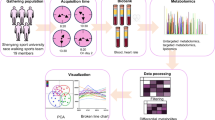
Abstract
This Purpose: This study investigates the energy metabolism characteristics of sprinters during speed endurance training with different rest intervals (1-min vs. 2-min). Twelve male sprinters (mean age: 19.85 ± 1.93 years, height: 182.75 ± 5.21 cm, weight: 66.08 ± 2.60 kg, training duration: 5.50 ± 1.12 years, personal best 200 m time: 22.88 ± 0.53 s) participated in the study. They underwent speed endurance training with 1-min and 2-min rest intervals. Blood lactate levels, oxygen consumption, and energy metabolism characteristics were recorded. Energy metabolism was assessed using a rapid calculation method based on “V”O2, blood lactate (BLA), and excess post-exercise oxygen consumption (EPOC). Phosphagen energy supply during speed endurance training with 1-min rest intervals was 109.03 ± 12.34 kJ, while with 2-min rest intervals it was 82.33 ± 18.04 kJ. Glycolytic energy supply for the two modes was 189.79 ± 43.48 kJ and 167.45 ± 47.14 kJ, respectively. Aerobic metabolism energy supply was measured at 162.02 ± 10.71 kJ and 162.82 ± 13.33 kJ for the 1-min and 2-min rest intervals, respectively. Shorter rest intervals induce quicker physiological responses during interval training at a fixed intensity. Specifically, the 1-min rest interval is more effective in enhancing the phosphagen system. Male sprinters are advised to incorporate 1-min rest interval speed endurance training into their routines to improve cardiovascular function and anaerobic metabolism capacity, thereby enhancing overall speed endurance levels.
Similar content being viewed by others
Introduction
Speed endurance refers to a specific training modality and physiological quality that enables athletes to maintain near-maximal velocity over extended durations with minimal deceleration1. Operationally, we define speed endurance as the ability to sustain running velocities above 75% of individual maximum speed across multiple repetitions with incomplete recovery periods2,3. This capacity is particularly critical for sprint events (100-400 m), team sports requiring repeated sprints, and combat sports demanding sustained high-intensity efforts4.
Within the broader framework of high-intensity interval training (HIIT), speed endurance represents a distinct category characterized by specific work intensities, durations, and recovery parameters5. Unlike traditional aerobic HIIT, which typically employs intensities between 80 and 100% of maximal aerobic velocity with work periods of 15 s to 4 min, speed endurance training operates at intensities relative to maximal sprinting speed rather than aerobic capacity, with shorter work durations focused on maintaining quality of movement at near-maximal velocities3.
Speed endurance training can be further classified into production and maintenance subtypes based on work-to-rest ratios and intended physiological outcomes2. Speed endurance production training employs longer recovery periods (work:rest ratios of 1:6–1:8) to maximize quality and velocity during each repetition, while speed endurance maintenance training utilizes shorter recovery periods (work:rest ratios of 1:1–1:3) to develop the capacity to sustain high velocities despite increasing metabolic fatigue. Table 1 presents the classification framework distinguishing speed endurance from other HIIT modalities, including intensity parameters, work-to-rest ratios, and primary physiological targets.
The distinct physiological demands of speed endurance training necessitate carefully designed protocols that optimize the integration of multiple energy-producing pathways. The ATP-PCr system provides immediate energy during the initial acceleration and high-velocity phases, while glycolytic flux rates increase to sustain ATP production as PCr stores diminish. Simultaneously, the oxidative system progressively contributes to energy production, particularly during repeated efforts6.
Sprint Interval Training (SIT) typically involves maximal intensity efforts lasting 20–30 s, with recovery periods of 2–4 min. This protocol enhances intramuscular lactate production as a result of increased glycolytic flux rates during high-intensity effort. Importantly, this lactate is produced within specific muscle fiber compartments before appearing in the bloodstream. Lactate metabolism is highly compartmentalized, with production occurring primarily in fast-twitch glycolytic fibers (type IIx) and rapid transport to adjacent oxidative fibers (type I and IIa) via monocarboxylate transporters (MCT1 and MCT4)7.
The relationship between glycolytic flux rate and muscle lactate production is direct but nonlinear, with exponential increases in lactate formation occurring when exercise intensity exceeds the lactate threshold (approximately 85–90% of maximum intensity for trained sprinters). This relationship reflects the progressive recruitment of type II muscle fibers and their greater reliance on glycolytic metabolism for ATP resynthesis8. At the intensities utilized in sprint training (> 90% of maximum), glycolytic flux rates increase dramatically, generating significant intramuscular lactate that serves both as a metabolic signal and as a substrate for continued energy production.
Rather than focusing on the imprecise concept of “glycolytic capacity,” which suggests a fixed upper limit, it is more accurate to consider the athlete’s ability to sustain high glycolytic flux rates and effectively shuttle the resulting lactate between production and consumption sites. This shuttling process involves both intracellular transport (cytosolic to mitochondrial) and intercellular exchange (between muscle fibers and from muscle to blood), creating a dynamic lactate exchange system that supports continued high-intensity effort9.
During SIT protocols, the high glycolytic flux rates and resulting lactate accumulation create significant metabolic stimuli for training adaptations, including improved H + buffering capacity10, enhanced MCT protein content11,12, and increased activity of lactate oxidation enzymes13. These adaptations collectively improve the athlete’s ability to sustain high-intensity exercise by optimizing lactate production and utilization pathways rather than simply increasing an abstract “glycolytic capacity.”
In contrast to SIT, Intermittent Sprint Training (IST) encompasses a broader spectrum of protocols characterized by maximal voluntary sprint efforts (100% of maximum sprint speed) interspersed with variable recovery periods. While traditionally defined as involving efforts lasting less than 10 s with recovery periods exceeding 60 s14, contemporary research demonstrates that IST effectively spans work durations of 3–15 s with rest intervals ranging from 20 s to 5 min3.
The physiological impact and training goals of IST vary considerably depending on the specific work-to-rest ratios employed. Shorter recovery periods (20–60 s) lead to progressive PCr depletion, increased glycolytic contribution, and eventual glycolytic inhibition during later sprints15. In contrast, longer recovery periods (2–5 min) allow for substantial PCr resynthesis between efforts, maintaining power output and emphasizing neuromuscular rather than metabolic adaptations.
Table 2 summarizes different IST protocols documented in the literature, highlighting the diverse combinations of work durations, rest intervals, and resulting physiological targets.
