Abstract
Anticoagulants were suggested to influence cancer survival by possible immunomodulatory effect. However, it remained unclear if concomitant use of anticoagulants and various types of them could influence the efficacy of immune checkpoint inhibitors (ICIs) in cancer patients. Accordingly, the meta-analysis was performed to systematically evaluate the effect of concomitant anticoagulants in cancer patients receiving ICIs. Relevant studies were obtained by literature search in PubMed, Embase, and Web of Science databases. A conservative random-effect model was used to combine the results. A total of 2686 patients were enrolled, including all the patients analyzed for overall survival (OS) and 2457 patients for progression-free survival (PFS). The publication types of these 5 studies were retrospective studies. We found that concomitant use of anticoagulants significantly impaired the PFS (hazard ratio (HR): 1.29, 95% confidence interval (CI): 1.10–1.51, p = 0.002) and OS (HR:1.26, 95% CI:1.07–1.47, p = 0.004) of cancer patients receiving ICIs. Subgroup analyses showed that there was worse OS in heparin product subgroup (HR, 2.90; 95%CI: 1.71–4.92, p < 0.001). Our findings suggested that concomitant use of anticoagulants seemed to significantly impair the survival of cancer patients treated with ICIs.
Similar content being viewed by others
Introduction
Currently, immune checkpoint inhibitors (ICIs) are revolutionizing the treatment of advanced cancers1,2,3,4. The prognosis of cancer patients undergoing systemic treatment may be influenced by multiple factors3,5,6,7. ICIs can inhibit immune-negative regulatory cells or molecules and reactivate immune cells including CD8 + T cells through block of the PD-1/PD-L1 signaling pathway to exert anti-tumor effects8. Therefore, immunotherapy has demonstrated significant efficacy in the clinical application of various malignant tumors9,10. However, in clinical practice, immunotherapy currently faces challenges from various factors that affect its efficacy in cancer patients. Multiple combined use of medications may produce drug-drug interactions with ICIs. Drug-drug interactions (DDIS) play an important role in safe and effective systemic anticancer therapies, which can alter drug efficacy or exacerbate the toxicity of systemic therapies through pharmacodynamic (PK) and pharmacokinetic (PD) interactions11. At the same time, these drugs can also exert immunomodulatory effects in the systemic and tumor microenvironment.
Venous thromboembolism (VTE) is the main cause of incidence rate and mortality in cancer patients12, and anticoagulants are now commonly used for prevention and therapy of VTE in these patients13. Several preclinical studies have revealed the correlation between the coagulation pathway and tumor immune evasion, providing a theoretical basis for cautious combined application of ICIs and anticoagulants in the treatment of cancer patients in clinical practice. Metelli et al. revealed that thrombin cleaves glycoprotein A repetitions predominant (GARP), which could mediate the release of transforming growth factor-ß (TGF-ß) from platelets to downregulate CD8 + T cells, upregulate CD4 + T cells and reduce immune cell infiltration of tumors by inducing collagen and fibroblast barriers14. Another study led by Graf uncovered that Factor Xa produced in the tumor microenvironment (TME) could facilitate tumor immune evasion, through further research in mouse models of colon cancer and fibrosarcoma, the study also found that rivaroxaban could synergize with anti-PD-L1 to improve antitumor immunity15.
However, results of recent observational studies evaluating the influence of concomitant anticoagulants use on survival of cancer patients treated with ICIs were not consistent16,17,18,19,20. One study suggested that concomitant anticoagulants may impair the survival of these patients16, while others did not show a significant influence17,18,19,20. Meanwhile, different types of anticoagulants on the efficacy of the cancer patients receiving immunotherapy have been controversial in known clinical studies. We hypothesized that concomitant use of anticoagulants could impair survival outcomes in cancer patients treated with ICIs. Therefore, this meta-analysis was conducted to systematically evaluate the effect of concomitant anticoagulants on the therapeutic efficacy of ICIs in cancer patients.
Methods
Protocol and guideline
Registration of the full protocol was completed on dedicated websites (https://www.crd.york.ac.uk/prospero/) as CRD42022372636. The whole process of this study followed the statement of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklist.
Search strategy
Studies evaluating the prognoses of anticoagulants-used versus anticoagulants-free in cancer patients receiving ICIs treatment from inception to 1 May 2024 were retrieved by searching PubMed, Cochrane Library, Web of Science, Embase, and major conference proceedings. Studies in English reporting hazard ratios (HRs) with 95% confidence intervals (CIs) for overall survival (OS) and/or progression-free survival (PFS) were included. The search queries were built with Mesh terms and free texts (Supplementary Table 1). The following broad terms were used to build the search queries: ‘immune checkpoint inhibitor’, ‘immunotherapy’, ‘anticoagulants’, ‘neoplasm’ and ‘cancer’.
