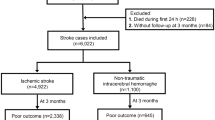
Abstract
Ischemic stroke is a major health concern, particularly in patients with type 2 diabetes mellitus (T2DM), who are at elevated risk. This study aimed to evaluate the prognostic impact of glibenclamide pretreatment on patients with acute ischemic stroke and T2DM. A total of 122 patients with acute ischemic stroke and T2DM were retrospectively analyzed. Based on medication history, patients were categorized into the glibenclamide group (group 1, n = 54) and the control group (group 2, n = 68). Stroke severity at admission was assessed using the National Institutes of Health Stroke Scale (NIHSS), and functional status was evaluated with the Barthel Index (BI) and modified Rankin Scale (mRS). Patients were followed for 3 months. At admission, group 1 demonstrated significantly better neurological function (lower NIHSS) and higher BI scores compared to group 2 (P < 0.05). At 3 months, mortality was 1.9% in group 1 and 5.9% in group 2, though this difference was not statistically significant (χ2 = 0.430, P = 0.512). Adverse events occurred in 7.4% and 10.3% of patients in groups 1 and 2, respectively (P > 0.05), with no drug-related deaths or serious safety concerns. The incidence of hemorrhagic transformation also showed no significant difference (P > 0.05). However, group 1 showed significantly better functional outcomes at 3 months, with higher BI scores and lower mRS scores (P < 0.05), suggesting improved independence and neurological recovery. Glibenclamide pretreatment in patients with acute ischemic stroke and T2DM may confer clinical benefits by reducing stroke severity and enhancing post-stroke functional outcomes, without increasing adverse events.
Similar content being viewed by others
Introduction
Ischemic stroke is caused by cerebral blood flow disruption, leading to ischemia and hypoxic necrosis of brain tissue, which results in corresponding neurological deficits. It is the most prevalent form of stroke, accounting for approximately 60–80% of cases1,2,3, particularly those related to large-artery atherosclerosis4. Stroke is characterized by high incidence, mortality, and disability rates5, and has become a major public health challenge in China6,7, in which diabetes was recognized as an independent risk factor for its occurrence8. Specifically, patients with type 2 diabetes (T2DM) have a 2.27-fold increased risk of ischemic stroke compared with non-diabetic individuals9. Currently, the prevalence of diabetes in China exceeds 10%, and a growing number of diabetics experience strokes each year, thereby intensifying the national stroke burden. Evidence suggests that the prognosis of acute ischemic stroke in patients with T2DM is generally unfavorable10. Therefore, identifying effective strategies to improve survival and reduce both mortality and disability in this population is of critical importance.
Glibenclamide (GBC), a second-generation sulfonylurea hypoglycemic agent, exerts its therapeutic effects primarily by inhibiting sulfonylurea receptor 1 (Sur1)11. Recent studies have demonstrated that glibenclamide can mitigate brain injury and enhance survival following ischemic stroke12,13. However, the prognostic impact of glibenclamide pretreatment in patients with acute ischemic stroke and T2DM remains elusive. Compared with other sulfonylureas, glibenclamide has shown to possess distinct neuroprotective properties beyond glycemic control, particularly through its potent inhibition of the Sur1–Trpm4 channel complex, playing a critical role in cerebral edema and secondary injury following ischemic stroke14,15. This mechanism is not shared by all sulfonylureas, making glibenclamide uniquely positioned for potential therapeutic benefit in diabetic patients experiencing acute ischemic stroke.
The present retrospective study examined the association between glibenclamide pretreatment and outcomes in this patient population, with the objective of providing evidence-based guidance for clinical management.
