Abstract
Sepsis is a life-threatening condition triggered by a dysregulated immune response to bloodstream infection. Patients with solid and hematologic malignancies are at increased risk of severe infections and the onset of sepsis. Due to the limitations of blood cultures, particularly in culture-negative sepsis, multiple serological biomarkers, such as C-reactive protein (CRP), interleukin-6 (IL-6), procalcitonin (PCT), white blood cells (WBC), lymphocytes (LYM), neutrophils (NEU), and monocytes (MON), are frequently used to diagnose infections. This prospective observational study aims to evaluate the efficacy of these biomarkers in distinguishing sepsis in patients with hematologic and solid tumors. It was observed that hematologic cancer patients exhibited significantly elevated IL-6, PCT, and MON levels, indicating their strong potential for sepsis detection. However, this difference was not statistically significant in patients with solid cancers and sepsis. Specifically, patients with blood cancer at the onset of sepsis have elevated levels of IL-6, PCT and MON, with AUCs exceeding 0.75, indicating strong predictive value. In contrast, patients with solid tumors had a moderate but not statistically significant increase in PCT (AUC = 0.693). Biomarker combinations enhanced the diagnostic power for hematologic cancers, but their performance in solid tumors remained limited. The findings underscore the need for cancer-specific sepsis diagnostic approaches, with a particular focus on the unique immune dynamics of hematologic versus solid tumors.
Similar content being viewed by others
Introduction
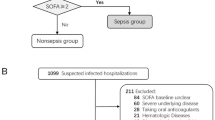
Patients with solid and hematologic cancers are at increased risk of severe infections and the onset of sepsis1. Septicemia is characterized as a life-threatening condition resulting from organ dysfunction triggered by a dysregulated immune response to infection1. This condition can be identified in two scenarios: (1) if sepsis is explicitly documented as the primary diagnosis upon hospital admission or (2) if the primary diagnosis at admission is an infection accompanied by organ dysfunction, as indicated by a Sequential Organ Failure Assessment (SOFA) score of 2 or higher, according to the criteria established by the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)2,3,4. To enhance clinical applicability in every-day settings, a qSOFA assessment was developed, indicating organ dysfunction with a qSOFA > 2 1,3,4,5.
This infection-related complication, especially prevalent among patients suffering from hematologic malignancies, is generally caused by both the immunosuppressive effects inherent to the cancer itself and the immunosuppressive nature of cancer treatments such as chemotherapy6,7.
Patients who meet the criteria for the SOFA score are expected to have a mortality rate exceeding 10% 8. Early identification and proper treatment of sepsis can help lower both mortality and morbidity. Several tests are available to detect sepsis and monitor its progression9. Owing to the limitations of blood cultures in diagnosing sepsis, particularly in culture-negative cases, several biomarkers, such as procalcitonin (PCT), interleukin-6 (IL-6), and C-reactive protein (CRP), are frequently employed. These methods have proven to be highly promising in enhancing sensitivity and shortening the time required for diagnosis; however, their specificity and sensitivity can vary depending on the infection type and the patient’s individual characteristics10,11. Moreover, advancements in technology have made blood cell parameter testing more efficient, thus providing faster and more precise results12.
Parameters related to the white blood cell (WBC) count and differential counts of neutrophils, monocytes, and lymphocytes also play critical roles in diagnosing infection and have been actively investigated for the early detection of sepsis13. Chemotherapy-induced neutropenia significantly impairs the body’s immune defense, reducing the ability to respond promptly to bacterial infections, thereby increasing the risk of sepsis. Neutrophil counts, alongside other WBC subtypes, provide important information about the body’s immune status and potential for fighting infection14. This susceptibility is further exacerbated during episodes of febrile neutropenia, where the combination of fever and a critically low neutrophil count (< 500 cells/µL) could lead to a life-threatening infection15. Studies have shown that the mortality rate in patients with febrile neutropenia can reach 20–30% during the course of sepsis16. Additionally, the neutrophil-to-lymphocyte ratio (NLR) has gained attention as a prognostic marker in cancer patients, where a high NLR reflects both inflammation and immune suppression, conditions often associated with poor outcomes in sepsis patients17. Lymphocytes, including T cells, B cells, and natural killer (NK) cells, are essential for both innate and adaptive immune responses, particularly in cases of viral infections and malignancies. However, cancer patients frequently exhibit lymphocytopenia (low lymphocyte counts), which can result from the direct effects of cancer (e.g., bone marrow infiltration) or from side effects of chemotherapy and radiation therapy. Empirical evidence underlines that lymphocyte depletion, particularly in septic cancer patients, leads to an increased risk of secondary infections, prolonged hospital stays, and increased mortality rates18.