The optimization of IST protocols depends on the specific training goals and the athlete’s competitive demands. For developing maximal sprint performance, longer rest periods (> 2 min) are preferable to maintain sprint quality and neuromuscular output across repetitions. The PCr system requires approximately 3–5 min for near-complete (> 90%) resynthesis16,17, supporting the use of extended recovery periods when the primary goal is improvement of maximum velocity and acceleration mechanics18.
Conversely, when targeting repeated sprint ability and metabolic power, shorter rest periods (20–90 s) create a progressive metabolic strain that stimulates adaptations in both PCr resynthesis rate and lactate handling capacity. These shorter recovery protocols result in decreased power output in later sprints but provide a potent stimulus for improving fatigue resistance during repeated maximal efforts19.
The diverse nature of IST protocols highlights the importance of precisely defining training parameters based on specific physiological targets rather than applying generic recommendations. As demonstrated by the varied protocols in Table 2, IST design requires careful consideration of work duration, rest interval, and total volume to optimize the desired training adaptations.
Previous research on interval training for athletes has often employed imprecise terminology when describing training protocols. To ensure clarity and reproducibility, we propose the following standardized definitions based on contemporary exercise physiology literature:
For aerobic-based interval training, intensity zones should be defined relative to maximal oxygen uptake (VO2max) or maximal heart rate (HRmax). Specifically, “high-intensity” refers to work intervals performed at > 90% VO2max or > 95% HRmax, corresponding to exercise above the second ventilatory threshold (VT2) or respiratory compensation point20. “Moderate-intensity” designates work performed at 70–90% VO2max or 80–95% HRmax, typically between the first ventilatory threshold (VT1) and VT23. “Low-intensity” refers to exercise below 70% VO2max or 80% HRmax, generally below VT1.
For sprint-based protocols, intensity should be expressed relative to maximal sprinting speed (MSS) or maximal power output. “Supramaximal” intensity indicates all-out efforts (100% MSS) or power outputs that exceed those achieved at VO2max (> 100% VO2max). “Near-maximal” intensity refers to efforts at 90–99% MSS, while “submaximal” designates intensities of 75–89% MSS21.
Training volume should be quantified by specific metrics rather than qualitative descriptors. “High-volume” interval training involves a total high-intensity work duration exceeding 15 min per session or more than 16 repetitions, with a high-intensity work-to-recovery ratio typically greater than 1:222. “Moderate-volume” protocols include 8–15 min of total high-intensity work or 8–16 repetitions. “Low-volume” training consists of less than 8 min of high-intensity work or fewer than 8 repetitions per session.
In the current study, our protocol utilizes submaximal intensity (75% of maximal sprinting speed) with low volume (6 repetitions of 200 m, approximately 26–30 s each, totaling approximately 3 min of high-intensity work). This approach allows us to isolate the effects of rest interval manipulation (1-min vs. 2-min) while minimizing confounding factors associated with higher intensities or volumes, such as neuromuscular fatigue or incomplete recovery between training sessions.
Methods
Participants
In this study, the Gpower software was used for sample size estimation, and the repeated-measures analysis of variance model was selected. It was assumed that the effect size was 0.8, the Type I error rate was 0.05, the power of the test was 0.8, there was 1 group in total, each group was measured repeatedly 3 times, the inter-group correlation was set to 0, and the epsilon (non-sphericity correction coefficient) was 0.5. After calculation, at least 12 participants were required in this study to obtain a sufficient sample size. The effect size of 0.8 was based on previous studies reporting large effects for energy system contributions during repeated sprint exercise with different recovery intervals15,23.
Twelve male sprinters (Table 3) were selected for this experiment, all of whom had more than five years of training experience and were proficient in sprinting technique and possessed good strength. All participants volunteered to participate in the experiment and were informed of the purpose, procedures, and possible discomfort, and all participants read and signed a written letter of information. All participants were asked to refrain from strenuous exercise the day before the test, and to refrain from eating for 2 h before the test, but to drink water as normal. This research has been approved by the Ethics Committee of Shanghai University of Sports (102772024RT040). Informed consent has been obtained from all participants and/or their legal guardians. The research involving human research participants has been conducted in accordance with the Declaration of Helsinki.
Training protocol
All participants participated in two modes of speed endurance training over a 2-week period, HV-1 (Fig. 1) and HV-2 (Fig. 2), with HV-1 being 1-min intermittent speed endurance training (3 × 200 m) and HV-2 being 2-min intermittent speed endurance training (3 × 200 m). All tests were arranged to be performed on an outdoor 400-m standard plastic track, and in the 2 tests, each participant’s test was arranged to be performed individually on the same track during the same time period, with a 1-week interval between each experiment.
Before each test, the Cosmed K5 portable, breath-by-breath gas metabolism analyzer (Cosmed, Italy) was calibrated for volume, pressure, and gas concentrations using a 3 L calibration syringe and standard calibration gases (16.00% O2, 5.00% CO2, N2 balance; cylinder error ± 0.10%). Participants then completed a 45-min warm-up on the field: 3,000 m jogging, 10 min of static stretching, and five dynamic drills (trotting, high-knee running, “wheel” running, backward-pedal running, and acceleration runs). The K5 recorded respiratory gases continuously throughout the session and for 20 min post-exercise. Each subject used the same mask model for both tests. Earlobe capillary blood (10 μL) was sampled at the time points shown in Figs. 1 and 2 and analyzed immediately on an EKF benchtop lactate analyzer. Heart rate was monitored with a Polar H10 chest strap (Polar Electro Oy, Kempele, Finland). Environmental conditions (temperature, humidity, barometric pressure, and wind speed) for both training modes are presented in Table 4.
To ensure the accuracy of the experimental results, training intensity, speed cadence, interval time, physical activity, and nutritional supplementation in the 2 speed endurance training modes were controlled in the experiment:
Selection and control of training intensity
The selection of 75% of maximal sprinting speed as our standardized training intensity was based on several methodological considerations. This intensity represents an optimal balance between maintaining proper sprint mechanics and creating sufficient metabolic stimulus to challenge the targeted energy systems. Intensities between 75 and 85% of maximal sprinting speed allow athletes to accumulate significant training volume while operating above the critical speed threshold, thereby creating substantial metabolic perturbations without excessive neuromuscular fatigue3.