Materials and methods
A total of 122 patients with T2DM and acute ischemic stroke were retrospectively selected from the Neurology Departments of Minzu Hospital Affiliated to Guangxi Medical University (Nanning, China), the Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University (Luzhou, China), and Ganxian District People’s Hospital of Ganzhou City (Ganzhou, China), between July 2021 and June 2023. Patients were categorized into two groups based on their antidiabetic treatment. Group 1 (n = 54) consisted of patients who had been taking glibenclamide for at least 1 month prior to stroke onset and continued its use afterward. The glibenclamide regimen was 1.25 mg orally, three times daily, administered half an hour before meals, with dosage adjustments made according to clinical blood glucose level. Group 2 (n = 68) included patients treated with other antidiabetic medications. All patients received standard medical management for cerebral infarction, including antiplatelet agents and statins. The glibenclamide dosage of 1.25 mg three times daily was selected based on commonly prescribed clinical regimens for glycemic control in Chinese patients with type 2 diabetes, as well as prior pharmacodynamic studies demonstrating effective blood glucose regulation at this dose with a lower risk of hypoglycemia15,16. A minimum exposure duration of 1 month before stroke onset was chosen to ensure adequate drug accumulation and potential modulation of cerebrovascular and neuroinflammatory pathways.
This research protocol was approved by the Ethics Committee of Ganxian District People’s Hospital (No. MR-20230316) and adhered to ethical guidelines and standards set forth by relevant academic and scientific communities. As this study was retrospective, written informed consent was deemed unnecessary by an Institutional Review Board (IRB), the Ethics Committee of Ganxian District People’s Hospital.
Previous experimental and clinical studies have shown that glibenclamide, a sulfonylurea-class antidiabetic agent, can reduce cerebral edema and the risk of hemorrhagic transformation following ischemic stroke. This protective effect may be mediated through the inhibition of SUR1-TRPM4 channels, which are upregulated in ischemic brain tissue and contribute to the formation of vasogenic edema and capillary fragmentation. By blocking these channels, glibenclamide may preserve microvascular integrity, attenuate blood–brain barrier (BBB) disruption, and reduce the extent of secondary hemorrhagic injury following severe ischemia. Based on this pathophysiological rationale, this study aimed to investigate whether prior use of glibenclamide in diabetic patients could influence stroke outcomes and reduce the incidence of hemorrhagic transformation.
Inclusion and exclusion criteria
Inclusion criteria
Patients were included if they met the following criteria:
-
1.
Diagnosed with ischemic stroke according to established criteria14, confirmed by head MRI to be cerebral infarction, and aged ≥ 18 years14.
-
2.
TOAST classification of ischemic stroke: large-artery atherosclerosis and small-vessel occlusion.
-
3.
A history of T2DM, treated with medication, and baseline HbA1c < 8.0%.
-
4.
All patients experienced their first episode of cerebral infarction or had previous episodes without neurological deficits.
Exclusion criteria
Patients were excluded if they had any of the following conditions:
-
1.
Allergies to sulfonylurea drugs.
-
2.
History of hypoglycemia.
-
3.
Significant history of bleeding or bleeding tendency.
-
4.
Severe systemic diseases such as intracranial hemorrhage, malignant tumors, atrial fibrillation, diseases of blood system, or severe cardiopulmonary, hepatic, or renal dysfunction.
-
5.
Patients who are lactating or pregnant.
Clinical evaluation
All patients were carefully screened based on predefined inclusion and exclusion criteria to ensure homogeneity in the study population. General information of the participants, including gender, age, hypertension, hyperlipidemia, baseline HbA1c, smoking and alcohol consumption history, infarction site, TOAST classification, intravenous thrombolysis, endovascular interventional treatment, hospital stay, and medication history, was recorded. Additionally, routine blood tests, coagulation function, liver and kidney function, blood glucose, and blood lipid levels were monitored. The severity of neurological impairment at admission was assessed using the National Institutes of Health Stroke Scale (NIHSS), while the Barthel Index was utilized to estimate the patients’ daily living abilities.
