Abstract
The combined effects of climate change and human activities are reshaping species distributions, with many valuable plant species also being affected. This study uses the Maximum Entropy (Maxent) model to assess the current and future distribution of Lophatherum gracile Brongn. in China under different environmental and anthropogenic scenarios. A dataset comprising 142 occurrence records and 13 selected environmental variables, including bioclimatic, soil, and solar radiation factors, was used to predict suitable habitats. The model achieved high accuracy (AUC > 0.9, TSS > 0.7, CBI > 0.7), with precipitation during the driest month, elevation, and soil saturation identified as key factors influencing habitat suitability. The results indicate that under current conditions, L. gracile is predominantly distributed in southern China, including provinces such as Hunan, Guangxi, and Fujian. Future climate scenarios (SSP245 and SSP585) predict significant shifts in habitat suitability, with an overall reduction in highly suitable areas and a westward shift in the distribution centroid. Human activities exacerbate habitat fragmentation, resulting in a 2.18% reduction in suitable areas. These findings underscore the vulnerability of L. gracile to both environmental and anthropogenic pressures, highlighting the need for targeted conservation strategies. This research provides a scientific foundation for the conservation of L. gracile and informs adaptive management strategies to mitigate the adverse effects of climate change and human disturbance.
Similar content being viewed by others
Introduction
The combined effects of human activities and climate change are significantly altering plant growth and distribution patterns1. With the intensification of global warming, rising global temperatures have led to plant migration towards higher latitudes and altitudes, extended growing seasons for many plant species, and shifts in phenology2,3. A meta-analysis by Parmesan and Yohe revealed that over 80% of species exhibit phenological changes, such as earlier flowering or leaf drop4. However, plants have limited adaptive capacity to climate change, which may result in the loss of genetic diversity and further weaken ecosystem resilience5. Future climate change is expected to cause significant shifts in plant distribution patterns globally. This is particularly true in tropical and subtropical regions, where locally adapted plant species face a heightened risk of extinction6. Additionally, human activities such as agricultural expansion, urbanization, and deforestation are transforming habitats, further exacerbating changes in plant distribution7. The interplay between land-use change and climate change results in habitat fragmentation, which in turn disrupts plant population connectivity and threatens their long-term viability. Additionally, this fragmentation poses significant risks to the provision of essential ecosystem services8,9. Human-driven global warming has exacerbated these challenges, with extreme weather events, such as droughts and floods, posing direct threats to the growth of many plant species10. Therefore, understanding the combined effects of climate change and human activities on plant distributions is crucial for assessing their impacts on biogeographic patterns.
Lophatherum gracile Brongn. is a herbaceous plant belonging to the Poaceae family. Its dried stems and leaves have a long history of use in traditional Chinese medicine as a medicinal material11. This species is distributed in mountainous forest margins and understories across China, Japan, Korea, and Southeast Asia12. As a traditional herbal medicine, L. gracile is rich in bioactive compounds such as flavonoids13polysaccharides14and coumarins15. Research has shown that it possesses significant anti-inflammatory16antioxidant17and antiviral18 properties. In traditional practice, it is commonly used to treat conditions such as fever, thirst, and urinary tract infections19. Biodiversity threats, driven by both natural processes and human activities, have become one of the primary concerns among scholars today20. In particular, human activities—recognized as a critical environmental issue—have adverse impacts not only on humans but also on other forms of life. Currently, L. gracile does not receive adequate protection. Given its remarkable medicinal value, it is essential to conduct in-depth research on the distribution of L. gracile resources and to predict future suitable areas that could serve as plantation sites or climate refugia. Achieving this goal requires a comprehensive analysis of its growing environment, along with the application of modern ecological modeling tools, such as the Maxent model, to predict future potential distribution patterns. Incorporating environmental factors such as climate and soil conditions allows researchers to better understand the suitable growth conditions for L. gracile across different regions, providing a scientific foundation for its sustainable use and preservation as a valuable medicinal resource. These predictions will not only facilitate the rational cultivation and effective protection of L. gracile but also help address the challenges posed by external factors, such as climate change, on its future distribution.
Species distribution models (SDMs) serve as critical tools for delineating suitable habitats and forecasting the potential ecological consequences of climate change21. Among the array of available SDMs, the Maxent model has gained widespread recognition for its robust predictive performance, capacity to incorporate both continuous and categorical environmental predictors, and its unique presence-background framework that contrasts species occurrence records against environmentally stratified background samples22. As a presence-background algorithm, Maxent identifies environmental conditions (e.g., temperature thresholds, precipitation gradients, soil characteristics) that maximize the divergence between the species’ occupied habitats and the broader available environmental space, avoiding the ecological uncertainties associated with true absence data23. This methodological framework renders Maxent particularly advantageous in contexts where true absence data are lacking or species occurrence records are geographically sparse. Moreover, Maxent produces not only spatial projections of suitable habitat, but also continuous probability surfaces that reflect habitat suitability24. In light of the limited distributional data available for L. gracile and the extensive application of Maxent in similar biogeographic and conservation studies, we selected this approach as the most appropriate tool for modeling the species’ current and future potential distribution.