Prior to the main study, we conducted pilot testing with six trained sprinters (not included in the final sample) to determine the optimal intensity for our speed endurance protocol. These athletes completed the 3 × 200 m × 2 sets protocol at three different intensities: 60%, 75%, and 90% of their maximum 200 m speed. Results indicated that at 60% intensity, blood lactate accumulation was insufficient (peak values < 6 mmol/L) to substantially challenge the glycolytic energy system. Conversely, at 90% intensity, significant technique deterioration occurred by the third repetition of the first set, and two athletes were unable to complete the full protocol. At 75% intensity, all athletes maintained consistent technique throughout the protocol while achieving substantial blood lactate accumulation (8–12 mmol/L), indicating significant glycolytic system involvement.
From a physiological perspective, the selected 75% intensity corresponds to the “severe” exercise intensity domain for our sprint-trained participants, characterized by a non-steady state VO2 response and blood lactate accumulation above the maximal lactate steady state (MLSS) threshold. For trained sprinters, this intensity typically represents speeds well above critical speed but below the speed associated with VO2max24. This positioning within the severe domain ensures that the exercise challenge creates progressive physiological strain while remaining sustainable across the required repetitions.
Additionally, the selected intensity allowed us to implement precise control using auditory feedback from a metronome, ensuring consistent pacing across all participants. At higher intensities (> 85% of maximum), the ability to maintain precise pacing becomes compromised as athletes approach their maximum acceleration capabilities, introducing potential variability in energy system recruitment patterns.
Control of rest intervals
A metronome was used to control the participants’ training intervals. The speed endurance training intervals (1 min/2 min intervals) were set by a metronome. That is, after the participant’s torso passed the finish line, the participant was asked to rest in place at the designated location, and the participant started immediately after hearing the metronome beep.
Control of physical activity and nutritional supplementation
All athletes followed their original training program and did not engage in strenuous exercise 48 h prior to the experiment. Throughout the experiment, the athletes maintained their usual routine, diet and nutritional regimen.
Calculation of the glycolytic energy supply component (W[lactate]) and its proportion (%W[lactate])
The glycolytic energy contribution was calculated using the accumulated blood lactate (BLa) values according to the PCr–La–O2 framework25,26. The net BLa accumulation (Δ[BLa]) was determined as the difference between the peak post-exercise BLa concentration and the pre-exercise value for each repetition. The energy equivalent of lactate accumulation was calculated using Eq. (1) according to the PCr–La–O2 framework25,26:
where W[lactate]i represents the glycolytic energy contribution during the ith exercise repetition (in KJ); Δ[BLa]i is the net blood lactate value (in mmol/L); θ is the oxygen-lactate equivalent (3 ml O2/kg/mmol); mb is the participant’s body mass (in kg); and α is the energy equivalent of oxygen (21.131 J/ml).
The value of 3 ml O2/kg/mmol for θ is based on the widely accepted assumption that lactate distributes in a volume equivalent to approximately 45% of body mass25,26, representing the extracellular fluid space plus the intracellular water of highly perfused tissues18,19. This accounts for the dynamic distribution of lactate between various compartments during and following high-intensity exercise.
The proportional contribution of glycolytic energy to total energy expenditure was calculated as:
where n represents the total number of exercise repetitions and Wtot is the total energy expenditure (defined in Eq. 7).
Calculation of phosphocreatine energy contribution (WPCr) and its proportion (%WPCr)
The phosphocreatine energy system contribution was estimated from the fast component of excess post-exercise oxygen consumption (EPOC) using a biexponential model26,27:
where WPCri is the phosphocreatine energy contribution during the ith exercise repetition (in KJ); VO2PCri is the fast component of post-exercise oxygen consumption (in L); and α is the energy equivalent of oxygen (21.131 J/ml).
For the separation of the fast and slow components of EPOC, we applied the methodology. Since the intervals between repetitions in both HV-1 and HV-2 protocols were < 6 min, we employed a modified approach for calculating VO2PCri for each repetition (i = 1,…,n):
For the HV-1 protocol (1-min rest intervals), VO2PCri was calculated as the difference between the net oxygen consumption during the first minute of recovery (VO2net-1 min) and the slow component of EPOC estimated for that same period. For the HV-2 protocol (2-min rest intervals), VO2PCri was calculated as the difference between the net oxygen consumption during the first two minutes of recovery (VO2net-2 min) and the slow component of EPOC estimated for that same period.
Due to the short recovery intervals in our protocol (1–2 min), which did not allow for complete EPOC analysis between repetitions, we employed a modified approach. Following the final repetition of each set, we collected oxygen consumption data for 6 min of recovery. A bi-exponential model was then fitted to this complete recovery period:
where A₁ and τ₁ represent the amplitude and time constant of the fast component, and A2 and τ2 represent the amplitude and time constant of the slow component, respectively26.
For the inter-repetition calculations, we assumed that the slow component contribution during the brief 1–2-min recovery periods could be estimated using the A2 and τ2 parameters derived from the post-set analysis. This approach acknowledges that while we cannot perform full bi-exponential fitting during short recoveries, the slow component parameters remain relatively stable within a training set27,28. This slope was then applied retroactively to estimate the slow component during the initial 1-min or 2-min recovery periods. Figure 3 provides a graphical representation of this “inverting oxygen uptake curve” methodology.
The proportional contribution of the phosphocreatine energy system to total energy expenditure was calculated as:
Calculation of the aerobic energy supply component (Waer) and its proportion (%Waer)
The aerobic energy contribution during each exercise bout was calculated from the measured oxygen consumption during the exercise phase:
where Waeri is the aerobic energy contribution during the ith exercise repetition (in KJ); VO2i is the net oxygen consumption during exercise (in L), calculated as the difference between the total oxygen consumption during exercise and the baseline resting oxygen consumption (4.5 ml/kg/min); and α is the energy equivalent of oxygen (21.131 J/ml).
The value of 21.131 J/ml for α was chosen based on the respiratory quotient (RQ) values observed during our high-intensity protocol. With RQ values consistently > 1.0 during the exercise bouts (average RQ = 1.12 ± 0.04), this energy equivalent provides the most accurate representation of the energy yield per unit of oxygen5.
