Abstract
IgA nephropathy (IgAN) is a prevalent primary glomerular disease and a major contributor to end-stage renal disease (ESRD). Budesonide Enteric Capsules (Nefecon®) are the first treatment approved by the U.S. Food and Drug Administration (FDA) targeting galactose-deficient IgA1 (Gd-IgA1) synthesis in the terminal ileum for IgAN. However, the detailed molecular mechanisms underlying its efficacy remain to be fully elucidated. In this study, we utilized bioinformatics approaches to integrate data from the GeneCards, CTD, and OMIM databases, identifying 787 IgAN-related targets. Additionally, we employed the PharmMapper, CTD and TargetNet databases to predict 384 budesonide targets, ultimately pinpointing 122 common targets. GO and KEGG enrichment analyses indicated that these targets are significantly enriched in biological processes such as apoptosis, inflammation responses, and cell proliferation, as well as in key pathways, including pathways in cancer, the PI3K-Akt signaling pathway, and the IL-17 signaling pathway. Protein–protein interaction (PPI) network analysis identified 10 core targets, including IL-6, TNF, AKT1, and TGF-β1, all of which exhibited strong binding affinity with budesonide in molecular docking studies. This study suggests that budesonide may exert its therapeutic effects on IgAN through multi-target and multi-pathway synergistic actions, offering valuable insights for its application in the clinical management of IgAN.
Similar content being viewed by others
Introduction
IgA nephropathy (IgAN), the most prevalent primary glomerulonephritis globally, is a leading cause of end-stage renal disease (ESRD)1. The disease is pathologically characterized by glomerular mesangial IgA deposition along with mesangial cell proliferation and matrix expansion. Clinical manifestations include microscopic or macroscopic hematuria, often accompanied by varying degrees of proteinuria, hypertension, and edema2. Nearly half of patients develop ESRD within 25 years of diagnosis, creating substantial socioeconomic burdens3. The “four-hit” hypothesis elucidates the potential etiological mechanisms involved in the pathogenesis, including the abnormal production of substantial amounts of galactose-deficient IgA1 (Gd-IgA1) by mucosal immunity in the gut; the generation of IgG or IgA autoantibodies that recognize Gd-IgA1; and the deposition of IgA1 and Gd-IgA1-containing immune complexes in the kidney, which activate the complement system, leading to renal inflammation and injury4. The Kidney Disease: Improving Global Outcomes (KDIGO) 2024 guidelines recommend that the treatment focus for IgAN is to optimize supportive care, including lifestyle modifications, blood pressure management, and the combined use of renin-angiotensin system inhibitors (RASi) and sodium-glucose cotransporter-2 inhibitors (SGLT2i), etc. For IgAN patients who are still at high risk of disease progression after receiving optimized supportive treatment, the addition of systemic glucocorticoid therapy may be considered5. However, the therapeutic outcomes of supportive care, RASi, and SGLT2i are limited. And systemic glucocorticoid therapy significantly increases the risk of adverse events6.
Recent progress in elucidating the pathogenesis of IgAN has spurred the development of innovative therapies. In December 2023, the U.S. Food and Drug Administration (FDA) granted full approval for the first gut-targeted treatment for IgAN—Budesonide Enteric Capsules, marketed as Nefecon7. Subsequently, the European Medicines Agency (EMA) and China’s National Medical Products Administration (NMPA), two highly authoritative regulatory bodies, also approved Nefecon for the treatment of IgAN8. Budesonide is a corticosteroid exhibiting strong glucocorticoid but minimal mineralocorticoid activity. Nefecon delivers budesonide specifically to terminal ileal mucosal B cells (including Peyer’s patches), modulating Gd-IgA1 production through localized immunomodulation9,10. This mechanism reduces the formation of Gd-IgA1 antibodies and immune complexes in the systemic circulation, consequently diminishing their glomerular deposition and associated renal injury11,12. Phase III clinical trials have demonstrated that Nefecon exhibits a significant advantage in reducing proteinuria and maintaining stable renal function, with a favorable safety profile13. Although multiple clinical trials have demonstrated the significant efficacy of Nefecon in treating IgAN7, the precise molecular mechanisms underlying its therapeutic effects have yet to be fully elucidated. There is a notable deficiency in systematic molecular-level research elucidating the mechanisms by which Nefecon exerts its therapeutic effects in IgAN, including detailed analyses of potential targets, biological processes, and metabolic pathways. Further analytical approaches and multidimensional research are necessary.
