Abstract
Cadmium contamination in agricultural soils poses significant risks to crop safety and ecosystem health. This study investigated the efficacy of modified biochar for Cadmium immobilization in mining-impacted soils through a pot experiment with Chinese cabbage (Brassica pekinensis (Lour.) Rupr.). Four treatments were evaluated: Control (CK), Biochar (6 g/kg, T1), KMnO₄-modified biochar (6 g/kg, T2), and H₃PO₄-modified biochar (6 g/kg, T3). The results showed that both T1 and T2 treatments significantly enhanced the pH and organic matter content of the contaminated soil in the mining area. Specifically, the T2 treatment improved the soil pH from 6.3 to 6.7 and the organic matter content from 29.3 g/kg to 34.3 g/kg. Furthermore, the T2 treatment significantly reduced the available cadmium content in the contaminated soil by 43.4%, the cadmium content in the edible parts of Chinese cabbage by 66.4%, the cadmium accumulation coefficient in the edible parts by 45.5%, the cadmium accumulation coefficient in the roots by 40%, and the transport coefficient by 33.9% compared to the control (CK). In conclusion, the treatment with KMnO₄-modified biochar outperformed both regular biochar and H₃PO₄-modified biochar treatments. The results of the study provide a theoretical basis for the production of vegetables grown in heavy-metal-contaminated soils and promote the recycling of resources and the environment.
Similar content being viewed by others
Introduction
With the rapid industrialization in China, issues of heavy metal pollution in soil have become particularly severe due to factors such as mineral resource exploitation, wastewater irrigation, and the improper use of fertilizers and pesticides1,2. This is especially true for areas surrounding metal mining sites, where cadmium and lead contamination is prevalent3. As one of the world’s major mining countries, China has a total mining area of approximately 104,000 km², with over 300 mining cities focused primarily on mining activities4. The large-scale extraction of mineral resources generates significant amounts of mine tailings, placing immense pressure on the environment5. The Honghe Hani and Yi Autonomous Prefecture in Yunnan Province, China, has a mining history of more than 2000 years. According to the environmental (groundwater, farmland cultivation and crops) monitoring data around the mine, there is a certain risk of environmental pollution in the surrounding area, especially heavy metal pollution, and cadmium pollution in the soil is more serious.
Heavy metal pollution not only affects the soil environment, but also may have negative effects on the quality and yield of agricultural products6,7,8. Therefore, the remediation of mining-contaminated soil and the restoration of its original function are of great significance in guaranteeing the sustainable development of agriculture, safeguarding human health, protecting the ecological environment and promoting socio-economic development.
Biochar can immobilize heavy metal elements in soil through various mechanisms such as adsorption, precipitation, complexation, and ion exchange. For example, corn straw biochar is a carbon-rich material produced by the pyrolysis of corn straw in oxygen-limited or anaerobic conditions9. Due to its high specific surface area, porosity, and abundance of carbon-containing functional groups and mineral nutrients, it has found widespread application in enhancing soil fertility, remediating contaminated sites, and improving agricultural soil environments10,11,12.
However, the effectiveness of adsorption and fixation of heavy metal ions in the soil varies depending on the biochar material used13,14,15. In real-world environments with complex contamination conditions, biochar often struggles to achieve the anticipated remediation effects16. A primary solution to this issue is the modification of raw biochar. Modified biochar has shown promising applications in soil improvement, heavy metal removal, wastewater treatment, and agricultural yield enhancement17. Previous studies have indicated that potassium permanganate-modified biochar and phosphoric acid-modified biochar exhibit good adsorption properties for cadmium. For example, Xie et al.18 showed that potassium permanganate-modified biochar had a higher adsorption capacity for Cd²⁺ compared to unmodified biochar, and its adsorption stability was also significantly improved. Guo et al.19 demonstrated that phosphoric acid-modified biochar effectively passivates single-contaminated Pb²⁺ and Cd²⁺, facilitating the transformation of heavy metal ions from their available to stable forms. The passivation effect was positively correlated with the amount of phosphoric acid-modified biochar added.
