Abstract
This study investigated the effects of NaOH molarity (6–14 M) and ground granulated blast furnace slag (GBFS) content (0–45%) on the properties of lithium slag (LS)-based cold-bonded lightweight aggregates. Bulk density, water absorption, porosity, and cylinder compressive strength were evaluated, and microstructural characterization was conducted using SEM, XRD, FTIR, MIP, and TG/DTG. Results showed that increasing NaOH molarity and GBFS content reduced water absorption (from 15.87 to 5.88%) and porosity (from 34.79 to 13.39%), while enhancing bulk density (731–1074 kg/m³) and compressive strength. At 30% GBFS, the 28-day strength increased by 224.48%, from 3.35 MPa (M6-30) to 10.87 MPa (M14-30). At 12 M NaOH, raising GBFS content from 0 to 45% increased strength by 435.62%, from 2.33 MPa to 12.48 MPa. LS without GBFS achieved 2.33 MPa, indicating inherent pozzolanic activity. Microstructural analysis revealed that performance improvement was due to enhanced geopolymerization and reduced harmful pores (> 200 nm). The M8-30 mix (915.68 kg/m³, 5.98 MPa) showed potential for meeting high-strength lightweight aggregate criteria with mix optimization. These findings demonstrate the feasibility of valorizing LS into high-performance lightweight aggregates, contributing to waste utilization and low-carbon construction.
Similar content being viewed by others
Introduction
Under the global demand for electric vehicles and renewable energy storage, China’s lithium-ion battery industry has rapidly expanded, leading to extensive lithium resource extraction1,2. As the world’s largest producer and consumer of lithium resources, China accounts for approximately 60% of global lithium refining capacity in 2023, generating significant amounts of lithium slag (LS) as a byproduct during lithium carbonate production from spodumene and lepidolite ores3,4. According to its high alkalinity and residual hazardous impurities, LS is classified as solid waste5,6. China’s annual and cumulative emissions of LS exceeded 1.2 million tons and 10 million tons, respectively, posing severe environmental challenges related to its disposal7,8.
Currently, LS is primarily landfilled or stored in open-air sites, carrying significant risks of soil alkalization, groundwater contamination, and resource wastage9,10. In recent years, numerous efforts have been made to resource utilization of LS in various fields, such as construction materials, ceramics, and soil remediation11,12,13. However, the utilization rate of LS remains below 30%, largely due to technical bottlenecks in hazardous component removal and value-added product development. Meanwhile, the depletion of natural construction resources and the demand for low-carbon building materials have sparked the interest in repurposing solid waste for construction applications. Among these, the cold bonding technique for manufacturing artificial aggregates from solid waste not only effectively immobilizes hazardous components but also produces high-performance construction materials14. Similar studies have achieved promising results in recycling red mud15,16phosphogypsum17,18sewage sludge ash19,20municipal waste incineration ash21,22and tailings23,24. Nevertheless, research on LS-based lightweight aggregates via cold bonding is still limited.
Extensive studies have shown that the performance of cold-bonded artificial aggregates depends on binder dosage, curing methods, and the inherent reactivity of raw materials25,26. For instance, Liu et al.27 used alkali activators to prepare lightweight aggregates from a mixture of granulated blast furnace slag (GBFS) and fly ash (3:7 mass ratio), achieving a 28-day cylinder compressive strength of up to 10 MPa. In contrast, under similar alkali activator conditions and GBFS content, aggregates made from sludge ash or incineration ash exhibited significantly lower strengths of only 1.0 MPa and 4.5 MPa, respectively, attributable to their lower pozzolanic activity compared to fly ash20,28. Increasing binder dosage or adopting complex curing methods inevitably raises production costs, hindering large-scale application. Therefore, solid wastes with higher reactivity, such as LS, hold greater promise for cold-bonded lightweight aggregates.
Fortunately, LS contains substantial SiO2 and Al2O3, indicating considerable reactivity, as confirmed by studies on its use as a supplementary cementitious material and geopolymer precursor. For example, replacing 40% of cement with LS in concrete significantly reduced chloride ion permeability and steel corrosion rates29. Additional applications of LS as a supplementary cementitious material include backfill and road construction12,30. Luo et al.31,32,33 demonstrated that thermal treatment could increase the amorphous phase content in LS, thereby improving the strength of LS-based geopolymer pastes, whose 28-day compressive strength is up to 53.1 MPa. Thus, compared to other low-reactivity solid wastes, LS offers superior potential for enhancing the performance and cost-efficiency of cold-bonded lightweight aggregates. Moreover, similar to the influence of water-cement ratio on cement-based materials, the parameters of alkali activators significantly affect the properties of geopolymer-based cold-bonded lightweight aggregates or mortars. Risdanareni found that the performance of fly ash-based alkali-activated aggregates was less sensitive to NaOH molarity in the activator solution34whereas Liu et al. discovered that the optimal NaOH concentration was 8 M for cold-bonded lightweight aggregates prepared from a 7:3 mass ratio mixture of alkali-activated municipal solid waste incineration ash and GBFS28. These findings suggest that the ideal NaOH molarity varies depending on the solid waste used. However, research on alkali-activated LS-based lightweight aggregates is limited, and the optimal NaOH molarity remains undetermined.
