Abstract
While cardiac myosin-binding protein-C (cMyBP-C) is a promising biomarker for acute coronary syndrome, its clinical utility in stable coronary artery disease (CAD) remains poorly defined. To investigate the association of cMyBP-C with the presence and angiographic severity of stable CAD, and to evaluate its prognostic value for 1-year major adverse cardiovascular events (MACEs). This study enrolled 367 patients undergoing coronary angiography for suspected CAD. We performed a cross-sectional analysis for CAD presence and severity (quantified by Gensini score [GS]) and a prospective follow-up of stable CAD patients for 1-year MACEs. Of 367 patients, cMyBP-C levels were significantly higher in those with stable CAD (n = 195) versus non-CAD (n = 172) (median 28.0 vs. 6.0 pg/ml, P < 0.001) and correlated positively with GS (r = 0.44, P < 0.001). In multivariate analysis, cMyBP-C was an independent predictor of both CAD presence (odds ratio [OR] 1.06, 95% confidence interval [CI] 1.05–1.08) and severe CAD (GS ≥ 18) (OR 1.03, 95% CI 1.02–1.04; both P < 0.001). The area under the curve for predicting CAD presence and severity was 0.827 and 0.778, respectively. In the prognostic cohort of 190 stable CAD patients, 56 MACEs (29.5%) occurred. As the proportional hazards assumption was violated, an Accelerated Failure Time (AFT) model was used. This model confirmed that a high cMyBP-C level (≥ 27 pg/ml) was independently associated with an accelerated time to MACEs (Acceleration Factor 0.66, 95% CI 0.46–0.94, P = 0.02). Circulating cMyBP-C is an independent predictor for the presence and angiographic severity of stable CAD, and an elevated level identifies patients at higher risk for 1-year MACEs. While these single-center findings require confirmation in larger, external validation cohorts, they position cMyBP-C as a promising biomarker for comprehensive risk stratification in this population.
Similar content being viewed by others
Introduction
Coronary artery disease (CAD) is a prevailing global health challenge, contributing substantially to cardiovascular morbidity and mortality. While there have been significant advancements in the management of acute coronary syndrome (ACS), which represents a life-threatening manifestation of CAD, the underlying chronic disease process often continues to progress. Therefore, proactive interventions and improved risk stratification in the earlier, stable phases of CAD are crucial for mitigating its long-term burden1. The progression of CAD is driven by myocardial fibrosis and cell death triggered by coronary artery stenosis, with the severity of the stenosis itself being a pivotal factor in establishing cardiovascular risk stratification and prognosis assessment2. While the Gensini score (GS) is widely employed in clinical practice to define CAD severity, its dependence on coronary angiography limits its effectiveness in screening high-risk populations.
Cardiac myosin-binding protein-C (cMyBP-C) is a protein located on the thick filament of the myocardium, participating in the regulation of cardiac structure and function3. As a cardiac-specific protein, cMyBP-C is exceptionally abundant, with a myocardial concentration at least twofold higher than that of cardiac troponin4. It is detectable at low levels in the healthy population and is rapidly released into the bloodstream following myocardial ischemic injury. Previous research has substantiated the ability of cMyBP-C to serve as an indicator of ongoing cardiovascular stress and disease. Specifically, it independently predicts long-term major adverse cardiovascular events (MACEs) in individuals without a history of cardiovascular disease (CVD)5. In addition, cMyBP-C may offer a more accurate quantification of cardiomyocyte injury than high-sensitivity cardiac troponin (hs-cTn), a finding supported by numerous studies confirming its efficacy in the early diagnosis and prognosis of ACS6,7,8,9. However, the role of cMyBP-C in CAD patients before the occurrence of acute events remains to be further explored10.
Considering that the severity of coronary stenosis is a major driver of chronic myocardial injury, and given the sensitivity of cMyBP-C in detecting such injury, we hypothesized that circulating cMyBP-C levels would correlate with the angiographic severity of coronary artery lesions and predict adverse prognosis in patients with stable CAD. Therefore, the objectives of this study are as follows: (1) to investigate whether cMyBP-C can independently predict the presence of CAD in high-risk individuals; (2) to explore the potential correlation between cMyBP-C and the angiographic CAD severity using GS; and (3) to examine whether cMyBP-C is associated with future cardiovascular events in patients diagnosed with stable CAD.
Methods
Study population and design
This study incorporates both a cross-sectional analysis and a prospective cohort study (ChiCTR2100043234), conducted in accordance with the principles of the Helsinki Declaration, STROBE guidelines, and approved by the hospital ethics committee ([2021]-KY-001–01). Written informed consent was obtained from all patients.