Currently, considerable research has been conducted on the use of biochar derived from various materials for remediating heavy metal-contaminated agricultural soils. However, there is a lack of direct comparative studies on the remediation effectiveness of biochars modified by different materials, particularly in the context of mining-affected soils. Based on this, in this study, corn stover biochar was modified with phosphoric acid and potassium permanganate as modifiers through potting tests to investigate the passivation effect of the two modified biochars on cadmium in polluted soils in mining areas and its inhibition effect on transfer and enrichment in cabbage. By comparing the effects of these two modified biochars, this research provides more targeted recommendations for practical applications, and aims to provide a scientific theoretical basis for the use of modified biochar in the remediation of cadmium-contaminated soils.
Methods
Test material
Preparation of biochar
The preparation of the biochar involved the following steps: First, corn stover was ground through a 100 mesh sieve, and then this powdered corn stover was placed in a tube furnace, heated to 500 °C, and held for 2 h. Throughout the preparation process, nitrogen (N₂) was introduced to ensure that the pyrolysis occurred in an oxygen-free environment. The resulting corn straw biochar was then sealed in wide-mouth jars for storage and later use20.
Modification of biochar
The preparation of potassium-permanganate-modified biochar was based on the method described by Mo et al.21. First, 2.0 g of biochar was placed in a 250 mL conical flask, followed by the addition of 100 mL of 0.1 mol/L potassium permanganate solution. After thorough stirring, the flask was sealed with a membrane and placed in a constant temperature shaker, set to 200 r/min at 25 °C for 6 h. Following this, the mixture was allowed to stand for 1 h before being filtered through a 0.45-micron membrane filter. The resulting biochar was repeatedly rinsed with deionized water until the filtrate was colorless. Finally, the filtered biochar solid was placed in an oven and dried at 75 °C until a constant weight was achieved, resulting in potassium-permanganate-modified biochar.
The preparation of the phosphoric-acid-modified biochar followed the method outlined by Peng et al.22. A 14% phosphoric acid solution was used to modify the corn stover biochar. Under 25 °C conditions, 20 g of biochar was soaked in 40 mL of the 14% phosphoric acid solution for 24 h. After soaking, the biochar was washed with deionized water until the pH of the supernatant stabilized at around 7.0 ± 0.2. The supernatant was then discarded, and the biochar was dried at 105 °C to obtain phosphoric-acid-modified biochar.
Biochar surface morphology analysis
The pore structure of the biochar’s surface was observed using a scanning electron microscope (SEM) in secondary electron imaging mode, with an operating voltage of 5–15 kV (SEM model: FlexSEM 1000). The specific procedure was as follows: First, a suitable amount of biochar was evenly distributed on the surface of a copper stub coated with conductive adhesive. Afterward, a gold sputter coating was applied. Finally, the sample’s pore structure was examined under the SEM at various magnifications23.
The specific surface area and pore size distribution were measured using a Micromeritics ASAP 2460 surface area analyzer. The biochar and modified biochar samples were degassed under vacuum at 180 °C for 6 h to remove surface impurities and moisture from the pores. Subsequently, the specific surface area and pore size distribution of the samples were determined in a nitrogen (N₂) atmosphere24.
Potting trials
Test material
Test soil: The potting soil used in this study was collected from Datun Town, Honghe Hani and Yi Autonomous Prefecture, Yunnan Province (23°25’37” N, 103°14’12” E). This area is characterized by acidic red soil, where soil cadmium contamination is severe. Soil samples were taken from the top 0–20 cm layer of farmland. The soil properties are detailed in Table 1.
Test vegetables: The vegetable used in this study was Chinese cabbage, specifically the “Jingyan Kuaicai No. 6 F1” variety, with seeds purchased from a nearby farmer’s market.
Test biochar: The study included corn straw biochar and two types of biochar modified with potassium permanganate and phosphoric acid.
Experimental design
Potting trials were conducted in the greenhouse of Yunnan Agricultural University from April to June 2023 (the temperature in the greenhouse was (25 ± 3) ◦C and (20 ± 2) ◦C during the day and at night, respectively; the light cycle was 13 h). A total of four treatments were established: a control group (CK: no biochar application), unmodified corn straw biochar (T1: 6 g/kg), potassium-permanganate-modified biochar (T2: 6 g/kg), and phosphoric-acid-modified biochar (T3: 6 g/kg)25. Each treatment was replicated three times, resulting in a total of 12 pots. A specific ratio of biochar, base fertilizer (15-15-15 NPK fertilizer at a rate of 450 kg/hm²), and sieved soil was mixed uniformly in plastic pots (diameter 21 cm, height 33 cm). Deionized water was added to adjust the moisture content to 65% of the field capacity. After a two-week equilibration period, Chinese cabbage seeds were sown, with 30 seeds per pot. Thinning was performed seven days post-emergence to maintain five seedlings per pot. During the growth period, regular watering and pest control were conducted, and the Chinese cabbage samples were harvested after 30 days.