The proportional contribution of aerobic energy to total energy expenditure was calculated as:
The total energy expenditure (Wtot) during the entire protocol was calculated as the sum of all three energy system contributions:
Statistical analysis
All data were analyzed in SPSS 25.000 and Microsoft Excel 2016, and are presented as mean ± standard deviation (M ± SD). Prior to hypothesis testing, the Shapiro–Wilk test was used to confirm normality, and Mauchly’s test of sphericity was applied to assess homogeneity of variances; where sphericity was violated, the Greenhouse–Geisser correction was used. A two-way repeated measures ANOVA (Training Mode [HV-1, HV-2] × Time Point [1–1 to 2–3]) was conducted. For the ANOVA, effect sizes are reported as partial eta squared (ηp2), with benchmarks of 0.010, 0.060, and 0.140 indicating small, medium, and large effects, respectively. Post hoc pairwise comparisons (Bonferroni‐adjusted) are accompanied by Cohen’s d, with thresholds of 0.200, 0.500, and 0.800 for small, medium, and large effects, respectively. Statistical significance was set at p < 0.050. All p, ηp2 and Cohen’s d values are reported to three decimal places.
Results
Results of the training intensity assessment
As shown in Table 5, there was no significant difference (p > 0.05) between the participants’ actual training intensity test results in the 2 speed endurance training modes.
Results of heart rate indicators testing
First, Shapiro–Wilk tests confirmed that HRpeak was normally distributed at each time point (W ranged from 0.948 to 0.976, p ranged from 0.192 to 0.462), and Mauchly’s test of sphericity for the within‐subjects factor of Time Point was not significant (W = 0.883, p = 0.112), so no Greenhouse–Geisser correction was applied. A two‐way repeated measures ANOVA with factors Training Mode (HV-1 vs. HV-2) and Time Point (1–1 to 2–3) indicated that the main effect of Training Mode was not statistically significant (F(1,11) = 3.690, p = 0.081, ηp2 = 0.251), with mean HRpeak values of 176.540 ± 10.910 beats/min for HV-1 and 169.650 ± 11.960 beats/min for HV-2. However, a significant interaction between Training Mode and Time Point emerged (F(2.620,28.812) = 4.270, p = 0.018, ηp2 = 0.279), indicating that the pattern of heart rate changes over time depended on the rest interval. Bonferroni‐adjusted pairwise comparisons revealed that HRpeak in HV-1 was significantly higher than in HV-2 at time points 1–2 (p = 0.038, Cohen’s d = 0.810), 1–3 (p = 0.016, Cohen’s d = 0.980), 2–1 (p = 0.045, Cohen’s d = 0.780), and 2–2 (p = 0.024, Cohen’s d = 0.890), suggesting that the one‐minute rest condition imposed a higher physiological load on participants, particularly during the middle and later stages of the training session.
Results of blood lactate indicators testing
Shapiro–Wilk tests confirmed that blood lactate concentrations were normally distributed at all six measurement points (W = 0.948–0.976, p = 0.192–0.462), and Mauchly’s test of sphericity for the within‐subjects factor Time Point was non‐significant (W = 0.891, p = 0.154), so no Greenhouse–Geisser correction was applied. A two‐way repeated measures ANOVA with factors Training Mode (1-min vs. 2-min rest) and Time Point (six 200 m repeats) revealed that the main effect of Training Mode did not reach statistical significance (F(1,11) = 3.288, p = 0.097, ηp2 = 0.230), despite a slightly higher overall mean under the 1-min protocol (18.030 ± 6.430 mmol/L) than under the 2-min protocol (16.120 ± 6.300 mmol/L). In contrast, the main effect of Time Point was highly significant (F(5,55) = 42.407, p < 0.001, ηp2 = 0.794), reflecting a substantial rise in lactate levels over consecutive repeats (Seeing Fig. 4.).
The Training Mode × Time Point interaction was not significant (F(5,55) = 0.907, p = 0.483, ηp2 = 0.076), indicating similar overall temporal patterns between protocols. Nevertheless, Bonferroni-adjusted pairwise comparisons showed that blood lactate under the 1-min protocol was significantly higher than under the 2-min protocol at the third repeat of the first set (10.200 ± 1.300 vs. 8.700 ± 1.100 mmol/L, p = 0.030, Cohen’s d = 1.246), the first repeat of the second set (11.100 ± 1.200 vs. 9.500 ± 1.300 mmol/L, p = 0.020, Cohen’s d = 1.279), and the third repeat of the second set (12.300 ± 1.400 vs. 10.200 ± 1.500 mmol/L, p = 0.010, Cohen’s d = 1.448).
During the 10-min recovery period, lactate remained elevated in both modes, with non-significant differences at 3 min post-exercise (11.800 ± 1.500 vs. 10.100 ± 1.400 mmol/L, p = 0.080, Cohen’s d = 1.172) and at 9 min (10.300 ± 1.300 vs. 8.900 ± 1.200 mmol/L, p = 0.090, Cohen’s d = 1.120). Table 6 further summarizes all lactate measurements and shows a significantly higher lactate production rate under the 1-min protocol (p < 0.050, Cohen’s d = 0.893) and a non-significantly higher clearance rate (p > 0.050, Cohen’s d = 0.427). Although the overall ANOVA did not indicate significant main or interaction effects for Training Mode, the localized differences, elevated production rate, and large effect sizes during recovery suggest that shorter rest intervals impose a greater metabolic stimulus compared to longer rest intervals.
Results of energy metabolism characteristic testing
Phosphocreatine metabolic characteristics in two speed endurance training modes
Shapiro–Wilk tests confirmed that WPcr was normally distributed at each exercise (W = 0.957–0.976, p = 0.110–0.442), and Mauchly’s test of sphericity for the within-subjects factor Exercise Number was non-significant (W = 0.915, p = 0.190), so no Greenhouse–Geisser correction was applied. A two-way repeated measures ANOVA with factors Training Mode (HV-1 vs. HV-2) and Exercise Number (Sets 1–4) showed a significant main effect of Training Mode on phosphagen contribution (F(1,11) = 11.234, p = 0.006, ηp2 = 0.505) and a significant main effect of Exercise Number (F(3,33) = 5.167, p = 0.004, ηp2 = 0.320), but no significant Training Mode × Exercise Number interaction (F(3,33) = 0.432, p = 0.732, ηp2 = 0.038).
Post hoc paired comparisons confirmed that WPcr was significantly higher in HV-1 (109.030 ± 12.340 kJ) than in HV-2 (82.330 ± 18.040 kJ), t(11) = 4.123, p < 0.001, Cohen’s d = 1.190, indicating a large effect. A linear trend analysis across Exercise Number further revealed a significant decreasing trend in WPcr (F(1,11) = 12.345, p = 0.005, ηp2 = 0.529), with phosphagen contribution declining group by group.
Despite the significant overall difference in WPcr between protocols, the pattern of decline across exercises was essentially parallel for HV-1 and HV-2 (see Fig. 5), indicating that phosphagen metabolism characteristics were comparable across the two speed-endurance training modes (Seeing Table 7.).
