Abstract
Heart failure is a significant global health challenge with high mortality rates. This study examines the association between glycemic variability and short-term mortality in critically ill heart failure patients. Data from the eICU Collaborative Research Database (eICU-CRD) and the Medical Information Mart for Intensive Care (MIMIC-IV) database were analyzed, including 23,744 heart failure patients. Glycemic variability, measured by the coefficient of variation of glucose levels during ICU stay, was categorized into quartiles. Multivariable logistic regression and Cox proportional hazards models were used to assess associations with in-hospital and 30-day mortality. Linear regression models were employed to evaluate the association with ICU length of stay. Dose-response relationships were explored using restricted cubic splines. The in-hospital and 30-day mortality rates were 15.0% and 17.6%, respectively. The median ICU length of stay was 3.1 days (IQR: 1.9–5.4). Patients in the highest glycemic variability quartile had a significantly higher risk of in-hospital mortality (OR: 1.77, 95% CI: 1.54–2.04) and 30-day mortality (HR: 1.37, 95% CI: 1.23–1.53) compared to the lowest quartile. Additionally, higher glycemic variability was associated with prolonged ICU stays, with each unit increase resulting in a 2.57-day extension (95% CI: 2.03–3.10, P < 0.001) after adjustment for covariates. A U-shaped association was observed for in-hospital mortality, while a linear relationship was seen for 30-day mortality. Sensitivity and subgroup analyses confirmed the robustness of these findings. Elevated glycemic variability is independently associated with increased short-term mortality and prolonged ICU stays in critically ill heart failure patients, highlighting the importance of managing blood glucose fluctuations to improve outcomes and reduce healthcare resource utilization.
Similar content being viewed by others
Introduction
Heart failure (HF) represents a major global health challenge, characterized by the heart’s reduced capability to effectively circulate blood. This condition arises from either systolic dysfunction, where the heart’s contraction is impaired, or diastolic dysfunction, where the heart does not fill adequately, both of which lead to inadequate metabolic supply12. According to the World Health Organization, HF currently affects 64.3 million people globally, adding significant strain on healthcare systems as the prevalence continues to rise34. HF is most commonly caused by conditions such as coronary artery disease, hypertension, cardiomyopathy, and valvular diseases.
Glycemic variability (GV) describes the oscillations in blood glucose levels, commonly measured by standard deviation or coefficient of variation. Increasingly, GV is recognized for its potential impact on cardiovascular health due to its relationship with oxidative stress and inflammation5. It plays a particularly critical role in diabetes management, as elevated GV levels heighten the risk of various complications67. Research has also shown a correlation between increased GV and worse outcomes in conditions like acute coronary syndrome8 and myocardial infarction910. In intensive care unit (ICU) settings, elevated GV has been associated with increased mortality and prolonged hospital stays11. Moreover, GV has been implicated in promoting left ventricular remodeling, fibrosis, and impaired diastolic function, thereby complicating existing cardiovascular conditions12,13,14,15.
The association between GV and mortality in HF patients is gaining attention, with evidence suggesting that those with higher GV have an increased risk of short-term mortality, particularly in non-diabetic populations1617. However, there is inconsistency in the findings across various studies due to differences in patient characteristics and GV measurement methods. These variations contribute to discrepancies in the conclusions. Although the exact mechanisms remain unclear, oxidative stress, inflammation, and endothelial dysfunction have been proposed as critical pathways through which GV affects prognosis.
However, the inconsistencies across studies regarding GV’s impact on prognosis arise from differences in study design and measurement techniques. The biological pathways by which GV influences prognosis, while not fully understood, likely involve oxidative stress, inflammatory processes, and endothelial dysfunction. Additionally, many studies suffer from small sample sizes or focus on specific subgroups, limiting the generalizability of their findings.
This study aims to assess the relationship between GV and mortality among HF patients, drawing data from the eICU Collaborative Research Database (eICU-CRD) and the Medical Information Mart for Intensive Care IV (MIMIC-IV) database. By utilizing these large, high-quality datasets, this research aims to enhance understanding of how GV affects HF outcomes, ultimately contributing to the development of glucose management strategies and setting the stage for future investigations into GV’s role in HF prognosis.
Materials and methods
Data sources
This retrospective cohort study utilized data from the eICU-CRD and MIMIC-IV databases (version 3.1). The eICU-CRD database contains historical data on more than 200,000 ICU patients from over 200 medical centers, recorded between 2014 and 201518. The MIMIC-IV database includes critical care information for 94,458 patients admitted to ICUs at Beth Israel Deaconess Medical Center (Boston, MA, USA) from 2008 to 202219.