Although previous studies have explored the use of LS in cementitious materials, road bases, and geopolymer systems, research on its application in cold-bonded lightweight aggregates remains limited. Most existing studies focus on low-reactivity wastes such as red mud, phosphogypsum, or incineration ash, where high binder dosages or complex curing methods are often required to achieve adequate strength, increasing production costs35. Moreover, the optimal NaOH molarity for alkali-activating LS-based aggregates has not been established, and the combined effect of alkali concentration and high-reactivity precursors such as GBFS on LS aggregate performance is still unclear. There is also a lack of systematic quantitative evaluation linking mix parameters to both physical and mechanical properties, and correlating these with pore structure and thermal stability. Addressing these gaps is essential for developing cost-effective, high-strength LS-based lightweight aggregates that meet performance standards and support large-scale industrial adoption.
Given the dual benefits of waste valorization and low-carbon material synthesis, the development of LS-based lightweight aggregates aligns with circular economy principles and sustainable development goals. To this end, this study employs LS as the primary raw material and GBFS as a supplementary high-reactivity precursor to prepare alkali-activated LS-based lightweight aggregates via cold bonding technology. The effects of NaOH molarity (6, 8, 10, 12, and 14 M) and GBFS content (0%, 15%, 30%, and 45%) on aggregate properties are evaluated through bulk density, water absorption, and cylinder compressive strength tests. The underlying mechanisms are further elucidated by characterizing mineral composition, chemical bonding, microstructure, thermal stability, and porosity. The results aim to identify optimal preparation parameters and potential applications for alkali-activated LS-based lightweight aggregates.
Experimental program
Raw materials
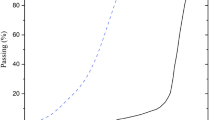
The LS used in this study is obtained from Sichuan Tianhua Lithium Industry Co., Ltd. Prior to experimentation, the LS is dried in an oven at 105 °C until constant weight is achieved, followed by ball milling for 1 h to ensure uniform particle size distribution. Grade S95 GBFS is selected as the supplementary precursor material for geopolymer synthesis. Both LS and GBFS undergo comprehensive physicochemical characterization including chemical composition using X-ray fluorescence test, microstructure using scanning electron microscopy technique, mineralogy and particle size distribution before being employed in lightweight aggregate production. The chemical compositions of LS and GBFS are presented in Fig. 1. The microstructure, mineral composition, and particle size distribution of LS and GBFS are presented in Fig. 2. LS primarily consists of SiO2 and Al2O3, while GBFS is mainly composed of SiO2 and CaO. Mineralogical analysis reveals that LS predominantly contains lithium aluminum silicate (LAS) and leached spodumene (LSP), whereas GBFS exhibits an amorphous phase, as indicated by its broad diffraction hump. Morphological examination via scanning electron microscopy (SEM) demonstrates that both LS and GBFS particles possess irregular shapes, with LS exhibiting a rougher surface texture compared to the relatively smoother GBFS particles. Particle size distribution analysis indicates that GBFS particles are more uniformly sized, while LS displays a broader size distribution range. The specific surface areas of LS and GBFS are determined to be 359.78 m2/kg and 450 m2/kg, respectively. The alkaline activator was prepared by first dissolving solid sodium hydroxide pellets (98% purity) in deionized water under constant stirring for 5 min until fully dissolved. The dissolution process was carried out in a polypropylene container and cooled to room temperature (25 ± 2 °C) to avoid premature reaction with sodium silicate. The liquid sodium silicate has a chemical composition of 8.54% Na2O and 27.3% SiO2, with a modulus of 3.3. Based on preliminary studies and pilot tests, five different NaOH molarities (6, 8, 10, 12, and 14 M) and four GBFS incorporation levels (0%, 15%, 30%, and 45%) were selected to investigate their effects on the properties of LS-based aggregates. For convenience, the mixtures are denoted as Mx–y, where x is the NaOH molarity (M) and y is the GBFS content (wt%). The detailed mix proportions and alkaline activator parameters are summarized in Table 1, where the nomenclature (e.g., M6-30) denotes the NaOH molarity (6 M) and GBFS content (30%) in the mixture.