In clinical practice, monitoring the complete blood count (CBC), including the absolute counts of neutrophils, monocytes, and lymphocytes, provides vital information about a patient’s immune status and capacity to combat infection. This traditional approach, when integrated with newer biomarkers, enhances the clinician’s ability to diagnose infections early and assess the risk of progression to severe conditions such as sepsis. Nonetheless, it is crucial to remember that no single test is sufficient for diagnosing sepsis. Indeed, clinical judgment and thorough evaluation of the patient’s medical history and symptoms, in association with a multibiomarker approach, are vital for an accurate and timely diagnosis.
In this study, we prospectively monitored sepsis-correlated serological biomarkers and white blood cell differentiation counts to identify quantitative differences between patients with hematological and solid cancers with probable case of sepsis. The aim of this study was to define an early detection tool that is useful for both cancer groups in everyday practice.
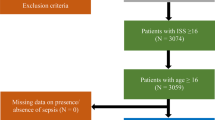
Materials and methods
In this study, we enrolled 48 adult patients (23 hematological, 25 oncological, Table 1) admitted to the Mediterranean Oncology Institute between February 2022 and March 2024 as probable sepsis, who had at least one fever episode during inpatient hospitalization and/or at least two of clinical signs of sepsis even in the absence of fever, such as hypotension, tachypnea, altered consciousness, oliguria/anuria. During the same period, a total of 738 patients were hospitalized in the Hematology unit and 1315 in the Oncology unit, providing a broader context to the representativeness of the study cohort. Given the frailty of the abovementioned setting of patients and impaired clinical conditions qSOFA was used as a diagnostic tool in order to evaluate the probability of sepsis and risk for in-hospital mortality due to organ dysfunction2. Patient inclusion and exclusion criteria along with the monitoring flow are further detailed in the CONSORT diagram (Fig. 1). In case of positive blood cultures, the episode was labeled as confirmed sepsis. The hematological group included 10 females and 13 males, while the oncological group included 12 females and 13 males. Laboratory tests using peripheral blood samples at fever onset that were repeated daily until at least 48 h after the last fever episode according to the internal protocol included the following: complete blood cell count, including white blood cell differentiation; serum creatinine levels; blood urea nitrogen concentrations; electrolyte measurement; hepatic transaminase enzymes; total bilirubin determination; and CRP, PCT and IL-6 levels. Furthermore, no fewer than three sets of blood cultures were taken, with a set collected simultaneously from each lumen of an existing central venous catheter and from a peripheral vein site during fever spike; furthermore, stool and urine cultures were analyzed from each patient. The study was approved by an independent ethics committee of the coordinating center (Catania 2, n.118/2022/ Catania 2) and was conducted in accordance with International Conference on Harmonization Guidelines on Good Clinical Practice and the principles of the Declaration of Helsinki. All enrolled patients have provided written informed consent to data recording.
PCT levels were quantified via an enzyme-linked fluorescence assay with bioMérieux kits on a VIDAS immunoassay system. The upper limit of serum PCT for high risk of sepsis was considered 2 ng/mL, given that the study population was immunocompromised both due neoplastic disease and treatment and lower levels of PCT could be due to other factors.
The immuno-rate format for CRP was based on an enzymatic heterogeneous, sandwich immunoassay format by VITROS 5600 (QuidelOrtho). The precision of the method was evaluated with quality control materials on a VITROS 5600 Integrated System following the NCCLS protocol EP5. The upper limit of the normal range for serum CRP was 1 ng/mL.
The IL-6 test was performed via the VITROS5600 (Ortho Clinical Diagnostic) with a two-step immunometric technique. The precision of the method was evaluated with quality control materials on a VITROS 5600. The upper limit of the normal range for serum IL-6 was 6.65 pg/mL. For WBC counting, the SYSMEX XN 1000 was used. It is a fully automated 5-part differential hematology analyzer.
Depending on the cellular characteristics, different intensities of the signals were collected, and scattergrams of the respective measuring channels were populated. These scattergrams are used for the classification of the cells as well as flagging the abnormal population. WBC differentiation was assessed via fluorescence flow cytometry.
Characteristics of the study cohort
Forty-eight enrolled patients had fever episodes during inpatient care and were evaluated with serological biomarkers for sepsis. The cohort was divided based on tumor status into 23 hematological patients, with a median age of 57 years (range 54–63 years), and 25 oncological patients, with a median age of 67 years (range 55–74 years). The patients were classified on the basis of their tumor diagnosis in Table 1, and the most frequent were multiple myeloma, followed by Hodgkin lymphoma in hematologic (respectively 56% and 17%) and the gastrointestinal tract (48%) in solid tumors (table available in the supplementary materials). With respect to the antineoplastic treatment used, all hematological patients had at least a partial response and were treated with early high-dose conditioning chemotherapy followed by autologous stem cell transplant (ASCT). Among the oncologic patients, seventeen (68%) received chemotherapy during the course of active disease (clinical details for the two cohorts are provided in the supplementary file).