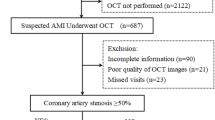
Initially, 414 consecutive patients who presented with chest pain to the Cardiovascular Department outpatient clinic of the Second Affiliated Hospital of Nanjing Medical University from December 2021 to June 2023, suspected of CAD and subsequently admitted for coronary angiography, were included. CAD was defined as the presence of at least one major coronary vessel (left main, left anterior descending, left circumflex, or right coronary artery) with ≥ 50% diameter stenosis, as assessed by coronary angiography. The diagnosis of stable CAD was based on the 2019 European Society of Cardiology guidelines11. Patients without luminal narrowing on coronary angiography or with major coronary luminal narrowing < 30% were considered for inclusion in the non-CAD group. The final determination of the underlying cause of chest pain was made by two independent cardiologists with access to all patient medical records, except for the cMyBP-C levels. In cases of diagnostic discordance, a third cardiologist resolved the disagreement. Exclusion criteria were: coronary luminal narrowing 30% ~ 50%; a diagnosis of ACS; a history of acute myocardial infarction (AMI), coronary stent implantation, or coronary artery bypass grafting; severe heart failure defined as left ventricular ejection fraction (LVEF) < 30%, non-ischemic cardiomyopathy, or valvular heart disease; severe hepatic or renal dysfunction, infectious diseases, autoimmune diseases, malignancies, or incomplete clinical data. A total of 47 patients were excluded. Ultimately, 367 patients were included in the study, categorized into the stable CAD group (n = 195) and the non-CAD group (n = 172). Clinical characteristics, including gender, age, body mass index (BMI), current smoking status, history of hypertension, history of diabetes, and family history of CVD, were collected via in-person interviews at baseline. Additionally, blood test results and LVEF assessed by echocardiography were recorded within 24 h of admission. BMI was calculated as weight (kg) divided by the square of height (m). Current smoking was defined as smoking at least one cigarette per day for a minimum of one year. Hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg on three separate measurements or current use of antihypertensive medication. Diabetes was defined as fasting blood glucose ≥ 7.0 mmol/L or random blood glucose ≥ 11.0 mmol/L or current use of antidiabetic medication. Family history of CVD was defined as the occurrence of a CVD event in a first-degree relative (female ≤ 65 years old or male ≤ 55 years old). The GS was calculated to quantify the severity of coronary artery stenosis.
Subsequently, stable CAD patients were enrolled in a prospective cohort, with periodic follow-ups to assess the occurrence of MACEs for prognostic analysis. All medication treatments and interventions were conducted in accordance with guidelines11. Major coronary stenosis exceeding 70% required stent implantation, and the decision for percutaneous coronary intervention (PCI) was based on coronary angiography results, ischemic symptoms, clinical evaluation, and patient preferences12.
Biochemical analysis and measurement of cMyBP-C level
Venous blood samples were collected from all participants after an overnight fast. Standard hematological and biochemical analyses for plasma hemoglobin, platelets, D-dimer, uric acid (UA), creatinine, and lipid profiles, including total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), were conducted at the Clinical Laboratory Center of the Second Affiliated Hospital of Nanjing Medical University. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) equation. Fasting arterial blood samples were obtained during cardiac catheterization, centrifuged at 3000 RPM for 15 min, and the collected plasma was stored at − 80 °C until blinded analysis in a specialized laboratory. Plasma cMyBP-C levels were determined using a commercially available colloidal gold immunochromatographic analyzer (cMyBP-C test kit, Botian Kezhi, Nanjing, China), with a detection range of 0–5000 pg/ml and an interassay coefficient of variation < 15%. The concentration of cardiac troponin I (cTnI) was determined using the enzyme-linked immunosorbent assay (ELISA) method, employing cTnI-specific antibodies from Nanjing Botian Kezhi for detection. cMyBP-C and cTnI levels are expressed in picograms per milliliter (pg/ml). The laboratory technicians measuring the concentrations of cMyBP-C and cTnI were blinded to the clinical data of the participants.
Assessment of CAD severity
Angiographic data were systematically collected and reviewed by two interventional cardiologists blinded to patients’ clinical and laboratory data. Experienced cardiologists calculated the GS for each patient in the stable CAD group. This scoring system allocates weights to different coronary artery segments based on the degree of luminal narrowing and the anatomical importance of the affected vessels. The total score is obtained by summing the individual scores for each segment, providing a comprehensive assessment of the CAD severity13. Subsequently, patients in the stable CAD group were stratified into 2 groups based on the median GS.