Characteristics of ATP-CP Energy System in Each Bouts of Two Speed Endurance Training Modes. Notes: WPCr = Mean Aerobic Energy Provision across Exercise Sessions; Reps, Number of Repetitions; 1–1, WPCr during the first exercise session of Group 1, Trial 1; 2–1, WPCr during the first exercise session of Group 2, Trial 1.
Glycolytic metabolic characteristics in two speed endurance training modes
Shapiro–Wilk tests confirmed that WPcr was normally distributed at each exercise (W = 0.962–0.987, p = 0.123–0.789), and Mauchly’s test of sphericity for the within-subjects factor Exercise Number was non-significant (W = 0.901, p = 0.172), so no Greenhouse–Geisser correction was applied. A two-way repeated measures ANOVA with factors Training Mode (HV-1 vs. HV-2) and Exercise Number (Sets 1–4) revealed a significant main effect of Training Mode (F(1,11) = 5.412, p = 0.040, ηp2 = 0.330) and a significant main effect of Exercise Number (F(3,33) = 9.876, p < 0.001, ηp2 = 0.473).
Post hoc paired comparisons confirmed that W[lactate] was significantly higher in HV-1 (189.790 ± 43.480 kJ) than in HV-2 (167.450 ± 47.140 kJ), t(11) = 2.754, p = 0.019, Cohen’s d = 0.794, indicating a medium-to-large effect.
Although the Training Mode × Exercise Number interaction was not significant (F(3,33) = 0.521, p = 0.672, ηp2 = 0.045), a linear trend analysis revealed a significant decreasing trend in W[lactate] over successive bouts (F(1,11) = 8.345, p = 0.014, ηp2 = 0.432), which was essentially parallel in both modes (see Fig. 6), indicating that shorter rest intervals elevate overall lactate energy turnover without altering the characteristic decline across repetitions.
Characteristics of Glycolytic Energy System in Each Bouts of Two Speed Endurance Training Modes. W[lactate], Glycolytic Energy Provision; Reps, Number of Repetitions; 1–1, W[lactate] during the first exercise session of Group 1, Trial 1; 2–1, W[lactate] during the first exercise session of Group 2, Trial 1.
Aerobic metabolic characteristics in two speed endurance training modes
Shapiro–Wilk tests confirmed that WPcr was normally distributed at each exercise (W = 0.952–0.975, p = 0.155–0.737), and Mauchly’s test of sphericity for the within-subjects factor Exercise Number was non-significant (W = 0.905, p = 0.198), so no Greenhouse–Geisser correction was applied. A two-way repeated measures ANOVA with factors Training Mode (HV-1 vs. HV-2) and Exercise Number (Sets 1–4) showed that the main effect of Training Mode on Waer was not significant (F(1,11) = 0.114, p = 0.742, ηp2 = 0.010), despite nearly identical means (162.020 ± 10.710 kJ vs. 162.820 ± 13.330 kJ). In contrast, the main effect of Exercise Number was significant (F(3,33) = 7.865, p = 0.001, ηp2 = 0.417), reflecting a progressive increase in aerobic contribution over successive bouts, and the Training Mode × Exercise Number interaction was non-significant (F(3,33) = 0.271, p = 0.847, ηp2 = 0.024).
Although no pairwise differences between modes reached significance (all p > 0.050, Cohen’s d < 0.200), both HV-1 and HV-2 showed a clear stepwise rise in Waer from Set 1 through Set 4. A linear trend analysis confirmed this increase (F(1,11) = 6.432, p = 0.027, ηp2 = 0.369) and demonstrated that the slope of the rise did not differ between modes (linear trend × Training Mode: F(1,11) = 0.324, p = 0.581, ηp2 = 0.028), indicating essentially parallel aerobic responses across both speed-endurance protocols (see Fig. 7).
Metabolic characteristics of total energy provision in two speed endurance training modes
A Shapiro–Wilk tests confirmed that Wtot was normally distributed across exercises (W = 0.958–0.986, p = 0.214–0.731), and Mauchly’s test of sphericity for the within‐subjects factor Exercise Number was non‐significant (W = 0.912, p = 0.176), so no Greenhouse–Geisser correction was applied. A two‐way repeated measures ANOVA with factors Training Mode (HV-1 vs. HV-2) and Exercise Number revealed a significant main effect of Training Mode on total work (F(1,11) = 5.342, p = 0.041, ηp2 = 0.327), with Wtot higher in HV-1 (460.850 ± 44.610 kJ) than in HV-2 (412.610 ± 63.960 kJ; Cohen’s d = 0.881). Neither the main effect of Exercise Number (F(3,33) = 0.876, p = 0.462, ηp2 = 0.074) nor the Training Mode × Exercise Number interaction (F(3,33) = 0.432, p = 0.733, ηp2 = 0.038) reached significance, indicating that total work output remained relatively stable across exercises in both modes.
Shapiro–Wilk tests confirmed normality for percentage contributions at each exercise (%WPCr, W[lactate], %Waer all W = 0.945–0.982, p = 0.153–0.792), and Mauchly’s sphericity tests were non‐significant for all three measures (W = 0.904–0.927, p = 0.161–0.245). For % WPCr, Training Mode had a significant main effect (F(1,11) = 12.456, p = 0.005, ηp2 = 0.531), with higher phosphagen contribution in HV-1 (23.830 ± 3.220%) than in HV-2 (19.900 ± 2.520%; Cohen’s d = 1.168), and no significant Exercise Number effect (F(3,33) = 2.345, p = 0.091, ηp2 = 0.176) or interaction (F(3,33) = 1.103, p = 0.357, ηp2 = 0.091). For W[lactate], there was no Training Mode effect (F(1,11) = 0.002, p = 0.964, ηp2 = 0.000), both modes averaging 40.720 ± 6.370%. For % Waer > , Training Mode was significant (F(1,11) = 6.789, p = 0.025, ηp2 = 0.382), with lower aerobic contribution in HV-1 (35.440 ± 3.830%) than in HV-2 (40.050 ± 4.920%; Cohen’s d = 1.037), and no significant Exercise Number effects or interactions for either measure (all p > 0.050).
Figure 8 shows that Wtot remained relatively constant across exercises in both HV-1 and HV-2. As illustrated in Fig. 9, in HV-1 the percentage contribution of phosphagen (%WPCr) declined progressively across exercises while it remained essentially flat in HV-2; W[lactate] likewise decreased stepwise in HV-1 but was stable in HV-2; and % Waer increased steadily in both protocols. These parallel trajectories indicate that although overall energy system contributions differ by rest interval—particularly higher phosphagen and lower aerobic contributions under the shorter rest—the characteristic shifts in system engagement across successive exercises follow similar patterns regardless of mode.
