Abstract
Callosobruchus maculatus (F.) is a serious pest that causes post-harvest losses, which is a threat to global food security, therefore there is need to develop sustainable pest management strategies. This study investigates the synergistic insecticidal effects of zinc-loaded zeolite nanoparticles in combination with essential oils from Rosmarinus officinalis (L.) and Pimpinella anisum (L.) against C. maculatus adults and their progeny. Zeolite-A and zeolite-X were synthesized and characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS), and found to be highly crystalline and successfully zinc functionalized. The chemical profiles of the essential oils were elucidated by gas chromatography-mass spectrometry (GC-MS). The results showed that zeolites alone had moderate insecticidal activity against the tested insect. Zeolites loaded with zinc enhanced insecticidal activity on C. maculatus. Combining zeolites with essential oils further increases insecticidal activity, with LC50 values ranging from 161 to 306 mg/kg. Zeolite nanoparticles and P. anisum essential oil formulation was the most effective in killing C. maculatus adults and progeny. Co-toxicity factor analysis indicated that there were synergistic effects between the essential oils and zeolites, especially between P. anisum and Zn-zeolite-A. Morphological examination of treated C. maculatus adults via SEM revealed cuticle abrasions, desiccation areas, and damage to sensilla, indicating a physical mode of action for the zeolites. This study suggests that zeolite nanoparticles and essential oil combinations can be used as eco-friendly insecticides for the management of C. maculatus in stored cowpea seeds.
Similar content being viewed by others
Introduction
Grain legumes are crucial in providing nutrition to both humans and animals across the globe1. Cowpeas, Vigna unguiculata (L.) (Fabales: Fabaceae), stand out as an essential grain legume, offering a rich energy source, protein, vitamins, minerals, and dietary fibre. Sprouting legumes improves nutritional absorption and digestion, making them significant in human nutrition2. Incorporating whole-grain cereals and grain legumes can act as a meat alternative in terms of being a protein, zinc, and iron source. Furthermore, this combination can enhance dietary fibre and folate intake, which are often inadequately consumed3. Cowpeas are an essential crop for food security but are vulnerable to insect damage4. Infestations by Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae) can lead to post-harvest losses that threaten sustainable food security and cause significant economic harm to farmers worldwide. This pest damages stored seeds, resulting in reduced nutritional quality, decreased seed viability, dry weight loss, and diminished market value5.
The predominant approach for managing cowpea bruchid in storage places is through the use of fumigants such as phosphine and other chemical insecticides. Although synthetic insecticides are considered effective in controlling insect pests, their long-term environmental impact, leftover toxicity in food, and the development of insect resistance have necessitated the development of safer alternatives6. Additionally, these insecticides can be hazardous to users7. Consequently, there has been a shift toward exploring natural products as substitutes for traditional insecticides8,9. Research has focused on the potential of plant essential oils and inert dust as viable options for controlling pests instead of relying on chemical control methods10. Plant-derived essential oils are organically produced secondary metabolites currently employed in various applications, including fragrance, cosmetics, herbal medicine, and nutrition.
These oils can also serve as eco-friendly insecticides11. Various studies have concentrated on utilizing essential oils extracted from plants to manage insect pests in stored grains12,13,14. R. officinalis (L.) (Lamiales: Lamiaceae) and P. anisum (L.) (Apiales: Apiaceae) essential oils have been found to be effective against stored product insects as a repellent, fumigant, and contact insecticides due to their active ingredients like 1,8-cineole, camphor, and anethole15,16. However, plant essential oils have the potential to leave a long-lasting scent. Their high concentration could cause food to possess an overpowering aroma and unpleasant taste17. However, when essential oils are used in low quantities, the protective impact may be lessened since certain active elements may move into the environment. This poses a challenge when using botanical essential oils as grain treatments, as their efficacy against insects needs to be increased while decreasing residues in food commodities18.
Inert dusts are utilized to manage insects in stored grain. Unlike conventional contact insecticides, inert dusts work through their physical characteristics, which often results in a slower response time19,20. Zeolites are microporous, crystalline aluminosilicates that can be found naturally or synthesized21. They have a wide range of applications in agriculture, including improving soil properties, promoting plant growth, increasing metal content in plant aerial parts, serving as food additives for domestic animals, and acting as carriers for fertilizers22. Zeolites can effectively control insect populations by abrading or adsorbing the epicuticular lipids on their bodies. This causes the insects to lose water and eventually die from dehydration23. Hard, non-sorptive particles are responsible for the abrasion of epicuticular lipids, while sorptive particles are responsible for their adsorption6. Zeolites are generally considered safe for the environment, animals, and humans. They are listed as safe for human consumption by the FDA and are classified as non-toxic by the World Health Organization’s International Agency for Research on Cancer24,25. Like other inert dusts, zeolites take some time to cause insect mortality. Moreover, high application rates of zeolites can alter stored grains’ bulk density and physical properties22. One potential solution to this issue is combining essential oils with low concentrations of zeolites. Zinc is one of the most commonly used ions to be mixed with zeolite structure to produce biological activity, owing to its excellent stability and the broad spectrum of efficiency against numerous types of bacteria, viruses, germs, and fungi26.
In this study, zeolites were combined with essential oils as a novel approach to gain a rapid action of inert dusts against stored product insects. Also, the study aimed to mitigate odors caused by high concentrations of essential oils. Through comprehensive experimentation and analysis, this research aimed to evaluate the cumulative insecticidal mortality of two types of zeolites (zeolite-A and zeolite-X) and their zinc-loaded zeolites, as well as their formulations with R. officinalis and P. anisum essential oils, against the adults of C. maculatus and their progeny production.
Materials and methods
Plant materials and essential oil extraction
Rosemary leaves, R. officinalis and aerial parts of anise, P. anisum were collected from the medicinal and aromatic plant farm at the National Research Centre, Egypt (latitude 30° 30′ 1.4′′ N, longitude 30° 19′ 10.9′′ E) in May 2022. The plant materials were identified by Prof. Shoukry M. Selim of floriculture, Faculty of Agriculture, Fayoum University. The harvested plant components were washed with tap and distilled water and then dried in a shaded area at room temperature (26 ± 1 °C) for three days. To obtain the herbal oils, 1000 g of leaves from each plant were mixed with 2000 mL of distilled water and subjected to hydro-distillation using a Clevenger apparatus. The resulting oils were dehydrated with anhydrous sodium sulfate and stored in dark bottles at 4 °C until chemical analysis and insecticide formulations.