Sample collection and analysis
Plant and soil sample collection: When the cabbages were harvested, they were divided into two parts: their roots and their edible parts. Then, they were rinsed with tap water and drenched with distilled water three times, and finally the plant surface was blotted dry with dust-free paper and the processed cabbage samples were put into an oven at 105 °C for 60 min to kill the greening process, followed by drying to a constant weight, grinding, and sieving (0.5 mm). The soil around the inter-root area after harvesting the Chinese cabbage was collected, naturally air-dried, ground, sieved (2 mm and 0.149 mm), and left to be measured26.
Determination and analysis: Soil organic matter was determined according to the “NY 1121.6–2006 Agricultural industry standard Determination of soil organic matter”; soil pH was determined using the potentiometric method with a soil-to-water ratio of 1:2.5; the total soil cadmium was measured with reference to the “Determination of soil quality of lead, cadmium Graphite Furnace Atomic Absorption Spectrophotometric Method” (GB/T 17141 − 1997); the soil effective state cadmium content was determined with reference to the “soil weight effective state lead and cadmium determination of atomic absorption method” (GB/T 3739 − 2009); the Chinese cabbage cadmium content was determined according to the “food safety National Standard for the Determination of Cadmium in Food (GB 5009.15–2014), the plant standard material GBW100351 [(0.42 ± 0.02) mg/kg] was used for quality control, and the cadmium recovery was 80%–95%; and the bioconcentration factor (BCF) and transport factor (TF) of the Chinese cabbage were also calculated27.
where C1 is the cadmium content in the edible parts of Chinese cabbage, C2 is the cadmium content in the root parts of Chinese cabbage, and Csoil is the cadmium content in the inter-root soil.
Data analysis
Raw data were analyzed using Excel 2016 for data statistics, with SPSS Statistics 26 used for the mean, standard deviation, and significance of the data. Duncan’s multiple comparisons tests were used to analyze the differences between treatments, with a significance difference level of p < 0.05, and OriginPro 2021 was used for graphing.
Results
Characterization of modified biochar
The physicochemical properties of the three types of biochar are presented in Table 1. The surface area and pore size measurements of the biochar and the two types of modified biochar indicated that T2 had the highest specific surface area at 114.01 m²/g, followed by T3 at 97.67 m²/g, and the lowest was T1 at 92.86 m²/g (Table 2). Additionally, the total pore volume and peak pore size distribution for the three biochar samples showed the order T2 > T3 > T1 (Table 2) Scanning electron microscope (SEM) images at 10 μm and 50 μm wavelengths revealed that T1 exhibited a few pores on its surface, characterized by a rough texture and the presence of particles (Fig. 1), likely due to impurities on T1’s surface. After phosphoric acid modification, the surface of the biochar became smoother, and its pore structure was enriched (Fig. 1). Compared to T1, T3 exhibited more pronounced macropore structures, suggesting that the phosphoric acid treatment may have removed the surface-bound impurities from T1, resulting in more available adsorption sites on T3. Following potassium permanganate modification, T2 displayed significant changes in surface morphology, with numerous fractures and an increase in pore size and volume compared to T1, accompanied by the formation of fine particulate matter, which contributed to the increased specific surface area of T2 (Fig. 1).
Effect of modified biochar on pH of contaminated soil in mining areas
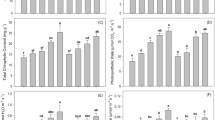
The effects of different treatments on the pH of polluted soils in the mining area varied, as shown in Fig. 2a. The application of biochar and potassium-permanganate-modified biochar significantly increased the pH of the contaminated soils. In all treatments, T1 and T2 significantly raised the pH by 0.5 and 0.4 units, respectively, compared to the control (CK). Specifically, T1 elevated the soil pH from 6.3 to 6.8, while T2 increased it from 6.3 to 6.7. In contrast, T3 did not show a significant difference in pH compared to CK. These results indicate that the application of biochar and potassium-permanganate-modified biochar was most effective in enhancing the pH of the polluted soils in the mining area.