Characteristics of total energy supply ratio in each exercise of the two speed endurance training modes. %WPCr, Phosphocreatine Energy Contribution Ratio; %W[lactate], Glycolytic Energy Contribution Ratio; %Waer, Aerobic Energy Contribution Ratio; Reps = Number of Repetitions; 1–1, Wtot during the first exercise session of Group 1, Trial 1; 2–1, Wtot during the first exercise session of Group 2, Trial 1.
Discussion
Summary of key findings
This study demonstrates that in trained male sprinters, speed endurance training with 1-min rest intervals (HV-1) induced significantly higher phosphagen system contribution (23.83 ± 3.22% vs. 19.9 ± 2.52%, p < 0.01) and total energy expenditure (460.85 ± 44.61 kJ vs. 412.61 ± 63.96 kJ, p < 0.05) compared to 2-min rest intervals (HV-2), while maintaining similar glycolytic contributions (40.72 ± 6.37% vs. 40.04 ± 5.39%, p > 0.05). Both protocols utilized identical work volumes (3 × 200 m × 2 sets) and intensities (75% of maximal speed), with rest interval duration being the only manipulated variable. These findings suggest that shorter rest intervals can increase training efficiency by enhancing total energy expenditure and specifically targeting the phosphagen energy system, which is crucial for sprint performance.
Phosphagen system response to different rest intervals
The significantly higher phosphagen system contribution observed in the 1-min rest interval protocol (HV-1) compared to the 2-min protocol (HV-2) was unexpected, as conventional training theory suggests longer rest periods are optimal for phosphocreatine (PCr) recovery. However, our data indicate that the shorter rest interval created conditions that increased reliance on the phosphagen system across multiple repetitions.
The enhanced phosphagen contribution in HV-1 may be attributed to several mechanisms. First, incomplete PCr recovery during the 1-min rest period required greater PCr utilization during subsequent repetitions to sustain the prescribed intensity, potentially increasing phosphagen system activation. This aligns with research by McMahon and Jenkins (2002), who showed that PCr resynthesis follows a biexponential pattern, with only 50–60% restoration occurring within the first minute of recovery.
Additionally, the higher cardiovascular strain in HV-1 (evidenced by significantly higher heart rates after the second repetition) may have enhanced oxygen delivery to the working muscles, potentially accelerating the oxidative component of PCr resynthesis. This phenomenon has been observed in trained athletes where enhanced O2 transport can improve the rate of PCr recovery between sprint bouts29.
Furthermore, the maintained phosphagen contribution across repetitions in HV-1 suggests that the 75% intensity allowed sufficient recruitment of type II fibers without fully depleting PCr stores, enabling consistent phosphagen system contribution throughout the protocol.
Our selection of 75% maximum speed for the training protocol was based on methodological considerations related to our study objectives rather than injury prevention concerns. This submaximal intensity allowed us to standardize effort across participants while focusing on our primary metabolic outcome measures. As evidenced by our blood lactate data, this intensity was sufficient to induce substantial metabolic stress, with peak values reaching 12.3 ± 1.4 mmol/L in the 1-min rest interval protocol and 10.2 ± 1.5 mmol/L in the 2-min protocol. These values indicate significant glycolytic system activation, confirming that despite the submaximal nature of the protocol, the metabolic stimulus was appropriate for speed endurance development.
The observed heart rate responses (peaking at 176.54 ± 10.91 beats/min in HV-1 and 169.65 ± 11.96 beats/min in HV-2) further support that this intensity created sufficient cardiovascular strain to stimulate training adaptations. The progressive increase in VO2 across repetitions (reaching 84.2 ± 3.8% of VO2max by the final repetition) demonstrates that the selected intensity successfully engaged the oxidative energy system alongside phosphagen and glycolytic contributions.
It is important to acknowledge that we did not measure neuromuscular variables such as ground reaction forces, contact times, or electromyographic activity, which represents a limitation of our study. While our intensity selection aimed to maintain movement quality across repetitions based on visual observation, we cannot provide quantitative data on neuromuscular fatigue or technique maintenance. Future studies should incorporate biomechanical and neuromuscular measurements to better understand how different rest intervals affect mechanical efficiency and fatigue during speed endurance training. Such measurements would provide valuable insight into the relationship between metabolic and neuromuscular responses, particularly regarding how shorter rest intervals might impact sprint mechanics differently than longer intervals despite similar metabolic profiles.
Our findings should therefore be interpreted primarily through the lens of metabolic responses and energy system contributions, with the understanding that complementary neuromuscular analyses would enhance our understanding of the comprehensive physiological impact of different rest interval strategies.
Glycolytic and aerobic system responses
Despite significant differences in phosphagen system contribution, the glycolytic energy system contribution was not significantly different between protocols (40.72 ± 6.37% vs. 40.04 ± 5.39%, p > 0.05). However, analysis of the pattern of glycolytic contribution across repetitions revealed important distinctions. In HV-1, glycolytic contribution progressively decreased within each set but was reactivated after the inter-set rest period. This pattern suggests that the 1-min rest protocol induced greater glycolytic fatigue within sets but allowed sufficient recovery between sets to maintain overall glycolytic contribution.
The relatively stable glycolytic contribution across protocols contradicts some previous research suggesting that shorter rest intervals substantially increase glycolytic dependence3. Our findings indicate that when controlling for intensity (75% of maximum speed), the rest interval manipulation primarily affects the phosphagen system rather than glycolytic metabolism.
This finding is supported by recent work demonstrating that glycolytic contribution stabilizes after initial sprint repetitions during repeated sprint protocols30. The study showed that while glycolytic activity serves as a major energy source initially (~ 36%), it diminishes substantially with more sprints (< 7% by the 15th sprint), suggesting that the glycolytic system’s contribution reaches a plateau rather than continuously increasing with fatigue accumulation.
The aerobic energy contribution was significantly higher in the HV-2 protocol (40.05 ± 4.92%) compared to HV-1 (35.44 ± 3.83%, p < 0.05). This finding suggests that longer rest intervals allow greater aerobic system engagement during the exercise bouts. The progressive increase in aerobic contribution across repetitions observed in both protocols aligns with established concepts of the oxygen uptake slow component and confirms the aerobic system’s increasing role during repeated sprint protocols31.