As for the probability of sepsis and risk for in-hospital mortality, qSOFA score equal or greater than 2 was present in around half of the oncologic patients, and in only 10% of hematologic ones. Small and extremely heterogeneous study population, composed from both hematological and oncological patients, treated with different treatment regimens, including chemo and immune therapy or no therapy, should be taken into account when evaluating the results observed.
Statistical analysis
In this study, statistical analysis was conducted to assess the ability of biomarkers (IL-6, PCT, CRP, LYM, MON, NEU and WBC) to predict sepsis status in hematologic and oncologic patients prior to blood culture results, while ICU admission, in-hospital mortality, length of in-patient stay and fever duration were used as clinical parameters of sepsis outcome. The data were tested for normality via the Shapiro‒Wilk test and log-scaled due to their skewed distributions. Since normality cannot be assumed for all groups, we decided to use the Wilcoxon signed rank test as a nonparametric test to evaluate differences in cohorts. To compare biomarker levels between patients who were positive or negative for sepsis in a more precise manner, we used the empirical cumulative distribution function (ECDF) to calculate the Kolmogorov‒Smirnov test. ROC curves were used to determine the performance of classification for single and combined biomarkers, and contingency tables were used for sensitivity and specificity. The dynamics of the combined biomarker distributions were explored by calculating the Pearson correlation coefficient and performing a principal component analysis (PCA). A p-value less than 0.05 was considered statistically significant. Statistical analyses were conducted using R19 and RStudio20. The following R packages were employed: pROC21 for analysis of ROC curves; corrplot22 for correlation analysis; gridges23 for density probability; factoextra24 for principal component analysis; and ggplot225 for data visualization.
Results
Diagnostic performance of biomarkers in sepsis
Upon conducting an in-depth analysis of the markers utilized to monitor inflammation and immune responses (specifically, CRP, IL-6, PCT, WBC, LYM, NEU and MON), substantial differences were identified in patients with hematological or solid tumors who developed sepsis (Fig. 2). To further clarify the role of these markers, we compared their levels on the basis of sepsis status, with either negative (−) or positive (+) blood cultures. CRP concentrations were higher in blood cancer patients with sepsis than in those without; however, this difference was not statistically significant in patients with solid tumors under similar conditions. Statistically significant differences were detected between patients in the Blood (−) and Solid (−) groups, whereas those with positive sepsis status were not significantly different. Figure 2 clearly shows that the CRP, IL-6, and PCT levels are elevated in blood cancer patients with sepsis compared to those with fever alone. In patients with hematologic malignancies, both the IL-6 and PCT levels were significantly different, a contrast not observed in patients with solid tumors, regardless of sepsis status. Additionally, PCT concentrations in sepsis-negative patients with blood cancers, compared to those with solid tumors, rejected the null hypothesis, an effect not replicated in patients with sepsis. A similar trend was observed for IL-6 levels, which were significantly different in patients who did not develop sepsis.
Comparison of the levels of CRP, IL-6 and PCT between blood and solid cancer samples. The horizontal axis represents groups, where the symbol (+) represents the sample with sepsis and (−) represents the sample with other causes of fever. The vertical axis represents the biomarker level expressed in log10. The p-value for statistically significant differences between groups is represented as follows: “−“ p-value > 0.05; “*” p-value ≤ 0.05; “**”p-value ≤ 0.01.
The analysis of different white blood cell concentrations presented in Fig. 3 revealed nonsignificant differences whensepsis-positive and sepsis-negative subjects were compared in terms of all cell types. Although these two groups did not exhibit notable variations, a marked disparity between negative (Blood (−) and Solid (−)) and positive (Blood (+) and Solid (+)) samples was shown. Within the hematological group, LYM, MON, NEU, and WBC levels were increased in Blood (−) compared to Blood (+); however, these differences were not statistically significant. Similarly, the concentrations of all white blood cell types in Solid (−) and Solid (+) categories were almost indistinguishable. When comparing cohorts negative to sepsis, some statistically significant distinctions emerged: Blood (−) samples had significantly higher concentrations of both LYM and MON compared to Solid (−); conversely, Blood (−) groups expressed lower values of NEU and total WBC than Solid (−) samples did, with significant differences in both cell types. The analysis of sepsis-positive samples generally revealed higher concentrations of white blood cells in solid cancers, with NEU, MON and WBC displaying significantly greater levels in Solid (+) compared to Blood (+).
Comparison of the level of lymphocyte, monocyte, neutrophil and WBC between blood and solid cancers. The horizontal axis represents groups, where the symbol (+) represents the sample with sepsis and (−) represents the sample with other causes of fever. The vertical axis represents the biomarker level expressed in log10. The p-value for statistically significant differences between groups is represented as follows: “–“ p-value > 0.05; “*”p-value ≤ 0.05; “**” p-value ≤ 0.01; “***” p-value ≤ 0.001; “****” p-value ≤ 0.0001.