(a) Effect of different treatments on soil pH; (b) Effect of different treatments on soil organic matter content; (c) Effect of different treatments on soil available cadmium content; (d) Cadmium in the edible parts of Chinese cabbage: no biochar applied (CK), biochar applied at 6 g/kg (T1), potassium-permanganate-modified biochar applied at 6 g/kg (T2), and phosphoric-acid-modified biochar applied at 6 g/kg (T3). The values are presented as means ± standard deviations (n = 3). Different small letters indicate significant differences at the level of p < 0.05.
Effect of modified biochar on the organic matter of contaminated soils in mining areas
Biochar is rich in organic matter and its application to soil effectively enhances the organic matter content, thereby improving soil productivity28. The effects of biochar and modified biochar on soil organic matter differ, as illustrated in Fig. 2b. Both biochar and modified biochar significantly increased the organic matter content of the polluted soils in the mining area. Among all treatments, T1 and T2 showed the most effective improvements compared to the control (CK). Specifically, T1 raised the soil organic matter from 29.3 g/kg to 34 g/kg, while T2 increased it from 29.3 g/kg to 34.3 g/kg. In contrast, T3 only increased the organic matter from 29.3 g/kg to 31.5 g/kg. These results indicate that the application of biochar and potassium-permanganate-modified biochar was most effective in enhancing the organic matter content of the polluted soils in the mining area.
Effect of modified biochar on soil available cadmium content in mining areas
The effects of different treatments on the available cadmium content in the polluted soils from the mining area varied, as shown in Fig. 2c. Both biochar and modified biochar applications significantly reduced the available cadmium levels in the contaminated soils. Among all treatments, T2 exhibited the most significant reduction in available cadmium compared to the control (CK), decreasing it by 43.4%. In contrast, T1 and T3 led to reductions of 30.4% and 33%, respectively, compared to CK. These results indicate that the application of potassium-permanganate-modified biochar was the most effective in lowering available cadmium levels in the polluted soils, outperforming both biochar and phosphoric-acid-modified biochar in this regard.
Effect of modified biochar on cadmium content in the edible parts of Chinese cabbage
The effects of different treatments on the cadmium content in the edible parts of Chinese cabbage varied, as shown in Fig. 2d. The application of both biochar and modified biochar significantly reduced the cadmium levels in the edible parts of the cabbage. Among all treatments, T2 demonstrated the most significant reduction, lowering the cadmium content by 66.4% compared to the control (CK). In comparison, T1 and T3 resulted in reductions of 29.6% and 33.3%, respectively, relative to CK. These findings indicate that potassium-permanganate-modified biochar was the most effective at reducing cadmium levels in the edible parts of Chinese cabbage. Furthermore, the reduction achieved with potassium-permanganate-modified biochar was superior to that of both biochar and phosphoric-acid-modified biochar. Importantly, all treatments significantly decreased the cadmium levels in the edible parts of Chinese cabbage, with all values remaining below the Chinese food safety standard limit of 0.2 mg/kg for cadmium.
Effect of modified biochar on cadmium uptake and translocation in Chinese cabbage
The effects of different treatments on the cadmium accumulation coefficients in Chinese cabbage varied, as shown in Table 3. Both biochar and modified biochar applications significantly reduced the cadmium accumulation coefficients in the edible parts and roots of Chinese cabbage. Among all treatments, T2 exhibited the most significant reduction in cadmium accumulation coefficients, decreasing them by 45.5% and 40% for the edible parts and roots, respectively, compared to the control (CK). T1 also led to significant reductions of 18.2% and 20% in the cadmium accumulation coefficients for the edible parts and roots compared to CK. T3 resulted in reductions of 37.5% and 25%, respectively. These results indicate that the potassium-permanganate-modified biochar was the most effective in lowering the cadmium accumulation coefficients in both the edible parts and roots of Chinese cabbage. Furthermore, the effectiveness of potassium-permanganate-modified biochar in reducing these coefficients surpassed that of both phosphoric-acid-modified biochar and unmodified biochar.