Test Preparation
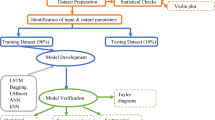
The raw materials were precisely weighed according to the designated GBFS content ratios and dry-mixed in a stainless-steel pan for 2 min to ensure homogeneity before transfer to a BY-300 granulator. The granulator was operated at a rotation speed of 36 rpm for 10 min to facilitate pelletization. The alkali activator, prepared in advance and cooled to room temperature, was sprayed uniformly onto the mixture surface at a rate of approximately 50 mL/min using a hand-operated spray nozzle while the granulator was in operation. Always control the mass ratio of the mixture to the alkaline activator solution at 2.31. After polishing, aggregates with particle sizes between 9.5 and 16 mm are collected and initially air-cured for 1 h before being sealed for storage. The sealed specimens were maintained under controlled ambient indoor conditions at 20 ± 2 °C and 60 ± 5% relative humidity until testing at 7-day and 28-day intervals. Figure 3 illustrates the experimental procedure.
Test methods
The physical and mechanical properties of the LS-based lightweight aggregates are evaluated in accordance with the Chinese standard GB/T 17431.2–2010 Lightweight aggregate and its test methods36. The aggregates are divided into two groups for testing cylinder compressive strength and physical properties respectively. Cylinder compressive strength tests are conducted at 7 days and 28 days using a testing apparatus as shown in Fig. 4, with a loading speed of 400 N/s and termination condition set at 20 mm displacement. For physical property testing after 28 days of curing, the aggregates are first measured for initial mass, then oven-dried at 50 °C to constant weight to determine dry mass, followed by bulk density and water absorption measurements. The drying temperature of 50 °C was selected to avoid decomposition of geopolymer gels, which can occur at temperatures above 60 °C, thereby preserving the integrity of the pore structure for accurate measurement37. All physical and mechanical property test results are averaged from three repeated measurements. Microstructural characterization of 28-day cured aggregates is performed using scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), mercury intrusion porosimetry (MIP), and thermogravimetric analysis (TG/DTG) to examine the microstructure, crystalline phases, chemical bonding, pore characteristics, and thermal stability.
Results and discussions
Water content
Figure 5 shows the effect of NaOH molarity in the alkaline activator and GBFS content on the water content of LS-based lightweight aggregates. The results indicate that increasing either the NaOH molarity or GBFS content reduced the water content of the aggregates. The water content of the aggregates mainly depends on the water content required in the alkaline activator during formation and the compactness of the aggregates themselves. The increase in NaOH molarity reduces both the water content in the alkaline activator and increases the compactness of the aggregates, thereby decreasing their water content. Similarly, the increase in GBFS content leads to reduced water content by enhancing the compactness of the aggregates.
Bulk density
Figure 6 illustrates the effects of NaOH molarity in alkaline activators and GBFS content on the bulk density of LS-based lightweight aggregates. All tested aggregates exhibit bulk densities below 1200 kg/m3, meeting the density requirements for lightweight aggregates. Notably, the M12-0 group demonstrates the lowest bulk density, corresponding to density grade 800. The M6-30 group shows bulk densities ranging between 800 and 900 kg/m3, belonging to density grade 900), while the M8-30, M10-30, M12-30 and M12-15 groups range between 900 and 1000 kg/m3, belonging to density grade 1000. The M14-30 and M12-45 groups exhibit bulk densities of 1000–1100 kg/m3, belonging to density grade 1100. The results clearly demonstrate that increasing either NaOH molarity or GBFS content leads to higher aggregate bulk density, indicating improved compactness of the aggregate structure.
Water absorption rate
Figure 7 shows the effects of NaOH molarity in alkaline activators and GBFS content on the water absorption of LS-based lightweight aggregates. The results demonstrate that increasing either NaOH molarity or GBFS content effectively reduces aggregate water absorption by enhancing structural compactness, as confirmed by bulk density measurements and MIP analysis. Notably, the 1-hour water absorption values for M12-30 (7.08%), M14-30 (5.88%), and M12-45 (6.67%) all meet the Chinese standard GB/T 17431.2–2010 requirement (≤ 10%). Other groups exhibit water absorption rates between 10 and 16%, still complying with the ACI-213R standard limit (≤ 25%)38. Of particular interest, the absorption rates of M8-30, M10-30, and M12-15 approach the threshold of Chinese Standard GB/T 17431.2–2010, suggesting their water absorption could be readily optimized to meet standard requirements through either mix proportion adjustment or low-cost modification techniques.
