Abstract
Salinity is one of the biggest limitations of agriculture in semi-arid regions of the world. It negatively impacts the growth and yield of Zea mays L. Especially the seedling stage is extremely sensitive to salt stress. Sargassum tenerrimum J. Agardh. is a macroalgae which produces a plethora of important secondary metabolites, micro and macro-nutrients, polyamines and natural phytohormones etc. These compounds have been reported to improve plant growth and alleviate the harmful effects of salt stress. The current study explores the effectiveness of algal based Zinc Oxide nanoparticles (ZnOSt-NPs) in alleviating NaCl stress in Zea mays. The study involved two levels of NaCl (0 = control and 200 mmol kg− 1) and four levels (0, 30, 60 and 80 ppm) of ZnOSt-NPs applied through foliar spray. A notable enhancement was observed for morphological parameters Shoot length: 18.92%; root length: 18.56%; shoot fresh weight: 38.94%; dry weight: 23.74% and root fresh weight: 21.43% dry weight (27.97%) photosynthetic pigments (chl a: 40.98%; chl b: 93.55%; total chlorophyll: 55.38% and carotenoid 43.5%) and chlorophyll fluorescence parameters also exhibited a remarkable enhancement at 80 ppm ZNOSt-NPs under salt stress. Decrease in the levels of antioxidant enzymes (POD: 25.67%; SOD: 25.67%; CAT: 59.85% and APX: 54.21%) as well as non-enzymatic anti-oxidants (GSH: 34.67%; GR: 52.80%; AsA: 10.47% and lycopene: 34.40%) were also observed at 80 ppm ZnOSt-NPs under salt stress. The foliar application of ZnOSt-NPs significantly decreased MDA and H2O2 levels 38.40% and 32.80% and uptake of Na⁺ (34.25%) and Cl− (38.65%) respectively. while an increase in the total soluble proteins (87.50%), K+ uptake (77.27%), water content (74.63%) and salt tolerance index (17.12%) was observed with application of 80 ppm ZnOSt-NPs. This study provides important insight into the potential of algal based ZnO-NPs as a low cost and ecofriendly method for managing salinized soil.
Similar content being viewed by others
Introduction
Salinity is an abiotic factor which is harmful to agricultural plant growth and development. In contrast, the increasing demand of agricultural products is necessary to nourish an additional 2 billion people by 2050 1. However, the continuously increasing salinization of arable land may make it more difficult for the globe to constantly supply food for its expanding population2. Salinity stress causes essential cereal crops like maize, rice, and wheat to yield less every year3. Pakistan around 8.6 million hectares (mha) of agricultural land is useless due to salinity4. Salinity adversely affects the plant’s cellular membranes, reduces photosynthetic efficiency, disturbs nutritional balance, alters the concentrations of growth regulators, inhibits enzyme activity, and causes metabolic disturbances5. Furthermore, ion toxicity within plant cells is triggered by excessive Na and Cl influx and uptake under salinity that alter nutrient homeostasis like K+/Na⁺ ratio6. Plants have an inherent defense system that limits the accumulation of harmful ions in cytoplasm via sequestering these ions in vacuoles or apoplast, producing osmoprotective substances, controlling the production of reactive species by both enzyme- and non-enzyme-based antioxidants and controlling stomatal functions to reduce water loss in plants under salinity stress7. The green synthesis of nanoparticles (NPs) is thought to be a simple, safe, and environmentally friendly process that uses non-toxic reducing agents instead of hazardous chemicals8. Nanoparticles have remarkable chemical and physical capabilities due to their minute size and have high surface area-to-volume ratio. Researchers are exploring the beneficial role of nanoparticles in agriculture focusing on enhancing yields, improving plant health and stress tolerance9. Zinc oxide nanoparticles (ZnO-NPs) are metallic NPs that have a vital and crucial role in plant growth under harsh environmental conditions especially salinity stress10. Previously reported that the application of ZnO-NPs increases salinity tolerance of cotton11sorghum12Abelmoschus esculentus L. Moench13rapeseed, Triticum aestivum L., Zea mays, sunflower and spinach14,15,16,17,18. ZnO-NPs are considered to improve plant growth, photosynthetic rates, and activity of antioxidant enzymes under salinity stress. Moreover, ZnO-NPs is a promising method in the release of osmoprotectants and phytohormones to relieve salt stress in plants. The utilization of algae as nanoparticles is considered one of the most effective methods because of their capacity to both accumulate and release metal ion19. Seaweed extract has been shown by numerous researchers to have stimulating effects on a wide range of vegetable crops, including seed germination, early seedling growth, vigor, and the improvement of seedling establishment. Sargassum is one of the biggest and most diverse genera of brown seaweed in the category of marine algae20. Rice productivity and grain quality have increased with the addition of algae, which has also lessened the negative impacts of salt and water stress21. Furthermore, Triticum aestivum L. has been shown to benefit from the use of Sargassum latifolium algal extracts in reducing the adverse effects of drought22. Similarly, Abelmoschus esculentus has shown enhanced salt stress tolerance when treated with Sargassum wightii aqueous extract23. Sargassum tenerrimum J. Agardh. extract applied to tomato plants increases their resistance to Macrophomina via controlling antioxidants and hormones, which in turn increases yield.