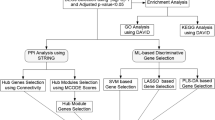
Bioinformatics integrates systems biology with network informatics to enable multi-level, systematic analysis of complex biological systems. It offers a novel theoretical framework for elucidating the molecular mechanisms of diseases and identifying therapeutic targets. Accordingly, this study aims to elucidate the molecular mechanisms of Nefecon in the treatment of IgAN using bioinformatics approaches, and provide new theoretical evidence for the clinical application of budesonide in managing IgAN. The workflow is outlined in Fig. 1.
Materials and methods
Acquisition of disease-related target genes
To obtain a comprehensive and reliable set of disease-related genes, three complementary databases were systematically queried using “IgA nephropathy” as the search term. The GeneCards database, a comprehensive and authoritative compendium of human gene annotations, provides detailed information on gene function, expression, and disease associations14. The CTD database offers high-quality experimental evidence by curating chemical-gene-disease relationships from a vast array of literature15. The OMIM database provides detailed information on the genetic basis of disease16. Genes with a Relevance Score of 1 or higher from GeneCards and those with a frequency above the median from CTD were selected. Genes appearing in at least two databases were classified as IgAN-related targets.
Screening of targets of the budesonide
The 2D structural data of budesonide (SDF format) were obtained from PubChem17 and submitted to PharmMapper for target prediction. The resulting gene names were standardized to human gene nomenclature using the UniProt database. Concurrently, the keyword “Budesonide” was searched in the CTD and TargetNet databases. Each of these databases employs a distinct approach to target prediction: PharmMapper18 utilizes molecular structure for target identification, CTD15 leverages toxicogenomic interactions to forecast targets, and TargetNet19 applies machine learning models to predict potential targets. Genes retrieved from these databases were merged and duplicates were removed to minimize bias and identify budesonide-related genes.
Prediction of common targets of drug and disease
A Venn diagram webtool was used to perform an intersection between human genes associated with IgAN and target genes of budesonide. The common targets identified from this analysis were defined as IgAN-budesonide-related targets. The procedure for gene selection is depicted in Fig. 2.
GO function and KEGG pathway enrichment analysis
GO analysis is a method that systematically reveals the distribution of gene functions and categorizes genes based on their biological functions, including biological processes (BP), cellular components (CC), and molecular functions (MF). Compared with other analytical methods, GO analysis is a widely accepted functional analysis method in the scientific community, offering more comprehensive and detailed functional annotations20. KEGG pathway enrichment analysis identifies key signaling pathways related to the actions of drugs and diseases21. To elucidate the functional distribution of intersecting targets across BP, CC, and MF, and to explore the potential signaling pathways of budesonide in treating IgAN, the intersecting targets were uploaded to the DAVID database with the identifier set as “OFFICIAL_GENE_SYMBOL” and the species designated as “Homo sapiens” for subsequent GO and KEGG pathway enrichment analyses. Results with P < 0.05 were considered statistically significant. Visualization of the analysis results was performed using the WeiShengXin and Hiplot platforms.
Construction of protein–protein interaction (PPI) network
The STRING database, an interactive tool, was used to perform PPI network analysis, revealing the interaction relationships among targets, which is essential for understanding protein function and identifying potential drug targets22. The species was set to “Homo sapiens,” and the medium confidence threshold was set to 0.4. Cytoscape 3.9.1 software was used for network visualization, and the CentiScape 2.2 plugin was employed to calculate the degree, betweenness centrality, and closeness centrality of each target. The top ten targets with the highest degree values were defined as core targets.
Molecular docking
The 3D structures of the core targets were obtained from the PDB database23. Utilizing a set of criteria—including human origin (Homo sapiens), presence of bound ligands, intact binding pockets, corroborating literature, and high-resolution data—we selected suitable structures for our key targets. The 3D structure of budesonide was retrieved from the PubChem database. Molecular docking is a computational technique that simulates interactions between drugs and their targets at the atomic level, calculating parameters to evaluate binding characteristics. Designate the core targets as the receptors and budesonide as the ligand. The AutoDockTools software was utilized to perform dehydration and hydrogenation on the structures of targets, respectively. Budesonide was hydrogenated and automatically distributed charges to construct the docking grid box for binding with the target proteins. We docked budesonide with each of the core targets 50 times to obtain their docking binding energies. The docking results were imported into the PyMOL software for 3D visualization and to analyze the relevant interactions between budesonide and the target proteins.
Results
Screening of IgAN-related targets
A total of 1378, 5886, and 573 disease targets were retrieved from GeneCards, CTD, and OMIM databases, respectively. Targets present in at least two databases were considered reliable IgAN-related targets. After eliminating duplicate target genes and taking intersections, 787 IgAN-related targets were obtained (Fig. 3).