Essential oil analysis
R. officinalis and P. anisum essential oils were analyzed using gas chromatography-mass spectroscopy (GC-MS). The plant essential oils were mixed with diethyl ether. Then 1 µL of the mixture was injected into the GC-MS, Trace GC Ultra coupled with single quadrupole mass spectrometry, and the TG-5MS fused silica capillary column (30 m, 0.25 mm, and 0.1 mm film thickness). Helium gas was used as a carrier gas at a regular flow rate of 1 mL min− 1. The injector and MS transfer line were both set at 280 °C while the oven temperature was programmed to start at 50 °C (held for 2 min), increase at a rate of 7 °C per minute to 150 °C, and then further increase at a rate of 5 °C per minute to 270 °C (held for 2 min), before finally increasing at a rate of 3.5 °C per minute to 310 °C (held for 10 min). All components were quantified using a percent comparative peak area. The compounds were tentatively identified based on comparing the retention index of the GC peaks obtained using a homologous series of n-alkanes (C8 to C20) with those reported in the literature27. The mass spectra of these components were also matched with those of the GC/MS system’s NIST 08 and WILLY 7 library data.
Insect rearing
Adult cowpea bruchids (C. maculatus) were gathered from a laboratory culture that has been kept free of insecticides since April 2017. A total of 20 female and 20 male bruchids were selected from the culture and placed into 1000 mL glass jars containing 500 g of clean, sterilized cowpea grains (V. unguiculata var. Dokki 331) with a moisture content of 9.7% at a temperature of 27 ± 1 °C, relative humidity of 65 ± 5%, and a light-dark photoperiod of 12 h each. The cowpea seeds were frozen for seven days at -13 °C to eliminate any live insects present before use. The jars were covered with cloth and perforated metal lids to prevent the beetles from escaping while allowing sufficient aeration. After seven days, the parental insects were removed by sieving, and the infested cowpea grains were kept in the laboratory until the F1 progeny emerged. For all bioassays, sexed insects between the ages of 1 to 2 days were utilized.
Preparation and functionalization of zeolites with ZnO nanoparticles
The synthetic zeolites utilized in this study were treated using the procedure described by Youssef, et al.28 under microwave conditions of 3.0 M NaOH, 80 °C, and 110 °C for 2 h to yield zeolite powders of zeolite-A and Faujasite-NaX (zeolite-X), respectively. The zeolite products that were previously obtained underwent functionalization through cation exchange processing, which involved doping the zeolite powder in a solution containing zinc. In a typical procedure, one gram of each zeolite type (zeolite-A and zeolite-X) was placed in 100 mL of a 0.1 M zinc chloride solution and stirred gently at 400 rpm for two hours at room temperature. The resulting mixture was then separated by solid/liquid separation using centrifugation at 3600 rpm and repeatedly washed with distilled water until the pH reached 7. The resulting clean powder was dried at 50 °C in an electric oven for two hours and stored in a dry container until characterization could be performed.
X-ray diffraction of prepared zeolites
The mineral composition of the synthetic product was characterized by the XRD method using BRUKUR D8 ADVANCE with secondary monochromatic beam Cu Kα radiation at KV = 40 and mA = 40.
Scanning electron microscope and energy dispersive X-ray identification for zeolites
The mineral composition and internal texture of zeolite-A and zeolite-X, along with their ZnO-exchanged materials, were identified by XRD and SEM model Quanta 250 FEG (Field Emission Gun) attached with EDX Unit (Energy Dispersive X-ray analyses), with accelerating voltage 30 KV, magnification 14 up to 1,000,000, and resolution for Gun.1n. Field Electron and Ion Company, Netherlands.
Toxicity bioassay
Efficiency of zeolites
The toxicity of inert dust, including zeolite-A, zeolite-X, and their ZnO-loaded counterparts, against the adult of C. maculatus was determined using a direct contact assay. Glass jars with a volume of 500 mL were filled with 100 g of clean cowpeas and treated with zeolites at rates of 250, 500, 750, and 1000 mg/kg. An untreated lot was used as a control. The jars were shaken by hand and then by a rotary mixer for 10 min. Thirty sexed adults of C. maculatus (15 males and 15 females) were added to each jar vessel. The jars were tightly closed with cotton cloth to prevent insect escape and ensure proper aeration. Each treatment was replicated four times. Mortality rates were recorded after 2, 5, and 7 days of contact with treated cowpeas. To assess the treatments’ efficacy on progeny production, live and dead individuals were discarded after 7 days, and the jars were kept under the same rearing conditions for 30 days. The number of emerged adults of C. maculatus was then counted and expressed as progeny production.
Efficiency of zeolites formulated with essential oils
Zeolite-A, zeolite-X, and their ZnO-loaded forms were combined with R. officinalis and P. anisum essential oils. The formulations were obtained by mixing 100 and 200 mg of each essential oil separately with four types of zeolites (zeolite-A, zeolite-X, Zn-zeolite-A, and Zn-zeolite-X) at 750 and 1000 mg/kg cowpea grains. To ensure even distribution of the zeolite formulations, 1 mL of acetone was added. A control treatment, consisting of cowpea grains treated with 1 mL of acetone only, was included for comparison. After solvent evaporation, the tested insect was exposed to each treatment, and cumulative mortality was recorded after 2, 5, and 7 days. Moreover, the effects of the formulations on insect progeny production were investigated.
Joint action studies
The co-toxicity factor (CTF) was used as a criterion to evaluate the combined lethal effect of the essential oils and zeolites. Mortality percentages corresponding to LC25 were determined from regression lines. The summation of mortality percentages of zeolites and the essential oils was considered as expected mortality. The tested insect was exposed to pairs of the essential oils and zeolite mixtures at their respective LC25 levels. Mortality (%) caused by these mixtures was recorded after 5 days of treatment and considered as observed mortality. A co-toxicity factor was taken as a criterion for evaluation of the joint toxic effect as follows:
CTF = (OM - EM)/EM × 100, where OM is the observed mortality (%) of the mixture and EM (expected mortality) is the sum of mortality (%) caused by each fraction of the mixture when tested individually. A positive CTF value + 20 or higher indicates a synergy effect, a negative CTF value of − 20 or lower indicates an antagonism effect, and values between − 20 and + 20 imply an additive effect29.
Scanning electron microscope for C. maculatus
SEM was used to examine C. maculatus adults treated with either zeolites or zeolites loaded with zinc at 1000 mg/kg. The dead adults were air-dried for 5 days without any further preparations to ensure the zeolites remained on them. The gold coating process was performed on the samples using Quorum Q 150 ES, United Kingdom, with a 20 nm thick layer of gold applied for 60 s. The untreated and treated dead adults were examined using the TESCAN VEGA 3 electron scan microscope, Czech Republic. The beetle’s whole body’s dorsal and ventral surfaces were observed and captured in photographs.