Patients in each group were followed up for 3 months, with mortality and complications recorded. Neurological function recovery was evaluated using the modified Rankin Scale (mRS), and the Barthel Index was used again to assess daily living abilities. Clinical assessments and follow-ups were conducted by dedicated personnel who were blinded to the patients’ group assignments to minimize bias.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD), and differences between groups were compared using a t test. The Chi-square (χ2) test was utilized for analyzing categorical variables and mortality. Statistical analysis was conducted using SPSS 17.0 software. P < 0.05 was considered statistically significant. The statisticians remained blinded to the subjects’ characteristics throughout the analysis.
To minimize confounding due to non-random treatment assignment, propensity score matching (PSM) was employed. A logistic regression model was used to estimate the propensity scores based on baseline covariates, including age, sex, hypertension, hyperlipidemia, baseline NIHSS score, HbA1c, infarction site, TOAST classification, smoking status, alcohol consumption, and prior cardiovascular history. Patients in the glibenclamide group were matched to control patients using 1:1 nearest-neighbor matching without replacement and a caliper width of 0.2 standard deviations of the logit of the propensity score. The adequacy of matching was evaluated using standardized mean differences (SMDs), with values < 0.1 indicating good balance between groups. Statistical comparisons of baseline characteristics post-matching were conducted using paired t tests or McNemar’s test as appropriate.
Results
Baseline characteristics of patients
A total of 122 acute ischemic stroke patients with T2DM, who met the inclusion criteria, were enrolled in the study. Patients were then divided into two groups based on their medication history: the glibenclamide group (group 1, n = 54) and the control group (group 2, n = 68). Before PSM, baseline characteristics between the two groups showed some imbalances. To control for these potential confounding factors, propensity score matching was performed. After PSM, 54 patients in each group were included, and no statistically significant differences were identified in baseline characteristics, including gender, age, hypertension, hyperlipidemia, baseline HbA1c, smoking and alcohol history, infarct location, TOAST classification, intravenous thrombolysis, endovascular treatment, and length of hospital stay (all P > 0.05, Table 1). The SMDs for all covariates were less than 0.1, confirming adequate balance between groups.
Data collection and Follow-up
The severity of neurological impairment and ability of daily living in stroke patients at admission
Compared to the control group (group 2), the glibenclamide group (group 1) exhibited significant improvements in neurological deficits and activities of daily living at admission. This was evident from the NIHSS scores (4.93 ± 4.91 in group 1 vs. 7.09 ± 6.62 in group 2, P = 0.047 < 0.05, Fig. 1a) and Barthel index scores (74.62 ± 21.87 in group 1 vs. 64.93 ± 22.77 in group 2, P = 0.019 < 0.05, Fig. 1b).
(a, b, c) The neurological function and daily living ability. (a) The activities of daily living in stroke patients at admission, Barthel index scores 74.62 ± 21.87 in group 1 vs. 64.93 ± 22.77 in group 2, P = 0.019 < 0.05; (b) The degree of neurological deficits at admission, as evidenced by NIHSS scores 4.93 ± 4.91 in group 1 vs. 7.09 ± 6.62 in group 2, P = 0.047 < 0.05; (c) The results (Barthel index) within 3 months after treatment, 82.32 ± 24.24 in group 1 vs. 72.13 ± 29.10 in group 2, and the difference between groups was statistically significant (P < 0.05).
Although the differences reached statistical significance, they may also reflect clinically meaningful improvements, as NIHSS reductions of 1–2 points can be associated with improved functional outcomes, and a 10-point increase in the Barthel index may translate to greater independence in daily activities.