The effects of different treatments on the cadmium translocation coefficient in the edible parts of Chinese cabbage varied, as shown in Table 3. The application of both biochar and modified biochar significantly reduced the cadmium translocation coefficient in the edible parts. Among all treatments, T2 demonstrated the most notable reduction, lowering the translocation coefficient by 33.9% compared to the control (CK). In contrast, T1 and T3 did not show significant differences in their effects on the translocation coefficient compared to CK. These results indicate that potassium-permanganate-modified biochar was most effective at inhibiting the transfer of cadmium from the roots of Chinese cabbage to its edible parts.
Discussion
The specific surface area, pore structure, and surface functional groups of biochar are key factors determining its adsorption capacity15. This study shows that the surface area of potassium permanganate-modified biochar (T2, 114.01 m²/g) is significantly higher than that of the original biochar (T1, 92.86 m²/g) and the phosphoric acid-modified biochar (T3, 97.67 m²/g) (Table 2). This result is consistent with the SEM observations, which show that the T2 surface has a more complex pore structure and more pronounced particle fragmentation (Fig. 1). From the perspective of molecular chemical mechanisms, potassium permanganate modification may induce the cleavage of aromatic rings on the surface of biochar through strong oxidation, forming more oxygen-containing functional groups (such as carboxyl and hydroxyl groups), while generating nano-scale manganese oxide (e.g., MnO₂) particles18. The oxygen-containing functional groups on the surface of these manganese oxides can combine with Cd²⁺ through chemical adsorption and coordination, forming stable complexes25. In contrast, the original biochar mainly relies on physical adsorption. Therefore, the chemical adsorption capacity of T2 for Cd²⁺ is significantly enhanced. In addition, the adsorption capacity of potassium permanganate-modified biochar is also related to the redox properties of MnO₂, which can convert part of Cd²⁺ into CdO through electron transfer, further reducing its bioavailability20. Sun et al.29 reported that the modification of biochar with potassium permanganate significantly improves its pore structure, increases the number of oxygen-containing functional groups, and efficiently loads manganese oxides onto the biochar surface. This enhancement strengthens biochar’s ability to adsorb heavy metal ions, further improving its performance in heavy metal adsorption. In contrast, phosphate-modified biochar (T3) may form insoluble cadmium phosphate precipitates (such as Cd₃(PO₄)₂) with Cd²⁺ through phosphate groups (PO₄³⁻), but its passivation effect is weaker than that of T2 (Fig. 2c). This may be because the loading amount of phosphate groups on the surface of T3 is lower than that of oxygen-containing functional groups on T2, and phosphate is prone to compete with Ca²⁺ in the soil for binding sites, resulting in a reduction in the proportion of available phosphorus actually involved in Cd²⁺ precipitation19. It is worth noting that some studies have pointed out that the precipitation effect of phosphate-modified biochar will be inhibited when the soil pH is < 6.5, and the soil pH (6.4) after T3 treatment in this study is close to this critical value, which may further weaken its effect..
Biochar’s enhancement of soil pH and organic matter is a key mechanism in reducing the bioavailability of cadmium (Cd)21. In this study, treatments T1 and T2 increased the soil pH from 6.3 to 6.8 and 6.7, respectively (Fig. 2a), while T3 had no significant effect on pH. The increase in soil pH can promote the hydrolysis of Cd²⁺ to form hydroxy complexes (e.g., Cd(OH)⁺), which, through electrostatic repulsion, reduces the competition for adsorption sites on soil colloid surfaces22. Additionally, elevated pH enhances the specific adsorption of cadmium by Fe/Mn oxides in the soil23. Treatments T1 and T2 significantly increased soil organic matter content (Fig. 2b), with humic acid, polysaccharides, and other organic compounds in the organic matter potentially forming complexes with Cd²⁺, thereby reducing its mobility24. Notably, while treatment T3 had a weaker effect on organic matter, it still significantly reduced the bioavailable cadmium content (by 33%). This may be attributed to the direct involvement of phosphate groups in precipitation reactions, rather than relying on the role of organic matter19.