Data analysis
Before analysis, the mortality data underwent Arcsin transformation. The data was then separately examined using one-way analysis of variance (ANOVA). Duncan’s New Multiple Range Test was utilized at a significance level of p < 0.05 to determine the significance of mean differences. To obtain the LC50 values, the concentration–mortality data from laboratory tests was analyzed after 5 d using Probit. All serial concentrations of the essential oils and zeolite combinations had 100 mg/kg of the essential oil. The values of LC50 were found to be significantly different if the 95% confidence limit was not crossed. Statistical analysis was performed using XLSTAT Addinsoft 2021.2.2 software.
Results
The chemical constituents of essential oils
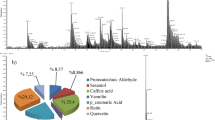
Table 1 shows the chemical composition of essential oils extracted from R. officinalis and P. anisum using GC-MS. Based on a fresh plant of R. officinalis weight of extract part, the hydro-distillation yielded about 0.3% w/w. Twenty compounds have been identified, representing 97.23% of the essential oil. These compounds were divided into 43.11% monoterpene hydrocarbons (α-thujene, α-pinene, Camphene, β-pinene, myrcene, γ-terpinene, terpinolene, α-phellandrene, and cymene); 47.81% oxygenated monoterpenes (1,8-cineole, linalool, trans-pinocarveol, Camphor, borneol, terpinen-4-ol, and α-terpineol); 4.59% of sesquiterpene hydrocarbons (caryophyllene, aromadendrene, and humulene); 1.72% of ketone (3-octanone). The major compounds of R. officinalis essential oil were 1,8-cineole (25.36%), α-pinene (23.75%), Camphor (12.66%), and Camphene (8.19%). For P. anisum essential oil, with a yield of 0.35% w/w based on the sample’s fresh weight, twenty-one compounds were recorded, accounting for 96.45%. The essential oil analysis revealed that the oil had a lower quantity of monoterpene hydrocarbons of 3.95% (pinene, carene, limonene, ocimene, and terpinene). The essential oil had rich amounts of oxygenated monoterpenes of 74.71% (linalool, Methyl chavicol, Z-anethole, and E-anethole). The amount of sesquiterpene hydrocarbons was 15.62% (isoledene, longifolene, cedrene, thujopsene, gurjunene, elemene, guaiene, himachalane, and E-isoeugenol), while the oxygenated sesquiterpenes recorded 2.17% (cis-sesquisabinene hydrate, spathulenol, and geranyl isovalerate). P. anisum essential oil contained a high percentage of E-anethole (64.23%), followed by methyl chavicol (8.69%) and longifolene (5.08%). The two oils also differed in their terpene hydrocarbon content. R. officinalis had a higher proportion of monoterpene hydrocarbons (43.11%) than P. anisum (3.95%). In contrast, P. anisum has a higher percentage of oxygenated monoterpenes (74.71%) than R. officinalis (47.81%).
Characterizations of the synthetic zeolites
Figure 1A and B presents the parent zeolite (zeolite-A and zeolite-X). Zeolites mineral profiles were compared to the XRD database and showed perfect matching with PDF card # 73-2340, with Na12Al12Si12O48.27H2O for zeolite-A (Fig. 1A), and PDF # 39-1380 (1), with Na2Al2Si4O12.8H2O composition for zeolite-X (Fig. 1B), in respective order. The distinct, sharp, and complete set of both zeolites’ peaks implied good crystallinity. Notably, the synthetic product contains some residue of quartz mineral, which was traced back to the kaolin precursor from which they were formed. Meanwhile, Faujasite-NaX showed a small number of nanoparticle zeolite peaks that seemed to accompany the originally developed zeolite-X material, and this could be seen in light of the similarity in preparation conditions of both zeolites.
Figure 1C and D shows XRD details for Zn-doped zeolites. Both charts compared to the standard references of the PDF-2 database and confirmed the evolution of Zn-doped types of Zn-zeolite-A and Zn-zeolite-X, having respective chemical compositions of Na50Zn23Al96Si96O384.216H2O (Fig. 1C) and (Zn, Na)2Al2Si2.5O9.72H2O (Fig. 1D), respectively. As shown in Fig. 1 (C-D) the XRD patterns for Zn-containing phases indicated sharper peaks with higher intensities than those recorded for their un-doped forms (Fig. 1A and B). In addition, zeolite-X implies the presence of minor amounts of zeolite nanoparticles as a secondary accompanying phase that can develop in the zeolite mixtures. This indicates very high crystallinity of many pure phases with no residues of the amorphous metakaolin precursor, which was preserved within the synthetic zeolite powders and appeared in the XRD patterns in the form of a humpy background in Fig. 1 (A and B).
Internal structure testing (SEM and EDS)
The internal textural analysis of zeolite product and its chemical microanalysis give a clear idea about the characteristic morphology and elemental contents of the contained crystals. The internal crystalline texture and the elemental micro-chemical analysis of the dry, unfunctionalized zeolite-A and zeolite-X products were examined using the SEM and EDS tools. The obtained data are given in Fig. 2A and B and Table 2. As can be noticed from Fig. 2A, zeolite-A exhibited its distinctive cubic-shaped crystal form with uniform grain particles in the range of 1–3 μm in size.
Figure 2B presents the obtained product of Faujasite-NaX (zeolite-X). The micrograph monitors large crystals with pyramidal epics of less than 1 μm in size, accompanied by an ample amount of zeolite nanoparticles (10–15%) and some scattered quartz particles (< 5%). The former SEM result for both zeolites is consistent with the previous XRD data. Table 2 demonstrates the elemental composition of the un-doped zeolites, where the calculated average Si/Al ratios of the crystal composition were 1.11 and 1.75 for zeolite-A and zeolite-X, respectively. Figure 3A and B monitors the SEM morphologies of the obtained zeolite-produced powders after the exchange of their sodium constituents by zinc. The micro-chemical composition is given in Table 2. Obviously, there were no destructive changes in the crystal configuration for both zeolites after doping since the crystal identities were preserved without shape alteration. The only notice was the clear crystal faces and edges. The results of the EDS analysis were collected from an average of three measurement detections for the crystal surfaces of three different crystal generations of the same zeolite species. The respective atomic ratio of Si/Al for zeolite-A was 1.07, and for zeolite-X was 1.6 (Table 2).
Efficiency of zeolite nanoparticles on C. maculatus
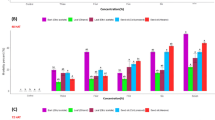
The mortality of C. maculatus treated with zeolites at different durations is presented in Table 3. The mortality of the beetles increased with increasing concentration and duration of exposure for all treatments. The results also showed significant differences in mortality between the different types and concentrations of zeolites at each time interval.