Currently, no studies have been reported on the distribution of L. gracile. Therefore, we selected variables likely to influence its growth and distribution, screened these variables, and applied the Maxent model to investigate the effects of climate change and human activities on the habitat distribution of L. gracile. The study is structured around two main comparisons: (1) Differences in the habitat distribution of L. gracile under conditions with and without human disturbance. (2) Shifts in the habitat distribution of L. gracile under various future climate scenarios. The results of this study will offer valuable insights into the distribution patterns and future trends of L. gracile in China. Additionally, they will provide scientific guidance for local authorities and policymakers in formulating effective conservation and management strategies.
Materials and methods
Geographic distribution data and processing for L. gracile
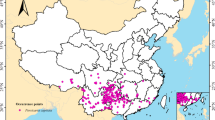
To obtain occurrence records of L. gracile for modeling, the research team retrieved latitude and longitude information from the National Specimen Information Infrastructure (http://www.nsii.org.cn/) and the Chinese Virtual Herbarium (https://www.cvh.ac.cn/). For records lacking coordinate data, we used Baidu Maps (https://map.baidu.com/) to manually geolocate the specimens based on the provided geographic information (e.g., province, city, county). To improve temporal consistency and data reliability, only occurrence records collected within the last 25 years were retained, based on their collection dates rather than upload dates. After filtering, the team collected a total of 169 occurrence records. To reduce sampling bias and spatial autocorrelation, we utilized the “Spatially Rarefy Occurrence Data for SDMs (Reduce Spatial Autocorrelation)” function in ArcGIS, with a minimum distance of 20 km between occurrence records. This threshold was selected based on the resolution of environmental variables, the spatial distribution of occurrence data, and the ecological characteristics of L. gracile. Similar filtering distances have been adopted in previous studies to minimize spatial clustering and improve model performance in large-scale species distribution modeling25,26. This process reduced the dataset to 142 distribution points, as shown in Fig. 1.
The distribution of L. gracile in China, with red dots indicating individual occurrence points. (Colors are for visual distinction only and do not represent any quantitative data.) (Created with ArcGIS 10.8.1, URL: https://www.esri.com).
Acquisition and processing of environmental and human factors data
At the outset, 37 environmental factors were selected for model development, encompassing climate, elevation, solar radiation, and soil data. Climate, elevation, and solar radiation data were sourced from the WorldClim website (https://worldclim.org/). For climate data, the research team selected the BCC-CSM2-MR global climate model under SSP245 and SSP585 scenarios for the periods 2041–2060 and 2061–2080. BCC-CSM2-MR is developed by the Beijing Climate Center and is specifically optimized for simulating climate processes over China and East Asia. It shows strong performance in reproducing key regional climate features such as the East Asian monsoon, precipitation patterns, and temperature gradients. SSP245 represents a “middle-of-the-road” development pathway, characterized by moderate population growth, technological development, and mitigation efforts that stabilize radiative forcing at 4.5 W/m² by 2100. In contrast, SSP585 reflects a high-emission trajectory associated with rapid economic growth, intensive fossil fuel use, and minimal climate mitigation, leading to radiative forcing levels of 8.5 W/m² by the end of the century. By incorporating both scenarios, we aimed to capture a range of possible future climate outcomes, from moderately controlled to extreme warming conditions—to better understand the potential impacts on L. gracile habitats under different socio-environmental futures27. Soil data were obtained from the Harmonized World Soil Database (HWSD) website (https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/harmonized-world-soil-database-v20/zh/). HWSD is a comprehensive global soil inventory that provides detailed insights into soil properties, including morphological, chemical, and physical characteristics. The data are compiled and organized using the Geographic Information System (GIS) by IIASA, ensuring the database’s accuracy and comprehensiveness28. Human footprint (Hf) data were derived from the Global Human Influence Dataset, Version 3 (http://www.ciesin.columbia.edu/wild_areas)29. This dataset combines information from 1993 to 2009 on land use, population density, railways, highways, electrical infrastructure, and cropland, with a spatial resolution of 1 km. It provides an objective and comprehensive representation of the intensity and spatial distribution of human activities, making it ideal for assessing the level of human disturbance. Base map data required for modeling were sourced from the National Fundamental Geographic Information System of China (http://nfgis.nsdi.gov.cn) at a scale of 1:400,000, used as the analytical base map. All the above datasets were subjected to cropping, reformatting, and resampling to a uniform spatial resolution of 1 km, ensuring consistency and compatibility with the Maxent model.
Building distribution model
The Maxent model is a tool for predicting species distribution based on the principle of maximum entropy. It is widely used in ecological and biodiversity research30,31. The model operates under the assumption that the optimal distribution pattern, under unknown conditions, is the one with the highest entropy—representing the least biased prediction for the species. Additionally, it assumes that occurrence records are randomly sampled and represent the species’ actual distribution proportional to its population density32,33. To examine the impact of environmental variables and human disturbances on the distribution patterns of L. gracile, the team developed three models: Model A: Forecast based on environmental variables (bioclimatic factors + topography + solar radiation) under current climate conditions. Model B: Prediction based on environmental variables (bioclimatic factors + topography + solar radiation) combined with human footprint under current climate conditions. Model C: Prediction based on environmental variables (future bioclimatic factors + topography + solar radiation) under future climate scenarios. It is noteworthy that in Model C, the future bioclimatic data are used as projection layer, indicating that the model is not re-calibrated future bioclimatic variables. Instead, the training model established under current conditions (Model A) is projected onto future bioclimatic variables via the “projection layers directory” function in Maxent. The Maxent modeling software was used to implement these models. The occurrence point data of L. gracile and the selected environmental variable datasets were imported into their respective fields in the software. The following options were selected: Create response curves, Make pictures of predictions, and Do jackknife to measure variable importance. These settings enabled a more intuitive presentation of the results and allowed for an evaluation of the model’s accuracy. The Random test percentage was set to 25%, with 75% of the data allocated for training. The Replicates parameter was set to 10 to improve model precision. The Write round predictions option was also selected to generate raw data for subsequent mapping. Other parameters were kept at their default values. The model was then run to generate predictions. To avoid multicollinearity and overfitting in the model, we first performed modeling using all 37 collected environmental variables to obtain their respective contribution rates. Subsequently, we used SPSS 27 to screen the variables. Specifically, a Pearson correlation matrix was calculated for all 37 variables, and variable selection was based on their contribution rates: for any pair of variables with a correlation coefficient greater than 0.8, only the variable with the higher contribution was retained. All variables with correlation coefficients less than 0.8 were included in the subsequent modeling process34. Ultimately, 13 environmental variables were chosen based on their contribution rates for the final modeling, as shown in Table 1.