Access to the databases was granted upon successful completion of the Collaborative Institutional Training Initiative certification (Certification numbers: 52219361 for Tang). Given the retrospective nature of this study and the use of publicly available data, informed consent was waived. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines20.
Study population and data exclusion
Upon receiving approval, the databases were downloaded and analyzed using Structured Query Language (SQL)21 with PostgreSQL (version 13.0) for database management and Navicat software (version 16.0) for data querying.
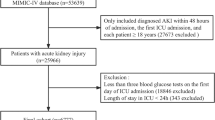
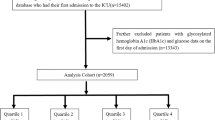
Patients admitted to the ICU for the first time were identified from the eICU-CRD and MIMIC-IV databases. The inclusion criteria involved adult patients diagnosed with heart failure, as defined by the International Classification of Diseases, Ninth or Tenth Revision (ICD-9 or ICD-10; see Table S1). Exclusion criteria included patients under 18 years of age, ICU stays shorter than 24 h (due to insufficient data for meaningful analysis), and fewer than three glucose measurements during the entire ICU stay (to ensure reliable glycemic variability assessment). After applying these criteria, 11,037 patients from the eICU-CRD database and 12,707 patients from the MIMIC-IV database remained, resulting in a final cohort of 23,744 patients for analysis (Fig. 1).
Clinical data collection
The following variables were included for all participants based on established literature and clinical expertise: (1) Demographic characteristics: age, gender, and ethnicity. (2) Physical examination parameters: heart rate, mean blood pressure (MBP), and body mass index (BMI), calculated as weight (kg) divided by height (m²). (3) Comorbidities: hypertension, diabetes, myocardial infarction, atrial fibrillation, chronic obstructive pulmonary disease (COPD), renal failure, acute kidney injury (AKI), severe liver disease, and cancer. (4) Laboratory tests: initial measurements after ICU admission, including glucose, hemoglobin, white blood cell (WBC) count, platelet count, creatinine, blood urea nitrogen (BUN), potassium, sodium, chloride, calcium, magnesium, and left ventricular ejection fraction (LVEF). (5) Treatments: angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB), beta-blockers, anti-platelet drugs, diuretics, digoxin, statins, insulin, oral antidiabetic agents, hormone therapies, vasoactive drugs (e.g., dobutamine, dopamine, vasopressin, phenylephrine, norepinephrine, nitrate, nesiritide, epinephrine), enteral nutrition support, parenteral nutrition support, device therapy (including implantable cardioverter-defibrillator [ICD], cardiac resynchronization therapy [CRT]), hemodialysis, and mechanical ventilation.
We also included the Sequential Organ Failure Assessment (SOFA) score, which evaluates organ function across six systems: respiratory, coagulation, liver, cardiovascular, central nervous, and renal, with scores ranging from 0 to 4, where higher scores indicate greater organ dysfunction. The SOFA score is assessed daily and is used to monitor the progression of organ failure22. The Simplified Acute Physiology Score II (SAPS II) was employed to assess illness severity and estimate the risk of in-hospital mortality. SAPS II incorporates 17 variables, including patient age, vital signs, laboratory values, and relevant clinical history. Higher SAPS II scores reflect greater disease severity and are associated with an increased risk of hospital mortality23.
Mean blood glucose for each subject was calculated using all available glucose measurements from the first day of ICU admission. GV was assessed by computing the coefficient of variation (CV) for glucose concentrations, defined as the ratio of the standard deviation (SD) to the mean blood glucose. GV values were stratified into quartiles. In our analysis, GV was evaluated both as a continuous variable and as a categorical variable using quartiles, with the first quartile serving as the reference.
Outcomes
The primary outcome of this study was in-hospital and 30-day mortality from the time of ICU admission. Secondary outcome was ICU length of stay (LOS).
Statistical analysis
The normality of the variable distributions was examined through histograms, Q-Q plots, and the Kolmogorov-Smirnov test. Continuous variables that followed a normal distribution were presented as means with standard deviation (SD), whereas those that did not follow a normal distribution were described using medians and interquartile ranges (IQR). Categorical variables were summarized by their frequencies and percentages. For comparing continuous variables between groups, either the independent samples Student’s t-test or the Mann-Whitney U test was used, based on the distribution of the data. Categorical data were compared using either the chi-square test or Fisher’s exact test, depending on the expected frequencies.