Acquisition of targets of budesonide
The PharmMapper platform yielded 292 potential targets, while the CTD and TargetNet databases provided 87 and 34 targets, respectively. After merging and deduplicating these results, a total of 384 drug targets associated with budesonide were obtained.
Screening of potential targets for budesonide treatment in IgAN
A Venn diagram was constructed with 787 IgAN-related targets and 384 budesonide-related targets, and the results are shown in Fig. 4. Genes associated with both budesonide and IgAN are likely to be potential therapeutic targets for budesonide treatment of IgAN. A total of 122 IgAN-budesonide-related targets were obtained.
GO and KEGG pathway enrichment analysis
To systematically characterize gene function distributions and identify relevant biological processes, we performed GO analysis on the 122 IgAN-budesonide-related targets. This analysis identified 907 GO enrichment terms, comprising 718 BP, 53 CC, and 136 MF. Among these, BP was predominantly enriched in inflammatory responses, cell proliferation, and apoptosis; CC mainly included the nucleus and cytoplasm; and MF primarily involved binding to proteins, metal ions, ATP, DNA, and cytokines. The top 10 enrichment results from each GO analysis are depicted in Fig. 5.
The KEGG pathway enrichment analysis identified 162 pathways associated with budesonide treatment in IgAN. Based on −log10(P-value) and gene counts, we selected the top 10 most enriched pathways (Fig. 6). Notably enriched pathways included “Pathways in cancer,” “Lipid and atherosclerosis,” “PI3K-Akt signaling pathway,” “AGE-RAGE signaling pathway in diabetic complications,” and “IL-17 signaling pathway”. These findings suggest that budesonide may ameliorate IgAN through multi-pathway mechanisms.
PPI network construction
The 122 IgAN-budesonide-related targets obtained through screening were employed to construct the PPI network. Following removal of isolated nodes, the final network comprised 121 nodes interconnected by 2681 edges. Cytoscape software was utilized to analyze the degree, betweenness centrality, and closeness centrality in the network. Betweenness centrality reflects the number of shortest paths passing through a node, with higher values indicating stronger control and greater influence on information transmission within the network24. Closeness centrality measures the average distance to all other nodes, where higher values suggest shorter average distances to other nodes and faster influence across the network25. Degree quantifies the number of connections a node has, with higher values signifying more interactions and a more central role in biological processes. The integration of the aforementioned network topology parameters enables a more comprehensive identification of key targets, taking into account factors such as network controllability, information propagation efficiency, and the number of node connections. Using degree as the primary criterion, we identified the top 10 targets: IL-6, ALB, TNF, AKT1, IL-1β, TP53, MMP9, CASP3, TGFB1, and PTGS2 (Fig. 7; Table 1). These targets likely represent crucial regulators in budesonide’s therapeutic mechanism for IgAN.
Molecular docking
Molecular docking validation was performed between the core targets and budesonide. The binding energy represents the energy released when a ligand (drug molecule) binds to a receptor (protein target), reflecting the strength and stability of the interaction between the ligand and receptor. A binding energy of less than -5.00 kcal/mol indicates a favorable binding interaction between the drug and the targets26. Our results demonstrated strong binding affinity between budesonide and all 10 core targets, with each binding energy < −5.00 kcal/mol. PyMOL-generated structural visualizations of these molecular interactions are presented in Fig. 8, with detailed energy values provided in Table 2.
Molecular docking models between core targets and budesonide. The numbering A-J corresponds to the numbers in Table 2. Yellow structure: budesonide; light-blue structure: core targets. Yellow dashed lines indicate hydrogen bonds with distances labeled. Green: amino acid residues with names labeled at binding sites.
Discussion
IgAN is a chronic immune-mediated kidney disease and a leading cause of renal failure worldwide. Patients who progress to ESRD often develop severe comorbidities, including infections, cardiovascular disorders, and secondary hyperparathyroidism, all of which are associated with increased mortality27. Thus, there is an urgent need to develop effective treatments for IgAN. Nefecon® (Budesonide Enteric Capsules), a novel oral corticosteroid with targeted-release properties, modulates the number and activity of Peyer’s patch B cells in the gut, thereby effectively suppressing the production of Gd-IgA1 and inhibiting the progression of IgAN28. Multiple clinical studies have demonstrated Nefecon’s efficacy in sustaining proteinuria reduction, minimizing microscopic hematuria, and preserving renal function29. However, to date, the underlying molecular mechanisms remain unclear. In this study, we aim to elucidate the potential molecular mechanisms of Nefecon on IgAN using bioinformatics approaches.