Cylinder compressive strength
At NaOH molarities of M6-30, M8-30, M10-30, M12-30, and M14-30, the 28-day compressive strengths are 3.35 MPa, 5.98 MPa, 7.34 MPa, 8.62 MPa, and 10.87 MPa, respectively. Corresponding results for GBFS variation at constant 12 M NaOH (M12-0, M12-15, M12-30, M12-45) are also presented in Fig. 8. All data shown correspond to the 7-day and 28-day testing periods. Clearly, increasing either the NaOH molarity or GBFS content can improve the compressive strength of aggregates, which is consistent with the physical property test results. The Fig. 8 shows that when NaOH molarity is higher or GBFS content is lower, strength development mainly occurs at early ages because the geopolymerization reaction rate is significantly affected by NaOH molarity and raw material activity39. With lower NaOH molarity, early strength development is slower but strength continues to increase at later ages. When higher-activity GBFS content increases, the early geopolymerization reaction does not completely consume the raw materials, allowing remaining materials to continue participating in later reactions. At NaOH molarities of M6, M8, M10, M12 and M14, the 28-day compressive strengths are 3.35 MPa, 5.98 MPa, 7.34 MPa, 8.62 MPa and 10.87 MPa respectively. A 224.48% increase from M6-30 to M14-30. When GBFS content increased from 0 to 45%, the 28-day strength rose from 2.33 MPa to 12.48 MPa, a remarkable 435.62% increase. The measurable strength of M12-0 group (2.33 MPa) demonstrates inherent pozzolanic activity of LS and its potential for cold-bonded artificial aggregates. Under similar mix proportions, aggregates made from sludge incineration residues or waste incineration ash show much lower strength than LS-based lightweight aggregates19,27. Notably, GB/T 17431.2–2010 requires cylinder compressive strengths of 6.0 MPa and 6.5 MPa for density grades 800 and 900 respectively to qualify as high-strength lightweight aggregates. The M6-30 (density grade 900) and M12-0 (density grade 800) groups clearly fail to meet these requirements. However, M8-30 with bulk density of 915.68 kg/m3 and compressive strength of 5.98 MPa could potentially meet high-strength lightweight aggregate requirements through mix optimization, thereby expanding the application range of LS-based lightweight aggregates. Previous studies have shown that fiber reinforcement can simultaneously reduce bulk density and enhance mechanical properties of cold-bonded lightweight aggregates20.
Microstructure and energy spectrum analysis
The fractured surfaces of aggregates after 28-day cylinder compressive strength testing are examined for microstructural characterization, with results shown in Fig. 9. Distinct geopolymer gel formation is observed in specimens containing 30% GBFS, while minimal gel is present in GBFS-free or low-GBFS (15%) samples, confirming that geopolymer gel primarily forms through GBFS activation and demonstrating significantly higher pozzolanic activity of GBFS compared to LS40. At constant GBFS content, aggregates prepared with different NaOH molarities exhibit similar microstructures consisting of geopolymer gel, partially encapsulated LS particles, and microcracks/voids, with increasing NaOH concentration yielding denser, more homogeneous structures that correlate with enhanced strength. The main reason for this phenomenon is that a higher molar concentration of NaOH can promote the formation of geopolymer gels, thereby resulting in a thicker phase layer of geopolymer gels41. Although the increase in the molar concentration of NaOH can enhance the compactness of the structure, it is also more likely to introduce some cracks caused by geopolymer gel drying or carbonization42. The increase in the number of micro-cracks in aggregates may lead to a loss in the durability of aggregates, as moisture and harmful substances in the environment can more easily enter. To alleviate this impact, measures such as introducing fibers can be considered. Although M12-0 and M12-15 specimens show negligible geopolymer gel, they maintain reasonable compactness due to inherent pozzolanic activity of LS. Energy-dispersive X-ray spectroscopy reveals consistent elemental composition (O, Si, Ca, Na, Al, S, Mg) across all samples, with Na content increasing proportionally with NaOH molarity but decreasing with higher GBFS content (30–45%) as rapid reaction of GBFS with alkali activators reduces activator demand, while Ca content rises significantly (from CaO in GBFS) and S decreases (from gypsum content in LS).
Mineral composition analysis
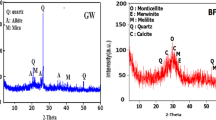
Figure 10 presents the XRD analysis of various aggregates after 28 days of curing. All samples exhibit identical crystalline phases including NaAlSi2O6, LiAlSi2O6, SiO2 and CaCO3, with no new mineral phases detected. Figure 10 (a) demonstrates negligible influence of NaOH molarity (6-14 M) on mineral composition because the amorphous phase geopolymer gel is difficult to detect. In contrast, Fig. 10 (b) shows progressively attenuated diffraction peaks for NaAlSi2O6 and LiAlSi2O6 with increasing GBFS content (0–45%), confirming these phases primarily originate from alkaline-activated decomposition and reorganization of LS components43. The inverse correlation between GBFS incorporation and LS-derived crystalline phase intensities substantiates the progressive consumption of LS reactants during geopolymerization.
Chemical bond types analysis
Figure 11 presents the FTIR analysis of 28-day cured aggregates to investigate the molecular structure evolution under varying NaOH molarities and GBFS contents. While neither parameter fundamentally alters the chemical bond types, distinct vibrational signatures can be observed. First of all, 3450 cm−1 and 1650 cm−1 peaks (-OH stretching/bending) confirm hydroxyl groups and free water44,45,46. Secondly, 880/1424/1430 cm−1 bands (CO32− asymmetric stretching) corroborate XRD-detected calcite47. It can also be observed that critical geopolymerization markers at 1010 cm−1 (Si/Al-O-Si asymmetric stretching) and 450 cm−1 (Si-O-Si bending)48. Moreover, 780 cm−1 (Al-O vibration) indicating alumina participation49. These findings collectively verify the dual role of LS as both reactant (through Al-O bonds) and filler (via carbonate phases).