Follow-up and endpoints
In this study, all patients in the stable CAD group were followed by trained research staff who were blinded to the study’s objectives. These personnel consistently monitored patients’ medical records and obtained follow-up information through face-to-face or telephone communication every two months. Survival time was recorded from the initiation of the study until the occurrence of MACEs or completion of the 12-month follow-up period. The study endpoint was defined as MACEs, encompassing all-cause death, non-fatal myocardial infarction, unplanned revascularization, and hospitalization due to any cardiovascular cause. Five participants were lost to follow-up during the study, leaving a cohort of 190 patients with stable CAD for inclusion in the prognostic cohort (Fig. 1). Non-fatal myocardial infarction was defined as elevated cTn levels accompanied by symptoms of myocardial ischemia and/or characteristic changes in electrocardiogram14. Unplanned revascularization was defined as any PCI or coronary artery bypass grafting performed on a patient who was admitted due to recurrent angina-related symptoms during the follow-up period. Participant death was reported by family members, treating physicians, or retrieved from medical records. Endpoint events were adjudicated by experienced cardiologists blinded to cMyBP-C levels.
Statistical analysis
We employed histograms, quantile–quantile plots, and the Shapiro–Wilk test to assess the normality of the data. Continuous variables are presented as mean ± standard deviation or median (interquartile range), while categorical variables are expressed as numbers (%). For comparisons between two groups, independent sample t-tests were applied for normally distributed continuous variables, and the Mann–Whitney U test was employed for non-normally distributed ones. Chi-square tests were used for categorical variables. Binary logistic regression analysis was utilized to evaluate independent predictors for the presence of stable CAD. The diagnostic accuracy of cMyBP-C levels for stable CAD was validated using the area under the receiver operating characteristic curve (AUC). The optimal cMyBP-C cut-off concentration and corresponding sensitivity and specificity were derived from the receiver operating characteristic (ROC) curve based on the maximum Youden index. The association between cMyBP-C concentration and angiographic CAD severity was analyzed using the Pearson correlation coefficient. The stable CAD group was stratified into high GS (≥ 18, n = 101) and low GS (< 18, n = 94) based on the median GS. Variables significantly associated with high GS in univariate analysis were incorporated into a multivariate logistic regression model to identify independent predictors of high GS. Similarly, ROC analysis was utilized to evaluate the diagnostic precision of cMyBP-C levels for high GS. The prognostic cohort was divided into two groups based on the median cMyBP-C concentration: a high cMyBP-C concentration group (≥ 27 pg/ml, n = 98) and a low cMyBP-C concentration group (< 27 pg/ml, n = 92). Survival curves for the two groups during follow-up were generated using the Kaplan–Meier method, and inter-group comparisons were made using the log-rank test. Cox proportional hazards regression analysis was applied to investigate the risk of cMyBP-C levels for MACEs. The proportional hazards (PH) assumption of the Cox models was assessed using Schoenfeld residuals. Variables that violated the PH assumption were modeled using Cox models with time-dependent covariates. Given that the dichotomized cMyBP-C did not satisfy the PH assumption even after adjustments, an Accelerated Failure Time (AFT) model was employed to evaluate its prognostic significance. To explore the dose–response relationship between cMyBP-C and 1-year MACEs, we fitted univariate and multivariate logistic regression models using restricted cubic splines (RCS) with 4 knots. Statistical analysis was conducted using Python (version 3.9) and R (version 4.4.1). A two-tailed p-value of < 0.05 was considered statistically significant.
Results
Baseline characteristics of the study population
Table 1 presents the baseline characteristics of all 367 patients. In terms of demographic features, patients with stable CAD were older and more likely to be male or smokers. They were also more likely to have a history of hypertension, diabetes, or a family history of CVD. Clinical characteristics indicated that, compared to the non-CAD group, the stable CAD group exhibited lower platelet count, eGFR, and LVEF, while the level of D-dimer was higher. Additionally, cMyBP-C levels were significantly elevated in patients with stable CAD (P < 0.001), with no difference in cTnI levels between the two groups.