Logistic regression models were used to estimate odds ratios (ORs) with corresponding 95% confidence intervals (CIs) to assess the relationship between GV, and linear regression models assessed the association with ICU LOS. For the analysis of 30-day mortality, Cox proportional hazards models were employed, providing hazard ratios (HRs) and 95% CIs. To verify the proportional hazards assumption, log-log plots were checked, and interaction terms with survival time were incorporated where necessary. Kaplan-Meier survival curves, categorized by GV quartiles, were compared using the log-rank test.
Potential confounders were identified based on clinical relevance, previous studies, statistical significance in univariate analysis, or if they resulted in a greater than 10% change in effect size. A total of five models were built: Model 1 was unadjusted; Model 2 adjusted for demographic factors; Model 3 further included vital signs and comorbidities; Model 4 additionally incorporated laboratory measurements; and Model 5, the primary model, included treatment-related variables. Stratified and interaction analyses were conducted across subgroups of interest.
To address missing data, multiple imputation using chained equations was applied with five imputations, based on the method described by van Buuren and Groothuis-Oudshoorn (2011)24which was executed using the R package mice. This approach was employed to enhance statistical power and mitigate bias resulting from missing data. The percentage of missing data for some variables was less than 20%, as detailed in Table S2. Additionally, comprehensive sensitivity analyses were conducted to assess the robustness of the main findings. These analyses included: (1) complete-case analysis after excluding patients with missing data; (2) adjustment for glucose measurement frequency to account for potential measurement bias; (3) inclusion of SAPS II scores as an additional severity adjustment; (4) exclusion of patients receiving device therapy; (5) exclusion of patients with oral antidiabetic agent usage; and (6) exclusion of patients with severe liver disease.
To assess the potential influence of unmeasured confounding, we performed a quantitative bias analysis using the E-value methodology as proposed by VanderWeele and Ding25. The E-value represents the minimum strength of association that an unmeasured confounder would need to have with both the exposure (GV) and the outcome (30-day mortality) to fully explain away the observed association. This provides a sensitivity analysis framework for interpreting the robustness of our findings to potential residual confounding.
All statistical analyses were performed using R Statistical Software (version 4.2.2, available at http://www.R-project.org, The R Foundation) and the Free Statistics Analysis Platform (version 2.0, Beijing, China, http://www.clinicalscientists.cn/freestatistics). Statistical significance was set at a two-sided P-value below 0.05.
Results
Baseline characteristics of study participants
Table 1 outlines the baseline characteristics of critically ill patients with HF, categorized by GV quartiles. The study included a total of 23,744 patients, 54.8% of whom were male, with an average age of 71.6 ± 13.5 years. It was observed that as GV increased, the mean age of patients tended to decrease, and the proportion of males slightly reduced. Higher GV was linked to increased heart rates and elevated SOFA score, suggesting more severe illness. Additionally, a higher prevalence of diabetes, myocardial infarction, and renal failure was noted among patients with elevated GV. These patients also had higher initial glucose levels, white blood cell counts, blood urea nitrogen, and creatinine levels. The proportion of patients undergoing hemodialysis increased with GV. Additional baseline characteristics and covariates used in sensitivity analyses are presented in Table S3.
Association between GV and mortality
Mortality analysis during the follow-up period showed overall in-hospital and 30-day mortality rates of 15.0% and 17.6%, respectively. Significant differences in survival curves were identified, with patients in the highest GV quartile demonstrating substantially lower 30-day survival rates compared to those with lower GV (Fig. 2, P < 0.001).
Treating GV as a continuous variable, multivariable logistic regression analysis indicated that each unit increase in GV was associated with an increased risk of in-hospital mortality (OR: 3.21, 95% CI: 2.38, 4.33). Patients in the highest GV quartile had significantly higher odds of in-hospital mortality compared to those in the lowest quartile (OR: 1.77, 95% CI: 1.54–2.04), as presented in Table 2.
To evaluate the independent effect of GV on 30-day mortality in critically ill heart failure patients, five multivariate Cox regression models were employed. The results indicated that higher GV was linked to increased risks of 30-day (HR: 1.95, 95% CI: 1.58, 2.40). Specifically, patients in the highest GV quartile showed a 1.37-fold (95% CI: 1.23, 1.52) increased risk of 30-day mortality compared to those in the lowest quartile (Table 2, Model 5).