The highest mortality rate achieved by zeolite-X was 48.3% after 7 days at 1000 mg/kg, while it was 43.3% for zeolite-A at the same concentration and time interval. The highest mortality was observed for Zn-zeolite-A (51.7%) at 1000 mg/kg after 7 days. Zeolite-X outperformed zeolite-A in insecticidal activity against C. maculatus, while zeolite-A loaded with zinc surpassed Zn-zeolite-X in insecticidal efficacy.
Efficiency of zeolite nanoparticles and R. officinalis combinations on C. maculatus
The mortality of zeolite and R. officinalis combinations after 2, 5, and 7 days against the tested insect is elucidated in Table 4. The concentration- and time-dependent mortality was evident in all treatments compared to the control group that had minimal mortality (3.3%) even after seven days of exposure. Use of R. officinalis essential oil at a single dose produced moderate insecticidal activity with the higher dose (200 mg/kg) causing a higher mortality of 63.3% on the seventh day, compared to 43.3% at the lower concentration (100 mg/kg). When R. officinalis essential oil was used together with zeolites, a high improvement in insecticidal activity was observed. In most cases, the higher the concentration of the essential oil and the zeolites, the higher the mortality. The mixtures of the high concentration of R. officinalis (200 mg/kg) with any of the tested zeolites at 750 or 1000 mg/kg were the most effective. Interestingly, several treatments (R. officinalis (200 mg/kg) combined with Zeolite-X (both 750 and 1000 mg/kg), Zeolite-A (1000 mg/kg), Zn-zeolite-X (1000 mg/kg), and Zn-zeolite-A (both 750 and 1000 mg/kg)) reached 100% mortality by day seven. The fastest and most efficient treatment was the combination of R. officinalis (200 mg/kg) and Zn-zeolite-A (1000 mg/kg) that led to 100% mortality in only five days of exposure.
Efficiency of zeolite nanoparticles and P. anisum combinations on C. maculatus
The mortality of zeolite and P. anisum combinations after 2, 5, and 7 days against the tested insect is presented in Table 5. The findings indicate that P. anisum and R. officinalis essential oils alone or in combination with zeolites were found to significantly increase the mortality of C. maculatus compared to the control. P. anisum was more effective than R. officinalis at the same concentrations. For example, P. anisum (200 mg/kg) produced 63.3% mortality after 2 days, compared to 43.3% in R. officinalis (200 mg/kg). This was consistent throughout the exposure periods, and the P. anisum treatments tended to produce more rapid and more severe lethal effects. The synergistic mixtures of P. anisum or R. officinalis with zeolites also increased the mortality, especially at increased concentrations (1000 mg/kg). It is worth noting that P. anisum-based formulations, such as P. anisum (200) + Zn-zeolite-A (1000) was able to kill 100% of the larvae in 5 days whereas the most effective R. officinalis combination, R. officinalis (200) + Zn-zeolite-A (1000) took 7 days to kill the larvae to the same extent.
Effect of zeolite nanoparticles on progeny production
The mortality of progeny of C. maculatus exposed to zeolites with and without loaded zinc is recorded in Table 6. The results showed that all zeolite treatments caused a significant increase in the mortality of C. maculatus offspring compared to the control. As the concentration of zeolite increases, the mean number of progeny decreases. None of the concentrations applied could suppress the progeny production. All zeolite treatments showed moderate effects on the mortality of progeny of C. maculatus. Zeolite-X and zeolite-A loaded zinc were the most effective treatments with mortality of offspring of 48.43 and 49.64%, respectively at the highest application rate of 1000 mg/kg.
Effect of zeolite nanoparticles and R. officinalis combinations on progeny production
Data presented in Table 7 show the mortality of progeny of C. maculatus treated with zeolite and R. officinalis combinations. All treatments significantly reduced the mean number of progeny and increased the percentage of offspring mortality of C. maculatus compared to the control. The treatment with R. officinalis essential oil (RO) alone at 100 mg/kg resulted in a mean number of progeny of 42, which is significantly lower than the control group (129). Additionally, the mortality of the progeny under this treatment was 35.7%. Increasing the concentration of R. officinalis essential oil to 200 mg/kg further reduced the mean number of progeny to 21 and increased the mortality percentage to 55.2%. The combination of zeolite and R. officinalis essential oil increased the mortality of progeny compared with zeolite alone. Zeolites loaded with zinc and R. officinalis oil mixtures could suppress progeny production at 200 mg/kg of the essential oil and 1000 mg/kg of zeolite.
Effect of zeolite nanoparticles and P. anisum oil combinations on progeny production
The results of Table 8 demonstrate that the progeny of C. maculatus mortality was greatly affected using P. anisum essential oil, either alone or in combination with various zeolites. The progeny mortality rate of the control group was low at 2.1%. On the other hand, the mortality rate was significantly higher when P. anisum essential oil at 100 and 200 mg/kg was used alone (55.0 and 64.6%, respectively). An interesting synergistic effect was also found when P. anisum essential oil was used together with zeolites. The combination of P. anisum at 200 mg/kg with different zeolites was the most effective treatments. A complete mortality was observed with P. anisum (200 mg/kg) and Zeolite-A (750 mg/kg). Moreover, a number of combinations such as P. anisum (200 mg/kg) and either Zeolite-X (750 and 1000 mg/kg), Zeolite-A (1000 mg/kg), or zinc-loaded zeolites (both 750 and 1000 mg/kg) totally inhibited the development of insect progeny.
Toxicity of zeolites, essential oils, and their combinations on C. maculatus
The results of contact toxicity of zeolites, essential oils, and their combinations against C. maculatus are recorded in Table 9. P. anisum oil exhibited higher toxicity than R. officinalis oil against the tested insect, with LC50 values of 126 and 200 mg/kg, respectively. Zeolite-X and zeolite-A had high LC50 values (1407 and 1658 mg/kg, respectively), suggesting lower toxicity than essential oils. Zinc loading in zeolite-X and zeolite-A showed a slight increase in toxicity. The combinations of essential oils (R. officinalis and P. anisum) with zeolites (zeolite-X, zeolite-A, zeolite-X loaded with zinc, and zeolite-A loaded with zinc) significantly lowered LC50 and LC95 values compared to the individual components alone. The LC50 values for the combinations ranged from 161 to 306 mg/kg. The combination of P. anisum oil with zeolite-A loaded with zinc exhibited the lowest LC50 value (161 mg/kg), suggesting the highest toxicity among the tested combinations..