In this study, we identified 122 common targets between budesonide and IgAN, which may serve as potential therapeutic targets for budesonide intervention. GO and KEGG enrichment analyses of these common targets shed light on the potential mechanisms of budesonide in IgAN. The GO analysis revealed that significantly enriched BP terms are primarily associated with apoptosis, inflammation responses, and cell proliferation, while MF terms mainly include binding to proteins and metal ions. Additionally, the CC terms are predominantly located in the nucleus. Concurrently, KEGG enrichment analysis suggests that the therapeutic action of budesonide in IgAN may be mediated through several key pathways: “Pathways in cancer,” “Lipid and atherosclerosis,” “PI3K-Akt signaling pathway,” “AGE-RAGE signaling pathway in diabetic complications,” and “IL-17 signaling pathway.” Hyperlipidemia, by disrupting lipid metabolism, induces atherosclerosis30 and subsequently exacerbates renal impairment through mechanisms that include triggering podocyte apoptosis and stimulating the proliferation of endothelial and mesangial cells31. Similar to its role in oncogenesis32, the PI3K-Akt signaling pathway regulates cellular proliferation, survival, and cycle progression, driving mesangial hyperplasia and subsequent renal fibrosis in IgAN, while also inducing the release of pro-inflammatory cytokines (e.g., IL-6) that exacerbate glomerular injury33. The AGE-RAGE signaling pathway induces oxidative stress and upregulates pro-inflammatory mediators, including TNF-α34. IL-17 signaling pathway mediates renal inflammation in IgAN35, triggering podocyte apoptosis and downregulating podocalyxin expression36. The synergistic action of these pathways ultimately leads to renal fibrosis and renal insufficiency. These findings suggest that budesonide may exert its therapeutic effects in IgAN by modulating the aforementioned pathways, thereby influencing apoptosis, inflammatory responses, and cell proliferation processes.
Network topology analysis of 122 targets identified 10 core proteins (IL-6, ALB, TNF, AKT1, IL-1β, TP53, MMP-9, CASP3, TGF-β1, and PTGS2) ranked by degree values. Molecular docking confirmed budesonide’s strong binding affinity to these targets, suggesting their central role in the treatment of IgAN. These targets functionally converge on oxidative stress, inflammatory responses, and fibrosis. ALB, the most abundant plasma protein, regulates osmotic pressure, exacerbating renal damage by increasing glomerular plasma volume37. Additionally, studies have shown that albumin may be a primary target of oxidative stress in the development of renal insufficiency and is associated with the severity of proteinuria38. AKT1, a key regulatory kinase, controls cell growth and survival through the PI3K-Akt pathway and is involved in antioxidant stress39. Elevated AKT activation has been documented in studies examining experimental renal interstitial fibrosis40,41. This activation is a central component of various signaling pathways that are implicated in renal injury and significantly contributes to the development of renal fibrosis42. IL-6, TNF, IL-1β, and PTGS2 are related proteins involved in the inflammatory process. IL-6 activates mesangial cell proliferation, releasing core proteins including TNF, IL-1β, and TGF-β, increasing extracellular matrix (ECM) and glomerulosclerosis, leading to renal failure43. It also contributes to Gd-IgA1 production in IgAN by activating signaling pathways like PI3K-Akt44,45. TNF, a cell signaling protein involved in systemic inflammation, activates renal tubular epithelial cells after IgA deposition and leads to the activation of mesangial cells and C3 deposition in IgAN, promoting disease progression46,47. Hypoglycosylation of IgA1 is a key pathogenic factor in IgAN48. IL-1β increases IgA concentration and decreases IgA1 glycosylation, leading to mesangial immune complex deposition49. PTGS2, also referred to as cyclooxygenase-2 (COX-2), plays a pivotal role in the production of prostaglandins during inflammatory responses50, and shows increased expression in IgAN intestinal mucosa51. Active inflammation in IgAN correlates with elevated serum IgA, proteinuria, and hematuria, and increased COX-2 expression in renal interstitium suggests its role in interstitial inflammation progression52. B cells and T cells participate in Gd-IgA1 production and inflammatory infiltration in IgAN53. Jun-Jian Li et al.54, demonstrated that Caspase-3 cleavage to Cleaved Caspase-3 induces T and B cell apoptosis, slowing IgAN progression. Additionally, TP53, MMP-9, and TGF-β1, along with the previously mentioned proteins involved in regulating inflammatory processes, collectively contribute to renal fibrosis. The p53 protein, encoded by TP53, acts as a tumor suppressor that can induce cell cycle arrest or apoptosis55. Its expression is elevated in kidneys affected by IgAN, where it mediates renal tubular interstitial injury and fibrosis56. MMP-9 exhibits functional synergy with TNF and MCP-1, promoting inflammatory cell infiltration into the renal endothelium57, and its aberrant expression induces renal fibrosis and proteinuria by disrupting type IV collagen balance58. TGF-β1 promotes ECM synthesis leading to glomerulosclerosis and drives the transition of renal tubular epithelial cells to mesenchymal cells, causing renal interstitial fibrosis59,60.