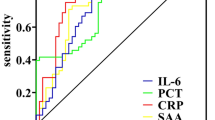
To evaluate the performance of each biomarker as a classifier for sepsis, we conducted a comprehensive ROC analysis examining all biomarkers across the two conditions under investigation (Fig. 4), blood and solid cancers. As shown in the figure, the area under the curve (AUC) was assessed, indicating a strong overall performance of the biomarkers in identifying sepsis within blood cancer patients (Fig. 4A), whereas such efficacy was not observed in solid cancers (Fig. 4B). Notably, for blood cancers (Fig. 4A), IL-6 and PCT had AUC of 0.804 and 0.777, respectively, followed by MON (0.759), LYM and NEU (0.741), CRP (0.710) and WBC (0.665). In solid cancers (Fig. 4B), the classification efficacy of biomarkers was generally lower than that in blood cancers. Nevertheless, PCT had a moderate classification potential with an AUC = 0.693, in contrast to other biomarkers that did not show any discriminatory power, such as WBC (0.596), MON (0.579), LYM (0.548), NEU (0.500), IL-6 (0.482) and CRP (0.447), which had a much more similar impact of a random guess.
To provide a more detailed evaluation of the classifier’s ability to distinguish between positive and negative sepsis cases, we included contingency tables for all biomarkers. Table 2 presents the results for IL-6 and PCT, while Tables S1-S2 in the supplementary materials cover CRP, MON, LYM, NEU, and WBC.
These tables were constructed using the optimal thresholds determined as the points closest to the top-left corner of each ROC curve in Fig. 4. The contingency tables for IL-6 in Table 2 highlight the critical differences in sepsis classification between blood and solid cancers. In blood cancer, IL-6 has a sensitivity of 1.0, whereas it has a sensitivity of 0.33 in solid cancers, underscoring its significant role in accurately detecting all sepsis cases. PCT is the most consistent biomarker, showing moderate overall performance across both cancer types (Table 2). The highest accuracy in classifying sepsis cases was achieved by NEU, MON and LYM in blood cancer (Table S1), with values of 0.87, 0.83 and 0.82, respectively, demonstrating their strong predictive ability in this context. In contrast, for solid cancers, the best-performing biomarker was LYM (Table S2), which achieved an accuracy of 0.76, indicating a relatively lower but still noteworthy ability to distinguish between sepsis-positive and sepsis-negative cases in solid tumor patients. These metrics should be interpreted considering values distribution (Figs. 2 and 3), which highlights the inherent high variability associated with blood cancers.
As shown in Fig. 4, certain biomarkers have demonstrated good classification potential in identifying patients with sepsis. In the subsequent analysis, certain biomarker combinations were identified with discriminative power in identifying sepsis, as shown in Fig. 5. The impact of biomarker combinations was especially evident when these combinations were applied to hematological cancer samples (Fig. 5A). Specific combinations such as “IL-6 + PCT” and “LYM + NEU” achieved an AUC of 0.866, indicating high diagnostic performance. These combinations were followed by the combinations “IL-6 + PCT + CRP” and “LYM + NEU + MON” with AUC = 0.839, “IL-6 + CRP” (0.804), “NEU + MON” (0.795), “LYM + MON” (0.741) and “CRP + PCT” (0.723). In the context of solid tumors, the efficacy of some biomarkers in combination was slightly improved compared with that of individual biomarkers, as shown in Fig. 5B; however, PCT alone had a better AUC. For the biomarker pairs in solid tumors, “CRP + PCT” was the most effective combination, with an AUC of 0.658, followed by “IL-6 + PCT + CRP”, with an AUC of 0.640, and “IL-6 + PCT” (0.623). The remaining combinations, “NEU + MON” (0.579), “IL-6 + CRP” (0.570), “LYM + NEU + MON” (0.561), “LYM + NEU” (0.535) and “LYM + MON” (0.465), had results similar to random guessing or worse.