Elevated cMyBP-C independently predicts the presence of CAD
We explored the relationship between cMyBP-C levels and the presence of CAD. Univariate logistic regression analysis revealed that cMyBP-C levels served as a predictor of CAD presence, with an unadjusted odds ratio (OR) of 1.07 (95% confidence interval [CI] 1.05–1.08, P < 0.001). After adjusting for variables identified as significant in univariate analysis (age, male gender, smoking, hypertension, diabetes, family history of CVD, D-dimer, eGFR, and LVEF), multivariate logistic regression demonstrated that cMyBP-C (OR 1.06, 95% CI 1.05–1.08, P < 0.001), age, male gender, diabetes, and LVEF were independent predictors of CAD presence (Table 2). AUC was utilized to evaluate the predictive efficacy of cMyBP-C for CAD presence, with an AUC of 0.827 (95% CI 0.786–0.866; Fig. 2A). ROC curve analysis indicated a sensitivity of 70.3% and specificity of 81.4% at the optimal cMyBP-C cut-off value of 19.5 pg/ml for predicting CAD presence.
ROC curves for cMyBP-C. (A) ROC curve of cMyBP-C for diagnosing the presence of stable CAD. (B) ROC curve of cMyBP-C for diagnosing the severity of stable CAD. AUC, area under the curve; CAD, coronary artery disease; cMyBP-C, cardiac myosin-binding protein-C; ROC; receiver operating characteristic curve.
Elevated cMyBP-C independently predicts the angiographic CAD severity
Supplemental Table S1 summarizes the baseline characteristics of the stable CAD population stratified by the median GS. Compared to the low GS group, patients in the high GS group exhibited a significant elevation in cMyBP-C levels (P < 0.001). Meanwhile, there was a significant increase in D-dimer (P = 0.028), UA (P = 0.007), and TG (P = 0.021) with increasing GS, while HDL-C demonstrated a significant decrease (P = 0.002). Additionally, GS was negatively related to LVEF (P = 0.002). Pearson correlation analysis revealed a moderate positive correlation between plasma cMyBP-C levels and GS (r = 0.44, P < 0.001), whereas cTnI showed no significant correlation with GS (r = 0.10, P > 0.05). The correlation matrix with significance levels is depicted in Fig. 3.
Correlation matrix illustrating the relationships between clinical variables in stable CAD patients. The matrix combines scatter plots with linear regression lines and confidence intervals, histograms on the diagonal, and Pearson correlation coefficients. Statistical significance is denoted as follows: P < 0.05*, P < 0.01**, and P < 0.001***. BMI, body mass index; cMyBP-C, cardiac myosin-binding protein-C; cTnI, cardiac troponin I; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; TC, total cholesterol; TG, triglycerides; UA, uric acid.
To further explore the relationship between cMyBP-C, other major CVD risk factors, and GS, binary logistic regression analysis was conducted (Table 3). The results demonstrated that cMyBP-C levels were positively associated with high GS in both univariate (OR 1.03, 95% CI 1.02–1.04, P < 0.001) and multivariate analyses (adjusted OR 1.03, 95% CI 1.02–1.04, P < 0.001), establishing it as an independent predictor of angiographic CAD severity. In contrast, HDL-C and LVEF exhibited negative correlations with high GS in both univariate and multivariate analyses (P < 0.05, respectively). Additionally, UA and TG were only significantly and positively correlated with high GS in univariate analysis (both with P < 0.05). ROC curve analysis indicated that the optimal diagnostic cut-off value for cMyBP-C in predicting GS ≥ 18 was 23.5 pg/ml, with a sensitivity of 80.2% and specificity of 68.1%. The diagnostic accuracy of cMyBP-C for high GS, quantified by the AUC, was 0.778 (95% CI 0.708–0.841; Fig. 2B).
Elevated cMyBP-C independently predicts 1-year MACEs
During the follow-up period, five participants (2.6%) were lost to follow-up, all of whom were in the high cMyBP-C group (≥ 27 pg/ml). Over the 12-month follow-up, 56 patients (29.5%) reached the study endpoint (MACEs), including 2 deaths, 3 non-fatal myocardial infarctions, 21 unplanned revascularizations, and 30 hospitalizations due to cardiovascular causes. Supplemental Table S2 illustrates the baseline characteristics of patients based on their survival status during the follow-up. Compared to the non-event group, patients in the event group had significantly higher plasma cMyBP-C levels (P = 0.001), higher GS (P < 0.001), and significantly lower LVEF (P = 0.021).
Kaplan–Meier survival analysis revealed a significantly higher cumulative incidence of 1-year MACEs in the high cMyBP-C group (≥ 27 pg/ml) compared to the low cMyBP-C group (< 27 pg/ml) (Log rank P = 0.0014; Fig. 4).