This study utilized bioinformatics approaches to identify several biological pathways and therapeutic targets implicated in the treatment of IgAN with budesonide, and validated these findings through molecular docking simulations. The identified pathways include the “Lipid and atherosclerosis”, the “PI3K-Akt signaling pathway” and the “IL-17 signaling pathway”, while core proteins include IL-6 and TNF, and others. These results are in concordance with prior studies. For instance, the Cox regression risk model developed by Syrjänen et al.61 for 223 IgAN patients suggests that hyperlipidemia is an independent risk factor for the progression of IgAN; Sharon Cox et al.62, highlighted the PI3K-Akt signaling pathway as an important pathway in IgAN; Wenhui Zhu et al.47, demonstrated that the IL-17 signaling pathway is enriched and associated with disease progression in IgAN; Jung Nam An et al.63,64,65, revealed that IL-6, TNF, IL-1β, and TGF play significant roles in the development and progression of IgAN. Additionally, a genome-wide association study (GWAS) encompassing 17 independent international IgAN case–control cohorts (comprising 10,146 biopsy-defined cases and 28,751 controls) indicated that disease risk loci are strongly enriched in inflammatory responses and signaling regions66, findings that are consistent with the BP identified in our GO analysis.
However, in contrast to Ting Gan et al.67, who identified NK cell-mediated cytotoxicity as a major factor in IgAN through transcriptomic analysis, our study found that pathways such as “Pathways in cancer” and the “AGE-RAGE signaling pathway in diabetic complications” also significantly influence the therapeutic effects of budesonide. These discrepancies may stem from differences in the datasets utilized, specific bioinformatics tools employed, and the inclusion of bioinformatics analysis specific to budesonide. Further validation is necessary to resolve these differences and to provide a more comprehensive understanding of the molecular mechanisms underlying budesonide’s treatment of IgAN, thereby laying the groundwork for the development of novel therapeutic strategies for IgAN.
It is worth noting that this study has several limitations. Firstly, the proposed molecular mechanisms of budesonide lack experimental validation, which underscores the necessity for experimental studies to corroborate the computational findings. Secondly, the selection of data from public databases may introduce bias, and the reliability and accuracy of the predictions are contingent upon the quality of the data. Finally, the results of this study, based on bioinformatics computational analysis, may not fully capture the complex biological interactions in vivo. This highlights the importance of further research, including in vitro experiment, animal models, and clinical studies, employing methods such as Western blotting for protein expression validation, quantitative real-time PCR (qPCR) for mRNA expression quantification, cell-based functional assays to assess biological effects, and luciferase reporter assays to study regulatory activity, to evaluate the biological effects of the identified targets and pathways, thereby elucidating the multi-target, multi-pathway, and synergistic mechanisms of action of Budesonide Enteric Capsules.
Conclusion
In this study, we utilized bioinformatics approaches to elucidate the molecular mechanisms underlying the therapeutic effects of Budesonide Enteric Capsules in IgAN treatment. Our findings indicate that budesonide exerts its pharmacological effects through a multi-target, multi-pathway mechanism. We propose that budesonide modulates key signaling pathways, such as “Pathways in cancer,” “Lipid and atherosclerosis,” “PI3K-Akt signaling pathway,” “AGE-RAGE signaling pathway in diabetic complications,” and “IL-17 signaling pathway,” thereby influencing apoptosis, inflammatory responses, and cell proliferation in IgAN. By targeting core nodes, including IL-6, ALB, TNF, AKT1, IL-1β, TP53, MMP-9, CASP3, TGF-β1, and PTGS2, Nefecon contributes to the therapeutic management of IgAN.