Selection criteria
The inclusion criteria for the study were as follows1: Studies focusing on patients with solid tumors or hematological malignancy treated with ICIs2; Studies involved the association between concurrent use of anticoagulants and ICIs efficacy in patients with cancer reporting OS or PFS3; Sufficient data were provided to calculate the HR and 95%CI. Studies with insufficient information to evaluate HRs, 95%CIs, or in languages other than English were excluded.
Study selection, data extraction, and quality assessment
Two investigators selected the studies that fulfilled our inclusion criteria and extracted the relevant information independently. Disagreements were resolved by discussion with an independent expert. The following information was extracted: first author’s name, publication year, country, type of cancer, ICIs agent, ICIs line of treatments, anticoagulants type, sample size, outcome, median PFS and OS, HRs for OS and PFS and 95%CIs between uses and non-users. Data from the multivariate analysis model was prior to adoption when both univariate and multivariate analysis model data were available. The Quality Assessment of Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of studies. This scale consists of three parameters: selection, comparability and outcome assessment. NOS scores ≥ 6 was considered high-quality studies.
Statistical analysis
All statistical analyses were calculated using the software Review manager 5.4. HRs with corresponding 95%CIs were synthesized to yield pooled results. Heterogeneity among the included studies was assessed by the Cochrane Q test and I2 value. I2 > 50% and P < 0.1 for the Q test were considered significant heterogeneity and a random effect model was adopted. Subgroup and meta-regression analyses were performed to identify the potential heterogeneity contributors among the included studies. A funnel plot with Egger’s regression test was constructed to evaluate publication bias. Sensitivity analyses were conducted to examine the stability of the outcomes by the leaving-one-out approach. A two-tailed P < 0.05 was defined as statistical significance.
Results
Selection and characteristics of studies
A flowchart showing our literature selection is shown as Fig. 1. Initially, 408 relevant records were retrieved from selected databases. A total of 365 records were retained after duplicate removal. Of these, 352 were excluded by screening the title and abstract, thereby leaving 13 potentially relevant full-text articles. Eventually, we selected 5 studies16,17,18,19,20 that evaluated the impact of related anticoagulants on the survival of patients with cancer treated with ICIs. All eligible studies were retrospective and involved 2686 patients, who were included in our meta-analysis. All studies were graded as “moderate” or “high” quality according to NOS criteria and qualified for a meta-analysis.
A total of 2,686 patients were included in the analysis, with OS data available for all patients and PFS data available for 2,457 of them. The publication types of these 5 studies were retrospective studies. There were 405 anticoagulants-used patients and 2281 anticoagulants-free patients. Multiple cancer types were reported in three studies16,17,20. The regimen of ICIs included anti-PD-L1 monoclonal antibody monotherapy, anti-PD-1 monoclonal antibody monotherapy, anti-CTLA-4 monoclonal antibody monotherapy and anti-PD-1 with anti-CTLA-4 monoclonal antibody (anti-PD-1/L1 + anti-CTLA-4). Anticoagulants types could be classified as four types: Direct thrombin inhibitor, Factor Xa inhibitor, Heparin product and Vitamin K antagonist. The time window of anticoagulants use included simultaneous use17,18 and baseline use16,19,20. All studies were graded as high methodological quality. The characteristics and quality assessment results of included studies are shown in Table 1.
Pooled OS and PFS
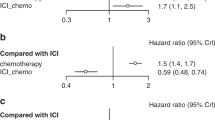
A total of 5 and 4 cohorts reported HR data for OS and PFS respectively. One cohort16 was observed a significantly negative effect on OS and PFS for the concomitant use of anticoagulants and ICIs. Four cohorts17,18,19,20 for OS and three cohorts17,18,19 for PFS were observed no significant impact on prognoses in cancer patients treated with ICIs and anticoagulants versus ICIs without anticoagulants. The above contradictory results confirmed the necessity of our study once again. Consequently, the method of integrating different studies through meta-analysis would be feasible to resolve this issue. Based on the clinical heterogeneity among included studies, random effect model was adopted to calculate pooled results. Meta-analysis showed that the pooled HR was 1.29 (95%CI, 1.10–1.51; p = 0.002) for OS (Fig. 2A) and 1.26 (95%CI, 1.07–1.47; p = 0.004) for PFS (Fig. 2B), which meant a significantly worse OS and PFS were found in the population receiving ICIs concomitant with anticoagulants.