Evaluation of model prediction results
The performance of the Maxent model was evaluated using the Area Under the Curve (AUC), True Skill Statistic (TSS), and Continuous Boyce Index (CBI). AUC, derived from the Receiver Operating Characteristic (ROC) curve, assesses model performance across all thresholds by measuring sensitivity (true positive rate) and specificity (false positive rate)35. As a threshold-independent metric, AUC is particularly effective for imbalanced datasets and allows comparison across models, with values ranging from 0.5 (random prediction) to 1 (perfect prediction)36. TSS, by contrast, is threshold-dependent and integrates sensitivity and specificity into a single value, offering a more practical evaluation of classification accuracy37. Following threshold recommendations by Fielding & Bell (1997) and Pearson et al.. (2007), this study implemented a TSS threshold of 0.25 for L. gracile to maximize sensitivity (minimizing false negatives), given its restricted occurrence data. This strategy aligns with the conservation paradigm that, under resource-constrained scenarios for species distribution, overestimation of suitable habitat may sometimes be more appropriate than underestimation of its extent38,39. It ranges from − 1 to + 1, where + 1 indicates perfect agreement and values ≤ 0 suggest performance no better than random40. The use of both AUC and TSS provides complementary insights into model performance41. In addition, the Continuous Boyce Index (CBI) was employed to assess model calibration. CBI measures the correlation between predicted suitability and the frequency of observed presences across suitability classes, with values closer to 1 indicating better model consistency. Unlike AUC, CBI is not affected by pseudo-absences and is especially suitable for presence-only data42. The AUC value was directly obtained from the Maxent output. The TSS was calculated in R (v4.3.3) using the PresenceAbsence package. The CBI was computed in R using the ecospat package42,43. To assess the degree of environmental extrapolation in model projections, a Multivariate Environmental Similarity Surface (MESS) analysis was conducted using the dismo package in R. This allowed identification of regions where environmental conditions in the projection fall outside the training range, highlighting areas of reduced model reliability.
Centroid shift analysis
To evaluate the spatial dynamics of L. gracile under climate change, we analyzed the centroid shifts of suitable habitats predicted by the Maxent model. These binary raster layers were then converted to polygon features using the “Raster to Polygon” tool in ArcGIS 10.8.1. All suitable areas were merged into a single polygon using the “Dissolve” tool. The geographic centroid of each scenario’s suitable habitat was calculated using the “Feature to Point” tool with the “Inside” option enabled. To visualize and measure spatial shifts, we connected the centroids across scenarios using the “XY to Line” tool and calculated the displacement distance and direction. This analysis allowed us to assess the trajectory of habitat suitability changes and infer potential migration trends of the species under future climate conditions.
Results
Model prediction results
In this study, all constructed models achieved AUC values greater than 0.9, indicating excellent predictive performance. When True Skill Statistic (TSS) and Continuous Boyce Index (CBI) were used as complementary evaluation metrics, all models yielded TSS values ranging from 0.783 to 0.805 and CBI values between 0.693 and 0.717. These results demonstrate that the models possess reliable discriminatory power in predicting the suitable habitat distribution of L. gracile. Detailed results are presented in Table 2. The MESS map indicated that the majority of predicted suitable habitats fall within the environmental space of the training data, with only minor regions showing negative MESS values, suggesting limited extrapolation. These results support the reliability of the Maxent projections in our study (Fig. 2).
Multivariate environmental similarity surface (MESS) analysis for L. gracile under different combinations of climate change scenarios. (A) SSP 245 2041–2060; (B) SSP245 2061–2080; (C) SSP 585 2041–2060; (D) SSP 585 2061–2080. (Created with ArcGIS 10.8.1, URL: https://www.esri.com)
Model analysis
Variables with a cumulative contribution exceeding 85% are generally considered the primary factors influencing a species’ distribution44. Maxent model predictions revealed that under environmental influences alone (Model A), the main variables driving the potential geographic distribution of L. gracile were bio14 (68.2%), t-bs (7%), bio2 (4.3%), elevation (3.9%), and srad5 (3.4%), with a cumulative contribution of 86.8%. When human disturbance factors were included (Model B), the main influencing factors were bio14 (66.2%), t-bs (6.3%), elevation (4.2%), bio2 (3.8%), srad5 (3.3%), and srad6 (3.2%), with a total cumulative contribution of 87%. After adding human disturbance, the contribution rate of bio14 decreased by 2%, t-bs by 0.7%, and bio2 by 0.5%, while the contribution rate of elevation increased slightly. These results suggest that the key environmental factors affecting the distribution of L. gracile include elevation, soil base saturation, precipitation of driest month, and May solar radiation. The response curves of the key factors are shown in Fig. 3.