Data availability
All data analyzed during this study are included in the above website. Specifically, IgAN-related targets were obtained from the GeneCards, CTD, and OMIM databases, which can be accessed at https://genecards.org/, https://ctdbase.org/, and https://omim.org/, respectively. Budesonide-related genes were retrieved from the PubChem, PharmMapper, UniProt, CTD, and TargetNet databases, available at https://pubchem.ncbi.nlm.nih.gov/, http://lilab-ecust.cn/pharmmapper/, https://uniprot.org/, https://ctdbase.org/, and http://targetnet.scbdd.com/. GO and KEGG pathway enrichment analyses were performed using the DAVID database, accessible at https://davidbioinformatics.nih.gov/. The PPI network for the IgAN-budesonide-related targets was constructed using the STRING database, available at https://cn.string-db.org/. The 3D structures of the top 10 core targets used for molecular docking were obtained from the PDB database at https://www.rcsb.org/. All data used in this study are publicly available and can be directly accessed through the provided links.
References
Zhang, H. & Barratt, J. Is IgA nephropathy the same disease in different parts of the world?. Semin Immunopathol. 43, 707–715 (2021).
Barbour, S. J. et al. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med. 179, 942–952 (2019).
Tamura, H. IgA nephropathy associated with Crohn’s disease. World J Methodol. 13, 67–78 (2023).
Nasri, H. Letter to the article: association of C1q deposition with renal outcomes in IgA nephropathy Clin Nephrol. 2013; 80: 98–104. Clin Nephrol. 81, 228–229 (2014).
Floege, J. et al. Executive summary of the KDIGO 2024 clinical practice guideline for the management of ANCA-associated vasculitis. Kidney Int. 105, 447–449 (2024).
Lv, J. et al. Effect of oral methylprednisolone on decline in kidney function or kidney failure in patients with IgA nephropathy: The TESTING randomized clinical trial. JAMA 327, 1888–1898 (2022).
Barratt, J., Kristensen, J., Pedersen, C. & Jerling, M. Insights on nefecon((R)), a targeted-release formulation of budesonide and its selective immunomodulatory effects in patients with IgA nephropathy. Drug Des Devel Ther. 18, 3415–3428 (2024).
Barratt, J., Lafayette, R. A. & Floege, J. Therapy of IgA nephropathy: time for a paradigm change. Front Med (Lausanne). 11, 1461879 (2024).
Smerud, H. K. et al. New treatment for IgA nephropathy: enteric budesonide targeted to the ileocecal region ameliorates proteinuria. Nephrol Dial Transplant. 26, 3237–3242 (2011).
Barratt, J., Lafayette, R. A., Rovin, B. H. & Fellstrom, B. Budesonide delayed-release capsules to reduce proteinuria in adults with primary immunoglobulin A nephropathy. Expert Rev Clin Immunol. 19, 699–710 (2023).
Barratt, J. et al. Why target the gut to treat IgA nephropathy?. Kidney Int Rep. 5, 1620–1624 (2020).
Smith, A. C., Molyneux, K., Feehally, J. & Barratt, J. O-glycosylation of serum IgA1 antibodies against mucosal and systemic antigens in IgA nephropathy. J Am Soc Nephrol. 17, 3520–3528 (2006).
Lafayette, R. et al. Efficacy and safety of a targeted-release formulation of budesonide in patients with primary IgA nephropathy (NefIgArd): 2-year results from a randomised phase 3 trial. Lancet 402, 859–870 (2023).
Barshir, R. et al. GeneCaRNA: A comprehensive gene-centric database of human non-coding RNAs in the genecards suite. J Mol Biol. 433, 166913 (2021).
Davis, A. P. et al. Comparative toxicogenomics database (ctd): update 2023. Nucleic Acids Res. 51, D1257–D1262 (2023).
Amberger, J. S. & Hamosh, A. Searching online mendelian inheritance in man (OMIM): A knowledgebase of human genes and genetic phenotypes. Curr Protoc Bioinf. 58, 1–2 (2017).
Kim, S. et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 49, D1388–D1395 (2021).
Wang, X. et al. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 45, W356–W360 (2017).
Yao, Z. J. et al. TargetNet: A web service for predicting potential drug-target interaction profiling via multi-target SAR models. J Comput Aided Mol Des. 30, 413–424 (2016).
Zhu, J., Zhao, Q., Katsevich, E. & Sabatti, C. Exploratory gene ontology analysis with interactive visualization. Sci Rep. 9, 7793 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–D677 (2025).
Szklarczyk, D. et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 49, D605–D612 (2021).
Velankar, S., Burley, S. K., Kurisu, G., Hoch, J. C. & Markley, J. L. The protein data bank archive. Methods Mol Biol. 2305, 3–21 (2021).
Nithya, C., Kiran, M. & Nagarajaram, H. A. Hubs and Bottlenecks in protein-protein interaction networks. Methods Mol Biol. 2719, 227–248 (2024).