Subgroup analysis of ACs types
To explore potential factors contributing to heterogeneity among the included studies, subgroup and meta-regression analyses were conducted by various types of anticoagulant agents. The population was divided into four subgroups based on anticoagulants types, comprising factor Xa inhibitor, heparin product, vitamin K antagonist, and direct thrombin inhibitor. Subgroup analyses showed significant worse OS in heparin product subgroup (HR, 2.90; 95%CI, 1.71–4.92; p < 0.001), while there was no trend towards significance for OS in the other three subgroups (Fig. 3). No statistical significance was observed for PFS in all subgroups (Fig. 4).
Publication bias
Figures 5(A) and 5(B) display the funnel plots for the outcomes of OS and PFS. Visual inspection revealed symmetry of the plots, reflecting a low risk of publication biases. Egger’s regression tests also indicated low risk of publication biases (p = 0.241 and 0.210, respectively).
Leave-one-out analyses were conducted to further assess the reliability of the results in our study. For the pooled OS and PFS, we found that only when the study led by Cortellini16 was omitted that the pooled HR became insignificant, which was 1.17 (95%CI, 0.95–1.45; p = 0.92) for OS and 1.13 (95%CI, 0.93–1.37; p = 0.67) for PFS respectively. For the Subgroup analyses of OS in heparin product subgroup, we found that when the study led by Johannet P17 was omitted that the pooled HR became insignificant, which was 1.12 (95%CI, 0.72–1.75; p = 0.62).
Discussion
To our knowledge, this study was the first to summarize the current evidence evaluating the prognostic effect of anticoagulants in cancer patients receiving ICIs treatment through meta-analysis. Compared with previous studies, this meta-analysis with the inclusion of multiple cancer types and more than 2500 patients made the results reliable and convincing.
In this meta-analysis, we pooled the results of five cohort studies and showed that concomitant use of anticoagulants in cancer patients receiving ICIs treatment was associated with significantly impaired survival outcomes, including PFS and OS. It was observed that the study led by Cortellini was the only study showing the poor OS and PFS in patients receiving anticoagulants, and it accounted for nearly half weight in pooled analysis for both PFS and OS. Whether this study will cause significant bias towards the results of this study was needed for further exploration. Firstly, before pooled effect analysis, all the other studies consistently indicated the trend of worse survival outcomes associated with the use of anticoagulants, although not significant. This may be due to the relatively small sample size and lower test efficacy, which were insufficient to reliably detect this effect. Secondly, in the sensitivity analysis, we found that only after excluding the study led by Cortellini did we obtain insignificant PFS and OS. However, the direction of the combined effect of other studies did not change, indicating the same potential effects and suggesting that the overall results were not solely driven by the study led by Cortellini. Therefore, we considered that the pooled analysis for PFS and OS were still relatively reliable to reflect the true impact of anticoagulants on survival prognosis.
Through further subgroup analysis of various types of anticoagulants on the efficacy in cancer patients receiving immunotherapy, heparin was found to be detrimental to OS, yet show no significant influence on PFS. Anticoagulants are believed to regulate immune balance and affect innate immune responses to antibiotics21. Heparin is a high molecular weight compound with a relative molecular weight of 2000 to 40,000 highly sulfated, heterogeneous linear glycosaminoglycans. There are some arguments about immunoregulation effect of heparin in some current preclinical researches. One study led by Fang Wei found combined treatment of heparin and anti-PD1 produced synergistic antitumor effects in Pancreatic ductal adenocarcinoma through tumor vascular normalization, hence increased antitumor T-cell responses due to reduced Treg infiltration and increased M1 macrophage polarization22. Another study focusing on colorectal carcinoma treatment also revealed that Low Molecular Weight Heparin (LMWH) could enhance ICIs-based immunotherapy by increasing lymphocyte infiltration into tumors, especially cytotoxic CD8 + T cells23. However, one research found that enoxaparin could alleviate colon inflammation in mice by reducing the number of M1 macrophages, inhibiting the expression of IL1 β and increasing the number of M2 macrophages24.Therefore, the application of enoxaparin might compromise the efficacy of immunotherapy which relied on the inflammatory tumor microenvironment25.
However, the poor prognosis of cancer patients receiving anticoagulants treatment may not be solely related to its inhibitory effect on the efficacy of immunotherapy. Patients receiving anticoagulants typically suffered higher burden of diseases, had worse performance status than their counterparts, received other medicines or undergoing surgical operation, which may confound the analyzes when evaluating PFS and OS. Furthermore, we observed the fact that the OS but not the PFS was wore in patients receiving heparin. Heparin is the most commonly used anticoagulant for cancer patients with severe venous thromboembolism especially for patients with pulmonary thromboembolism, meanwhile, these patients may show worse systemic condition due to worse physical condition, higher bleeding risks and relatively higher age17,19. Therefore, the causality between heparin and worse OS may be a reflection of the indication bias and it suggested that heparin effect may be less of an immune modulatory effect and more of general survival. However, the limitations of inference in retrospective observational meta-analyses made it relatively difficult to exclude the influence, it was still vital for us to ascertain that heparin should be cautiously applied in the cancer patients receiving immunotherapy.