Adjusted smoothed plots demonstrated a non-linear, U-shaped relationship between GV and in-hospital mortality (Fig. 3A; P for non-linearity < 0.001), with the highest 0.5% of data points for each GV measure excluded. In contrast, a linear relationship was observed between GV and 30-day mortality (Fig. 3B; P for non-linearity = 0.151), also excluding the highest 0.5% of data points. Elevated GV levels were associated with an increased risk of both in-hospital and 30-day mortality, suggesting that larger fluctuations in blood glucose are indicative of poorer clinical outcomes.
Sensitivity and subgroup analysis
The sensitivity analysis results align with the initial findings, confirming that the association between GV and mortality (both in-hospital and 30-day) remains significant (Table 3). This reinforces the independent effect of GV on adverse clinical outcomes, particularly highlighting the elevated risk of mortality in patients with the highest GV levels.
Subgroup analyses, including stratification by database, age, gender, race, BMI, myocardial infarction, renal failure, AKI, diabetes, AF, cancer, enteral nutrition support, parenteral nutrition support, LVEF (≤ 50 vs.> 50), and primary diagnosis (Figure S1) were conducted to further explore the relationship between GV and in-hospital mortality (Figure S2) and 30-day mortality (Figure S3). These analyses revealed no significant effect modification or interaction for most subgroups, as indicated by P values for interaction > 0.05. However, in the diabetes subgroup, a significant interaction was observed for in-hospital mortality (P = 0.005), indicating that the association between GV and in-hospital mortality may differ in patients with diabetes.
The E-value for the observed association between high GV and 30-day mortality was 2.08. This indicates that an unmeasured confounder would need to be associated with both GV and 30-day mortality by a risk ratio of at least 2.08, beyond the measured covariates, to explain away the observed effect. For the lower bound of the 95% confidence interval (HR = 1.76), the minimum required risk ratio would be 1.76 (Figure S4). These results suggest that only unmeasured confounders with moderately strong associations could potentially account for the findings.
Association between GV and ICU LOS
Linear regression analysis demonstrated a significant positive association between GV and ICU LOS (Fig. 4). In the crude regression model, each unit increase in glycemic variability was associated with a 3.29-day prolongation in ICU length of stay (95% CI: 2.82–3.77, P < 0.001). After adjustment for covariates including demographics, vital signs, comorbidities, laboratory tests, and treatments, this association remained statistically significant with an adjusted coefficient of 2.57 (95% CI: 2.03–3.10, P < 0.001).
When GV was analyzed by quartiles, all quartile groups showed significantly prolonged ICU LOS compared to the lowest quartile (Q1). In the adjusted model, the regression coefficients were 1.62 (95% CI: 1.40–1.84, P < 0.001) for Q2, 2.07 (95% CI: 1.86–2.29, P < 0.001) for Q3, and 1.69 (95% CI: 1.46–1.91, P < 0.001) for Q4. These results demonstrated a dose-response relationship between glycemic variability and ICU length of stay, with higher glycemic variability associated with longer ICU stays.
Discussion
In this retrospective multi-cohort study, we found that elevated GV was independently associated with higher in-hospital, 30-day mortality and prolonged ICU length of stay in critically ill patients with heart failure. Specifically, patients in the highest GV quartile demonstrated significantly increased odds of both in-hospital (OR: 1.77, 95% CI: 1.54–2.04) and 30-day mortality (HR: 1.37, 95% CI: 1.23–1.53) compared to those in the lowest quartile. Additionally, higher GV was associated with extended ICU stays, with each unit increase in GV resulting in a 2.57-day prolongation of ICU length of stay (95% CI: 2.03–3.10, P < 0.001) after adjustment for covariates. A non-linear, U-shaped relationship was observed between GV and in-hospital mortality, suggesting that both low and high GV levels contribute to increased mortality risk. Conversely, a linear association was found between GV and 30-day mortality, indicating that progressively higher GV is directly proportional to worse outcomes at 30 days. These findings remained robust even after adjusting for potential confounders and conducting sensitivity analyses to account for missing data. Subgroup analyses further supported the consistency of the results across different patient characteristics. However, in the diabetes subgroup, a significant interaction was identified specifically for in-hospital mortality (P = 0.005), suggesting that the relationship between GV and mortality may be more pronounced in none-diabetic patients.
Temporal dynamics of mortality outcomes
The differential patterns between in-hospital and 30-day mortality provide important insights into glycemic variability’s temporal impact. In-hospital mortality (15.0%) was lower than 30-day mortality (17.6%), with 85.5% of 30-day deaths occurring during hospitalization, indicating concentrated acute-phase risk.
The dose-response relationships differed markedly between endpoints. The U-shaped relationship for in-hospital mortality (P for non-linearity < 0.001) suggests both extremely low variability—reflecting over-aggressive control—and high variability are acutely harmful. Conversely, the linear 30-day mortality relationship (P for non-linearity = 0.151) indicates cumulative metabolic instability effects extending beyond hospitalization.