Chea, E. & Livesay, D. R. How accurate and statistically robust are catalytic site predictions based on closeness centrality?. BMC Bioinf 8, 153 (2007).
Hsin, K. Y., Ghosh, S. & Kitano, H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS ONE 8, e83922 (2013).
Deng, X. et al. Unraveling the mechanism of zhibaidihuang decoction against IgA nephropathy using network pharmacology and molecular docking analyses. Tohoku J Exp Med. 259, 37–47 (2022).
Lai, K. N., Leung, J. C., Tang, S. C. Recent advances in the understanding and management of IgA nephropathy. F1000Res. 5 (2016).
Barratt, J. et al. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int. 103, 391–402 (2023).
Wang, J. et al. The role of hypertriglyceridemia and treatment patterns in the progression of IgA nephropathy with a high proportion of global glomerulosclerosis. Int Urol Nephrol. 52, 325–335 (2020).
Hua, W. et al. CD36 mediated fatty acid-induced podocyte apoptosis via oxidative stress. PLoS ONE 10, e0127507 (2015).
Si, S., Liu, H., Xu, L. & Zhan, S. Identification of novel therapeutic targets for chronic kidney disease and kidney function by integrating multi-omics proteome with transcriptome. Genome Med. 16, 84 (2024).
Tamouza, H. et al. The IgA1 immune complex-mediated activation of the MAPK/ERK kinase pathway in mesangial cells is associated with glomerular damage in IgA nephropathy. Kidney Int. 82, 1284–1296 (2012).
Zhang, L. et al. Exploring the mechanisms underlying the therapeutic effect of Salvia miltiorrhiza in diabetic nephropathy using network pharmacology and molecular docking. Biosci Rep. 41(6), 202 (2021).
Matsumoto, K. & Kanmatsuse, K. Interleukin-17 stimulates the release of pro-inflammatory cytokines by blood monocytes in patients with IgA nephropathy. Scand J Urol Nephrol. 37, 164–171 (2003).
Wang, L. et al. The role of Th17/IL-17 in the pathogenesis of primary nephrotic syndrome in children. Kidney Blood Press Res. 37, 332–345 (2013).
Li, X. et al. Identifying potential biomarkers for the diagnosis and treatment of IgA nephropathy based on bioinformatics analysis. BMC Med Genomics. 16, 63 (2023).
Camilla, R. et al. Oxidative stress and galactose-deficient IgA1 as markers of progression in IgA nephropathy. Clin J Am Soc Nephrol. 6, 1903–1911 (2011).
Chen, S., Li, B., Chen, L. & Jiang, H. Uncovering the mechanism of resveratrol in the treatment of diabetic kidney disease based on network pharmacology, molecular docking, and experimental validation. J Transl Med. 21, 380 (2023).
Kim, I. Y. et al. Role of Akt1 in renal fibrosis and tubular dedifferentiation during the progression of acute kidney injury to chronic kidney disease. Korean J Intern Med. 36, 962–974 (2021).
Lan, A. & Du, J. Potential role of Akt signaling in chronic kidney disease. Nephrol Dial Transplant. 30, 385–394 (2015).
Gaumond, L. et al. Identification of inflammatory biomarkers in IgA nephropathy using the NanoString technology: A validation study in Caucasians. Inflamm Res. 73, 447–457 (2024).
Groza, Y., Jemelkova, J., Kafkova, L. R., Maly, P. & Raska, M. IL-6 and its role in IgA nephropathy development. Cytokine Growth Factor Rev. 66, 1–14 (2022).
Nagy, J., Per, B., Trinn, C., Nagy, G. & Burger, T. Incidence of IgA (monomer-dimer, IgA1-IgA2) and IgG producing cells in the tonsils of patients with IgA nephropathy. Orv Hetil. 129, 1481–1485 (1988).
Lai, A. S. & Lai, K. N. Molecular basis of IgA nephropathy. Curr Mol Med. 5, 475–487 (2005).
Webster, J. D. & Vucic, D. The balance of TNF mediated pathways regulates inflammatory cell death signaling in healthy and diseased tissues. Front Cell Dev Biol. 8, 365 (2020).
Zhu, W. et al. Ferroptosis-related genes in IgA nephropathy: Screening for potential targets of the mechanism. Int J Genomics. 2024, 8851124 (2024).
Nakazawa, S. et al. Difference in IgA1 O-glycosylation between IgA deposition donors and IgA nephropathy recipients. Biochem Biophys Res Commun. 508, 1106–1112 (2019).
Liu, C. et al. microRNA-630 regulates underglycosylated IgA1 production in the tonsils by targeting TLR4 in IgA nephropathy. Front Immunol. 11, 563699 (2020).