In solid cancer, cancer patients had a higher risk of developing thromboembolism events, especially during anti-cancer therapy, leading to deterioration in quality of life, increased healthcare costs and shortened survival26. However, anticoagulants treatment also associated with the increased bleeding risk in cancer patients, hence the potential of therapeutic synergy between anticoagulants and ICIs needs to be comprehensively evaluated before prospective trials27,28. Based on our analysis, we revealed that concomitant use of Factor Xa inhibitor showed the trend of improving the PFS and OS of cancer patients receiving immunotherapy though there was no significance. The results also reminded us that proper and reasonable use of Factor Xa inhibitor may benefit cancer patients receiving immunotherapy and we may balance the efficacy and the risk of the anticoagulants to reach the optimum therapeutic strategy.
Our research provided evidence-based medicine for the cautious use of anticoagulants in cancer patients receiving immunotherapy in future clinical practice, and also provided theoretical basis for future clinical research and trials. There was the need for more basic researches on the impact of anticoagulants on the tumor immune microenvironment, and more clinical studies focusing on practical application of anticoagulants in cancer patients receiving ICIs to achieve more comprehensive interpretation of this theory. Currently, researches of the impact of combined medication on the efficacy of immunotherapy was increasing and deepening as related studies were showing more significant evidences. Therefore, the interaction between anticoagulants and immunotherapy may become important research direction in the future.
This meta-analysis also had some limitations. Firstly, most of the studies were retrospective and of limited sample sizes, results of which may be affected by possible recall and selection biases. Despite adjustment methods, unmeasured variables or the lack of individual patient data such as performance status, comorbidities, concurrent medications, PD-L1 expression or treatment intent, which may be the source of heterogeneity and influenced the observed associations. Secondly, some results of subgroup analyses should also be interpreted with caution because of the undesired sample size and study heterogeneity. Accordingly, Meta-analysis based on individual-patient data should be performed for further evaluation and large-scale prospective cohort studies were needed to validate the findings.
Conclusion
This meta-analysis of retrospective studies suggests that concomitant use of anticoagulants, particularly heparin products, may be associated with inferior survival outcomes in cancer patients receiving ICIs. Prospective validation and mechanistic investigations are warranted. In view of the possible increased risk of worse survival outcomes, concomitant use of anticoagulants in cancer patients receiving ICIs should be cautious.
Data availability
The data adopted in this meta-analysis are available from the corresponding author on reasonable request.
References
Pardoll, D. M. The Blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 12 (4), 252–264 (2012).
Ribas, A. & Tumeh, P. C. Cancer therapy: tumours switch to resist. Nature 490 (7420), 347–348 (2012).
Rizzo, A. et al. KEYNOTE-522, IMpassion031 and geparnuevo: changing the paradigm of neoadjuvant immune checkpoint inhibitors in early triple-negative breast cancer. Future Oncol. 18 (18), 2301–2309 (2022).
Ricci, A. D., Rizzo, A. & Brandi, G. DNA damage response alterations in gastric cancer: knocking down a new wall. Future Oncol. 17 (8), 865–868 (2021).
Bas, O., Sahin, T. K., Karahan, L., Rizzo, A. & Guven, D. C. Prognostic significance of the cachexia index (CXI) in patients with cancer: A systematic review and meta-analysis. Clin. Nutr. ESPEN. 68, 240–247 (2025).
Sahin, T. K., Ayasun, R., Rizzo, A. & Guven, D. C. Prognostic value of Neutrophil-to-Eosinophil ratio (NER) in cancer: A systematic review and Meta-Analysis. Cancers (Basel). 16(21), 3689 (2024).
Vitale, E., Rizzo, A., Santa, K. & Jirillo, E. Associations between cancer risk. Inflamm. Metabolic Syndrome: Scoping Rev. Biology (Basel). 13(5), 352 (2024).
Wu, M. et al. Improvement of the anticancer efficacy of PD-1/PD-L1 Blockade via combination therapy and PD-L1 regulation. J. Hematol. Oncol. 15 (1), 24 (2022).
Rizzo, A. & Ricci, A. D. Challenges and future trends of hepatocellular carcinoma immunotherapy. Int. J. Mol. Sci. 23(19), 11363 (2022).