Habitat prediction for L. gracile under current climate and human disturbance
The study predicted the distribution range of L. gracile under scenarios with and without human disturbance, calculating the habitat area for each suitability level (Table 3).
Under the current climate scenario, the potential habitat area for L. gracile is approximately 181.64 × 10⁴ km², primarily concentrated in southern China. including Taiwan, Hainan, Guangdong, Guangxi, Fujian, Jiangxi, Chongqing, Hunan, southeastern Sichuan, and southern Yunnan. The highly suitable habitat covers an area of approximately 49.78 × 10⁴ km², mainly distributed in eastern Guizhou, southeastern Sichuan, most of Guizhou, Fujian, Zhejiang, and scattered areas in Guangdong, Hunan, and Taiwan. The moderately suitable habitat spans 64.01 × 10⁴ km², predominantly located in western Guangxi, northern Guangdong, most parts of Hunan, Jiangxi, and western Guizhou. The low-suitability habitat comprises 67.85 × 10⁴ km², mainly distributed in southern Jiangsu, southern Yunnan, Hubei, Jiangsu, Anhui, and southern Shaanxi (Fig. 4). When human disturbance factors are included, the suitable habitat for L. gracile shows a contraction trend, primarily concentrated in regions such as Guizhou, Chongqing, Guangxi, Hunan, Fujian, Jiangxi, Zhejiang, Guangdong, Hainan, and Taiwan. Compared to Model A, the total suitable distribution area is reduced by 3.96 × 10⁴ km², with the highly suitable habitat shrinking by 3.28 × 10⁴ km², the moderately suitable distribution area increasing slightly by 0.23 × 10⁴ km², and the generally suitable habitat declining by 0.91 × 10⁴ km².
Predicted distribution of L. gracile under different scenarios. (1) With human influence (Model B); (2) Without human influence (Model A). Suitability levels are based on the Maxent default thresholds: Unsuitable (0–0.124), Generally Suitable (0.124–0.388), Moderately Suitable (0.388–0.664), and Highly Suitable (0.664–1). (Created with ArcGIS 10.8.1, URL: https://www.esri.com)
Shifts in the spatial distribution of L. gracile under various climate change scenarios
Under future climate conditions, the predicted suitable habitat area for L. gracile remains largely consistent with its current distribution, primarily located in southern China (Fig. 5). However, both the area and the spread of suitable habitats undergo changes under different future climate scenarios.
Under future climate scenarios, L. gracile is projected to experience a substantial reduction in highly suitable habitats across all time periods and emission pathways, posing serious challenges for conservation planning (Fig. 6). Under the SSP2-4.5 scenario, the total suitable habitat area is predicted to increase slightly from current levels, reaching 208.05 × 10⁴ km² in 2041–2060 and 210.87 × 10⁴ km² in 2061–2080. However, the highly suitable habitats decline markedly, shrinking to 34.82 × 10⁴ km² and 29.60 × 10⁴ km² in the respective periods—representing losses of 14.96 × 10⁴ km² and 20.18 × 10⁴ km² relative to the baseline. In contrast, under the high-emission SSP5-8.5 scenario, although the total suitable area initially expands to 215.17 × 10⁴ km² by 2041–2060, the highly suitable habitat contracts significantly to 26.45 × 10⁴ km², a net loss of 23.33 × 10⁴ km². By 2061–2080, intensified warming leads to a further collapse in habitat quality, with highly suitable areas reduced to just 15.59 × 10⁴ km², accounting for only 8.38% of the total suitable range (186.06 × 10⁴ km²), and representing a 68% decrease compared to current conditions. As shown in Fig. 5, these highly suitable areas become increasingly fragmented, particularly under SSP585 in the late century. These findings underscore the urgency of implementing targeted conservation strategies to safeguard residual refugia under accelerating climate change.
Predicted distribution of L. gracile at different time periods under two different future emission scenarios. (A) SSP 245 2041–2060; (B) SSP245 2061–2080; (C) SSP 585 2041–2060; (D) SSP 585 2061–2080. (Created with ArcGIS 10.8.1, URL: https://www.esri.com)
Shift in the distribution centroid of L. gracile
The study utilized the geographic distribution measurement tool in ArcGIS to calculate the distribution centroids of L. gracile for different time periods (Fig. 7). Under the current climate scenario, the distribution centroid is located in western Hunan Province, with coordinates at 111.02°E, 27.61°N. Under the SSP245 scenario, the distribution centroid remains in Hunan Province, leaning toward the western region. During the 2041–2060 period, the centroid shifts to 110.88°E, 27.59°N, migrating 13.97 km westward compared to the current climate scenario. In the 2061–2080 period, the centroid moves further to 110.72°E, 27.41°N, representing a 25.49 km shift southwestward compared to the 2041–2060 period. Under the SSP585 scenario, the centroid migration pattern is slightly different from that of SSP245 but still trends westward overall. During the 2041–2060 period, the centroid shifts 25.75 km southwestward, with coordinates at 110.91°E, 27.40°N. In the 2061–2080 period, the migration direction changes toward the northwest, resulting in an additional 41.32 km shift from the 2041–2060 period, with the centroid located at 110.60°E, 27.65°N.