Notably, while previous studies reported enhanced 3450 cm−1 peaks with increasing NaOH molarity28this trend was not observed in the results shown in Fig. 11 (a), likely due to the reduced water content and optimized alkali activator dosage at higher NaOH concentrations. In contrast, GBFS content significantly influenced the FTIR spectra, as shown in Fig. 11 (b). The characteristic peaks at 3450 cm−1, 1650 cm−1, and 1430 cm−1 show marked increases in intensity with higher GBFS incorporation, attributable to the elevated Ca²⁺ concentration and enhanced alkalinity from CaO content of GBFS, thereby enhancing the vibrational amplitude of both -OH bonds and O-C-O groups in calcium carbonate. Furthermore, the 1010 cm⁻¹ peak exhibited both intensity growth (~ 38% increase at 30% GBFS) and a distinct red-shift (~ 15 cm−1), clearly demonstrating superior reactivity of GBFS in promoting geopolymer gel formation through enhanced crosslinking density and Al3+ incorporation into silicate networks compared to LS. These spectral modifications provide molecular-level evidence for GBFS more efficient consumption of reactive species and greater amorphous phase formation during geopolymerization.
Pore characteristics analysis
Figure 12 illustrates the effects of NaOH molarity in alkaline activators and GBFS content on the pore characteristics of sealed-cured (28 days) LS-based lightweight aggregates. The cumulative pore content data shown in Fig. 12 (a) and (b) reveal that for fixed 30% GBFS content, increasing NaOH molarity of M6-30, M8-30, M10-30, M12-30 and M14-30 reduced pore content progressively (0.166 mg/L, 0.133 mg/L, 0.093 mg/L, 0.091 mg/L, 0.085 mg/L), with the most significant reduction occurring below 10 M NaOH. Secondly, at fixed 12 M NaOH, increasing GBFS content from 0 to 45% substantially decreased pore content (0.238 mg/L, 0.118 mg/L, 0.091 mg/L, 0.070 mg/L for M12-0, M12-15, M12-30 and M12-45, respectively). These trends show the 49% and 71% reductions respectively, correlating perfectly with microstructural observations, confirming that both higher NaOH molarity (≥ 10 M) and GBFS content (≥ 30%) effectively enhance matrix densification by promoting geopolymer gel formation and reducing void spaces.
Figure 12 (c) to (f) present the pore size distribution, pore volume, and total porosity of the aggregates, with pores classified into six ranges: <20 nm, 20–50 nm, 50–200 nm, 200–1000 nm, 1000–10,000 nm, and > 10,000 nm. Notably, pores > 200 nm are considered harmful as they may compromise performance and durability50,51. Increasing NaOH molarity reduced pores < 20 nm but initially decreased and then increased pores of higher 200 nm due to intensified geopolymer reactions that entrap air and release H₂, creating microcracks52. However, although the proportions of pores larger than 200 nm in M10-30, M12-30, and M14-30 are greater than those in M6-30 and M8-30, their total porosities are lower (M6-30: 25.69%, M8-30: 22.10%, M10-30: 15.97%, M12-30: 16.05%, M14-30: 15.49%), resulting in superior mechanical performance. Increased GBFS content promotes the formation of additional geopolymer gel, which increases the proportion of small pores while reducing large pores, as evidenced by the decreasing total porosity with higher GBFS content (M12-0: 34.79%, M12-15: 19.36%, M12-30: 16.05%, M12-45: 13.39%). Consequently, considering both pore characteristics and economic factors, the NaOH molarity in the alkaline activator for LS-based lightweight aggregate production should not be excessively high. Combined with the results in Sect. ❝Cylinder compressive strength❞, it is clear that an NaOH molarity of 8 M balances pore structure refinement and cost-effectiveness for high-strength lightweight aggregates.
TG/DTG analysis
Figure 13 demonstrates the thermal stability of LS-based lightweight aggregates through thermogravimetric analysis, showing two distinct mass loss stages: 30–200 °C (attributed to water evaporation and geopolymer gel decomposition) and 600–800 °C (due to calcium carbonate decomposition)53,54. The sealed curing condition effectively minimized carbonation, rendering carbonate-related mass loss insignificant. Therefore, the mass loss of different aggregates mainly depends on the content of free water and geopolymer gel within the aggregates. Figure 13 results specifically demonstrate that increasing both the NaOH molarity in alkaline activators and GBFS content can effectively enhance the aggregates’ resistance to high-temperature decomposition, which is attributed to the increased geopolymer gel formation and reduced free water content within the aggregates. Particularly, the residual mass percentages at 800 °C are as follows: for M6-30 78.37%, M8-30 79.35%, M10-30 80.80%, M12-30 80.97%, and M14-30 81.34% with 30% GBFS content; and for M12-0 74.57%, M12-15 78.31%, M12-30 80.97%, and M12-45 82.92% with 12 M NaOH concentration. These data clearly show the progressive improvement in thermal stability with increasing NaOH molarity and GBFS content.