The stronger in-hospital effect size (OR: 1.77 vs. HR: 1.37) reflects more pronounced immediate impact through acute glucose fluctuation complications. These temporal differences suggest acute care should avoid glucose control extremes, while longer-term management should emphasize consistent stability.
Clinical interpretation: disease severity marker versus independent risk factor
A fundamental question is whether elevated GV represents a disease severity marker or an independent risk factor. Our findings suggest both aspects. While GV correlates with SOFA scores (Q4: 5.0 vs. Q1: 4.0) and treatment intensity (insulin use: Q1 36.4% to Q4 59.2%), the persistence of associations after comprehensive adjustment suggests independent pathogenic effects.
Lessons from the NICE-SUGAR trial, showing increased mortality with intensive glucose targets (81–108 mg/dL versus ≤ 180 mg/dL), emphasize avoiding overly aggressive interventions26. Our U-shaped relationship finding indicates extremely low GV may reflect harmful over-control, while excessive GV indicates metabolic instability. This supports an optimal therapeutic range rather than pursuing extreme glucose stability.
Given the U-shaped relationship with in-hospital mortality, clinical strategies should focus on maintaining glucose stability within an optimal range balancing metabolic control with hypoglycemia avoidance. Applications should emphasize: (1) Risk stratification using GV monitoring as an early warning system; (2) Quality improvement through consistent, protocol-driven management minimizing dangerous fluctuations without extreme targets; (3) Individualized care considering the significant diabetes subgroup interaction effects. Integration into existing ICU protocols is feasible but should prioritize balanced control over aggressive variability minimization.
The results underscore the critical need for diligent monitoring and management of blood glucose fluctuations to improve survival rates in this vulnerable patient population. The non-linear relationship suggests that maintaining a narrow range of glucose levels, avoiding both hypoglycemia and hyperglycemia, is crucial. The study adds to the growing body of evidence on the impact of GV on clinical outcomes, emphasizing the importance of developing targeted therapeutic strategies to mitigate these effects.
In comparison to previous studies, our findings align with the broader literature indicating that elevated GV is linked to poor clinical outcomes, particularly in critically ill patients. For example, Krinsley et al. consistently demonstrated that increased GV is linked to higher mortality rates in ICU settings, regardless of diabetes status27. Egi et al. emphasized that GV, rather than average glucose levels, is a more critical predictor of mortality in critically ill patients28. Our study supports these findings, further substantiating the association between elevated GV and negative outcomes in HF patients. However, our research also highlights some differences in the extent of GV’s impact compared to previous studies. While earlier research often concentrated on general ICU populations, our study specifically focuses on HF patients, providing a more nuanced understanding of GV’s effects in this subgroup. Moreover, the non-linear, U-shaped relationship observed in our study, showing that both low and high GV levels are associated with increased mortality, adds a novel aspect to the understanding of GV’s impact, which was not extensively reported in earlier works. This finding suggests that both hyperglycemic and hypoglycemic fluctuations can be harmful, underscoring the need for balanced glucose management strategies. Thus, while our findings are consistent with the general consensus on the dangers of GV, they also offer new insights specific to the HF patient population. Importantly, our study also identified significant interaction effects in the diabetes subgroup, a nuance that warrants further investigation.
The possible mechanisms through which GV may contribute to an increased risk of mortality in patients with heart failure (HF) involve multiple pathways:: (1) Oxidative stress: Glucose fluctuations induce oxidative stress, leading to cellular damage that exacerbates heart failure progression29. (2) Inflammatory response: Elevated GV can trigger inflammatory pathways, raising levels of markers like CRP and IL-6, which are linked to adverse cardiovascular outcomes30. (3) Endothelial dysfunction: GV impairs endothelial function, reducing vasodilation capacity, which can precipitate cardiovascular events in vulnerable HF patients31. (4) Increased sympathetic nervous system activity: Glucose variability can activate the sympathetic nervous system, increasing cardiac workload and risk of arrhythmias in HF patients32. (5) Glucotoxicity Chronic hyperglycemia exacerbates myocardial cell apoptosis through mechanisms such as endoplasmic reticulum stress33. This toxic effect of glucose fluctuations on cardiomyocytes has been demonstrated in experimental models, as shown by L.-D. Wu et al., who found accelerated apoptosis in diabetic rats with glucose fluctuations compared to sustained hyperglycemia34. These pathophysiological cascades can be triggered early in the ICU course and have sustained effects on clinical outcomes, supporting the temporal validity of early GV measurements in predicting mortality outcomes.