Predicted distribution of L. gracile at different time periods under two different future emission scenarios (SSP245 and SSP585) (Created with ArcGIS 10.8.1, URL: https://www.esri.com))
Discussion
As global warming intensifies, climatic conditions continue to deteriorate, making it critical to predict species distribution trends under future climate change, particularly for biodiversity conservation45. Human activities significantly influence plant distributions, and understanding these impacts is essential for protecting species diversity. Such predictions enable a better understanding of species’ adaptability and vulnerabilities, which in turn facilitates the implementation of appropriate conservation measures. Given its medicinal importance, predicting the potential distribution of L. gracile is of high practical value. This study utilized the Maxent model and ArcGIS tools to predict the potential distribution of L. gracile in China, highlighting the impacts of climate change and human activities on its distribution. It also identified optimal habitats and priority areas for conservation. These results are vital for resource preservation and maintaining ecological balance.
The Maxent models developed in this study have demonstrated robust predictive performance, with AUC values exceeding 0.9 and high TSS and CBI scores, indicating reliable identification of suitable habitats for L. gracile. MESS analysis further confirmed that model extrapolation was limited, with most projections falling within the range of training environmental conditions, supporting the credibility of our predictions under current and future scenarios.
The results of the Maxent model indicate that, under the combined effects of climate change and human disturbances, the most critical variables affecting L. gracile’s potential distribution are bio14, elevation, t-bs, srad4, and bio2. Response curves show that optimal precipitation in the driest month for L. gracile is above 15 mm, with a favorable range of 15–19 mm; beyond 19 mm, the marginal benefits plateaus. Southern China experiences dry winters, and studies have shown that a winter precipitation increase of just 6.5 mm can boost the biomass of Bromus tectorum L. by 40%46, highlighting the importance of even slight increases in precipitation for the growth of Poaceae during drought months. Yang et al. demonstrated that precipitation in the driest month significantly limits the global distribution of Setaria italica (a species within the Poaceae family)47. This finding highlights the potential importance of aridity thresholds for other Poaceae plants, suggesting that drought resistance may be a critical adaptive trait across the family.
Additionally, the optimal mean diurnal temperature range (bio2) for L. gracile lies between 5 °C and 8.5 °C. In a study by Cordobés et al.., higher suitability of the Poaceae species Imperata was observed in regions with smaller diurnal temperature fluctuations, suggesting a preference for more thermally stable environments48. Following the principle of ecological similarity, this may also indicate that L. gracile is better adapted to habitats with reduced daily temperature variability. Research indicates that the suitable elevational range for L. gracile spans from 80 to 1200 m. Correspondingly, Hasigerili et al.. reported that L. gracile exhibits a stress-tolerant (S-selected) adaptive strategy, suggesting considerable ecological plasticity in response to varying altitudinal microenvironments49. This broad elevational distribution likely reflects the species’ adaptability to diverse habitat conditions and offers important insights into its ecological amplitude across altitude gradients.
Soil saturation is another key factor, with an optimal range of 7–54%. Within 16–24%, its effect on L. gracile growth is minimal; between 24 and 34%, suitability improves; but beyond 34%, saturation becomes unfavorable. Related research on the rice variety Mazandaran has shown that treatments involving no-tillage with permanent soil saturation, stacked 60 cm water levels, and continuous irrigation under no-tillage conditions produced optimal growth outcomes, further emphasizing the importance of soil moisture conditions for Poaceae plants50. Solar radiation, as the primary driver of photosynthesis, also significantly influences energy fluxes and plant productivity51. Studies have shown that solar radiation impacts dry matter accumulation in Pennisetum purpureum Schum., and that the stem elongation rate of this species varies with seasonal changes in solar radiation52. For L. gracile, the optimal solar radiation levels in May and June are below 17,000 and 18,200 KJ/m²/day, respectively. Maximum suitability occurs at approximately 15,000 KJ/m²/day in May and 15,950 KJ/m²/day in June, beyond which the positive influence declines.
Human activities are reshaping the globe, serving as a significant factor in species distribution changes. For different species, human activities may either promote or inhibit distribution shifts53. L. gracile primarily grows in densely populated southern China, where human disturbance is severe. The study shows that under human disturbances, suitable habitats for L. gracile decreased by 2.18%. This may result from habitat degradation caused by rapid population growth and economic development, leading to biodiversity loss and reduced habitat quality54,55.
The Maxent model predicts that the potential habitats of L. gracile are mainly located in provinces such as Hunan, Guizhou, Guangxi, Guangdong, Zhejiang, Chongqing, Fujian, Hainan, and Taiwan. Under future climate scenarios, the area of suitable habitat is expected to shrink. As species adapt to climate change, many tend to migrate to higher latitudes56,57. A study on European forest plants found that 39% of plant species migrated westward, with an average speed of 3.56 km per year. The study points out that this migration is mainly related to environmental changes such as nitrogen deposition, not just climate change58. This study examined the centroid of L. gracile’s suitable habitats using ArcGIS. Under SSP245 and SSP585 scenarios, the centroid of L. gracile’s suitable habitats exhibited a general westward and higher-latitude migration trend, consistent with predictions.