To comprehensively explain the results observed in the ROC curves and contingency tables, we conducted an in-depth analysis of the variable distributions independently and jointly (Fig. 6 and Fig. S1-S2 of supplementary materials). The density plot structure revealed different patterns that enhance the understanding of their discriminatory power. In the case of blood cancer patients (Fig. 6A), where the WBC count, with the lowest AUC (0.665), was compared with other biomarkers (Fig. 4A), a poorly separated distribution according to sepsis status was observed, and furthermore, negative sample values were widely dispersed. CRP, with a slightly improved performance (AUC = 0.710), had similar issues that affected WBC. The subgroups of white blood cells, namely, the MON, LYM, and NEU, had comparable abilities to discriminate sepsis, as they presented a distribution with distinct peaks and less overlap. PCT and IL-6 are the most useful markers for identifying patients on the basis of sepsis status. Even though their distributions seemingly overlapped, the values between the two groups were well separated, and their ranks differed systematically. Similar to Fig. 3, the WBC count demonstrates limited utility in accurately classifying sepsis. The Empirical Cumulative Distribution Function (ECDF) for blood cancers in Figure S1 highlights the strong discriminative power of IL-6, PCT, and CRP, along with the significant variability observed in the MON, LYM, and NEU. To understand overall group separation, we performed PCA on blood cancer patients (Figure S3–S4) and analyzed sepsis status separately and in combination. The explained variance for hematologic cancer was as follows: in sepsis-positive patients, 92.6%, with a strong contribution from IL-6, PCT, CRP, NEU, and LYM; in sepsis-negative patients, 79.4%, driven primarily by LYM, followed by CRP, WBC, and NEU (in decreasing order of contribution); and in the entire group, 78%, with Dim1 dominated by CRP, PCT, IL-6, and LYM (in opposing directions), while Dim2 was influenced by MON and NEU. Unfortunately, in the cohort with solid cancers (as depicted in Fig. 6B), the distributions frequently exhibited complete overlap, accompanied by similar variance, and were not able to distinguish sepsis status. PCT was the only one able to achieve a moderate AUC of 0.693 for sepsis classification among solid tumor patients. Its density distribution was similar to that of other biomarkers, but the values in the sepsis cohort were slightly greater (Fig. 6B). All the other independently taken biomarkers were similar to the random prediction of sepsis (Fig. 6B). The ECDF for the solid cancer group, presented in Figure S2, demonstrated a lack of discriminatory power between sepsis-positive and sepsis-negative samples, as suggested by previous results. As for blood cancer, we also performed PCA for the solid cancer group (Figure S4), where the explained variance was 88.8% for sepsis-positive patients (Dim1 driven by PCT, CRP, WBC, and IL-6, and Dim2 by LYM and MON) and 73.7% for sepsis-negative patients (Dim1 dominated by WBC, PCT, and NEU, and Dim2 dominated by LYM and IL-6). In the combined group, 72.6% of the variance was captured, with CRP, IL-6, and PCT driving Dim1 and MON and NEU contributing to Dim2.
The integration of multiple biomarkers produced density plots that reflected the ability of the individual biomarkers, as indicated by the ROC curves (Fig. 5). The sum of biomarkers in blood samples (Fig. 6A) significantly improved the discriminatory power in blood cancer patients, as highlighted in Fig. 5A. Conversely, the biomarker combinations in the solid tumor cohort (Fig. 6B) generally did not improve or possibly degrade the classification performance, except for combinations such as “CRP + PCT” and “IL-6 + PCT + CRP”, which achieved fair discrimination power.
Moreover, we analyzed the Pearson correlations among biomarkers across all four patient categories. As illustrated in Fig. 7, there was an abundance of moderate-to-strong relationships between these variables, as indicated by \(\:\left|\varvec{r}\right|\ge\:0.4\) (highlighted by red and blue squares), with some of these associations in opposite directions according to sepsis status. This complementary nature was helpful for choosing biomarkers that capture different aspects of the data, enhancing feature creation for improved classification accuracy. IL-6, PCT and CRP demonstrated particular efficacy in discriminating between sepsis status in blood cancer (Fig. 4A). The combination ultimately improved the discrimination accuracy owing to strong and negative relationships, contrasting information essential for further dividing density plots.
Clinical outcomes
To further assess the clinical utility of the studied biomarkers, we analyzed key clinical outcomes including ICU admission, in-hospital mortality, length of hospital stay and fever duration in our patient cohort (Table 3). Among the 48 patients included in the study, no patients required ICU admission during hospitalization. The overall in-hospital mortality rate was 14.6%, with a marked difference between cancer types: patients with solid tumors exhibited a significantly higher mortality rate (24.0%) compared to those with hematologic malignancies (4.3%) as expected given the higher percentage of qSOFA score equal or greater than 2.
The median length of hospital stay was notably longer in hematologic patients (20 days, IQR 18–23) than in solid tumor patients (8 days, IQR 7–16), likely reflecting the intensive treatment regimens such as high-dose chemotherapy and autologous stem cell transplantation in the more fit hematologic group. Fever duration was relatively short across both groups, with medians of 2 days (IQR 2–4) and 1 day (IQR 1–2) for hematologic and solid tumor patients, respectively.
These clinical outcomes provide important context for interpreting the biomarker performance results. The higher mortality observed in solid tumor patients, despite the superior biomarker discriminatory power in hematologic patients, underscores the complex interplay between biomarker expression and clinical outcomes in different cancer populations. The absence of ICU admissions suggests that the biomarkers may be particularly useful for early sepsis detection, associated with upfront antibiotic therapy, before progression to critical illness requiring intensive care.
Discussion
This study investigated the diagnostic utility of inflammatory and immune biomarkers in detecting sepsis among patients with hematologic and solid cancers and revealed critical differences in biomarker performance and immune response characteristics between these two cancer types.