Xia, M. et al. Based on network pharmacology tools to investigate the mechanism of tripterygium wilfordii against IgA nephropathy. Front Med (Lausanne). 8, 794962 (2021).
Honkanen, T. et al. Small bowel cyclooxygenase 2 (COX-2) expression in patients with IgA nephropathy. Kidney Int. 67, 2187–2195 (2005).
Hartner, A., Pahl, A., Brune, K. & Goppelt-Struebe, M. Upregulation of cyclooxygenase-1 and the PGE2 receptor EP2 in rat and human mesangioproliferative glomerulonephritis. Inflamm Res. 49, 345–354 (2000).
Liang, Y., Zeng, Q., Wang, X. H., Yan, L. & Yu, R. H. Mechanism of Yiqi Yangying Heluo formula in the treatment of IgA nephropathy by affecting Gd-IgA1 based on BAFF molecular level and T lymphocyte immunity. Biomed Res Int. 2023, 5124034 (2023).
Li, J. J. et al. Sinomenine hydrochloride protects IgA nephropathy through regulating cell growth and apoptosis of T and B lymphocytes. Drug Des Devel Ther. 18, 1247–1262 (2024).
Liu, H. et al. The mechanism of Shenbing decoction II against IgA nephropathy renal fibrosis revealed by UPLC-MS/MS, network pharmacology and experimental verification. Heliyon. 9, e21997 (2023).
Yang, R. et al. p53 induces miR199a-3p to suppress SOCS7 for STAT3 activation and renal fibrosis in UUO. Sci Rep. 7, 43409 (2017).
Wang, Y. et al. Intermedin ameliorates IgA nephropathy by inhibition of oxidative stress and inflammation. Clin Exp Med. 16, 183–192 (2016).
Neprasova, M. et al. Serum and urine biomarkers related to kidney fibrosis predict kidney outcome in Czech patients with IgA nephropathy. Int J Mol Sci. 24(3), 2064 (2023).
Yang, L. et al. Effects of periostracum cicadae on cytokines and apoptosis regulatory proteins in an IgA nephropathy rat model. Int J Mol Sci. 19(6), 1599 (2018).
Li, Z., Li, Y. & Chen, L. Association between transforming growth factor-beta1gene-509C/T polymorphism and susceptibility of IgA nephropathy: A meta-analysis. Ren Fail. 36, 1473–1480 (2014).
Syrjanen, J., Mustonen, J. & Pasternack, A. Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant. 15, 34–42 (2000).
Cox, S. N. et al. Altered modulation of WNT-beta-catenin and PI3K/Akt pathways in IgA nephropathy. Kidney Int. 78, 396–407 (2010).
An, J. N. et al. Urinary cMet as a prognostic marker in immunoglobulin A nephropathy. J Cell Mol Med. 24, 11158–11169 (2020).
Cao, Y. et al. BAFF is involved in the pathogenesis of IgA nephropathy by activating the TRAF6/NF-kappaB signaling pathway in glomerular mesangial cells. Mol Med Rep. 21, 795–805 (2020).
Yeo, S. C. & Barratt, J. The contribution of a proliferation-inducing ligand (APRIL) and other TNF superfamily members in pathogenesis and progression of IgA nephropathy. Clin Kidney J. 16, ii9–ii18 (2023).
Kiryluk, K. et al. Genome-wide association analyses define pathogenic signaling pathways and prioritize drug targets for IgA nephropathy. Nat Genet. 55, 1091–1105 (2023).
Gan, T. et al. Unveiling biomarkers and therapeutic targets in IgA nephropathy through large-scale blood transcriptome analysis. Int Immunopharmacol. 132, 111905 (2024).
Acknowledgements
The author would like to express gratitude to the researchers and staff involved in the software and databases utilized in this study.
Funding
This work was supported by the Zhongshan Hospital of Xiamen University Discipline Reserve Talent Training Program 2024 and the Fujian Provincial Nature and Science Foundation [Grant No. 2025J01120421].
Author information
Authors and Affiliations
Contributions
Mengshu Lin and Shengji Chen designed the experiments, and Mengshu Lin collected the data and wrote the main manuscript text. Shengji Chen analyzed the data and Yixuan Chen drew the pictures. Qing Gao critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, M., Chen, S., Chen, Y. et al. Exploring the molecular mechanism of budesonide enteric capsules in the treatment of IgA nephropathy based on bioinformatics. Sci Rep 15, 30795 (2025). https://doi.org/10.1038/s41598-025-16380-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16380-z