Despite incorporating environmental factors such as climate, terrain, and solar radiation, the study has limitations. Human activities and climate warming may elevate the frequency and intensity of extreme weather events, resulting in habitat degradation and loss. However, the Maxent model, which is based on species presence-only data and assumes environmental stability, may have limited ability to account for such abrupt and unpredictable changes. This could lead to an overestimation of suitable habitats, as the model does not fully capture the impacts of short-term climatic extremes, such as heatwaves, droughts, or sudden floods. In addition, uncertainties inherent in future climatic datasets—including variability among emission scenarios, model structures, and downscaling methods—may also affect habitat projections and should be considered when interpreting the model outputs. As a consequence, the actual distribution of L. gracile’s suitable habitats may be smaller than predicted. Based on these findings, bio14, elve, bio2, and solar radiation in May and June are key factors that should be prioritized in future conservation and cultivation efforts. These insights can guide the development of physiological regulation strategies to better protect wild L. gracile germplasm resources under both current and projected climate conditions.
Conclusion
This study employed the Maxent model to assess the combined impacts of climate change and human activities on the potential distribution of the medicinal plant L. gracile. Our results revealed that key environmental variables influencing its distribution include precipitation during the driest month, elevation, soil saturation, and solar radiation. Currently, L. gracile is predominantly found in southern China, with Hunan, Guangxi, and Fujian identified as highly suitable regions for cultivation. However, projections under future climate scenarios indicate a significant reduction in suitable habitat, especially in areas currently classified as highly suitable. In addition, human disturbances have exacerbated habitat fragmentation and environmental degradation, further limiting the species’ viable range.
These findings highlight the need for specific conservation actions and policy interventions. Priority should be given to establishing or expanding protected areas in core high-suitability zones, especially in Hunan and Guangxi, to preserve remnant natural populations. Restoration of degraded habitats—such as reforestation with native species and water retention management in southern hills—can help enhance microclimatic stability and soil moisture, improving resilience to climate extremes. In agricultural contexts, sustainable land-use strategies like agroforestry integration and seasonal shading or irrigation adjustments may support the cultivation of L. gracile in marginally suitable areas. Moreover, incorporating species distribution predictions into regional land-use planning can help avoid infrastructure expansion into future refugia.
By providing both ecological insights and practical guidance, this study not only supports the long-term conservation and utilization of L. gracile, but also contributes to broader biodiversity protection strategies under the pressures of global environmental change.
Data availability
The datasets generated during and/or analysed during the current study are available in the GitHub repository, [https://github.com/rongchunhan/Lophatherum-gracile].
References
Xu, L. et al. Impacts of climate change and human activity on the potential distribution of Aconitum Leucostomum in China. Sci. Total Environ. 912 https://doi.org/10.1016/j.scitotenv.2023.168829 (2024).
Walther, G. R. et al. Ecological responses to recent climate change. Nature 416, 389–395. https://doi.org/10.1038/416389a (2002).
Hughes, L. Biological consequences of global warming: is the signal already apparent? Trends Ecol. Evol. 15, 56–61. https://doi.org/10.1016/S0169-5347(99)01764-4 (2000).
Parmesan, C. & Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. https://doi.org/10.1038/nature01286 (2003).
Jump, A. S. & Peñuelas, J. Running to stand still: adaptation and the response of plants to rapid climate change. Ecol. Lett. 8, 1010–1020. https://doi.org/10.1111/j.1461-0248.2005.00796.x (2005).
Wiens, J. J. Climate-Related local extinctions are already widespread among plant and animal species. PLoS Biol. 14, e2001104. https://doi.org/10.1371/journal.pbio.2001104 (2016).
Scanes, C. G. Human activity and habitat loss: destruction, fragmentation, and degradation. In Animals and Human Society (ed Scanes, C. G.) 451–482 (Academic, (2018).
Sala, O. E. et al. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774. https://doi.org/10.1126/science.287.5459.1770 (2000).
Foley, J. A. et al. Global consequences of land use. Science 309, 570–574. https://doi.org/10.1126/science.1111772 (2005).
Hansen, J. E. & Sato, M. Trends of measured climate forcing agents. Proc. Natl. Acad. Sci. U. S. A. 98, 14778–14783, (2001). https://doi.org/10.1073/pnas.261553698
Chinese academy of sciences editorial committee of flora of China. In flora of China. (ed. Wu, Z. Y.) 9, 35. Beijing, (2002).
Zhang, R., Wang, Y. & Shen, S. Chloroplast genome structure and phylogenetic position of Lophatherum gracile. Mitochondrial DNA B Resour. 5, 2958–2959. https://doi.org/10.1080/23802359.2020.1845577 (2020).
Li, Q., Liu, Y., Bao, H. & Zhang, H. The application of Lophatherum gracile Brongn flavonoids in wheat flour products: effects on the structural and functional characteristics of wheat dough. Foods 13 https://doi.org/10.3390/foods13162556 (2024).
He, H. et al. Effects of pretreatment, NaOH concentration, and extraction temperature on the cellulose from Lophatherum gracile Brongn. Int. J. Biol. Macromol. 190, 810–818. https://doi.org/10.1016/j.ijbiomac.2021.09.041 (2021).
Tang, Q. et al. Simultaneous determination of 10 bioactive components of Lophatherum gracile Brongn by HPLC-DAD. J. Chromatogr. Sci. 53, 963–967. https://doi.org/10.1093/chromsci/bmu160 (2015).