Kaplan–Meier survival curves depicting the association between dichotomized cMyBP-C levels and 1-year MACEs in patients with stable CAD. The curve illustrates the significantly increased risk of 1-year MACEs in patients with cMyBP-C concentrations above the median (cMyBP-C ≥ 27 pg/ml) compared to the low cMyBP-C concentration group (cMyBP-C < 27 pg/ml) (Log-rank P = 0.0014). CAD, coronary artery disease; cMyBP-C, cardiac myosin-binding protein-C; MACEs, major adverse cardiovascular events.
To evaluate the independent prognostic value of cMyBP-C levels for MACEs, we initially conducted Cox proportional hazards regression analyses (Supplemental Table S3). However, the PH assumption was formally tested for the dichotomized cMyBP-C variable. The Schoenfeld residual analysis (Fig. 5A) revealed a clear violation of this assumption. Visually, the smoothed residual line exhibited a significant positive slope over time, rather than the expected horizontal line around zero. This observation was statistically confirmed by the global test, which yielded highly significant p-values (P < 0.001). This indicates that the effect of dichotomized cMyBP-C on MACEs risk is not constant over the follow-up period. An attempt to address this by modeling a time-dependent covariate interaction also failed to satisfy the PH assumption (Fig. 5B, P < 0.001). Given this persistent violation, we concluded that the Cox proportional hazards model was not appropriate for this analysis.
Scaled Schoenfeld residuals for dichotomized cMyBP-C in multivariate and time-dependent Cox models. (A) Scaled Schoenfeld residuals for the dichotomized cMyBP-C group in the multivariate Cox regression model. (B) Scaled Schoenfeld residuals for the dichotomized cMyBP-C group in the time-dependent Cox regression model. Both models demonstrate significant violations of the PH assumption for dichotomized cMyBP-C (A: P = 0.0007; B: P < 0.0001). cMyBP-C, cardiac myosin-binding protein-C; PH, proportional hazards.
Consequently, an AFT model was employed to assess the prognostic significance of cMyBP-C levels. The Weibull AFT model demonstrated that a high cMyBP-C level was significantly associated with a shorter time to MACEs (Acceleration Factor [AF] 0.66, 95% CI 0.46–0.94, P = 0.02). An AF < 1 indicates that the event time was accelerated, signifying a worse prognosis for patients in the high cMyBP-C group. Other variables included in the AFT model did not show significant associations with MACEs (all P > 0.05; Table 4).
To assess the robustness of our findings, a multivariate logistic regression analysis was performed. This confirmed that a high cMyBP-C level, when dichotomized, remained a strong and independent predictor for 1-year MACEs after adjusting for diabetes, TC, and LVEF (OR 2.92, 95% CI 1.14–7.52, P = 0.026; Supplemental Table S4).
To further understand the underlying dose–response relationship, we conducted RCS analyses. The univariate RCS analysis revealed a significant, non-linear association (P = 0.018, Fig. 6A), with the risk curve plateauing at higher concentrations. Subsequently, in a multivariate RCS model adjusting for the same confounders, the overall association for the continuous cMyBP-C variable was attenuated and no longer statistically significant (P = 0.088, Fig. 6B). This contrast underscores that our primary dichotomized approach was a more statistically powerful method for capturing the prognostic signal in this multivariate context, providing a clear rationale for its use.
Univariate and multivariate dose–response relationship between cMyBP-C levels and the risk of 1-year MACEs. The curves show the estimated log odds of 1-year MACEs according to cMyBP-C concentration, based on restricted cubic spline analyses. (A) The univariate model reveals a significant non-linear association (P = 0.018). (B) The multivariate model, adjusted for diabetes, total cholesterol (TC), and left ventricular ejection fraction (LVEF), shows an attenuated association that did not reach statistical significance (P = 0.088). Both models were fitted using 4 knots placed at the 5th, 35th, 65th, and 95th percentiles of the cMyBP-C distribution. In both panels, the solid line represents the estimated log odds, and the shaded area represents the 95% confidence interval (CI). CI, confidence interval; cMyBP-C, cardiac myosin-binding protein-C; LVEF, left ventricular ejection fraction; MACEs, major adverse cardiovascular events; TC, total cholesterol.