Three-month outcomes: mortality, complications, functional status, and neurological recovery
Within 3 months after treatment, one patient in Group 1 died (mortality rate, 1.9%), compared with 4 patients in Group 2 (mortality rate, 5.9%). Although Group 1 showed a lower mortality rate, the difference was not statistically significant (χ2 = 0.430, P = 0.512, Table 2), and no deaths were attributed to drug-related causes. Adverse events occurred in 4 patients (7.4%) in Group 1, including three cases of hypoglycemia, one stress ulcer, and one hemorrhagic transformation, versus 7 patients (10.3%) in Group 2, which included three stress ulcers, two cases of hypoglycemia, and two hemorrhagic transformations. The overall incidence of adverse events did not significantly differ between groups (χ2 = 0.306, P = 0.580, Table 2). Although hemorrhagic transformation was more frequent in Group 2, the difference approached, while did not reach statistical significance (χ2 = 3.567, P = 0.059, Table 2). Notably, patients in the glibenclamide group demonstrated significantly better functional outcomes on the Barthel Index at 3 months (82.32 ± 24.24 vs. 72.13 ± 29.10, P < 0.05), indicating improved independence in daily living activities. Clinically, this improvement is notable, as higher Barthel index scores correspond to better self-care ability and reduced long-term dependency, which are key rehabilitation goals in stroke recovery. Regarding neurological function recovery (mRS score, Fig. 2), the mRS score was 2.17 ± 1.37 in group 1 vs. 2.77 ± 1.57 in group 2, demonstrating a statistically significant difference between the two groups (t = −2.261, P < 0.05). A difference of 0.6 in the mRS score suggests a shift toward more favorable outcomes on this ordinal disability scale, potentially translating into better quality of life and lower caregiver burden.
To account for the observed difference in admission NIHSS and Barthel scores, multivariable linear regression was conducted in the propensitymatched cohort, with 3 months mRS and Barthel Index as dependent variables and including admission NIHSS (or Barthel) and treatment group as independent variables. Glibenclamide pretreatment remained significantly associated with lower 3 months mRS (adjusted β = −0.45, 95% CI −0.82 to −0.08, P = 0.02) and higher Barthel Index (adjusted β = +5.8, 95% CI + 1.1 to + 10.5, P = 0.016), indicating that the benefit of glibenclamide cannot be attributed solely to differences in baseline stroke severity.
Discussion
Cerebral infarction, characterized by a high incidence and disability rate, is a prevalent neurological disease that significantly contributes to the overall disease burden15. In China, strokes are the leading cause of morbidity, with an increasing prevalence among younger individuals, presenting a formidable challenge for both preventative measures and therapeutic interventions16,17. The primary etiology of ischemic stroke is attributed to the rupture and detachment of atherosclerotic plaques. T2DM is a prominent risk factor for the development of atherosclerosis in the cardiovascular and cerebrovascular systems, leading to ischemic stroke with a heightened incidence rate and unfavorable prognosis18,19,20. Consequently, the primary objective for individuals with T2DM who are also affected by acute ischemic stroke is to mitigate disease severity, enhance prognosis, and elevate overall quality of life.
Glibenclamide, a commonly prescribed oral hypoglycemic medication for T2DM, operates by inhibiting the sulfonylurea receptor 1 (Sur1), making it valuable in clinical settings due to its effective glucose-lowering capabilities and its ability to mitigate microvascular complications21,22. Research has highlighted its neuroprotective potential, particularly in mitigating central nervous system disorders, by impeding the Sur1-regulated transient receptor potential M4 (Sur1-Trpm4) channel23,24,25,26.