Comparison to contemporary work
Compared with other waste-derived cold-bonded aggregates such as red mud15,16phosphogypsum17,18sewage sludge ash19,20municipal waste incineration ash21,22mine tailings23,24and fly ash/GBFS blends27the LS-based aggregates in this study achieved comparable or higher performance under moderate activator dosages and ambient curing. The 28-day compressive strength reached 10.87–12.48 MPa with 30–45% GBFS, exceeding values commonly reported for sludge- and MSWI-based aggregates (≤ 4.5 MPa) and matching those of optimized fly ash/GBFS systems (~ 10 MPa). Several mixes met the ≤ 10% water absorption criterion with lower porosity (13.39–16.05%), indicating that LS, aided by GBFS, can produce high-strength lightweight aggregates without energy-intensive curing, offering a competitive and scalable route for large-scale waste valorization.
Conclusions
This study thoroughly investigated the effects of NaOH molarity in alkaline activators and GBFS content on the performance and mechanisms of LS-based cold-bonded lightweight aggregates. The main conclusions are as follows:
(1) Increasing both NaOH molarity (6 M-12 M) and GBFS content (0%−45%) in LS-based lightweight aggregates reduced the water content (12.12%−19.60%) and water absorption (5.88%−15.87%) of the aggregates, while increasing their bulk density (731 kg/m3−1074 kg/m3). Except for M6-30 and M12-0, the 1-hour water absorption of other aggregates met or approached the requirements of the Chinese standard GB/T 17431.2–2010.
(2) When GBFS content was 30%, increasing NaOH molarity from M6 to M14 raised the cylinder compressive strength of aggregates from 3.35 MPa to 10.87 MPa, representing a 224.48% improvement. When NaOH molarity was M12, increasing GBFS content from 0 to 45% increased the cylinder compressive strength from 2.33 MPa to 12.48 MPa, showing a remarkable 435.62% enhancement. The M8-30 aggregate, with bulk density of 915.68 kg/m³ and cylinder compressive strength of 5.98 MPa, showed the most promise for meeting high-strength lightweight aggregate requirements.
(3) The performance of LS-based lightweight aggregates mainly depended on the amount of geopolymer gel generated by the highly reactive GBFS and the compactness of the structure. Increasing either NaOH molarity or GBFS content reduced the porosity (13.39%−34.79%) of aggregates, thereby enhancing their performance.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wang, Y. R., Wang, D. M., Cui, Y., Zheng, D. P. & Liu, Z. Micro-morphology and phase composition of lithium slag from lithium carbonate production by sulphuric acid process. Constr. Build. Mater. 203, 304–313 (2019).
Wang, J. X., Han, L., Liu, Z. & Wang, D. M. Setting controlling of lithium slag-based geopolymer by activator and sodium tetraborate as a retarder and its effects on mortar properties. Cem. Concrete Comp. 110, 103598 (2020).
He, Y. et al. Mechanical and environmental characteristics of cemented paste backfill containing lithium slag-blended binder. Constr. Build. Mater. 271, 121567 (2021).
Zhao, F. et al. Resource utilization of the waste lithium slag in oil-well cement under high temperature: mechanical property, hydration behavior and environmental effect. J. Environ. Chem. Eng. 13, 116513 (2025).
Gu, T. et al. The formation, characteristics, and resource utilization of lithium slag. Constr. Build. Mater. 432, 136648 (2024).
Zheng, C. W. et al. Leaching behavior of lithium slag at various pH conditions. Cem. Concrete Comp. 159, 105985 (2025).
Cao, X. H. et al. Mechanical properties and heavy metals immobilization of lithium slag stabilized by magnesium slag as road subbase material. J. Clean. Prod. 505, 145484 (2025).
Zhou, S. Z., Zhang, Z. C. & Zhu, Y. Effect of lithium slag on hydration behavior of Portland cement paste. Constr. Build. Mater. 463, 138909 (2025).
Javed, U., Shaikh, F. U. A. & Sarker, P. K. Microstructural investigation of lithium slag geopolymer pastes containing silica fume and fly Ash as additive chemical modifiers. Cem. Concrete Comp. 134, 104736 (2022).
Javed, U., Shaikh, F. U. A. & Sarker, P. K. Microstructural investigation of thermo-mechanically processed lithium slag for geopolymer precursor using various characterization techniques. Constr. Build. Mater. 342, 127952 (2022).
Yang, B. H. et al. Synergistic effects of lithium slag and coarse limestone powder as supplementary cementitious materials: hydration and microstructure. J. Build. Eng. 99, 111608 (2025).
Yuan, L. Q. et al. Feasibility study of lithium slag as cementitious material with high-content application in cement stabilized macadam bases. Constr. Build. Mater. 457, 139224 (2024).