While our study provides significant insights into the association between GV and mortality in critically ill patients with HF, it has several limitations. First, as a retrospective cohort study, our research is limited by its observational design, which does not allow for establishing causality. This approach may also introduce selection bias and residual confounding. Our observational design cannot establish causality or determine optimal therapeutic interventions. The complex interplay between disease severity and GV requires carefully designed interventional studies to establish evidence-based treatment targets. Although we conducted comprehensive multivariable adjustments, residual confounding due to unmeasured variables cannot be entirely excluded. However, the results of our E-value analysis suggest that an unmeasured confounder would need to have a risk ratio of at least 2.08 with both GV and 30-day mortality to fully nullify the observed association. For the lower confidence interval limit, the corresponding E-value was 1.76. These thresholds exceed the strength of most known clinical confounders included in our model, thereby enhancing confidence in the robustness of our conclusions. Future research should consider a prospective design to more clearly determine the causal relationship between GV and mortality in HF patients and to assess whether interventions targeting GV can improve patient outcomes. Second, although GV was measured using the coefficient of variation (CV), more detailed data, such as those from continuous glucose monitoring (CGM), were not available. This limitation could affect the precision and detail of GV assessment. An important limitation of our study is the temporal assessment of GV patterns. Our retrospective design limits our ability to assess GV patterns over extended periods or to evaluate the optimal timing for GV measurement. While our early GV measurements demonstrated strong predictive validity, we acknowledge that they may not fully represent the complete ICU glycemic trajectory. The balance between early prediction utility and comprehensive temporal assessment represents an important methodological consideration for future research. Utilizing advanced glycemic monitoring technologies like CGM could offer a more nuanced understanding of GV’s impact on clinical outcomes. Future prospective studies utilizing continuous glucose monitoring throughout the entire ICU stay would provide more comprehensive temporal GV assessment and better address questions regarding the optimal timing and duration of GV measurements for mortality prediction. Additionally, investigating the role of interventions designed to minimize both hyperglycemic and hypoglycemic episodes could be beneficial. Finally, although we performed comprehensive adjustments for confounders, the potential for residual confounding cannot be completely ruled out.
Critical priorities include: (1) randomized trials targeting GV reduction while avoiding hypoglycemia; (2) biomarker studies distinguishing pathogenic from severity marker effects; (3) personalized glucose management approaches; and (4) mechanistic studies investigating optimal GV assessment timing.
Conclusions
This study highlights the critical importance of monitoring and managing GV in critically ill patients with HF. Both in-hospital and 30-day mortality were significantly associated with elevated GV, highlighting its potential as an important clinical parameter that should be closely monitored and managed in the ICU setting. The non-linear relationship between GV and in-hospital mortality, along with the linear association with 30-day mortality, underscores the complex role of blood glucose fluctuations in patient outcomes. These results suggest that routine monitoring and control of GV could play a pivotal role in improving patient prognosis, particularly in those with severe cardiovascular conditions.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- eICU-CRD:
-
the eICU Collaborative Research Database
- MIMIC-IV:
-
the Medical Information Mart for Intensive Care
- HF:
-
Heart failure
- GV:
-
Glycemic variability
- ICU:
-
intensive care unit
- SQL:
-
Structured query language
- ICD:
-
International classification of diseases
- MBP:
-
mean blood pressure
- BMI:
-
body mass index
- COPD:
-
chronic obstructive pulmonary disease
- AKI:
-
acute kidney injury
- WBC:
-
white blood cell
- BUN:
-
blood urea nitrogen
- LVEF:
-
left ventricular ejection fraction
- ACEI/ARB:
-
angiotensin converting enzyme inhibitors/angiotension receptor antagonists
- ICD:
-
implantable cardioverter-defibrillator
- CRT:
-
cardiac resynchronization therapy
- SOFA:
-
Sequential organ failure assessment score
- SAPS II:
-
the simplified acute physiology score II
- LOS:
-
length of stay
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- OR:
-
odds ratio
- CI:
-
Confidence intervals
- HR:
-
Hazard ratio
- CGM:
-
continuous glucose monitoring
References
Christiansen, M. N. et al. Age-Specific trends in incidence, mortality, and comorbidities of heart failure in denmark, 1995 to 2012. Circulation 135 (13), 1214–1223. https://doi.org/10.1161/circulationaha.116.025941 (2017). [published Online First: 2017/02/09].