The bioconcentration factor (BCF) indicates the ability of bok choy to absorb and accumulate cadmium (Cd) from the soil; the higher the BCF, the greater the plant’s ability to accumulate cadmium. The transfer factor (TF) describes the movement of heavy metals from the root system to the aerial parts of the plant, with a higher TF indicating a stronger ability to transfer heavy metals to the above-ground organs30. Biochar reduces the bioavailability of cadmium in the soil, thus inhibiting the absorption and translocation of cadmium by plants. In this study, the T2 treatment reduced the BCF and TF of cadmium in the edible parts of bok choy by 45.5% and 33.9%, respectively (Table 3), which was significantly better than treatments T1 and T3. This result is consistent with the study by Xie et al.18 who found that potassium permanganate-modified biochar increased the adsorption of Cd²⁺ by 1.8 times compared to unmodified biochar. The reduction in BCF indicates that biochar treatment decreased the root uptake of cadmium, which could be attributed to the increased pH in the rhizosphere and the release of competitive ions such as Ca²⁺ and Mg²⁺26. The decrease in TF is related to the weakened binding capacity of cadmium with organic acids (e.g., citric acid) during xylem transport27. In contrast, Guo et al.19 reported that phosphoric acid-modified biochar reduced the BCF of cadmium by about 40%, which is similar to the 37.5% reduction observed in this study for T3, but the effect on TF was weaker. This difference may be due to variations in the cadmium transport mechanisms between different crops (e.g., rice vs. bok choy)31. Previous studies have shown that silicon-modified peanut shell biochar significantly reduced the Cd content in spinach by 54.42%32. Additionally, magnesium-modified peanut shell biochar notably decreased the accumulation of Cd²⁺ in spinach shoots and roots. Compared with the control group (CK), after treatments with 1% and 2% of original biochar and modified biochar, the Cd²⁺ content in the above-ground parts decreased by 7.0% − 46.8%, and that in the roots decreased by 7.3% − 52.7%33. There are differences between these findings and the results of this study, which may be attributed to the variations in modifiers, materials, and crop species (This study and other studies on reducing cadmium accumulation in vegetables are shown in Table 4).
In future studies, green technologies, such as the use of Ascophyllum nodosum biostimulants34, can be combined to enhance the efficiency of heavy metal extraction from mining-contaminated soils, providing new green pathways for soil heavy metal remediation. Additionally, with the aid of plant transcriptome sequencing techniques35, the gene expression changes in plants under biochar-amended soil conditions can be explored. This approach will highlight the complex issues of biochar supplementation in soils and provide a molecular-level understanding of how biochar affects plant growth and heavy metal absorption and metabolism. This will further clarify the mechanisms and potential issues of biochar in practical applications, offering theoretical support for the more precise and efficient use of modified biochar in the remediation of mining-contaminated soils.
Conclusion
The application of biochar (T1), potassium permanganate-modified biochar (T2), and phosphoric acid-modified biochar (T3) can all effectively increase the pH and organic matter content of cadmium-contaminated soil in mining areas, reduce the available cadmium content in the soil and the cumulative cadmium content in the edible parts of Chinese cabbage. Among them, the T2 treatment shows the most significant effect, which can reduce the available cadmium content in the soil by 43.4% and the cadmium content in the edible parts of Chinese cabbage by 66.4%. This indicates that the above-mentioned materials have practical application potential in remediating cadmium-contaminated soil in mining areas and controlling cadmium enrichment in vegetables, providing a theoretical reference for the safe production of heavy metal-contaminated farmland and the recycling of resources.
However, this study has certain limitations. The experimental design lacks multi-gradient dose-effect research, making it difficult to reveal the quantitative relationship between dosage and remediation effect. There is a lack of long-term stability data of modified biochar in the soil, and its long-term impact on the soil-plant system needs to be verified. Therefore, future research should further optimize the experimental design, carry out multi-gradient application rate experiments to determine the optimal application range; establish a long-term positioning observation system to explore the long-term action mechanism of modified biochar; at the same time, strengthen the systematic evaluation of the practical application potential of remediation technologies, including cost-benefit analysis and secondary environmental risk prevention and control, and expand the research objects to different soil types and crop varieties, so as to improve the universality and practical value of the research results.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to privacy restrictions but are available from the corresponding author on reasonable request.