Chen, Y. L., Chen, C. Y., Lai, K. H., Chang, Y. C. & Hwang, T. L. Anti-inflammatory and antiviral activities of flavone C-glycosides of Lophatherum gracile for COVID-19. J. Funct. Foods. 101, 105407. https://doi.org/10.1016/j.jff.2023.105407 (2023).
Liu, X. et al. Spectrum-effect relationship between ultra-high-performance liquid chromatography fingerprints and antioxidant activities of Lophatherum gracile Brongn. Food Sci. Nutr. 10, 1592–1601. https://doi.org/10.1002/fsn3.2782 (2022).
Chen, L. F. et al. Antiviral activity of ethanol extract of Lophatherum gracile against respiratory syncytial virus infection. J. Ethnopharmacol. 242 https://doi.org/10.1016/j.jep.2018.10.036 (2019).
Kim, A., Im, M., Gu, M. J. & Ma, J. Y. Ethanol extract of Lophatheri herba exhibits anti-cancer activity in human cancer cells by suppression of metastatic and angiogenic potential. Sci. Rep. 6 https://doi.org/10.1038/srep36277 (2016).
Prakash, S. & Verma, A. K. Anthropogenic activities and biodiversity threats. Int. J. Biol. Innov. 4, 94–103. https://doi.org/10.46505/IJBI.2022.4110 (2022).
Srivastava, V., Lafond, V. & Griess, V. C. Species distribution models (SDM): applications, benefits and challenges in invasive species management. CABI Reviews. 1–13. (2019). https://doi.org/10.1079/PAVSNNR201914020 (2019).
Phillips, S. J. et al. Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol. Appl. 19, 181–197. https://doi.org/10.1890/07-2153.1 (2009).
Heikkinen, R. K. et al. Methods and uncertainties in bioclimatic envelope modelling under climate change. Prog Phys. Geogr. Earth Environ. 30, 751–777. https://doi.org/10.1177/0309133306071957 (2006).
Guisan, A. & Thuiller, W. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 8, 993–1009. https://doi.org/10.1111/j.1461-0248.2005.00792.x (2005).
Aiello-Lammens, M. E., Boria, R. A., Radosavljevic, A., Vilela, B. & Anderson, R. P. SpThin: an R package for Spatial thinning of species occurrence records for use in ecological niche models. Ecography 38, 541–545. https://doi.org/10.1111/ecog.01132 (2015).
Boria, R. A., Olson, L. E., Goodman, S. M. & Anderson, R. P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 275, 73–77. https://doi.org/10.1016/j.ecolmodel.2013.12.012 (2014).
Pinochet, F. & Thesis, P. D. Regional Climate under SSP245 and SSP585. St. Francis Xavier University (2024). https://hdl.handle.net/20.500.14648/65356
Nachtergaele, F. et al. FAO,. Harmonized world soil database version 2.0. (ed. Nachtergaele, F) 1–3 (2023).
Venter, O. et al. Global terrestrial human footprint maps for 1993 and 2009. Sci. Data. 3 https://doi.org/10.1038/sdata.2016.67 (2016).
Liu, L. A. O. et al. Predicting the potential distribution of Aralia chinensis L. (Wild vegetable) in China under different climate change scenarios. Biology 13 https://doi.org/10.3390/biology13110937 (2024).
Cao, G. et al. Prediction of the potentially suitable areas of eucommia ulmoides Oliver in China under climate change based on optimized Biomod2 and maxent models. Front. Plant. Sci. 15 https://doi.org/10.3389/fpls.2024.1359271 (2024).
Phillips, S. J., Anderson, R. P. & Schapire, R. E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 190, 231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026 (2006).
Merow, C., Smith, M. J. & Silander, J. A. Jr A practical guide to maxent for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography 36, 1058–1069. https://doi.org/10.1111/j.1600-0587.2013.07872.x (2013).
Dormann, C. F. et al. Collinearity: a review of method8s to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x (2013).
Lobo, J. M., Jiménez-Valverde, A. & Real, R. AUC: a misleading measure of the performance of predictive distribution models. Glob Ecol. Biogeogr. 17, 145–151. https://doi.org/10.1111/j.1466-8238.2007.00358.x (2008).
Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob Ecol. Biogeogr. 21, 498–507. https://doi.org/10.1111/j.1466-8238.2011.00683.x (2012).
Wunderlich, R. F., Lin, Y. P., Anthony, J. & Petway, J. R. Two alternative evaluation metrics to replace the true skill statistic in the assessment of species distribution models. Nat. Conserv. 35, 97–116. https://doi.org/10.3897/natureconservation.35.33918 (2019).
Fielding, A. H. & Bell, J. F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 24, 38–49. https://doi.org/10.1017/S0376892997000088 (1997).
Pearson, R. G., Raxworthy, C. J., Nakamura, M. & Townsend Peterson, A. ORIGINAL ARTICLE: predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J. Biogeogr. 34, 102–117. https://doi.org/10.1111/j.1365-2699.2006.01594.x (2007).
Shabani, F., Kumar, L. & Ahmadi, M. Assessing accuracy methods of species distribution models: AUC, specificity, sensitivity and the true skill statistic. GJHSS 18 (1) (2018). https://globaljournals.org/GJHSS_Volume18/2-Assessing-Accuracy-Methods.pdf
Allouche, O., Tsoar, A. & Kadmon, R. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 43, 1223–1232. https://doi.org/10.1111/j.1365-2664.2006.01214.x (2006).