Santoni, M. et al. Real-world outcome of patients with advanced renal cell carcinoma and Intermediate- or Poor-risk international metastatic renal cell carcinoma database consortium criteria treated by Immune-oncology combinations: differential effectiveness by risk group?? Eur. Urol. Oncol. 7 (1), 102–111 (2024).
Scripture, C. D. & Figg, W. D. Drug interactions in cancer therapy. Nat. Rev. Cancer. 6 (7), 546–558 (2006).
Muñoz Martín, A. J. et al. SEOM clinical guideline of venous thromboembolism (VTE) and cancer (2019). Clin. Transl Oncol. 22 (2), 171–186 (2020).
Martins, M. A., Silva, T. F. & Fernandes, C. J. An update in anticoagulant therapy for patients with Cancer-Associated venous thromboembolism. Curr. Oncol. Rep. 25 (5), 425–432 (2023).
Metelli, A. et al. Thrombin contributes to cancer immune evasion via proteolysis of platelet-bound GARP to activate LTGF-β. Sci. Transl Med. 12(525), eaay4860 (2020).
Graf, C. et al. Myeloid cell-synthesized coagulation factor X dampens antitumor immunity. Sci. Immunol. 4(39), eaaw8405 (2019).
Cortellini, A. et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J. Immunother Cancer. 8(2), e001361 (2020).
Johannet, P. et al. Treatment with therapeutic anticoagulation is not associated with immunotherapy response in advanced cancer patients. J. Transl Med. 19 (1), 47 (2021).
Nichetti, F. et al. Is there an interplay between immune checkpoint inhibitors, thromboprophylactic treatments and thromboembolic events?? Mechanisms and impact in Non-Small cell lung cancer patients. Cancers (Basel): 12(1), 67 (2019).
Haist, M. et al. Anticoagulation with factor Xa inhibitors is associated with improved overall response and Progression-Free survival in patients with metastatic malignant melanoma receiving immune checkpoint inhibitors-A retrospective, Real-World cohort study. Cancers (Basel). 13(20), 5103 (2021).
Gutierrez-Sainz, L. et al. Incidence of venous thromboembolic events in cancer patients receiving immunotherapy: a single-institution experience. Clin. Transl Oncol. 23 (6), 1245–1252 (2021).
Strobel, L. & Johswich, K. O. Anticoagulants impact on innate immune responses and bacterial survival in whole blood models of neisseria meningitidis infection. Sci. Rep. 8 (1), 10225 (2018).
Wei, F. et al. Anticoagulants enhance molecular and cellular immunotherapy of cancer by improving tumor microcirculation structure and function and redistributing tumor infiltrates. Clin. Cancer Res. 29 (13), 2525–2539 (2023).
Quan, Y. et al. Low molecular weight heparin synergistically enhances the efficacy of adoptive and anti-PD-1-based immunotherapy by increasing lymphocyte infiltration in colorectal cancer. J. Immunother Cancer. 11(8), e007080 (2023).
Lean, Q. Y. et al. Orally administered Enoxaparin ameliorates acute colitis by reducing Macrophage-Associated inflammatory responses. PLoS One. 10 (7), e0134259 (2015).
Teng, M. W., Ngiow, S. F., Ribas, A. & Smyth, M. J. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 75 (11), 2139–2145 (2015).
Lyman, G. H., Eckert, L., Wang, Y., Wang, H. & Cohen, A. Venous thromboembolism risk in patients with cancer receiving chemotherapy: a real-world analysis. Oncologist 18 (12), 1321–1329 (2013).
Angelini, D. E., Radivoyevitch, T., McCrae, K. R. & Khorana, A. A. Bleeding incidence and risk factors among cancer patients treated with anticoagulation. Am. J. Hematol. 94 (7), 780–785 (2019).
Johnstone, C. & Rich, S. E. Bleeding in cancer patients and its treatment: a review. Ann. Palliat. Med. 7 (2), 265–273 (2018).
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (No. 82304072, 2024.01-2026.12)
Author information
Authors and Affiliations
Contributions
Lei Xia and Zhenzhou Yang designed the study. Jin Xiong and Hongmei Jian performed database search, data extraction, and study quality evaluation. Jin Xiong and Hongmei Jian performed statistical analyses and interpreted the results. Jin Xiong drafted the manuscript. Lei Xia revised the manuscript. All authors approved the submission of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiong, J., Jian, H., Yang, Z. et al. Concomitant anticoagulants and the survival of cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Sci Rep 15, 30606 (2025). https://doi.org/10.1038/s41598-025-16236-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16236-6