Zea mays L., or maize, is a crop of great significance known for its high yield24. This adaptable crop is both a staple food source and a vital raw ingredient for many industrial items produced worldwide. Maize seeds contain 72% carbohydrate, 10% protein, 3% sugar, 4.8% oil and 5.8% fiber25. 60% of Pakistan’s poultry industry depends on maize26. An elevated level of Na is a significant threat to maize growth by disrupting potassium balance and stomatal regulation27. Maize plants respond to salt stress by producing reactive oxygen species (ROS), which in turn creates oxidative stress that impedes in plant’s important metabolic functions and damages DNA and causes peroxidation of membranes28. Previously, reported the beneficial impact of ZnO-NPs on growth and antioxidant enzyme activity in Maize when exposed to salinity, the foliar application of ZnO-NPs improved growth attributes, physiological performance, nutrient profiles, antioxidant activity, and yield-contributing characteristics29. Moreover, seaweed based ZnO-NPs are reported to have a positive effect on biomass development, photosynthetic pigments and reduce damage. However, there is still no information regarding the seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of maize especially under salinity stress. Moreover, to keep in mind the importance and demand of maize especially in future, this experiment is planned to evaluate and determine the foliar application of Sargassum tenerrimum based ZnO-NPs to enhance the morphological, physiological and biochemical characteristics of Zea mays under saline conditions.
Materials and methods
Collection and preparation of Sargassum tenerrimum extract
Sargassum tennerimum J. Agardh. was collected from the coastal areas of Karachi and identified by herbarium located in University of Karachi (specimen voucher no. KUH-ST No. 150-x a). Hot water algal extract was prepared by following the procedure described30. First algae were washed with fresh water then shade dried in air at daytime temperature reaching up to 40°C. Dry algae were grounded to particle size less than 2 mm and stored at room temperature in airtight plastic bottles. Algal powder (5 g) was added to 20 ml distilled water (DW) to prepare extract, the grout was incubated at 50°C for 3 h and stirred continuously at 140 rpm. The slurry was subjected to centrifugation at 10,000 g for 15 min. The filtrate was scooped, and the remaining pellet was extracted three times. Every time the supernatants were collected and freeze dried. The stock solution of algal extract was synthesized by mixing the freeze-dried extract in distilled water. Required dilutions were prepared accordingly.
Synthesis of S. tenerrimum based zinc oxide nanoparticles
For the synthesis of S. tenerrimum extract based ZnO-NPs (ZnOSt-NPs), deionized H2O as a solvent was used in the whole procedure. Zinc acetate dehydrate/Zn (CH3CO2)2.2H2O (4.5 g) of 100 ml of deionized water and heated on hot plate with continuous mixing. Stock solution of 0.25 M Zn was prepared following this procedure. The resulting algae extract was added to the stock solution of zinc acetate. The mixture was heated at 70°C with continuous stirring on a magnetic stirrer for 2 h. The remaining solution was evaporated using a hot plate, in a ceramic crucible cup the residue was placed and was calcined in a muffle furnace for 2 h at 200°C to remove contamination. The mixture changed color which was an indication of synthesis of ZnOSt-NPs31 (See supplementary Fig. S1 online). Solid pale brown colored matter was isolated and powdered in a sterilized mortar/pestle. Many pilot experiments were conducted to achieve the standardized concentration of both Zinc NPs and algal extract.