Combined toxic effect of essential oils and zeolites
The results in Table 10 show the effectiveness of two essential oils, R. officinalis and P. anisum, in combination with different zeolite substrates (natural and Zn-loaded Zeolite). All the binary combinations showed positive co-toxicity factors of 20.9 to 30.0, which is a.
sign of synergism. The combination P. anisum + Zn-zeolite-A was the one that produced the highest observed mortality (65%), and the highest co-toxicity factor (30), thus indicating a very strong joint effect. As a rule, the Zn-modified zeolites proved to be more effective in enhancing mortality compared to their non-modified counterparts. Moreover, mixtures of P. anisum oil always had greater co-toxicity factors than the respective combinations of R. officinalis essential oil, indicating greater synergistic effects.
Effect of zeolite nanoparticles on C. maculatus morphology examined by SEM
The SEM images of untreated and treated adults exposed to cowpea seeds treated with zeolite compounds (1000 mg/kg) are shown in Figs. 4 and 5. Zeolite particles revealed a homogeneous distribution of zeolite particles on the cuticle of C. maculatus adults and aggregation between the thorax and abdomen joints compared with untreated adults. Image analysis showed that zeolite particles adhered to all body parts. The results also showed that zeolite treatments induced scratches on the elytra and clear damage in sensilla scatters in some points and absent in others, leaving spaces between these parts in the ventral surface, compared with the normal cuticle shape in untreated beetles of C. maculatus. Zeolite treatments revealed scratches and splits on the cuticle, leading to water loss through dehydration as the water barrier was damaged and died out of desiccation.
SEM images of Callosobruchus maculatus adults. (A) and (B) Untreated adults’ dorsal and ventral surfaces showing normal cuticle and sensilla shapes. (C) The dorsal surface of adults treated with zeolite-A shows desiccation areas (arrows). (D) The vertical surface shows the aggregation of zeolite-A particles (arrow 1) and desiccation areas (arrow 2). (E) The dorsal surface of adults treated with zeolite-X shows the absence and reduction of the number of sensilla (arrow 1) and desiccation areas on the pronotum (arrow 2). (F) Ventral surface showing aggregation of zeolite-X particles on all body surface.
SEM images of Callosobruchus maculatus adults. (A) The dorsal surface of adults treated with Zn-zeolite-A showed abrasion and distribution of zeolite on the elytra surface and antennae (arrows). (B) The ventral surface shows an aggregation of Zn-zeolite-A particles and desiccation areas on the abdomen (arrows). (C) The head surface shows an aggregation of Zn-zeolite-X particles. (D) The ventral surface of an adult treated with Zn-zeolite-X particles shows the absence and reduction of the number of sensilla (arrows) on the abdomen cuticle.
Discussion
The essential oils of R. officinalis and P. anisum had different chemical profiles, with R. officinalis being rich in oxygenated monoterpenes such as 1,8-cineole, α-pinene, and camphor and P. anisum being dominated by E-anethole, an oxygenated monoterpene with a phenylpropanoid structure. These compounds have been reported as insecticidal, repellent, antifeedant, or oviposition deterrent properties against various insect pests30,31. Previous studies showed that 1,8-cineole demonstrated considerable contact and fumigant toxicity against various insects, like Sitophilus spp, Tribolium castaneum, Rhyzopertha dominica32. Additionally, α-pinene has been found to be more effective than certain commercial products like Detech® (a diatomaceous earth product) against various beetle species33. Wang, et al.34 stated that trans-anethole had insecticidal efficacy and repellent properties against major stored grain pests.
Zeolites are microporous crystalline aluminosilicates stemming from the reaction of volcanic rocks, ash strata, and alkaline underground water19,35. Natural zeolites (alkaline aluminum silicates), depending on their physical characteristics, are the most comparable to diatomaceous earth. Therefore, they can be categorized in the same group as dusts, which contain natural silicates36. The synthetic zeolites prepared from kaolin using the microwave technique had high crystallinity and purity, as confirmed by XRD and SEM analyses. The uniformity of zeolite-A in the present study is due to the microwave synthesis, which resulted in homogeneous heating of all reagents at the same time and produced a notable grain-sized regularity37. The Si/Al ratios of the synthesized zeolites were consistent with the literature values for zeolite-A and zeolite-X38. The cation exchange process successfully doped zeolites with zinc, creating clear crystal faces and edges. This could be due to the corrosive action of acidic Zn-solution on any amorphous relics of metakaolin during the cation exchange process of replacing Na+ with Zn+ 2. The Si/Al atomic ratio data for zeolite-A and zeolite-X concurred with Youssef, et al.39. The diminished amounts of Na+ in both samples have been compensated by the high presence of zinc cations. This can indicate a successful and nearly complete replacement procedure between zeolites’ sodium and the cations of Zn+ 2 in the immersed solution. The preservation of the crystalline identity of each zeolite type is evidence of the high stability of the framework structure of zeolite-A and zeolite-X under the acidic conditions of the cation exchange process. The zinc loading enhances the catalytic activity of zeolite, improves the porosity, and increases the surface area40,41. Zeolitization processing of natural resources usually produces microporous materials containing some relics of the originally treated raw materials37. The advantages of using these natural products are their low toxicity to humans and the environment, their biodegradability, and their potential to reduce insect resistance42,43.
The biological efficiency of zeolite on stored products has been articulated by many authors6,19,44. Zeolite could be used in both open fields and storage facilities45. The current research revealed that the mortality of C. maculatus adults exposed to zeolite-treated cowpeas increased with increasing concentration and exposure time6. Moreover, all the tested zeolite nanoparticles had detrimental effects on the mortality of the tested insect. Zeolites alone or loaded with zinc had moderate insecticidal activity against C. maculatus adults. Zeolite-X was more effective than zeolite-A. Data from SEM showed that zeolite-X contained more silica than zeolite-A. Moreover, it possessed a smaller average particle size compared to the larger crystals of Zeolite-A. This implies that the greater efficacy of Zeolite-X can be explained not only by its chemical composition but also by its physical morphology. The smaller particle size gives a higher surface area-to-volume ratio, which probably increases its capacity to abrade the insect cuticle and adsorb epicuticular lipids, thus hastening the lethal process of desiccation. Our findings are consistent with Julbe and Drobek46who reported that zeolite-A has a lower Si/Al ratio than zeolite-X, which means it has more cation exchange sites and higher exchange capacities. On the other hand, data from XRD demonstrated that zeolite-X contained minor amounts of zeolite nanoparticles, which means lower Zn substitution than zeolite-A. These results emphasize the mortality results for the insect, as Zn-zeolite-A was more efficient than Zn-zeolite-X. The current research elucidated that loading zinc into zeolites enhanced the insecticidal activity against C. maculatus. Zinc loaded on the zeolite surface could exchange with ions in the insect’s body, disrupting the insect’s ionic balance and interfering with their metabolic processes47.
