoparticles of Microbacterium proteolyticum LA2 (R) and Streptomyces rochei LA2 (O) against biofilm forming meningitis causing microbes. Sci. Rep. 13, 4150 (2023).
Dhanasekaran, D. & Jiang, Y. (eds) Actinobacteria: Basics and Biotechnological Applications (BoD–Books on Demand, 2016).
Khan, A., Ali, S., Khan, M., Hamayun, M. & Moon, Y. S. Parthenium hysterophorus’s endophytes: the second layer of defense against biotic and abiotic stresses. Microorganisms 10, 2217 (2022).
Chaves Neto, A. et al. Phytotoxic potential of Streptomyces spp. And metabolites against Echinochloa crus-galli in sustainable weed management. PLoS One. 16, e0222933 (2021).
Li, X. et al. Phytotoxicity of silver nanoparticles synthesized using Zanthoxylum nitidum extract against Bidens Pilosa. Plants 11 (10), 1377 (2022).
Mehta, C. M. et al. Green synthesis and herbicidal activity of AgNPs using Azadirachta indica against Echinochloa crus-galli. Environ. Nanotechnol Mon Manage. 15, 100432 (2021).
Abd-Elhady, H. M. et al. Biosynthesis and characterization of extracellular silver nanoparticles from Streptomyces aizuneusis: antimicrobial, anti-larval, and anticancer activities. Molecules 27, 212 (2021).
Zhang, J. et al. Identification of allelopathic compounds from rice and their inhibitory effects on weed species. BMC Plant Biol. 20, 246 (2020).
Manivasagan, P. et al. Biogenic silver nanoparticles from marine actinobacteria: characterization and application for control of harmful microbes. Microbial Pathogenesis, 118, 113–120 (2018). (2018).
Ahmed, S. et al. Characterization and antimicrobial potential of silver nanoparticles synthesized by endophytic actinomycetes. J. Nanobiotechnol. 21, 112 (2023).
Singh, P. et al. Biological synthesis of nanoparticles: a green approach for the development of antimicrobial agents. Front. Microbiol. 7, 1209 (2016).
Baker, S., Aziz, M. H. E., Abouzaid, A. M. & Z. K. A. & Efficacy of extracellular metabolite produced by Streptomyces Levis strain LX-65 as a potential herbicidal agent. J. Am. Sci. 10, 169–180 (2021).
Elshafie, H. S. et al. Actinobacteria-based bioproducts for weed management and plant growth promotion. Agronomy 12 (3), 729 (2022).
Kumari, N., Pandey, S. & Menghani, E. Evaluation of actinomycetes isolated antimicrobial metabolites as potent inhibitor of multidrug resistant organisms. Indian J. Geo-Mar. Sci. 50, 29–36 (2022).
Al-Dhabi, N. A. et al. Bioactivity assessment of the Saudi Arabian marine Streptomyces sp. Al-Dhabi-90, metabolic profiling and its in vitro inhibitory property against multidrug resistant and extended-spectrum beta-lactamase clinical bacterial pathogens. J. Infect. Public. Health. 12, 549–556 (2019).
Vurukonda, S. S. K. P. et al. Growth-promoting properties of actinobacteria in agriculture. Environ. Sustain. 2, 65–76 (2018).
Kumar, S. et al. Biocontrol of weeds using actinobacteria: A sustainable approach in crop protection. Microbiol. Res. 245, 126–135 (2022).
Ling, L. et al. Herbicidal activity of secondary metabolites isolated from Streptomyces sp. NEAU-HV44 on selected weeds and crops. J. Agric. Food Chem. 71, 17056–17066 (2023).
Umurzokov, M. et al. Herbicidal characteristics and structural identification of a potential active compound produced by Streptomyces sp. KRA18–249. Pestic Biochem. Physiol. 187, 105213 (2022).
Bo, A. B. et al. Isolation, identification and characterization of Streptomyces metabolites as a potential bioherbicide. PLoS One. 14, e0222933 (2019).
Bano, N. et al. Anticancer potential of synthesized silver nanoparticles using bioactive metabolites of actinobacteria against A549 lung cancer cells. Sci. Rep. 11, 12345 (2021).
Balagurunathan, R. et al. Sample collection, isolation, and diversity of actinobacteria. In Protocols in Actinobacterial Research 1–24 (Springer, (2020).
Passos, M. L. & Saraiva, M. L. M. Detection in UV-visible spectrophotometry: detectors, detection systems, and detection strategies. Measurement 135, 896–904 (2019).
Flower, N. A. et al. Characterization of synthesized silver nanoparticles and assessment of its genotoxicity potentials using the alkaline comet assay. Mutat. Res. /Genet Toxicol. Environ. Mutagen. 742, 61–65 (2012).
Al-Sheddi, E. S. et al. Anticancer potential of green synthesized silver nanoparticles using extract of Nepeta deflersiana against human cervical cancer cells (HeLA). Bioinorg. Chem. Appl. 9390784 (2018). (2018).
Dhanasekaran, D., Thajuddin, N. & Panneerselvam, A. Herbicidal agents from actinomycetes against selected crop plants and weeds. Nat. Prod. Res. 24, 521–529 (2010).
Khosravi Babadi, Z. et al. Isolation and identification of Streptomyces sp. Act4Zk, a good producer of staurosporine and some derivatives. Lett. Appl. Microbiol. 72, 206–218 (2021).
Azman, A. S. et al. Antibacterial, anticancer and neuroprotective activities of rare actinobacteria from Mangrove forest soils. Indian J. Microbiol. 57, 177–187 (2017).
Hamedi, J., Papiran, R. & Moghimi, H. Isolation and screening of phytotoxin-producing actinomycetes for biological control of Cardaria Draba. Biol. Control. 72, 77–84 (2014).
Yandigeri, M. S. et al. Drought-tolerant endophytic actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant. Growth Regul. 68, 411–420 (2012).
Gupta, A. et al. Comparative evaluation of different salt-tolerant plant growth-promoting bacterial isolates in mitigating the induced adverse effect of salinity in Pisum sativum. Biointerface Res. Appl. Chem. 11, 13141–13154 (2021).
Nongmaithem, D. & Pal, D. Effect of weed management practices on soil actinomycetes and fungi population under different crops. J. Crop Weed. 12, 120–124 (2016).
Acknowledgements
The authors are grateful to Integral University for providing Financial Assistance to the Department of Biosciences, Integral University, Lucknow, Uttar Pradesh, India.
Funding
Open access funding provided by Parul University.
Author information
Authors and Affiliations
Contributions
S.K.I., N.B., S.F.R., A.G., and U.A. conceived and designed this study. P.P., S.L., A.S., F.K., and V.J.U. supervised this study. S.K.I., N.B., S.F.R., A.G., and U.A performed most of the experiments and analyzed the data. P.P., S.L., A.S., F.K., and V.J.U. provided technical assistance. S.K.I., N.B., S.F.R., A.G., and U.A. prepared the manuscript. P.P., S.L., A.S., F.K., and V.J.U. revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Izhar, S.K., Bano, N., Rizvi, S.F. et al. Actinobacteria mediated synthesis of silver nanoparticles and their weedicidal potential against Parthenium hysterophorous Linnaeus. Sci Rep 15, 31540 (2025). https://doi.org/10.1038/s41598-025-15657-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15657-7