Characterization
The UV-vis absorption spectra of pure S. tenerrimum extract and aqueous solution of ZNOSt-NPs were recorded in 300–700 nm spectral regions via Shimadzu-UV 2600 spectrophotometer. Using a Perkin Elmer branded FTIR spectrophotometer, the vibrational spectra of S. tenerrimum extract and ZNOSt-NPs were obtained throughout the 400–4000 cm− 1 frequency range. The surface structure of S. tenerrimum extract and morphology of ZnOSt-NPs were examined by the ZEISS scanning electron microscope (SEM). The SEM was equipped with energy-dispersive X-ray (EDX) spectroscopy which was employed to check the produced NPs’ chemical composition and purity32. To measure the surface charge (stability) of Algae-produced ZnO NPs, Zeta (ζ) potential value was measured using Malvern ZETASIZER MAL1285161 brand while these NPs were dispersed in deionized water.
Experimental design
The seeds of Zea mays L. FH-1046 hybrid were collected from Maize and Millets Research Institute, Sahiwal. The green house experiment was performed at the department of Botany, The Islamia university of Bahawalpur, Pakistan (29° 24’ N and 71° 40’ E). The experiment was carried out in a completely randomized matter (CRD). The temperature (day and night), humidity and light intensity of the greenhouse were recorded daily. The temperature ranges between 24.5 and 52°C in summer and between 10.9 and 20.3 °C in winter, while 28.62%-59.92% of humidity Each pot was filled with about 4 kg of loamy soil (pH: 8.44; ECe: 3.33 mS cm2; Organic matter: 0.41%; Available Phosphorus: 5.78 mg kg− 1; Available Potassium: 112 mg kg− 1; Available Na⁺: 3.1 ppm).
Soil analysis
Soil analysis was done before and after treatment application. The methodology explained by Hardie & Doyle33 a very important parameter is the soil moisture content, which was measured first, and dry soil was used in the 1:2 method. Loamy soil was used for the experiment.
Salinity treatment
Seeds of Zea mays were first sterilized using HgCl2 and then washed five times with double distilled water (DDW). Seven seeds were sown in each pot and at the time of four-leaf stage (30 days old seedlings) only four similar seedlings were selected in each pot for experiment. Salinity treatment was gradually (@ 50 mM to avoid osmotic shock) increased up to 200 mM. All the possible controls were set up. The pots were divided into two groups, salt stress and control (without salinity). Pots were watered once daily until they reached 70% field capacity moisture. Equal quantity and desired salinity levels were maintained by measuring electrical conductivity at regular intervals34 until plants were harvested.
Foliar application of ZnOSt-NPs
When the desired salinity was maintained, four different concentrations (0, 30, 60 and 80 ppm) of ZnOSt-NPs solutions were sprayed with foliar spray (25 ml for each concentration after every 24 h) to the seedlings. After 20 days of salinity treatment, plants were harvested.
Growth parameters
Shoot length (SL), root length (RL), number of leaves, shoot dry weight, root dry weight of all treated maize plants was measured. Plants of each treatment were dried by oven drying method at a constant temperature of 100–105°C for 4 h to measure tissue dry weight.
Photosynthetic attributes
To estimate the chlorophyll in leaves, Brougham’s method35 was employed. About 1 g green leaf was ground in a mortar and pestle then cool. The chlorophyll content was extracted through repeated homogenization using 80% cooled acetone (20 ml DW + 80 ml acetone). The supernatant was filtered and diluted to measure photosynthetic pigments via absorbance in a double-beam UV spectrophotometer (Systronics 128) at 663, 645, 537 and 480 nm using the Arnon method36. Lycopene was measured by Amiri-Rigi and Abbasi37.
Determination of chlorophyll fluorescence parameters
Chlorophyll fluorescence parameters (Phi2, PhiNPQ, qL, NPQt, PhiNO and Fv′/Fm′,) were measured in light using a Portable synQ device.