The results showed that zeolites and essential oils’ combinations increased the mortality of C. maculatus adults and progeny production. The results also clarified that the simultaneous application of essential oils and zeolites significantly reduced the required application rate of zeolites. Korunic and Fields48 found that diatomaceous earth and Anethum graveolens L. essential oil combination was more effective at lower concentrations against various stored product insects than diatomaceous earth applied alone. At the same time, these lower concentrations of inert dust caused a much smaller reduction in bulk density than the diatomaceous earth used alone. Our results showed that loading essential oils to zeolite exhibited a longer duration of activity and suppressed egg emergence compared to the use of zeolite alone. The combination of essential oils and mesoporous silicates has been recently introduced to enhance the persistence and toxicity of pest management. For example, Ebadollahi, et al.49 examined the effect of loading Eucalyptus largiflorens essential oil into zeolite as an encapsulated formulation. It was found that the persistence increased from 6 to 17 days for pure and capsulated essential oils, respectively. The current research demonstrated that P. anisum essential oil was more efficient to enhance zeolite efficiency than R. officinalis essential oil. It might be related to the difference in their chemical constituents, where P. anisum essential oil has a higher percentage of oxygenated monoterpenes than R. officinalis essential oil. Moreover, P. anisum essential oil contained high amounts of E-anethole and methyl chavicol. Wang, et al.34 mentioned that trans-anethole recorded 100% mortality against the rusty grain beetle adults Cryptolestes ferrugineus (Stephens) at 30 mL/L. The effectiveness of traditional synthetic insecticides in the control of C. maculatus was emphasized in several studies. These insecticides are usually limited by the development of resistance and environmental hazards. A commercial formulation of β-cyfluthrin had an EC50 of 0.51 mg/L against C. maculatus on the first day of application50. Spinosad dust at 0.3 g/kg (300 mg/kg) reduced progeny by up to 94%51. In another study, fumigant Phostoxin was observed to have 86.7% mortality after 48 h at 0.20 mg/kg52.
The findings of the current research indicate that binary formulations that include essential oils and zeolites, especially P. anisum and Zn-zeolite-A, provide potential directions in biopesticide management of C. maculatus. Such synergistic effects are probably multifactorial. Zeolites can act as sustained-release carriers, thereby extending the exposure to volatile compounds. They may trap components of essential oils in their microporous structure to create microenvironments of high concentration that enhance contact toxicity. At the same time, zeolite structures can preserve the volatiles against degradation or evaporation, thus maintaining biological activity during long periods. Milićević, et al.53 encapsulated clove essential oil using different types of zeolites to create eco-friendly biopesticides. The formulations showed prolonged efficacy and strong biological activity against various pests and pathogens.
The morphological changes in C. maculatus adults exposed to cowpea seeds treated with zeolites showed that zeolites caused scratches, splits, damage, or loss of sensilla on the cuticle, elytra, or ventral surface of C. maculatus adults. These changes could impair the cuticle’s protective function and lead to insect dehydration or desiccation. Abdelgaleil, et al.54 mentioned that treatment of C. maculatus adults with inert dust of diatomaceous earth and kaolin induced general damage to the insect body, like scratches on the elytra and sensilla and traces in scattered areas of the cuticle. Similarly, the SEM results of S. oryzae adults treated with zeolite particles showed that zeolite particles adhered to all body parts, including the head, thorax, abdomen, elytra, and legs55.
In conclusion, our findings indicated that synthetic zeolites generated from kaolin under microwave conditions had high crystallinity and stability and increased cation exchange capacity after zinc loading. C. maculatus mortality rose as zeolite and essential oil concentrations increased. Zeolite-X was more potent than zeolite-A against C. maculatus, while loading zinc into zeolites resulted in Zn-zeolite-A being more efficient than Zn-zeolite-X. Combining P. anisum essential oil with zeolite caused higher potency against the mortality and progeny production of C. maculatus compared to zeolite alone or zeolite and R. officinalis combinations at the same concentrations and time durations. Elytra scratches and severe damage to the sensilla spreading of C. maculatus were also caused by zeolite treatments. Using zeolite nanoparticles loaded with zinc and combined with essential oils can control C. maculatus in stored cowpea seeds. The practical value of these zeolite-based insecticides warrants further research before large-scale implementation.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on request.
References
Bohra, A. et al. in Advancement in Crop Improvement Techniques (eds Narendra Tuteja, Renu Tuteja, Nishat Passricha, & Shabnam K. Saifi) 129–139 (Woodhead Publishing, 2020). https://doi.org/10.1016/B978-0-12-818581-0.00009-7.
Devi, C. B., Kushwaha, A. & Kumar, A. Sprouting characteristics and associated changes in nutritional composition of Cowpea (Vigna unguiculata). J. Food Sci. Technol. 52, 6821–6827. https://doi.org/10.1007/s13197-015-1832-1 (2015).
Röös, E., de Groote, A. & Stephan, A. Meat tastes good, legumes are healthy and meat substitutes are still strange—the practice of protein consumption among Swedish consumers. Appetite 174, 106002. https://doi.org/10.1016/j.appet.2022.106002 (2022).
Stathers, T. E., Arnold, S. E., Rumney, C. J. & Hopson, C. Measuring the nutritional cost of insect infestation of stored maize and Cowpea. Food Secur. 12, 285–308 (2020).
Hamdi, S. H. et al. Nutritional alterations and damages to stored chickpea in relation with the pest status of Callosobruchus maculatus (Chrysomelidae). J. Asia-Pacif Entomol. 20, 1067–1076. https://doi.org/10.1016/j.aspen.2017.08.008 (2017).
Lü, J., Sehgal, B. & Subramanyam, B. Insecticidal potential of a synthetic zeolite against the Cowpea weevil, Callosobruchus maculatus (Fabricius)(Coleoptera: Bruchidae). J. Stored Prod. Res. 72, 28–34. https://doi.org/10.1016/j.jspr.2017.03.001 (2017).
Abdelgaleil, S. et al. Monoterpenes for management of field crop insect pests. J. Agr Sci. Tech. 25, 769–784. https://doi.org/10.22034/jast.25.4.1 (2023).
Campolo, O. et al. Effects of inert dusts applied alone and in combination with sweet orange essential oil against Rhyzopertha Dominica (Coleoptera: Bostrichidae) and wheat microbial population. Ind. Crops Prod. 61, 361–369. https://doi.org/10.1016/j.indcrop.2014.07.028 (2014).