Ponikowski, P. et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC)Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur. Heart J. 37 (27), 2129–2200. https://doi.org/10.1093/eurheartj/ehw128 (2016). [published Online First: 20160520].
Vasan, R. S., Zuo, Y. & Kalesan, B. Divergent Temporal trends in morbidity and mortality related to heart failure and atrial fibrillation: age, sex, race, and geographic differences in the united states, 1991–2015. J. Am. Heart Assoc. 8 (8), e010756. https://doi.org/10.1161/jaha.118.010756 (2019). [published Online First: 2019/04/09].
Savarese, G. & Lund, L. H. Global public health burden of heart failure. Card Fail. Rev. 3 (1), 7–11 (2017). 10.15420/cfr.2016:25:2 [published Online First: 2017/08/09].
Monnier, L., Colette, C. & Owens, D. R. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? J. Diabetes Sci. Technol. 2 (6), 1094–1100. https://doi.org/10.1177/193229680800200618 (2008). [published Online First: 2009/11/04].
Ceriello, A., Monnier, L. & Owens, D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol. 7 (3), 221–230. https://doi.org/10.1016/s2213-8587(18)30136-0 (2019). [published Online First: 2018/08/18].
Su, G. et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc. Diabetol. 10, 19. https://doi.org/10.1186/1475-2840-10-19 (2011). [published Online First: 20110225].
Xia, J. et al. The correlation between glucose fluctuation from self-monitored blood glucose and the major adverse cardiac events in diabetic patients with acute coronary syndrome during a 6-month follow-up by WeChat application. Clin. Chem. Lab. Med. 56 (12), 2119–2124. https://doi.org/10.1515/cclm-2018-0220 (2018). [published Online First: 2018/07/18].
Moghissi, E. S. et al. American association of clinical endocrinologists and American diabetes association consensus statement on inpatient glycemic control. Diabetes Care. 32 (6), 1119–1131. https://doi.org/10.2337/dc09-9029 (2009). [published Online First: 2009/05/12].
Su, G. et al. Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care. 36 (4), 1026–1032. https://doi.org/10.2337/dc12-0925 (2013). [published Online First: 2013/01/26].
Krinsley, J. S. et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit. Care. 17 (2), R37. https://doi.org/10.1186/cc12547 (2013). [published Online First: 2013/03/05].
Zhang, Z. Y. et al. Glucose fluctuations promote aortic fibrosis through the ROS/p38 MAPK/Runx2 signaling pathway. J. Vasc Res. 57 (1), 24–33. https://doi.org/10.1159/000503608 (2020). [published Online First: 2019/11/13].
Zhang, Z. Y. et al. Glucose fluctuations promote vascular BK channels dysfunction via PKCα/NF-κB/MuRF1 signaling. J. Mol. Cell. Cardiol. 145, 14–24. https://doi.org/10.1016/j.yjmcc.2020.05.021 (2020). [published Online First: 2020/06/09].
Wakasugi, S. et al. Associations between continuous glucose monitoring-derived metrics and arterial stiffness in Japanese patients with type 2 diabetes. Cardiovasc. Diabetol. 20 (1), 15. https://doi.org/10.1186/s12933-020-01194-2 (2021). [published Online First: 2021/01/09].
Saito, S. et al. Glucose fluctuations increase the incidence of atrial fibrillation in diabetic rats. Cardiovasc. Res. 104 (1), 5–14. https://doi.org/10.1093/cvr/cvu176 (2014). [published Online First: 2014/08/02].
Chun, K. H. et al. In-hospital glycemic variability and all-cause mortality among patients hospitalized for acute heart failure. Cardiovasc. Diabetol. 21 (1), 291. https://doi.org/10.1186/s12933-022-01720-4 (2022). [published Online First: 2022/12/28].
Cunha, F. M. et al. Glucose variability predicts 6-month mortality in patients hospitalized with acute heart failure. Intern. Emerg. Med. 16 (8), 2121–2128. https://doi.org/10.1007/s11739-021-02719-7 (2021). [published Online First: 2021/04/06].
Pollard, T. J. et al. The eICU collaborative research database, a freely available multi-center database for critical care research. Sci. Data. 5, 180178. https://doi.org/10.1038/sdata.2018.178 (2018). [published Online First: 20180911].
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data. 10 (1), 1. https://doi.org/10.1038/s41597-022-01899-x (2023). [published Online First: 20230103].
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int. J. Surg. 12 (12), 1495–1499. https://doi.org/10.1016/j.ijsu.2014.07.013 (2014). [published Online First: 20140718].