Previous research had shown that glibenclamide could reduce cerebral edema and the incidence of hemorrhagic transformation in rodent models of severe ischemia-reperfusion injury27. Additionally, an exploratory analysis reported improved survival in patients who aged ≤ 70 years with large hemispheric infarction following acute intravenous glyburide therapy13. Furthermore, clinical research has demonstrated that oral glibenclamide is safe for the treatment of acute hemispheric infarction and may help prevent brain edema, thereby reducing the risk of severe disability and death28. In a retrospective study involving patients with acute ischemic stroke and T2DM, Hagen Kunte et al. demonstrated that glibenclamide reduced both the risk of hemorrhagic transformation and mortality rates following stroke29. Similarly, Igarashi et al. found that glibenclamide effectively decreased cerebral edema and hemorrhagic transformation after severe ischemia27, potentially through the inhibition of Sur1 expression, thereby mitigating ischemia-induced brain injury. In a rat model of middle cerebral artery occlusion, glibenclamide, a selective Sur1 inhibitor, attenuated perivascular tumor necrosis factor (TNF) labeling, indicating reduced inflammatory responses30. Ortega et al. pointed out that early blockade of Sur1 by glibenclamide facilitated the migration of neural progenitor cells to ischemic regions, enhanced cortical neurogenesis, and conferred long-term neuroprotection after cerebral ischemia. These findings suggest that glibenclamide may not only exert acute neuroprotective effects, but also support long-term brain repair and functional recovery31.
The results of this study demonstrated that acute ischemic stroke patients with T2DM who received glibenclamide treatment exhibited lower NIHSS scores and higher BI scores upon admission, as well as lower mRS scores and higher BI scores at 3 months compared to the control group. Although no drug-related deaths were observed, there was an increasing trend in mortality in the control group, although not statistically significant. Additionally, the incidence of adverse events, including the conversion of ischemic stroke to cerebral hemorrhage, did not significantly differ between groups, contrasting with previous findings27,29. This discrepancy may be attributed to the limited sample size and the inherent constraints of the retrospective study design. Consequently, further large-scale randomized controlled trials are warranted to validate the potential of glibenclamide in reducing mortality and adverse event rates. Despite the absence of significant differences in complications, the glibenclamide group exhibited a lower incidence of post-stroke hemorrhagic transformation compared with the control group, indicating a possible protective effect. These findings suggest that glibenclamide may contribute to improved clinical outcomes by attenuating stroke severity and enhancing patients’ quality of life.
The findings of this study are broadly consistent with those of Kunte et al.29, who reported that glibenclamide reduced the risk of hemorrhagic transformation and mortality in acute ischemic stroke patients with T2DM. Similarly, Igarashi et al.27 and Ortega et al.31 found a reduction in cerebral edema and improved neuroprotection in preclinical models following glibenclamide administration. However, in contrast to some of these prior studies, the present study did not show a statistically significant reduction in mortality or hemorrhagic transformation. This discrepancy may be attributed to differences in study design, population characteristics, and sample size. For instance, Kunte et al.29 employed a multicenter prospective design with a larger cohort, whereas the present study was a multi-center retrospective analysis, potentially limiting the statistical power to detect differences in rare outcomes such as mortality or hemorrhagic transformation. Additionally, the study population had relatively milder baseline stroke severity (as reflected by lower NIHSS scores) compared with the severe or large hemispheric infarctions described in earlier trials, which could attenuate the observable effect of glibenclamide. Nevertheless, the consistent directionality of improved neurological function and functional independence at 3 months may suggest a potential neuroprotective benefit of glibenclamide, warranting further validation in randomized trials.
Although this study applied strict inclusion and exclusion criteria and used PSM to balance baseline characteristics, the heterogeneity in antidiabetic agents used in the control group remains a potential confounding factor. Patients in the control group received various non-glibenclamide medications, which may have differing effects on stroke outcomes. While baseline HbA1c and other key variables were well-matched between groups, residual confounding cannot be completely excluded. Future prospective studies with standardized antidiabetic regimens are needed to validate the neuroprotective effects of glibenclamide in acute ischemic stroke.