Abdelmaksoud, N. M. et al. Potency of emulsifiable concentrate and nanoemulsion formulations as green insecticides against two insects, Aphis craccivora and Liriomyza trifolii. Ind. Crops Prod. 208, 117854. https://doi.org/10.1016/j.indcrop.2023.117854 (2024).
El-Bakry, A. M., Abdel-Aziz, N. F. & Sammour, E. A. Impact of Lavandula officinalis, inert dusts and their formulations on Sitophilus oryzae. AgricEngInt: CIGR J. 166–173 (2017).
Ríos, J. L. Essential oils: what they are and how the terms are used and defined in essential oils in food preservation, flavor and safety, 3–10 (Elsevier, 2016). https://doi.org/10.1016/B978-0-12-416641-7.00001-8.
Abdel-Aziz, N. F. et al. The effect of some green insecticides from essential oils on Aphis craccivora, and their side effects. J. Entomol. Res. 39, 275–286. https://doi.org/10.5958/0974-4576.2015.00034.1 (2015).
El-Bakry, A. M., Abdel-Aziz, N. F., Sammour, E. A. & Abdelgaleil, S. A. M. Insecticidal activity of natural plant essential oils against some stored product insects and their side effects on wheat seed germination. Egypt. J. Biol. Pest Control. 26, 83–88 (2016).
Abdelmaksoud, N. M., El-Bakry, A. M., Sammour, E. A. & Abdel-Aziz, N. F. Comparative toxicity of essential oils, their emulsifiable concentrates and nanoemulsion formulations against the bean aphid, Aphis fabae. Arch. Phytopathol. Plant. Protect 56, 187–208. https://doi.org/10.1080/03235408.2023.2178065 (2023).
El-Sayed, S. M. et al. Acaricidal and antioxidant activities of anise oil (Pimpinella anisum) and the oil’s effect on protease and acetylcholinesterase in the two-spotted spider mite (Tetranychus urticae Koch). Agriculture 12, 224. https://doi.org/10.3390/agriculture12020224 (2022).
Campolo, O. et al. Essential oils in stored product insect pest control. J. Food Qual. 6906105. (2018). https://doi.org/10.1155/2018/6906105 (2018).
Ahmad, F. et al. Insecticidal activity of some plant extracts against Trogoderma granarium (E). Agriculturists 11, 103–111 (2013).
Ziaee, M., Ebadollahi, A. & Wakil, W. Integrating inert dusts with other technologies in stored products protection. Toxin Rev. 40, 404–419. https://doi.org/10.1080/15569543.2019.1633673 (2021).
El-Bakry, A. M., Youssef, H. F., Abdel-Aziz, N. F. & Sammour, E. A. Insecticidal potential of Ag-loaded 4A-zeolite and its formulations with Rosmarinus officinalis essential oil against rice weevil (Sitophilus oryzae) and lesser grain borer (Rhyzopertha dominica). J. Plant. Prot. Res. 59, 324–333. https://doi.org/10.24425/jppr.2019.129741 (2019).
Liška, A. et al. Evaluation of the potential value of the F1H and F2H diatomaceous Earth formulations as grain protectants against Rhyzopertha Dominica (Fabricius)(Coleoptera: Bostrichidae). J. Henan Univ. Technol. (Natural Sci. Edition) 3, 29–41. https://doi.org/10.5073/jka.2018.463.118 (2018).
Möller, K. & Bein, T. Mesoporosity–a new dimension for zeolites. Chem. Soc. Rev. 42, 3689–3707. https://doi.org/10.1039/c3cs35488a (2013).
Eroglu, N., Emekci, M. & Athanassiou, C. G. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 97, 3487–3499. https://doi.org/10.1002/jsfa.8312 (2017).
Miranti, M. et al. Preparation and evaluation of zeolite nanoparticles as a delivery system for Helicoverpa armigera Nucleopolyhedrovirus (HaNPV) against the Spodoptera litura (Fabricius, 1775) larvae. Microorganisms 11, 847. https://doi.org/10.3390/microorganisms11040847 (2023).
Abdelgaleil, S. A. M., Gad, H. A., Atta, A. A. M. & Al-Anany, M. S. Control of Sitophilus granarius and Sitophilus oryzae on stored wheat using low-rate combinations of natural zeolite with three insecticides. J. Stored Prod. Res. 97, 101975. https://doi.org/10.1016/j.jspr.2022.101975 (2022).
Noviello, M. et al. Synthetic zeolite materials from recycled glass and aluminium food packaging as potential oenological adjuvant. Food Packaging Shelf Life. 26, 100572. https://doi.org/10.1016/j.fpsl.2020.100572 (2020).
Demirci, S. et al. Antimicrobial properties of zeolite-X and zeolite-A ion-exchanged with silver, copper, and zinc against a broad range of microorganisms. Appl. Biochem. Biotechnol. 172, 1652–1662. https://doi.org/10.1007/s12010-013-0647-7 (2014).
Adams, R. P. Identification of Essential Oil Components By Gas Chromatography/Mass Spectrometry. 4.1 edn. (2017).
Youssef, H., Abdel-Aziz, M. & Fouda, F. Evaluation of antimicrobial activity of different silver-exchanged nano and micronized zeolites prepared by microwave technique. J. Porous Mater. 24, 947–957. https://doi.org/10.1007/s10934-016-0334-5 (2017).
Mansour, N., Eldefrawi, M., Toppozada, A. & Zeid, M. Toxicological studies on the Egyptian cotton leaf worm, Prodenia litura. VI. Potentiation and antagonism of organophosphorus and carbamate insecticides. J. Econ. Entomol. 59, 307–311. https://doi.org/10.1093/jee/59.2.307 (1966).
Abdelgaleil, S. A. M. et al. Monoterpenes: chemistry, insecticidal activity against stored product insects and modes of action—a review. Int. J. Pest Manag. 1–23. https://doi.org/10.1080/09670874.2021.1982067 (2021).
Adouane, S. et al. Natural insecticides from native plants of the mediterranean basin and their activity for the control of the date moth Ectomyelois ceratoniae (Zeller)(Lepidoptera: Pyralidae). J. Plant. Dis. Prot. 129, 775–782. https://doi.org/10.1007/s41348-022-00593-9 (2022).
Polatoğlu, K. et al. Insecticidal activity of Salvia veneris hedge. Essential oil against coleopteran stored product insects and Spodoptera Exigua (Lepidoptera). Ind. Crops Prod. 97, 93–100. https://doi.org/10.1016/j.indcrop.2016.12.012 (2017).