Discussion
In this cross-sectional and prospective clinical study on patients undergoing coronary angiography for suspected CAD, we report four major findings. First, cMyBP-C concentrations were significantly elevated in patients with stable CAD, whereas conventional cTnI levels were not. This suggests that cMyBP-C may be a more sensitive biomarker for detecting the chronic, low-grade myocardial injury or stress characteristic of stable CAD compared to traditional troponins15,16. Second, cMyBP-C demonstrated promising diagnostic performance for CAD, with high specificity at 81.4%, indicating its practical clinical significance for early screening in high-risk individuals. Third, a positive correlation was observed between cMyBP-C concentration and CAD severity. Subsequent analyses further confirmed cMyBP-C as a strong predictor of advanced coronary artery lesions, suggesting its utility in improving risk stratification for CAD. Fourth, elevated cMyBP-C levels were independently associated with an increased risk of 1-year MACEs in stable CAD patients, as confirmed by the AFT model. This finding underscores the prognostic significance of cMyBP-C in predicting adverse cardiovascular outcomes, highlighting its potential to identify high-risk patients who may benefit from more intensive monitoring and therapeutic interventions.
The association between cMyBP-C and stable CAD has been relatively unexplored, with previous research predominantly focusing on its utility in ACS. In animal models of AMI, cMyBP-C levels exhibit a prompt elevation 30 min after left anterior descending coronary artery ligation, reaching a significant peak at 6 h17. Kaier et al. conducted a prospective diagnostic cohort study, revealing that cMyBP-C aids in risk stratification of chest pain patients and significantly enhances the pre-hospital diagnosis of AMI (average chest pain duration of 70 min), with cMyBP-C’s AUC at 0.839 compared to hs-cTn’s AUC at 0.813, P = 0.005. Furthermore, cMyBP-C demonstrates distinct concentration distributions in various presentations of ACS, with an average concentration of 306 pg/ml in ST-segment elevation myocardial infarction compared to 88 pg/ml in non-ST-segment elevation myocardial infarction and 19 pg/ml in unstable angina6. Considering the cardiac-specific domains of cMyBP-C, these findings suggest that cMyBP-C serves not only as a sensitive and cardiac-specific biomarker for the accurate diagnosis of ACS but also holds potential for assessing the severity of myocardial ischemic injury18. It is this high degree of cardiac specificity that becomes particularly salient when positioning cMyBP-C within the broader landscape of prognostic biomarkers. For instance, Growth Differentiation Factor-15 is well-established as a potent predictor of adverse outcomes in CAD, yet its clinical interpretation can be confounded by its role as a general marker of systemic inflammation and stress, being elevated in numerous non-cardiac conditions19,20. Similarly, the role of other biomarkers can be complex and controversial. A notable example is Growth Differentiation Factor 11 (GDF-11), another member of the transforming growth factor-β superfamily, which was initially suggested to have cardioprotective effects. However, recent evidence challenges this notion, demonstrating that high circulating levels of GDF-11 are associated with increased myocardial infarct size in humans and may even exacerbate myocardial injury21. In this context, cMyBP-C’s exclusive origin from cardiomyocytes means that its presence in the circulation of patients with stable CAD provides a more direct and unambiguous signal of ongoing, low-grade myocardial injury, rather than reflecting a non-specific systemic state. Our data build upon this by demonstrating that cMyBP-C is an independent predictor for both the presence and severity of stable CAD, positioning it as a potentially more targeted, cardiac-specific biomarker. Correlation analysis further confirms a positive association between cMyBP-C concentration and the quantitative severity of coronary stenosis determined by coronary angiogram. It is noteworthy that elevated circulating cMyBP-C levels do not necessarily imply myocardial cell death; rather, increased cardiac metabolic demand may lead to cMyBP-C cleavage and release22. Additionally, impaired coronary microcirculation could be a potential cause of elevated cMyBP-C levels, potentially explaining the observed association between more severe CAD and elevated cMyBP-C levels. Clinically, our study underscores that cMyBP-C is a valuable biomarker for screening high-risk CAD individuals and predicting the severity of CAD, underscoring its potential utility in clinical management.