Johnson, A. E. et al. The MIMIC code repository: enabling reproducibility in critical care research. J. Am. Med. Inf. Assoc. 25 (1), 32–39. https://doi.org/10.1093/jamia/ocx084 (2018). [published Online First: 2017/10/17].
Vincent, J. L. et al. The SOFA (Sepsis-related organ failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on Sepsis-Related problems of the European society of intensive care medicine. Intensive Care Med. 22 (7), 707–710. https://doi.org/10.1007/bf01709751 (1996).
Le Gall, J. R., Lemeshow, S. & Saulnier, F. A new simplified acute physiology score (SAPS II) based on a european/north American multicenter study. Jama 270 (24), 2957–2963. https://doi.org/10.1001/jama.270.24.2957 (1993).
van Buuren, S. & Groothuis-Oudshoorn, K. Mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45 (3), 1–67. https://doi.org/10.18637/jss.v045.i03 (2011).
Linden, A., Mathur, M. B. & VanderWeele, T. J. Conducting sensitivity analysis for unmeasured confounding in observational studies using E-values: the evalue package. Stata J. 20 (1), 162–175. https://doi.org/10.1177/1536867x20909696 (2020).
Finfer, S. et al. Intensive versus conventional glucose control in critically ill patients. N Engl. J. Med. 360 (13), 1283–1297. https://doi.org/10.1056/NEJMoa0810625 (2009). [published Online First: 20090324].
Krinsley, J. S. Glycemic variability and mortality in critically ill patients: the impact of diabetes. J. Diabetes Sci. Technol. 3 (6), 1292–1301. https://doi.org/10.1177/193229680900300609 (2009). [published Online First: 2010/02/11].
Egi, M. et al. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 105 (2), 244–252. https://doi.org/10.1097/00000542-200608000-00006 (2006). [published Online First: 2006/07/28].
Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414 (6865), 813–820 (2001). 10.1038/414813a [published Online First: 2001/12/14].
Esposito, K. et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106 (16), 2067–2072. https://doi.org/10.1161/01.cir.0000034509.14906.ae (2002). [published Online First: 2002/10/16].
Beckman, J. A. et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur. Heart J. 34 (31), 2444–2452. https://doi.org/10.1093/eurheartj/eht142 (2013). [published Online First: 2013/04/30].
Carnethon, M. R. et al. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the atherosclerosis risk in communities study, 1987–1998. Circulation 107 (17), 2190–2195. https://doi.org/10.1161/01.Cir.0000066324.74807.95 (2003). [published Online First: 2003/04/16].
Huo, J. L. et al. Diabetic cardiomyopathy: early diagnostic biomarkers, pathogenetic mechanisms, and therapeutic interventions. Cell. Death Discov. 9 (1), 256. https://doi.org/10.1038/s41420-023-01553-4 (2023). [published Online First: 2023/07/22].
Wu, L. D. et al. Glucose fluctuation promotes cardiomyocyte apoptosis by triggering Endoplasmic reticulum (ER) stress signaling pathway in vivo and in vitro. Bioengineered 13 (5), 13739–13751. https://doi.org/10.1080/21655979.2022.2080413 (2022). [published Online First: 2022/06/17].
Acknowledgements
We express our profound gratitude to the Massachusetts Institute of Technology and the Beth Israel Deaconess Medical Center for their invaluable contribution to the MIMIC project.
Author information
Authors and Affiliations
Contributions
S.T. contributed to the conception and design of the study,S.T. and P.L. are responsible for data extraction, data analysis, results visualization, and manuscript writing, Z.L. provided professional advice for the revision of the manuscript, W.D and Y.L. were responsible for the review and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the guidelines of the Helsinki Declaration. The MIMIC-IV database was supported by grants from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health (NIH) under award numbers R01-EB001659 (2003–2013) and R01-EB017205 (2014–2022) and approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA). The eICU-CRD Database was released under the Health Insurance Portability and Accountability Act (HIPAA) safe harbor provision. The re-identification risk was certified as meeting safe harbor standards by Privacert (Cambridge, MA) (HIPAA Certification no. 1031219-2). Informed consent was waived by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA) for the MIMIC-IV database, and by Philips Healthcare for the eICU-CRD database, due to the retrospective nature of the study, and the data are publicly available in the MIMIC-IV and eICU-CRD databases.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, P., Li, Z., Tang, S. et al. Association between glycemic variability and mortality in critically ill patients with heart failure. Sci Rep 15, 31021 (2025). https://doi.org/10.1038/s41598-025-16212-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16212-0