Our analysis revealed that across all biomarkers (CRP, IL-6, PCT, WBC, LYM, NEU and MON), the expression levels of IL-6 and PCT were the most discriminant in sepsis detection, which is in line with the known response in inflammation. In particular, sepsis-positive patients with blood and solid cancers presented higher levels of CRP, IL-6 and PCT than did those in sepsis-negative groups (Fig. 2). However, the significant distinctions found in the blood cancer groups were not maintained in terms of biomarker levels between the solid tumor groups (Solid (−) and Solid (+)).
The different white blood cells under study (LYM, MON, NEU, and WBC) showed limited discriminatory power for sepsis status (Fig. 3). This lack of differentiation may be attributed to the generalized inflammatory nature of sepsis, potentially masking significant changes in these markers. Specifically, for blood cancers, nonsignificant differences were observed between the Blood (+) and Blood (−) groups, yet significant disparities emerged when the Blood (−) and Solid (−) groups were compared. LYM and MON concentrations were higher in Blood (−), whereas NEU and WBC levels were elevated in Solid (−). This suggests inherent variability in immune responses between the two cancer types. Interestingly, the analysis revealed higher WBC, NEU, and MON counts in the Solid (+) group than in the Blood (+) group, which may reflect differences in immune system activation in response to sepsis.
ROC analysis revealed that IL-6 and PCT are the most reliable biomarkers of blood cancer, with high AUC values of 0.804 and 0.777 (Fig. 4), respectively, indicating strong potential for sepsis classification. While slightly less effective, the CRP also made a significant contribution to the diagnostic framework. PCA further emphasized the importance of these markers (Fig. S3–S4), with Dim1 being influenced primarily by IL-6, PCT, and CRP. In contrast, biomarker performance in solid cancers was lower, with PCT emerging as the only marker with moderate discriminatory power (AUC = 0.693), as reported in Fig. 4. Other markers, including WBC, MON, LYM, and NEU, presented AUC values close to random chance, reflecting their limited diagnostic utility in solid tumors.
Correlation analysis revealed moderate-to-strong relationships between biomarkers, particularly in blood cancer patients, where complementary biomarkers such as IL-6, PCT, and CRP exhibited opposing relationships with sepsis status. These correlations facilitated the selection of biomarker combinations that captured distinct aspects of the data, further enhancing classification accuracy. In solid cancers, weaker correlations and overlapping biomarker distributions limit the ability to create effective combinations. Driven by the mathematical characteristics of the biomarkers, we further studied the possibility of enhancing the classification power using new features that capture different types of biological information by combining their values. The combinations permit to stretch the distribution of biomarkers thanks to the analysis of both density (Fig. 6) and correlation plots (Fig. 7).
The integration of multiple biomarkers markedly increased diagnostic accuracy in blood cancer patients. Combinations such as IL-6 + PCT and LYM + NEU yielded high AUC values, reaching 0.866, highlighting the complementary nature of these biomarkers. Notably, combinations such as IL-6 + PCT + CRP and LYM + NEU + MON further improved diagnostic performance, emphasizing the utility of integrating biomarkers with contrasting relationships. In contrast, biomarker combinations in solid cancers demonstrated limited effectiveness, with only CRP + PCT and IL-6 + PCT + CRP achieving moderate discriminatory power.
In hematologic patients, our findings suggest specific biomarker thresholds that may serve as practical tools for early identification of higher sepsis risk. Notably, levels in log10 of IL-6 > 1.38 and PCT > 0.12 were associated with increased sepsis likelihood in hematologic cancer patients, consistent with previously reported IL-6 cutoffs and PCT thresholds in general sepsis populations26,27. Combined indices, such as IL-6 + PCT > 1.86 and CRP + PCT > 0.72 further enhanced risk stratification, aligning with literature demonstrating that combinations of inflammatory biomarkers improve diagnostic accuracy beyond single markers alone28,29.
Additionally, combined immune cell markers showed diagnostic relevance like LYM + NEU < 2.70 as well as the LYM + NEU + MON < 3.17, were indicative of elevated sepsis risk in this blood cancer group. While specific numeric cutoffs for combined leukocyte subsets are rarely standardized in the literature, the integration of lymphocyte, neutrophil and monocyte counts is increasingly recognized as valuable for sepsis diagnosis and prognosis30.
Conversely, in the solid tumor cohort, biomarker levels did not demonstrate significant differences between septic and non-septic patients, preventing the establishment of reliable diagnostic thresholds for this population.