Cumulative research has affirmed the prognostic significance of cMyBP-C for cardiovascular outcome. Lynch et al. identified a negative correlation between cMyBP-C levels and cardiovascular function in ACS patients, establishing it as an early biomarker for predicting cardiac function and patient outcome following ischemic injury23. Kaier et al. found that over a 2-year follow-up, cMyBP-C independently correlated with non-fatal AMI and all-cause mortality in AMI patients, aiding in prognostic risk assessment6. Moreover, a prospective multicenter cohort study confirmed cMyBP-C as an independent predictor of death in acute heart failure (AHF) patients (HR [hazard ratio] 2.19, 95% CI 1.66–2.89, P < 0.001) and of the composite endpoint of all-cause mortality and AHF rehospitalization (HR 1.63, 95% CI 1.31–2.02, P < 0.001), with prognostic accuracy comparable to N-terminal pro-B-type natriuretic peptide (NT-proBNP) and hs-cTn24. Consistent with prior studies, researchers have validated circulating cMyBP-C as a valuable predictor of clinical outcomes in a 7-year follow-up study of a baseline non-CVD population5. However, to date, there is a lack of research on the association between cMyBP-C and cardiovascular outcomes in stable CAD patients, prompting the design of our prospective cohort study. In our study, dichotomized cMyBP-C levels were independently associated with an increased risk of 1-year MACEs in stable CAD patients. To address concerns regarding the potential impact of the five patients lost to follow-up on our findings, we conducted sensitivity analyses. These analyses assumed both scenarios: all five patients experienced MACEs and none did during the follow-up period. We assigned the observed median follow-up time of the cMyBP-C ≥ 27 pg/ml group to these patients. The sensitivity analyses consistently indicated that the exclusion of these five patients did not materially alter the validity of our reported associations between dichotomized cMyBP-C levels and patient prognosis. While our observational study cannot establish a new therapeutic paradigm, it suggests that elevated cMyBP-C could identify high-risk patients who may warrant more intensive risk factor management, optimization of guideline-directed therapies, and shorter follow-up intervals to detect any progression of symptoms or adverse events early.
A key methodological finding of our study is the robust justification for our dichotomized analysis of cMyBP-C. The RCS analysis provides a compelling statistical rationale for this strategy. First, the univariate analysis revealed a pronounced non-linear relationship, which violates the linearity assumption of standard regression models and explains why a simple continuous term may fail10,22. More importantly, while the continuous association was attenuated in the multivariate RCS model, our dichotomized variable remained a robustly significant predictor within the identical adjusted model. This discrepancy highlights that dichotomization at 27 pg/mL, while a simplification, was a more statistically powerful and robust method for this dataset. It effectively captured the most salient feature of the risk curve, the substantial shift from a low-risk to a high-risk state, without being constrained by the assumption of linearity.
Our study has several limitations. Firstly, as a single-center investigation without an external validation cohort, the generalizability of our findings is limited. To mitigate this and to rigorously test the stability of our results, we conducted an internal validation using a 1,000-replicate bootstrapping procedure. This analysis confirmed the robustness of our primary prognostic finding, yielding a bootstrapped 95% CI of 1.22–10.77 for the adjusted OR of high cMyBP-C. Therefore, while the specific cut-off value we identified should be considered hypothesis-generating, our data provide strong, internally-validated evidence for the fundamental association between elevated cMyBP-C and adverse outcomes. Future validation in larger, multicenter populations remains essential before this biomarker can be considered for broad clinical implementation. Secondly, the selection and exclusion criteria may introduce a degree of selection bias. Specifically, our findings are primarily applicable to patients presenting with chest pain symptoms who were referred for coronary angiography, which may reduce their generalizability to asymptomatic individuals or broader population screening applications. Thirdly, our reliance on coronary angiography for CAD severity assessment, while standardized, may not capture the full picture of coronary pathophysiology, such as the impact of microvascular disease on cMyBP-C concentrations. Furthermore, the definition of severe CAD based on the median Gensini score, while practical, lacks a universally accepted threshold and may constrain the wider applicability of these specific results. Fourthly, our study did not include patients with end-stage renal disease requiring chronic hemodialysis. This is a critical group with a high burden of both cardiovascular disease and potentially altered biomarker levels, and the performance of cMyBP-C in this population remains to be explored.
Conclusion
In conclusion, this study identifies circulating cMyBP-C as an independent predictor for both the presence and angiographic severity of stable CAD. Furthermore, in patients with established stable CAD, an elevated cMyBP-C level, analyzed as a dichotomous variable, is independently associated with an increased risk of 1-year MACEs. These findings highlight the potential of cMyBP-C as a valuable biomarker to improve risk stratification and inform clinical management in this patient population.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Visseren, F. L. et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 42, 3227–3337 (2021).
Ambrose, J. A. et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J. Am. Coll. Cardiol. 12, 56–62 (1988).
Lynch, T. L. & Sadayappan, S. Surviving the infarct: A profile of cardiac myosin binding protein-C pathogenicity, diagnostic utility, and proteomics in the ischemic myocardium. PROTEOMICS-Clin. Appl. 8, 569–577 (2014).