Li, C. B., Zhang, G. F., Liu, D. Z. & Wang, M. T. Preparation of lightweight ceramsite from solid waste lithium slag and fly Ash. Constr. Build. Mater. 398, 132419 (2023).
Abdellatief, M., Baktheer, A., Shahin, M., Abadel, A. A. & Heniegal, A. M. Production and optimization of affordable artificial geopolymer aggregates containing crumb rubber, plastic waste, and granulated Cork based on machine learning algorithms. Case Stud. Constr. Mat. 22, e04725 (2025).
Zhang, C. et al. Investigation of hierarchical porous cold bonded lightweight aggregates produced from red mud and solid-waste-based cementitious material. Constr. Build. Mater. 308, 124990 (2021).
Yang, J. et al. Preparation and properties of alkali-activated red mud-based artificial lightweight aggregates. Constr. Build. Mater. 449, 138304 (2024).
Ding, C., Sun, T., Shui, Z. H., Xie, Y. F. & Ye, Z. Y. Physical properties, strength, and impurities stability of phosphogypsum-based cold-bonded aggregates. Constr. Build. Mater. 331, 127307 (2022).
Ouyang, G. S., Chen, J. J., Wang, Z. Y., Sun, T. & Xu, D. Valorization of alkali-activated fly ash-slag claddings to enhance the mechanical and leaching properties of phosphogypsum-based cold-bonded aggregates. Developments Built Environ. 18, 100464 (2024).
Tang, P. et al. Investigation of cold bonded lightweight aggregates produced with incineration sewage sludge Ash (ISSA) and cementitious waste. J. Clean. Prod. 251, 119709 (2020).
Zhou, X. L., Chen, Y. L., Liu, C. W. & Wu, F. Preparation of artificial lightweight aggregate using alkali-activated incinerator bottom Ash from urban sewage sludge. Constr. Build. Mater. 341, 127844 (2022).
Song, H. L., Liu, T., Gauvin, F. & Brouwers, H. J. H. Investigation of Sisal fiber incorporation on engineering properties and sustainability of lightweight aggregates produced from municipal solid waste incinerated bottom Ash. Constr. Build. Mater. 413, 134943 (2024).
Ferraro, A. et al. Production and characterization of lightweight aggregates from municipal solid waste incineration fly-ash through single-and double-step pelletization process. J. Clean. Prod. 383, 135275 (2023).
Li, Q. L., Wang, B. W., Yang, L. & Liu, C. Y. Preparation and characteristics of cold-bonded lightweight aggregates by recycling mine tailings and industrial waste residues based-binder. J. Build. Eng. 95, 110190 (2024).
Asadizadeh, M. et al. The impact of slag on the process of geopolymerization and the mechanical performance of mine-tailings-based alkali-activated lightweight aggregates. Constr. Build. Mater. 411, 134347 (2024).
Zhao, Q. X. et al. Investigation of various curing methods on the properties of red mud-calcium carbide slag-based artificial lightweight aggregate ceramsite fabricated through alkali-activated cold-bonded pelletization technology. Constr. Build. Mater. 401, 132956 (2023).
Tang, P., Xuan, D. X., Poon, C. S. & Tsang, D. C. W. Valorization of concrete slurry waste (CSW) and fine incineration bottom Ash (IBA) into cold bonded lightweight aggregates (CBLAs): feasibility and influence of binder types. J. Hazard. Mater. 368, 689–697 (2019).
Liu, X., Wen, Y., Chen, S. & Jiang, M. X. Geopolymer cold-bonded lightweight aggregate concrete: mechanical properties and microstructure. Constr. Build. Mater. 465, 140261 (2025).
Liu, J. et al. The performance and microstructure of alkali-activated artificial aggregates prepared from municipal solid waste incineration bottom Ash. Constr. Build. Mater. 403, 133012 (2023).
Amin, M. T. E., Sarker, P. K., Shaikh, F. U. A. & Hosan, A. Chloride permeability and chloride-induced corrosion of concrete containing lithium slag as a supplementary cementitious material. Constr. Build. Mater. 471, 140629 (2025).
Xue, Z. L. et al. Mechanical properties and damage evolution of lithium slag modified cemented tailings backfill under impact load. Constr. Build. Mater. 472, 140925 (2025).
Luo, X. F. et al. Preparation of geopolymers from thermally activated lithium slag: activity enhancement and microstructure. J. Build. Eng. 88, 109256 (2024).
Luo, X. F. et al. A technique for Preparing one-part geopolymers by activating alkali-fused lithium slag with solid sodium silicate. Constr. Build. Mater. 435, 136817 (2024).
Luo, X. F., Huang, L., Li, Y. & Chen, Z. J. Preparation of geopolymers from thermally activated lithium slag as sole precursor: mechanical properties and microstructure. Case Stud. Constr. Mat. 20, e03248 (2024).
Risdanareni, P., Schollbach, K., Wang, J. & De Belie, N. The effect of NaOH concentration on the mechanical and physical properties of alkali activated fly ash-based artificial lightweight aggregate. Constr. Build. Mater. 259, 119832 (2020).