This study has several inherent limitations. Firstly, its retrospective design might restrict the ability to establish causality and introduce selection bias despite efforts to minimize it through strict inclusion and exclusion criteria and PSM. Secondly, the relatively small sample size might reduce the statistical power to detect differences in mortality and adverse event rates. Thirdly, the control group consisted of patients using a heterogeneous mix of antidiabetic agents, which could have varied effects on stroke outcomes and act as potential confounders. Although baseline characteristics, including HbA1c, were balanced, residual confounding could not be fully excluded. Fourthly, the follow-up time was limited to 3 months, which might not capture long-term outcomes related to neurological recovery and complications. Finally, the study was conducted at only three centers, which might limit the generalizability of the findings. Future prospective, multicenter randomized controlled trials with standardized treatment regimens and longer follow-up are required to validate and extend these results.
In summary, glibenclamide pretreatment was found to be associated with improved clinical outcomes in patients with T2DM and acute ischemic stroke, including reduced post-stroke complication severity and enhanced recovery of neurological function and activities of daily living within 3 months. However, the retrospective nature of this study could introduce inherent limitations, and the inclusion of a specific patient population might lead to selection bias, potentially influencing the generalizability of the findings. Therefore, further multicenter, large-scale randomized controlled trials are required to confirm and validate these results.
Data availability
The data that support the findings of this study can be obtained through the corresponding authors.
References
Ntaios, G. et al. European stroke organisation (ESO) guidelines for the management of Tempe rature in patients with acute ischemic stroke. Stroke. 10 (6), 941–949 (2015).
Wang, W. et al. Prevalence, incidence, and mortality of stroke in china: Results from a nationwide population-based survey of 480687 adults. Circulation. 135 (8), 759–771 (2017).
Wang, D. et al. Patterns of stroke between university hospitals and nonuniversity hospitals in Mainland china: Prospective multicenter hospital-based registry study. World Neurosurg. 98, 258–265 (2017).
Nath, M. et al. Association of modifiable risk factors with ischaemic stroke subtypes in Asian versus Caucasian populations: A systematic review and meta-analysis. Eur. J. Clin. Invest. 52 (11), e13849 (2022).
Liu, J. et al. Trends in outcomes of patients with ischemic stroke treated between 2002 and 2016: Insights from a Chinese cohort. Circ. Cardiovasc. Qual. Outcomes. 12 (12), e005610 (2019).
Yang, G. et al. Rapid health transition in china, 1990–2010: Findings from the global burden of disease study 2010. Lancet. 381 (9882), 1987–2015 (2013).
Liu, L. et al. Chinese stroke association guidelines for clinical management of ischaemic cerebrovascular diseases: Executive summary and 2023 update. Stroke Vasc Neurol. 8 (6), e3 (2023).
Cao, Y. et al. Analysis of prognostic risk factors for ischemic stroke in china: A multicentre retrospective clinical study; a national survey in China. Curr. Neurovasc Res. 19 (1), 117–126 (2022).
Sarwar, N. et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. 375, 2215–2222 (2010).
Callahan, A. et al. Risk of stroke and cardiovascular events after ischemic stroke or transient ischemic attack in patients with type 2 diabetes or metabolic syndrome: Secondary analysis of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial. Arch. Neurol. 68, 1245–1251 (2011).
Woo, S. K. et al. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J. Biol. Chem. 288 (5), 3655–3667 (2013).
Wang, X. et al. Glimepiride and glibenclamide have comparable efficacy in treating acute ischemic stroke in mice. Neuropharmacology. 162, 107845 (2020).
Sheth, K. N. et al. Long-term outcomes in patients aged ≤ 70 years with intravenous glyburide from the phase II GAMES-RP study of large hemispheric infarction: An exploratory analysis. Stroke. 49 (6), 1457–1463 (2018).
Kleindorfer, D. O. et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American heart association/american stroke Association. Stroke. 52 (7), e364–e467 (2021).
Fan, J. et al. Global burden, risk factor analysis, and prediction study of ischemic stroke, 1990–2030. Neurology. 101 (2), e137–e150 (2023).
GBD 2016 Lifetime Risk of Stroke Collaborators. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N. Engl. J. Med. 379 (25), 2429–2437 (2018).