References
Han, F. et al. Simultaneous enhancement of soil properties along with water-holding and restriction of Pb–Cd mobility in a soil-plant system by the addition of a phosphorus-modified Biochar to the soil. J. Environ. Manage. 345, 118827 (2023).
Mahar, A. et al. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 126, 111–121 (2016).
L, G. Z. Upgrading and reshaping of mine geological restoration model in new era:conceptual analysis based on geological. Northwest. Geol. 52 (4), 270–278 (2019).
Wang, D. H. et al. Exploration and research progress on ion-adsorption type REE deposit in South China. China Geol. 1 (3), 415–424 (2018).
Gao, Y. et al. Biochar as a potential strategy for remediation of contaminated mining soils: mechanisms, applications, and future perspectives. J. Environ. Manag. 313, 114973 (2022).
Shao, Y. et al. Soil heavy metal lead pollution and its stabilization remediation technology. Energy Rep. 6, 122–127 (2020).
Kumar, V. et al. A review on the clean-up technologies for heavy metal ions contaminated soil samples. Heliyon 9 (5), e15472 (2023).
Lin, H., Wang, Z., Liu, C. & Dong, Y. Technologies for removing heavy metal from contaminated soils on farmland: A review. Chemosphere 305, 135457 (2022).
Lehmann, J. & Joseph, S. Biochar for Environmental Management: an introduction. In Biochar for Environmental Management (1–13). Routledge. (2015).
Sadegh-Zadeh, F., Parichehreh, M., Jalili, B. & Bahmanyar, M. A. Rehabilitation of calcareous saline‐sodic soil by means of biochars and acidified biochars. Land. Degrad. Dev. 29 (10), 3262–3271 (2018).
Islam, T., Li, Y. & Cheng, H. Biochars and engineered biochars for water and soil remediation: a review. Sustainability 13 (17), 9932 (2021).
Zhou, L. et al. Effects of applying Biochar on soil cadmium immobilisation and cadmium pollution control in lettuce (Lactuca sativa L). Agriculture 14 (7), 1068 (2024).
Wu, C. et al. Biological calcium carbonate enhanced the ability of Biochar to passivate antimony and lead in soil. Environ. Sci.: Process. Impacts. 25 (8), 1365–1373 (2023).
Chen, L. et al. Passivation and remediation of Pb and cr in contaminated soil by sewage sludge Biochar tubule. Environ. Sci. Pollut. Res. 28 (35), 49102–49111 (2021).
Luo, N., Wen, J., Li, Z., Huang, M. & Yang, R. Passivating effect of dewatered sludge and Biochar on as-contaminated soil. Water Air Soil Pollut. 231, 1–10 (2020).
Herath, I. et al. Microbe mediated immobilization of arsenic in the rice rhizosphere after incorporation of silica impregnated Biochar composites. J. Hazard. Mater. 398, 123096 (2020).
Li, H. et al. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 178, 466–478 (2017).
Xie, Y. F. et al. Study on the adsorption of Cd2 + by potassium permanganate modified wheat straw. J. Ecol. Rural Environ. 35 (5), 668–674 (2019).
Guo, D. D. & Zhai, X. W. Passivation characteristics of Pb2 + and Cd2 + in soil by phosphoric acid modified Biochar. Appl. Chemicals. 52 (1), 92–95 (2023).
Yang, X., Zhang, S., Ju, M. & Liu, L. Preparation and modification of Biochar materials and their application in soil remediation. Appl. Sci. 9 (7), 1365 (2019).
Mo, Z. L. et al. Adsorption characteristics of Pb (II) by potassium permanganate modified Eucalyptus Biochar. Environ. Sci. 42 (11), 5440–5449 (2021).
Peng, H. et al. Enhanced adsorption of Cu (II) and cd (II) by phosphoric acid-modified biochars. Environ. Pollut. 229, 846–853 (2017).
Xiao, N. C. et al. Effects of modified distiller ‘s grains Biochar on cadmium form in purple soil and cadmium uptake by rice. Environ. Sci. 45 (5), 3027–3036 (2024).
Zhao, Q. C. Preparation of Phosphoric Acid Modified Biochar and its Removal of Cr (VI) in Water [D] (North University of China, 2024).