Boyce, M. S., Vernier, P. R., Nielsen, S. E. & Schmiegelow, F. K. A. Evaluating resource selection functions. Ecol. Model. 157, 281–300. https://doi.org/10.1016/S0304-3800(02)00200-4 (2002).
Lu, Z. et al. TSSr: an R package for comprehensive analyses of TSS sequencing data. NAR Genom Bioinform. 3, lqab108. https://doi.org/10.1093/nargab/lqab108 (2021).
Yang, S. et al. Impacts of environment and human activity on grid-scale land cropping suitability and optimization of planting structure, measured based on the maxent model. Sci. Total Environ. 836 https://doi.org/10.1016/j.scitotenv.2022.155356 (2022).
Araújo, M. B. & Rahbek, C. How does climate change affect biodiversity? Science 313, 1396–1397. https://doi.org/10.1126/science.1131758 (2006).
Rinella, M. J., Vermeire, L. T. & Angerer, J. P. Integrating experiments and monitoring reveals extreme sensitivity of invasive winter annuals to precipitation. Ecol. Appl. 34, e3051. https://doi.org/10.1002/eap.3051 (2024).
Yang, J. et al. Potential global distribution of setaria italica, an important species for dryland agriculture in the context of climate change. PLoS ONE. 19, e0301751. https://doi.org/10.1371/journal.pone.0301751 (2024).
Cordobés, F. M., Robbiati, F. O., Anton, A. M. & Scrivanti, L. R. Phylogeny, evolution and ecological speciation analyses of imperata (Poaceae: Andropogoneae) in the neotropics. Syst. Biodivers. 19, 526–543. https://doi.org/10.1080/14772000.2021.1887959 (2021).
Hasigerili et al. Intraspecific variations of adaptive strategies of native and invasive plant species along an elevational gradient. Flora 304 https://doi.org/10.1016/j.flora.2023.152297 (2023).
Semeskandi, M. N., Mazloom, P., Arabzadeh, B., Moghadam, M. N. & Ahmadi, T. Evaluation of seedling cultivation and irrigation regimes on yield and yield components in rice plant. Braz J. Biol. 84, e266261. https://doi.org/10.1590/1519-6984.266261 (2022).
Durand, M. et al. Diffuse solar radiation and canopy photosynthesis in a changing environment. Agric. Meteorol. 311 https://doi.org/10.1016/j.agrformet.2021.108684 (2021).
Ferraris, R., Mahony, M. & Wood, J. Effect of temperature and solar radiation on the development of dry matter and attributes of elephant grass (Pennisetum purpureum Schum). Aust J. Agric. Res. 37, 621–632. https://doi.org/10.1071/AR9860621 (1986).
McKinney, M. L. & Lockwood, J. L. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14, 450–453. https://doi.org/10.1016/S0169-5347(99)01679-1 (1999).
Yohannes, H., Soromessa, T., Argaw, M. & Dewan, A. Spatio-temporal changes in habitat quality and linkage with landscape characteristics in the Beressa watershed, blue nile basin of Ethiopian highlands. J. Environ. Manage. 281, 111885. https://doi.org/10.1016/j.jenvman.2020.111885 (2021).
Sun, X., Li, Y., Hu, Y. & Li, Y. Human and natural factors affect habitat quality in ecologically fragile areas: evidence from Songnen plain, China. Front. Plant. Sci. 15, 1444163. https://doi.org/10.3389/fpls.2024.1444163 (2024).
Huntley, B. How plants respond to climate change: migration rates, individualism and the consequences for plant communities. Ann. Bot. 67, 15–22. https://doi.org/10.1093/OXFORDJOURNALS.AOB.A088205 (1991).
Muluneh, M. G. Impact of climate change on biodiversity and food security: a global perspective-a review Article. Agric. Food Secur. 10 https://doi.org/10.1186/s40066-021-00318-5 (2021).
Pieter, S. et al. Unexpected Westward range shifts in European forest plants link to nitrogen deposition. Science 386, 193–198. https://doi.org/10.1126/science.ado0878 (2024).
Funding
This work was supported by Anhui Provincial Education Department (2024AH040161), Anhui University of Chinese Medicine (2023LCTH18), Research Funds of Joint Research Center for Chinese Herbal Medicine of Anhui of IHM (yjzx2023004), Anhui Provincial Administration of Traditional Chinese Medicine (2024CCCX273), Fuyang Health Commission (FY2023-007), and Fuyang Science and Technology Bureau (FK20245529).
Author information
Authors and Affiliations
Contributions
Author Contributions: Conceptualization, X.T and X.R.; data curation, Z.L. and J.O.; formal analysis, Z.L., G.W. and L.Y.; funding acquisition, X.T. and C.Z.; investigation, Z.L., Y.S., L.Y., L.H., Z.F.; methodology, Z.L., S.H.; software, Z.L., L.H., R.H.; validation, X.T. and X.R.; supervision, X.T and C.Z.; writing—original draft, Z.L., G.W., L.Y., L.H., Z.F., S.H., J.O., C.Z.; writing—review and editing, G.W., Y.S., R.H., X.T. and X.R. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lu, Z., Wang, G., Shao, Y. et al. Assessing the impacts of climate change and human activities on distribution of Lophatherum gracile in China using the maxent model. Sci Rep 15, 29945 (2025). https://doi.org/10.1038/s41598-025-15693-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15693-3