Baker, J. O. et al. Cardiac myosin-binding protein C: a potential early biomarker of myocardial injury. Basic Res. Cardiol. 110, 1–14 (2015).
Shen, X. et al. Development and validation of a nomogram to predict the future risk of cardiovascular disease. Rev. Cardiovasc. Med. 24, 35 (2023).
Kaier, T. E. et al. Cardiac myosin-binding protein c to diagnose acute myocardial infarction in the pre-hospital setting. J. Am. Heart Assoc. 8, e013152 (2019).
Kaier, T. E. et al. Direct comparison of cardiac myosin-binding protein C with cardiac troponins for the early diagnosis of acute myocardial infarction. Circulation 136, 1495–1508 (2017).
Kaier, T. E. et al. A 0/1h-algorithm using cardiac myosin-binding protein C for early diagnosis of myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 11, 325–335 (2022).
Kaier, T. E., Alaour, B. & Marber, M. Cardiac myosin-binding protein C—from bench to improved diagnosis of acute myocardial infarction. Cardiovasc. Drugs Ther. 33, 221–230 (2019).
Karakayali, M. et al. Serum malondialdehyde levels at admission as a predictor of inhospital mortality in patients with acute coronary syndrome. Coron. Artery Dis. 36, 211–217 (2025).
Knuuti, J. et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The task force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 41, 407–477 (2020).
Thygesen, K. et al. Third universal definition of myocardial infarction. Circulation 126, 2020–2035 (2012).
Gensini, G. G. A more meaningful scoring system for determining the severity of coronary heart disease. Am J cardiol 51, 606 (1983).
Byrne, R. A. et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 44, 3720–3826 (2023).
Samman Tahhan, A. et al. High-sensitivity troponin I levels and coronary artery disease severity, progression, and long-term outcomes. J. Am. Heart Assoc. 7, e007914 (2018).
Kaier, T. E., Anand, A., Shah, A. S., Mills, N. L. & Marber, M. Temporal relationship between cardiac myosin-binding protein C and cardiac troponin I in type 1 myocardial infarction. Clin. Chem. 62, 1153–1155 (2016).
Kuster, D. W. et al. Release kinetics of circulating cardiac myosin binding protein-C following cardiac injury. Am. J. Physiol.-Heart Circul. Physiol. 306, H547–H556 (2014).
Govindan, S. et al. Increase in cardiac myosin binding protein-C plasma levels is a sensitive and cardiac-specific biomarker of myocardial infarction. Am. J. Cardiovasc. Dis. 3, 60 (2013).
Li, M. et al. Growth differentiation factor-15 is associated with cardiovascular outcomes in patients with coronary artery disease. Cardiovasc. Diabetol. 19, 120 (2020).
Wang, J., Wei, L., Yang, X. & Zhong, J. Roles of growth differentiation factor 15 in atherosclerosis and coronary artery disease. J. Am. Heart Assoc. 8, e012826 (2019).
Kraler, S. et al. Circulating GDF11 exacerbates myocardial injury in mice and associates with increased infarct size in humans. Cardiovasc. Res. 119, 2729–2742 (2023).
Tong, C. W. et al. Usefulness of released cardiac myosin binding protein-C as a predictor of cardiovascular events. Am. J. Cardiol. 120, 1501–1507 (2017).
Lynch, T. L. IV. et al. Cardiac myosin binding protein-C autoantibodies are potential early indicators of cardiac dysfunction and patient outcome in acute coronary syndrome. Basic to Translational Science 2, 122–131 (2017).
Kozhuharov, N. et al. Cardiac myosin-binding protein C in the diagnosis and risk stratification of acute heart failure. Eur. J. Heart Fail. 23, 716–725 (2021).
Funding
This study was funded by Beijing Hengji Health Management Development Foundation.
Author information
Authors and Affiliations
Contributions
X.C.S.—methodology, software, formal analysis and writing. J.Y.S.—software and formal analysis. Q.H.L.—software and formal analysis. Y.S.—Conceptualization and investigation. C.W.H.—investigation and data curation. H.C.Z.—investigation and data curation. M.X.Z.—investigation and data curation. Y.P.W.—investigation and data curation. R.Y.S.—investigation and data curation. H.Y.Z.—Conceptualization, formal analysis and project administration. M.Z.L.—Conceptualization, data curation and project administration.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shen, X., Sun, J., Long, Q. et al. Cardiac myosin-binding protein-C levels are associated with severity and prognosis in stable coronary artery disease. Sci Rep 15, 31768 (2025). https://doi.org/10.1038/s41598-025-16134-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16134-x