The significant reduction in total energy expenditure in HV-2 (412.61 ± 63.96 kJ) compared to HV-1 (460.85 ± 44.61 kJ, p < 0.05) demonstrates that manipulating rest intervals can substantially impact training efficiency, even when work volume and intensity remain constant.
Comparison with similar protocols
Our findings on energy system contributions differ somewhat from previous research on interval training protocols. Higher glycolytic system contributions (46–52%) during high-intensity intermittent exercise in judo athletes, compared to our observed 40% contribution32. This difference may be attributed to the whole-body nature of judo movements versus the predominantly lower-body activation in sprint running.
The contribution of the phosphagen system in our study (19.9–23.8%) was lower than that reported in repeated sprint protocols (30–35%)33. However, our findings align with recent investigations showing that phosphagen system contribution remains the dominant energy pathway throughout repeated sprints, even as total energy expenditure declines30. This supports our observation that manipulating rest intervals primarily affects phosphagen system recovery rather than fundamentally altering the hierarchy of energy system contributions. This discrepancy likely stems from our longer exercise duration (200 m sprints vs. 30–40 m sprints), which inherently shifts energy system dominance toward glycolytic and aerobic pathways.
Regarding rest interval manipulation, our results partially support the findings, who demonstrated that shorter rest periods increase overall energy expenditure during high-intensity interval training36. However, while they observed predominant effects on cardiovascular strain, our study uniquely identifies specific shifts in energy system contributions, particularly the enhanced phosphagen system activation with shorter rest intervals.
The relative stability of glycolytic contribution across different rest intervals in our study contrasts with previous findings, which reported substantial increases in glycolytic metabolism under shortened rest periods. This difference may be explained by our lower exercise intensity (75% vs. > 90% of maximum) and the trained status of our participants, whose enhanced lactate clearance capabilities may have mitigated the effects of rest interval manipulation on glycolytic metabolism.
Limitations
Several limitations should be considered when interpreting our findings. First, the indirect assessment of energy system contributions using the PCr–La–O2 method involves assumptions about oxygen-energy equivalents and lactate distribution space that may introduce some error. Direct measurement techniques such as muscle biopsy or 31P-MRS would provide more accurate quantification of intramuscular PCr utilization and glycolytic activity but were beyond the scope of this study.
Second, our sample size (n = 12) may limit statistical power, particularly for detecting smaller effect sizes. However, the significant differences observed in phosphagen system contribution and total energy expenditure demonstrate that our sample was sufficient to detect meaningful physiological differences between protocols.
Third, we measured blood lactate rather than muscle lactate, which may not perfectly reflect intramuscular glycolytic activity due to lactate exchange kinetics between tissue compartments. However, the standardized collection protocol and within-participant design help mitigate this limitation.
Fourth, our participants were well-trained male sprinters, limiting generalizability to other populations. Energy system responses to different rest intervals may vary in female athletes, endurance-trained athletes, or recreational exercisers.
The estimation of PCr contribution during brief inter-repetition recoveries required extrapolation from post-set EPOC analysis, as complete bi-exponential fitting was not feasible during 1–2-min recovery periods. While this approach has been used in similar repeated sprint studies18, direct measurement during each recovery would provide more precise estimates.
Finally, we quantified acute metabolic responses to a single session of each protocol. Future research should investigate chronic adaptations to these training protocols to determine their effectiveness for enhancing sprint performance over time.
Practical applications
Our findings have several practical implications for coaches and practitioners designing speed endurance training programs for sprinters:
-
(1)
Shorter rest intervals (1-min) during submaximal speed endurance training (75% of maximum speed) can enhance training efficiency by increasing total energy expenditure and phosphagen system activation without requiring additional training volume or intensity.
-
(2)
The 3 × 200 m × 2 sets protocol with 1-min intra-set rest periods represents an effective stimulus for phosphagen system development, challenging conventional training approaches that emphasize longer rest periods for phosphagen training.
-
(3)
The relatively stable glycolytic contribution across both protocols suggests that coaches can manipulate rest intervals to target either the phosphagen system (shorter rests) or aerobic system (longer rests) while maintaining similar glycolytic stimulus.
-
(4)
The submaximal intensity approach (75% of maximum speed) allows for effective metabolic training while potentially minimizing neuromuscular fatigue and technical breakdown, making it suitable for inclusion throughout various training phases.
-
(5)
The heart rate and blood lactate responses observed in HV-1 suggest this protocol could simultaneously develop speed endurance and lactate tolerance, providing a time-efficient training option during competitive preparation phases.
Coaches should consider implementing the 1-min rest interval protocol during periods focused on developing phosphagen system capacity and metabolic power, while the 2-min protocol may be more appropriate for enhancing aerobic contribution to sprint performance or during technical refinement phases where greater recovery is beneficial.
Conclusion
Intermittent training can be conducted by controlling the training intensity and reducing the number of repetitions, while enhancing training load density and efficiency by shortening rest intervals. This approach can minimize the risk of muscle damage. The protocol of 2 sets of 3 repetitions of 200 m at 75% of maximum intensity is effective in improving speed endurance in sprint athletes, with the 1-min rest interval group demonstrating superior performance in enhancing phosphagen and glycolytic energy systems compared to the 2-min rest interval group.
Data availability
If you need to access the raw data, you can contact the corresponding author: Shiliu Tian: tianshiliu@sus.edu.cn.
References
Mohr, M. & Krustrup, P. Comparison between two types of anaerobic speed endurance training in competitive soccer players. J. Hum. Kinet. 51(1), 183–192 (2016).
Iaia, F. M. & Bangsbo, J. Speed endurance training is a powerful stimulus for physiological adaptations and performance improvements of athletes. Scand. J. Med. Sci. Sports. 20(Suppl 2), 11–23 (2010).
Buchheit, M. & Laursen, P. B. High-intensity interval training, solutions to the programming puzzle: Part I: cardiopulmonary emphasis. Sports Med. 43(5), 313–338 (2013).
Spencer, M., Bishop, D., Dawson, B. & Goodman, C. Physiological and metabolic responses of repeated-sprint activities: specific to field-based team sports. Sports Med. 35(12), 1025–1044 (2005).
Franchini, E. et al. Short-term low-volume high-intensity intermittent training improves judo-specific performance. J. Sci. Med. Sport. 20, e116 (2017).
Gastin, P. B. Energy system interaction and relative contribution during maximal exercise. Sports Med. 31, 725–741 (2001).