The differences in biomarker performance between blood and solid cancers can arise from intrinsic variations in immune system cell dynamics associated with the cancer type, highlighting their potential link to sepsis. Among hematological and solid malignancies, distinctive variations in biomarker levels associated with septicemia have been demonstrated. Additionally, biomarker combinations improved the precision in diagnosing sepsis in patients with hematologic cancers; however, such combinations had impaired performance in solid ones. In terms of baseline demographic characteristics, oncologic patients were 10 years older than hematologic ones, and were generally treated in the course of active disease; therefore, they were more likely to have an impaired immune system. Thus, the highlighted difference between serological biomarkers was especially evident in Solid (−) samples compared to Blood (−) samples. On the other hand, no difference from PCT was present between Solid (+) and Solid (−) patients, which could also be explained by the presence of active disease in oncologic patients, with possible implications for the abovementioned values. Furthermore, more than 1/3 of the oncologic subpopulation with negative blood cultures did not receive chemotherapy, which could have an impact on the higher serological values of IL-6 and PCT. With respect to the differences in WBC and its subpopulations, namely, the NEU, LYM and MON, the hematological patients received high-dose chemotherapy prior to ASCT, and lower values of leukocytes were expected compared to the oncologic ones.
Our study has some major limitations, including a small and heterogeneous study population, composed from both hematological and oncological patients with different tumor type and consequential treatments, including chemo and immune therapy and no therapy, that probably had an influence on the aforementioned results. Furthermore, antimicrobic treatment used for each patient along with the type of bacteria present in the blood-stream had an impact on the outcome regarding the levels of the studied biomarkers. In addition, due to the nature of our study, we lack granular data on the precise timing of biomarker changes relative to the initial signs of sepsis, limiting our ability to assess the temporal dynamics of biomarkers variation. Finally, given that we described a single-center experience, it remains difficult to confirm the validity of our results in other healthcare settings with different microbiological epidemiology and nosocomial infections.
In light of the exploratory nature of our study, which is limited by a small sample size and significant patient heterogeneity, we did not apply formal corrections for multiple hypothesis testing. This decision was made to avoid an increased risk of Type II errors (false negatives), which could have masked potentially relevant signals in this fragile and complex population. Therefore, our findings should be interpreted as hypothesis-generating and serve as a foundation for future, more robust investigations.
Nonetheless, the results suggest that IL-6, PCT, and CRP may have superior performance for sepsis classification, both individually and in combination, in patients with hematologic malignancies. However, the reduced effectiveness of these markers in solid cancers emphasizes the need for cancer-specific approaches for sepsis diagnosis. Therefore, in case of clinical suspicion of sepsis, such as persistent fever or other clinical signs, addition of daily PCT and IL-6 tests together with CRP, which is already frequently monitored, could be of aid in interpreting and promptly acting with empiric antibiotic therapy, especially in hematologic ones, in order to avoid development of septic shock, thus increasing the mortality risk. Future research using independent validation cohort with larger number and greater homogeneity of patients in terms of both tumor type and treatment regimen could help in individualizing more precise sepsis biomarkers, thus improving patient outcome in this extremely fragile population. Furthermore, future studies should focus on identifying alternative biomarkers or novel combinations tailored to the unique inflammatory profiles of solid tumor patients in order to individualize in a more precise manner the potential differences between specific disease types.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Qiu, X., Lei, Y. P., Zhou, R. X., SIRS, SOFA, qSOFA, and NEWS in the diagnosis of sepsis and prediction of adverse outcomes: A systematic review and Meta-Analysis. Expert Rev. Anti Infect. Ther. 21(8), 891–900. https://doi.org/10.1080/14787210.2023.2237192 (2023).
Seymour, C. W. et al. Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8), 762. https://doi.org/10.1001/jama.2016.0288 (2016).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8), 801. https://doi.org/10.1001/jama.2016.0287 (2016).
Vincent, J. L. et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure: on behalf of the working group on sepsis-related problems of the european society of intensive care medicine (see contributors to the project in the appendix). Intens. Care Med. 22(7), 707–710. https://doi.org/10.1007/BF01709751 (1996).
Schertz, A. R. et al. Sepsis prediction model for determining sepsis vs SIRS, qSOFA, and SOFA. JAMA Netw. Open. 6(8), e2329729. https://doi.org/10.1001/jamanetworkopen.2023.29729 (2023).
Klastersky, J. et al. Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann. Oncol. 27, v111–v118. https://doi.org/10.1093/annonc/mdw325 (2016).
Kumar, G. et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 140(5), 1223–1231. https://doi.org/10.1378/chest.11-0352 (2011).
Angus, D. C. et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29(7), 1303–1310. https://doi.org/10.1097/00003246-200107000-00002 (2001).
Azoulay, E. et al. The intensive care medicine research agenda on critically ill oncology and hematology patients. Intens. Care Med. 43(9), 1366–1382. https://doi.org/10.1007/s00134-017-4884-z (2017).
Schuetz, P. et al. Serial procalcitonin predicts mortality in severe sepsis patients: results from the multicenter procalcitonin monitoring sepsis (MOSES) study. Crit. Care Med. 45(5), 781–789. https://doi.org/10.1097/CCM.0000000000002321 (2017).
Tsalik, E. L. et al. Discriminative value of inflammatory biomarkers for suspected sepsis. J. Emerg. Med. 43(1), 97–106.https://doi.org/10.1016/j.jemermed.2011.05.072 (2012).