Guo, B. Y. et al. Study on passivation remediation of lead and cadmium contaminated soil by manganese dioxide/amino modified Biochar. J. Ecol. Rural Environ. 39 (3), 422–428 (2023).
Zhou, H. Y. et al. Effects of different control measures on the accumulation of cadmium and lead in lettuce and its quality. Environ. Sci. 44 (9), 5196–5203 (2023).
Liu, Q. et al. Study on the difference of cd and Pb accumulation and transport in maize by different foliar inhibitors. J. Agricultural Environ. Sci. 42 (6), 1247–1256 (2023).
Wang, X. B. et al. Effects of amendments on soil available cadmium, lead and their accumulation in the below-ground biomass of tetrastigma Hemsleyanum. J. Ecol. Rural Environ. 40 (4), 565–571 (2024).
Sun, C. et al. Enhanced adsorption for Pb (II) and cd (II) of magnetic rice husk Biochar by KMnO4 modification. Environ. Sci. Pollut. Res. 26, 8902–8913 (2019).
Jiao, Z. Q. et al. Passivation remediation of Cd-contaminated soil by thiol-modified Biochar and soil microbial response. Environ. Sci. 45 (9), 5570–5577 (2024).
Rizwan, M. et al. Cadmium phytoremediation potential of brassica crop species: a review. Sci. Total Environ. 631, 1175–1191 (2018).
Sun, M. et al. Silicon-modified peanut shell biochar: novel passivation to mitigate cadmium bioaccumulation in spinach. J. Environ. Chem. Eng. 13 (1), 114995 (2025).
Shan, R., Li, W., Chen, Y. & Sun, X. Effects of Mg-modified Biochar on the bioavailability of cadmium in soil. BioResources 15 (4), 8008 (2020).
Jaskulak, M., Rostami, S., Zorena, K. & Vandenbulcke, F. Transcriptome sequencing of brassica Napus highlights the complex issues with soil supplementation with sewage sludge. Chemosphere 298, 134321 (2022).
Rostami, S., Akbari, H., Adibzadeh, A. & Akbari, H. Effects of ascophyllum nodosum-based biostimulants on improving phytoextraction of cadmium and lead in contaminated soils. Environ. Processes. 10 (2), 26 (2023).
Fu, Y. B., Dong, L. E. N. G. B. B. B. I. A. N. Q. Y., Liu, Z. D., Li, G. H. & Zhang, H. F. Passivation effect of Biochar on soil cadmium pollution and rape growth. Agricultural Sci. Technol. 26 (6), 183–190 (2024).
Wang, F. et al. Effects of Biochar application on cadmium transformation in brown soil and uptake by baby Bokchoi[J]. J. Agro-Environment Sci. 36 (5), 907–914 (2017).
Chen, W. R. et al. Research and application of low absorption rice barrier technology in cadmium contaminated soil. J. Zhejiang Normal Univ. (Natural Sci. Edition). 44 (4), 420–428 (2021).
Su, J. et al. Study on the adsorption and passivation ability of Struvite on cadmium. J. Ecol. Rural Environ. 1673–4831. (2024).
Funding
This research was funded the Yunnan Science Technology Talent and Platform Programme Project, grant number 202405AM340004.
Author information
Authors and Affiliations
Contributions
Conceptualization, Liyuan Mu; Data curation, Liyuan Mu; Formal analysis, Liyuan Mu; Funding acquisition, Naiming Zhang; Investigation, Liyuan Mu, Hongyin Zhou, Zhengli Lu , Junlei Wang , Sijing Sun and Ao Li; Methodology, Li Bao; Project administration, Li Bao; Resources, Liyuan Mu and Hongyin Zhou; Software, Liyuan Mu, Hongyin Zhou, Zhengli Lu , Junlei Wang , Sijing Sun and Ao Li; Supervision, Naiming Zhang; Validation, Liyuan Mu, Hongyin Zhou,Zhengli Lu , Junlei Wang , Sijing Sun and Ao Li; Visualization, Naiming Zhang; Writing – original draft, Liyuan Mu; Writing – review & editing, Hongyin and Zhou Li Bao. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mu, L., Zhou, H., Lu, Z. et al. Passivation effect of modified biochar on cadmium in mining contaminated soil. Sci Rep 15, 30402 (2025). https://doi.org/10.1038/s41598-025-15860-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15860-6