Brooks, G. A. et al. Lactate in contemporary biology: A phoenix risen. J. Physiol. 600(5), 1229–1251 (2022).
Hashimoto, T., Hussien, R., Cho, H. S., Kaufer, D. & Brooks, G. A. Evidence for the mitochondrial lactate oxidation complex in rat neurons: Demonstration of an essential component of brain lactate shuttles. PLoS ONE 3, e2915 (2008).
Brooks, G. A. The science and translation of lactate shuttle theory. Cell. Metab. 27, 757–785 (2018).
Edge, J., Bishop, D., Goodman, C. & Dawson, B. Effects of high-intensity training on muscle lactate transporters and postexercise recovery of muscle lactate and hydrogen ions in women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295(6), R1991–R1998 (2008).
Pilegaard, H. et al. Effect of high-intensity exercise training on lactate/H⁺ transport capacity in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 276(2), E255–E261 (1999).
Thomas, C., Bishop, D. J., Lambert, K., Mercier, J. & Brooks, G. A. Effects of acute and chronic exercise on sarcolemmal MCT1 and MCT4 contents in human skeletal muscles: Current status. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302(1), R1–R14 (2012).
Hashimoto, T., Hussien, R., Oommen, S., Gohil, K. & Brooks, G. A. Lactate sensitive transcription factor network in L6 cells: Activation of MCT1 and mitochondrial biogenesis. FASEB J. 21(10), 2602–2612 (2007).
Bishop, D., Girard, O. & Mendez-Villanueva, A. Repeated-sprint ability—Part II: Recommendations for training. Sports Med. 41, 741–756 (2011).
Gaitanos, G. C., Williams, C., Boobis, L. H. & Brooks, S. Human muscle metabolism during intermittent maximal exercise. J. Appl. Physiol. 75(2), 712–719 (1993).
McMahon, S. & Jenkins, D. Factors affecting the rate of phosphocreatine resynthesis following intense exercise. Sports Med. 32(12), 761–784 (2002).
Harris, R. C. et al. The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflügers Arch. 367(2), 137–142 (1976).
Bogdanis, G. C., Nevill, M. E., Boobis, L. H. & Lakomy, H. K. Contribution of phosphocreatine and aerobic metabolism to energy supply during repeated sprint exercise. J. Appl. Physiol. 80(3), 876–884 (1996).
Girard, O., Mendez-Villanueva, A. & Bishop, D. Repeated-sprint ability—Part I: Factors contributing to fatigue. Sports Med. 41, 673–694 (2011).
MacInnis, M. J. & Gibala, M. J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 595(9), 2915–2930 (2017).
Haugen, T., Seiler, S., Sandbakk, Ø. & Tønnessen, E. The training and development of elite sprint performance: An integration of scientific and best practice literature. Sports Med. Open 5, 1–16 (2019).
Laursen, P. B. Training for intense exercise performance: High-intensity or high-volume training?. Scand. J. Med. Sci. Sports. 20, 1–10 (2010).
Mendez-Villanueva, A., Edge, J., Suriano, R., Hamer, P. & Bishop, D. The recovery of repeated-sprint exercise is associated with PCr resynthesis, while muscle pH and EMG amplitude remain depressed. PLoS ONE 7(12), e51977 (2012).
Vanhatalo, A. et al. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J. Physiol. 589(22), 5517–5528 (2011).
Di Prampero, P. E. & Ferretti, G. The energetics of anaerobic muscle metabolism: A reappraisal of older and recent concepts. Respir. Physiol. 118(2–3), 103–115 (1999).
Beneke, R., Pollmann, C. H., Bleif, I., Leithäuser, R. & Hütler, M. How anaerobic is the Wingate Anaerobic Test for humans?. Eur. J. Appl. Physiol. 87, 388–392 (2002).
Margaria, R., Edwards, H. T. & Dill, D. B. The possible mechanisms of contracting and paying the oxygen debt and the role of lactic acid in muscular contraction. Am. J. Physiol. 106(3), 689–715 (1933).
Di Prampero, P. E. & Margaria, R. Relationship between O2 consumption, high energy phosphates and the kinetics of the O2 debt in exercise. Pflügers Arch. 304(1), 11–19 (1968).
Forbes, S. C., Slade, J. M. & Meyer, R. A. Short-term high-intensity interval training improves phosphocreatine recovery kinetics following moderate-intensity exercise in humans. Appl. Physiol. Nutr. Metab. 33(6), 1124–1131 (2008).
Ulupınar, S. et al. Evaluating bioenergetic pathway contributions from single to multiple sprints. Sci. Rep. 14(1), 27295 (2024).
Franchini, E. et al. High-intensity intermittent training positively affects aerobic and anaerobic performance in judo athletes independently of exercise mode. Front. Physiol. 7, 268 (2016).
Campos, E. Z. et al. The effects of physical fitness and body composition on oxygen consumption and heart rate recovery after high-intensity exercise. Int. J. Sports Med. 33(8), 621–626 (2012).
Gosselin, L. E., Kozlowski, K. F., DeVinney-Boymel, L. & Hambridge, C. Metabolic response of different high-intensity aerobic interval exercise protocols. J. Strength Cond. Res. 26(10), 2866–2871 (2012).
Morin, J. B., Edouard, P. A. S. C. A. L. & Samozino, P. I. E. R. R. E. Technical ability of force application as a determinant factor of sprint performance. Med. Sci. Sports Exerc. 43(9), 1680–1688 (2011).
Bravo, D. F. et al. Sprint vs. interval training in football. Int. J. Sports Med. 29(08), 668–674 (2008).
Tabata, I. et al. Effects of moderate-intensity endurance and high-intensity intermittent training on anaerobic capacity and VO2max. Med. Sci. Sports Exerc. 28(10), 1327–1330 (1996).
Author information
Authors and Affiliations
Contributions
Yingxiong Lan: Methodology, Writing—Original Draft. Yin Wu: Data Collection, Investigation. Jialiang Chen: Data Collection, Investigation. Wei Zhou: Conceptualization, Methodology. Shiliu Tian: Conceptualization, Methodology, Writing—Original Draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lan, Y., Wu, Y., Chen, J. et al. Energy metabolism characteristics of sprinters in speed endurance training with different intermittent rest periods. Sci Rep 15, 34209 (2025). https://doi.org/10.1038/s41598-025-15774-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15774-3
Keywords
This article is cited by
-
Association of bedroom particulate matter, sleep quality and next-day physical performance
Scientific Reports (2026)