Atay, T., Alkan, M., Ertürk, S. & Toprak, U. Individual and combined effects of α-Pinene and a native diatomaceous Earth product on control of stored product beetle pests. J. Asia-Pacif Entomol. 26, 102149. https://doi.org/10.1016/j.aspen.2023.102149 (2023).
Wang, Z. et al. Trans-anethole is a potent toxic fumigant that partially inhibits Rusty grain beetle (Cryptolestes ferrugineus) acetylcholinesterase activity. Ind. Crops Prod. 161, 113207. https://doi.org/10.1016/j.indcrop.2020.113207 (2021).
Eroglu, N. A review: insecticidal potential of zeolite (Clinoptilolite), toxicity ratings and general properties of Turkish zeolites. Arthur. Et Al Eds. 128, 755–767 (2015).
Subramanyam, B. & Roesli, R. in Alternatives to Pesticides in Stored-Product IPM (eds Bhadriraju Subramanyam & David W. Hagstrum) Ch. 321–380 (447 Springer US, 2000). https://doi.org/10.1007/978-1-4615-4353-4_12.
Youssef, H. F. et al. Preparation and characterization of different zeolites from andesite rock: Product evaluation for efficient dye removal. Microporous Mesoporous Mater. 328, 111485. https://doi.org/10.1016/j.micromeso.2021.111485 (2021).
Tantawy, H. A. & Ismail, N. Microwave synthesis of nano/micronized zeolites from natural source: Evaluation of energy storage capacities. Egypt. J. Chem. 63, 3669–3683. https://doi.org/10.21608/EJCHEM.2020.21421.2290 (2020).
Youssef, H., Ibrahim, D. & Komarneni, S. Microwave-assisted versus conventional synthesis of zeolite A from metakaolinite. Microporous Mesoporous Mater. 115, 527–534. https://doi.org/10.1016/j.micromeso.2008.02.030 (2008).
Inayat, A., Schneider, C. & Schwieger, W. Organic-free synthesis of layer-like FAU-type zeolites. Chem. Commun. 51, 279–281. https://doi.org/10.1039/c4cc07947g (2015).
Munguti, L. K., Dejene, F. B. & Muthee, D. K. High photodegradation performance of ZnO nanoparticles supported on porous zeolite Na-a: Effects of ZnO loading. Mater. Chem. Phys. 295, 127063. https://doi.org/10.1016/j.matchemphys.2022.127063 (2021).
Pavela, R. & Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant. Sci. 21, 1000–1007. https://doi.org/10.1016/j.tplants.2016.10.005 (2016).
Gupta, I. et al. Plant essential oils as biopesticides: applications, mechanisms, innovations, and constraints. Plants 12, 2916. https://doi.org/10.3390/plants12162916 (2023).
Subramanyam, B., Lu, J. & Sehgal, B. in 11th International Working Conference on Stored Product Protection. 894–902 (2015).
Karimzadeh, R., Salehpoor, M. & Saber, M. Initial efficacy of pyrethroids, inert dusts, their low-dose combinations and low temperature on Oryzaephilus Surinamensis and Sitophilus granarius. J. Stored Prod. Res. 91, 101780. https://doi.org/10.1016/j.jspr.2021.101780 (2021).
Julbe, A. & Drobek, M. in Encyclopedia of Membranes (eds Enrico Drioli & Lidietta Giorno) 2055–2056 (Springer Berlin Heidelberg, 2016). https://doi.org/10.1007/978-3-662-44324-8_604
Walton, H. F. Zeolite (2023). https://www.britannica.com/science/zeolite.
Korunic, Z. & Fields, P. G. Evaluation of three new insecticide formulations based on inert dusts and botanicals against four stored-grain beetles. J. Stored Prod. Res. 88, 101633. https://doi.org/10.1016/j.jspr.2020.101633 (2020).
Ebadollahi, A., Jalali Sendi, J., Setzer, W. N. & Changbunjong, T. Encapsulation of Eucalyptus largiflorens essential oil by mesoporous silicates for effective control of the cowpea weevil, Callosobruchus maculatus (Fabricius)(Coleoptera: Chrysomelidae). Molecules 27, 3531. https://doi.org/10.3390/molecules27113531 (2022).
Loha, K. M. et al. Bio-efficacy evaluation of nanoformulations of β-cyfluthrin against Callosobruchus maculatus (Coleoptera: Bruchidae). J. Environ. Sci. Health Part. B. 47, 687–691. https://doi.org/10.1080/03601234.2012.669254 (2012).
Khashaveh, A., Ziaee, M. & Safaralizadeh, M. H. Control of pulse beetle, Callosubruchus maculatus (F.)(Coleoptera: Bruchidae) in different cereals using spinosad dust in storage conditions. J. Plant. Prot. Res. 51. https://doi.org/10.2478/v10045-011-0014-z (2011).
Owolabi, M. S. et al. Insecticidal activity and chemical composition of the Morinda lucida essential oil against pulse beetle Callosobruchus maculatus. Sci. World J. 2014, 784613. https://doi.org/10.1155/2014/784613 (2014).
Milićević, Z. et al. Encapsulated clove bud essential oil: A new perspective as an eco-friendly biopesticide. Agriculture 12 (338). https://doi.org/10.3390/agriculture12030338 (2022).
Abdelgaleil, S. A., Gad, H. A., Hamza, A. F. & Al-Anany, M. S. Insecticidal efficacy of two inert dusts and Trichoderma harzianum, applied alone or in combination, against Callosobruchus maculatus and Callosobruchus chinensis on stored cowpea seeds. Crop Prot. 146, 105656. https://doi.org/10.1016/j.cropro.2021.105656 (2021).
Rumbos, C., Sakka, M., Berillis, P. & Athanassiou, C. Insecticidal potential of zeolite formulations against three stored-grain insects, particle size effect, adherence to kernels and influence on test weight of grains. J. Stored Prod. Res. 68, 93–101. https://doi.org/10.1016/j.jspr.2016.05.003 (2016).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was supported by the National Research Centre, Egypt, Project No. 13050117.
Author information
Authors and Affiliations
Contributions
A.M.E.: Conceptualization, Methodology, Formal analysis, Writing—Original Draft, Writing—Review & Editing. H.F.Y.: Methodology, Resources, Investigation, Writing—review & editing. N.M.A.: Methodology, Validation, Writing—original draft. N.F.A: Methodology, Investigation, Writing—Review & Editing. E.A.S.: Conceptualization, Methodology, Resources, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Bakry, A.M., Youssef, H.F., Abdelmaksoud, N.M. et al. Synergistic insecticidal effects of zinc-loaded zeolite nanoparticles combined with essential oils against Callosobruchus maculatus. Sci Rep 15, 31675 (2025). https://doi.org/10.1038/s41598-025-15752-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15752-9