Murali, G., Wong, L. S., Abdulkadir, I., Algaifi, H. A. & Abdellatief, M. Sustainable transformation of waste phosphogypsum into geopolymer concrete: comprehensive review on strength, durability, and microstructural characteristics. J. Build. Eng. 111, 113597 (2025).
17431.2–2010. G. T. Lightweight Aggregates and its Test Methods-Part 2: Test Meths for Lightweight Aggregates. (in Chinese).
Zhou, X. L., Tang, Z. P., Zheng, Y. T., Zhang, Y. D. & Wu, F. Research on the properties and mechanism of a fiber-reinforced alkali-activated lithium slag artificial lightweight aggregate. Constr. Build. Mater. 472, 140866 (2025).
ACI213R-03. Guide for structural lightweight-aggregate concrete (ACI 213R-03).
Swathi, B. & Vidjeapriya, R. Influence of precursor materials and molar ratios on normal, high, and ultra-high performance geopolymer concrete–A state of Art review. Constr. Build. Mater. 392, 132006 (2023).
García-Lodeiro, I., Palomo, A., Fernández-Jiménez, A. & Macphee, D. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concrete Res. 41, 923–931 (2011).
Xiao, L., Zhang, C., Zhang, H. E. & Jiang, Z. W. Optimization of alkali-activated slag for cold region construction: influence of primary parameters on its performance at subzero temperatures. J. Build. Eng. 106, 112556 (2025).
Chen, J. J., Thomas, J. J. & Jennings, H. M. Decalcification shrinkage of cement paste. Cem. Concrete Res. 36, 801–809 (2006).
Dong, J. L. et al. Investigation into the alkali-activation of lithium slag: A sustainable alternative to conventional cement with optimized mechanical properties. Constr. Build. Mater. 416, 135022 (2024).
Li, C., Sun, H. H. & Li, L. T. A review: the comparison between alkali-activated slag (Si + Ca) and Metakaolin (Si + Al) cements. Cem. Concrete Res. 40, 1341–1349 (2010).
Maraghechi, H., Rajabipour, F., Pantano, C. G. & Burgos, W. D. Effect of calcium on dissolution and precipitation reactions of amorphous silica at high alkalinity. Cem. Concrete Res. 87, 1–13 (2016).
Huang, G. D., Ji, Y. S., Li, J., Hou, Z. H. & Jin, C. Use of slaked lime and Portland cement to improve the resistance of MSWI bottom ash-GBFS geopolymer concrete against carbonation. Constr. Build. Mater. 166, 290–300 (2018).
Yu, L., Zhang, Z., Huang, X., Jiao, B. Q. & Li, D. W. Enhancement experiment on cementitious activity of copper-mine tailings in a geopolymer system. Fibers 5, 47 (2017).
Dong, J. L., Wang, L. J. & Zhang, T. T. Study on the strength development, hydration process and carbonation process of NaOH-activated Pisha sandstone. Constr. Build. Mater. 66, 154–162 (2014).
Rehman, M. U., Rashid, K., Haq, E. U., Hussain, M. & Shehzad, N. Physico-mechanical performance and durability of artificial lightweight aggregates synthesized by cementing and geopolymerization. Constr. Build. Mater. 232, 117290 (2020).
Chen, S. K. et al. Pore structure of geopolymer materials and its correlations to engineering properties: A review. Constr. Build. Mater. 328, 127064 (2022).
Liu, J. et al. The impact of cold-bonded artificial lightweight aggregates produced by municipal solid waste incineration bottom Ash (MSWIBA) replace natural aggregates on the mechanical, microscopic and environmental properties, durability of sustainable concrete. J. Clean. Prod. 337, 130479 (2022).
Yamaguchi, N., Nagaishi, M., Kisu, K., Nakamura, Y. & Ikeda, K. Preparation of monolithic geopolymer materials from urban waste incineration slags. J. Ceram. Soc. Jpn. 121, 847–854 (2013).
Chen, T. F. et al. The strength, reaction mechanism, sustainable potential of full solid waste alkali-activated cementitious materials using red mud and carbide slag. Constr. Build. Mater. 449, 138454 (2024).
Song, H. et al. Development of artificial leak-free phase change material (PCM) aggregates using emulsion technique, cementless binder, and cold-bonded pelletization. Constr. Build. Mater. 411, 134293 (2024).
Funding
Funding was provided by Guizhou Province Science and Technology Cooperation Achievement Project, [2023] General project:777.
Author information
Authors and Affiliations
Contributions
Y.D. and X.L. wrote the main manuscript text and Z.P and Y.T. prepared all figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Tang, Z., Zhou, X. et al. Effect of alkali activator and granulated blast furnace slag on the properties of lithium slag-based high-strength lightweight aggregates. Sci Rep 15, 30115 (2025). https://doi.org/10.1038/s41598-025-16048-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16048-8