Pierrakos, C. & Vincent, J. L. Sepsis biomarkers: A review. Crit. Care 14(1), R15. https://doi.org/10.1186/cc8872 (2010).
Urrechaga, E., Bóveda, O. & Aguirre, U. Role of leucocytes cell population data in the early detection of sepsis. J. Clin. Pathol. 71(3), 259–266. https://doi.org/10.1136/jclinpath-2017-204524 (2018).
MacPhail, A. et al. Sepsis mortality among patients with haematological malignancy admitted to intensive care 2000–2022: A binational cohort study. Crit. Care. 28(1), 148. https://doi.org/10.1186/s13054-024-04932-0 (2024).
Klastersky, J., Awada, A., Paesmans, M., & Aoun, M. Febrile Neutropenia: A critical review of the initial management. Crit. Rev. Oncol. Hematol. 78(3), 185–194. https://doi.org/10.1016/j.critrevonc.2010.03.008 (2011).
Klastersky, J. et al. The multinational association for supportive care in cancer risk index: A multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J. Clin. Oncol. Off J. Am. Soc. Clin. Oncol. 18(16), 3038–3051. https://doi.org/10.1200/JCO.2000.18.16.3038 (2000).
Forget, P. et al. What is the normal value of the Neutrophil-to-Lymphocyte ratio? BMC Res. Notes 10(1), 12. https://doi.org/10.1186/s13104-016-2335-5 (2017).
Hotchkiss, R. S., Monneret, G. & Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 13(3), 260–268. https://doi.org/10.1016/S1473-3099(13)70001-X (2013).
R Core Team. R: A language and environment for statistical computing. https://www.R-project.org/ (Accessed 18 Apr 2024).
RStudio Team. RStudio: Integrated Development Environment for R. http://www.rstudio.com/. (Accessed 18 Apr 2024).
Robin, X. et al. pROC: An open-source package for R and S + to analyze and compare ROC curves. BMC Bioinform. 12(1), 77. https://doi.org/10.1186/1471-2105-12-77 (2011).
Wei, T., Simko, V. R. & Package Corrplot: Visualization of a Correlation Matrix (2021).
Wilke, C. O. & Ggridges Ridgeline Plots in Ggplot2. https://wilkelab.org/ggridges/.
Kassambara, A. & Mundt, F.. Factoextra: Extract and visualize the results of multivariate data analyses (2020).
Wickham, H. Ggplot2: Elegant Graphics for Data Analysis (Springer, 2009). https://doi.org/10.1007/978-0-387-98141-3.
Song, J. et al. Diagnostic and prognostic value of Interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: A prospective controlled study according to the sepsis-3 definitions. BMC Infect. Dis. 19(1), 968. https://doi.org/10.1186/s12879-019-4618-7 (2019).
Varga, N. I. et al. IL-6 baseline values and dynamic changes in predicting sepsis mortality: A systematic review and Meta-Analysis. Biomolecules 15(3), 407. https://doi.org/10.3390/biom15030407 (2025).
Shao, W., Yu, D., Zhang, W. & Wang, X. Clinical significance of Interleukin-6 in the diagnosis of sepsis and discriminating sepsis induced by Gram-Negative bacteria. Pediatr. Infect. Dis. J. 37(8), 801–805. https://doi.org/10.1097/INF.0000000000001904 (2018).
Zeng, G. et al. Combination of C-reactive protein, procalcitonin, IL ‐6, IL ‐8, and IL ‐10 for early diagnosis of hyperinflammatory state and organ dysfunction in pediatric sepsis. J. Clin. Lab. Anal. 36(7), e24505. https://doi.org/10.1002/jcla.24505 (2022).
Hotchkiss, R. S., Monneret, G. & Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 13(12), 862–874. https://doi.org/10.1038/nri3552 (2013).
Acknowledgements
The authors have no acknowledgements to declare.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, D.C., P.C. and E.M.; methodology, D.C., P.C. and E.M.; software, E.M.; validation, P.C., and E.M.; formal analysis, P.C. and E.M.; investigation, D.C., V.I., F.G., U.M., L.G., D.S. and G.C.; resources, D.C., V.I. and L.G.; data curation, G.C., and E.M.; writing—original draft preparation, D.C., U.M., G.C., P.C. and E.M.; writing—review and editing, D.C., U.M., G.C., P.C. and E.M. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all the subjects involved in the study.
Institutional review board statement
The study was conducted in accordance with the International Conference on Harmonization Guidelines on Good Clinical Practice and the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Comitato Etico Catania 2 (protocol code 118).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Carcò, D., Iachelli, V., Markovic, U. et al. Sepsis detection in hematologic and solid tumor malignancies using quantitative inflammatory biomarker differences in a prospective single center study. Sci Rep 15, 31701 (2025). https://doi.org/10.1038/s41598-025-15784-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15784-1