Lindsay, M. P. et al. World stroke organization (WSO): Global stroke fact sheet 2019. Int. J. Stroke. 14 (8), 806–817 (2019).
Southerland, A. M. et al. Glucose control and risk of symptomatic intracerebral hemorrhage following thrombolysis for acute ischemic stroke: A SHINE trial analysis. Neurology. 102 (9), e209323 (2024).
Liu, J., Li, X. & Qu, J. Risk factors for acute ischemic stroke in patients with type 2 diabetes mellitus. Med. (Baltim). 102 (47), e36114 (2023).
Georgakis, M. K. et al. Diabetes mellitus, glycemic traits, and cerebrovascular disease: A Mendelian randomization study. Neurology. 96 (13), e1732–e1742 (2021).
Bell, D. S., Patil, H. R. & O’Keefe, J. H. Divergent effects of various diabetes drugs on cardiovascular prognosis. Rev. Cardiovasc. Med. 14 (2–4), e107–e122 (2013).
Hussien, N. R. et al. Sulfonylurea and neuroprotection: The bright side of the moon. J. Adv. Pharm. Technol. Res. 9 (4), 120–123 (2018).
Simard, J. M. et al. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke. 41 (3), 531–537 (2010).
Simard, J. M. et al. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J. Clin. Invest. 117 (8), 2105–2113 (2007).
Feng, X. et al. Safety and efficacy of glibenclamide on cerebral oedema following aneurysmal subarachnoid haemorrhage: A randomised, double-blind, placebo-controlled clinical trial. Stroke Vasc Neurol. 8, 2023 (2024).
Gerzanich, V. et al. Sulfonylurea receptor 1, transient receptor potential cation channel subfamily M member 4, and KIR6.2:Role in hemorrhagic progression of contusion. J. Neurotrauma. 36 (7), 1060–1079 (2019).
Igarashi, T. et al. Continuous Glibenclamide prevents hemorrhagic transformation in a rodent model of severe ischemia-reperfusion. J. Stroke Cerebrovasc. Dis. 30 (3), 105595 (2021).
Huang, K. et al. Exploratory analysis of oral Glibenclamide in acute ischemic stroke. Acta Neurol. Scand. 140 (3), 212–218 (2019).
Kunte, H. et al. Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann. Neurol. 72 (5), 799–806 (2012).
Mehta, R. I. et al. Sur-trpm4 cation channel expression in human cerebral infarcts. J. Neuropathol. Exp. Neurol. 74, 835–849 (2015).
Ortega, F. J. et al. Glibenclamide enhances neurogenesis and improves long-term functional recovery after transient focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 33 (3), 356–364 (2013).
Acknowledgements
This research was supported by the Sichuan Provincial Natural Science Foundation (No. 2024NSFSC1823), the project from Science and Technology Department of Sichuan Province (No. 2022YFS0613) and the project from Science and Technology of Ganzhou (No. 2023DXNS4747). This research protocol was approved by the Ethics Committee of Ganxian District People’s Hospital (No. MR-20230316), which also served as the overall coordinating body. As this study was retrospective, written informed consent was deemed unnecessary by an Institutional Review Board (IRB), the Ethics Committee of Ganxian District People’s Hospital.
Author information
Authors and Affiliations
Contributions
Li Chen and Kaimin Xiao designed and conceptualized the study. Ji Tang, Lihui Xie and Hongmei Chen acquired data. Kaimin Xiao, Ji Tang, Xinwei Yang and Hongmei Chen drafted the manuscript. Ping Liu and Rongxin He participated in the data analysis. Heling Chu, Li Chen and Yuping Tang revised the manuscript. The authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiao, K., Tang, J., Yang, X. et al. Impact of glibenclamide pretreatment on outcomes in acute ischemic stroke patients with type 2 diabetes: a retrospective case-control study. Sci Rep 15, 30666 (2025). https://doi.org/10.1038/s41598-025-15764-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15764-5