Total soluble proteins
The total soluble protein was determined with the help of a standard curve demonstrated by definite concentrations of bovine serum albumin (BSA). 1 ml extract of the third leaf of maize seedling was taken in a test tube. Phosphate buffer was prepared at 1 ml with pH 7.0 in log. Reagents containing test tubes were placed at room temperature for 1 min. Furthermore, 0.5 ml of Folin-phenol reagent was mixed and incubated for 30 min. The optical range was measured by a spectrometer at 620 nm38.
Determination of oxidative stress markers
Malondialdehyde (MDA) content was calculated by a modified thiobarbituric acid method39. Plant samples (0.5 g) were homogenized in chilled 4 ml of 1% (w/v) trichloroacetic acid (TCA) with a mortar and pestle at 4°C. Homogenates were centrifuged for 20 min at 12,000 g. A reaction mixture (3 ml) containing 20% (w/v) TCA and 0.5% (w/v) thiobarbituric acid (TBA) was added as a supernatant (1 ml). The mixture was incubated at 95 °C for 30 min before being swiftly placed in a cold bath to cease the process. The fraction’s absorbance was measured at 440, 532, and 600 nm. H2O2 content was measured by methodology described by Velikova et al.40. Fresh leaves (500 mg) were homogenized using a pre-chilled pestle and mortar with 5 mL of 0.1% (w/v) TCA. The mixture was then centrifuged for 15 min at 12,000×g. The resulting supernatant (0.5 ml) was combined with 1 ml of 1 M potassium iodide (KI) and 0.5 ml of 0.05 M phosphate buffer (pH 7.0). After shaking the mixture in a vortex, the absorbance was measured at 390 nm using a UV-visible spectrophotometer, with water as the blank.
Study of antioxidant enzymes
The antioxidant enzyme assays (peroxidases, superoxide dismutases, catalases, and ascorbate peroxidases) were measured by following their respective protocols. Distilled water inoculated samples will be used as a control group. About 1 gram of maize seedling was homogenized with 1 ml of 50 mM potassium-phosphate 7 pH buffer. The resulting mixture was then centrifuged at 12,000 rpm for 15 min at 4°C using.
Peroxidase assay
Peroxidase was evaluated using the Chance & Maehly41 approach. In 0.1 ml of sample enzyme extract, 50 mM potassium phosphate buffer pH 5, 20 mM guaiacol, and 40 mM H2O2 were mixed. Optical density was calculated at 470 nm every 20 s.
Catalase assay
The reaction mixture (3 ml) was tested, which contained 50 mM phosphate buffer (pH 6.0), 5.9 mM H2O2, and 0.1 mL enzyme extract. The CAT activity was determined by measuring the change in absorbance owing to H2O2 intake at 240 nm every 20 s41.
Superoxide dismutase assay
The ability of SOD to prevent the photochemical reduction of nitro blue tetrazolium (NBT). At 560 nm, the optical density (OD) was measured by using Giannopolitis method42. The reaction mixture comprises 50 mM sodium phosphate buffer (pH 7.8), 13 mM methionine, 2 mM riboflavin, 75 mM NBT, 100 mM EDTA, and 100 mL enzyme extract.
Ascorbate peroxidase assay
APX activity was assessed using a spectrophotometer set to 290 nm, with the reaction being observed for one minute at 30°C. The reaction mixture consisted of plant extract, 50 mM potassium phosphate buffer (pH 7.5), 0.5 mM ascorbic acid, 0.1 mM EDTA, and 0.1 mM hydrogen peroxide.
Glutathione reductase (GR) assay
The activity of GR was determined using the method of Foyer and Halliwell43by tracking the NADPH-dependent oxidation of glutathione at 340 nm. The assay solution contained 25 mM phosphate buffer (pH 7.8), 0.5 mM oxidized glutathione (GSSG), 0.2 mM NADPH, and the enzyme extract. GR activity was calculated with an extinction coefficient of 6.2 mM− 1 cm− 1. One unit of enzyme activity was defined as the amount required for oxidizing 1 µmol of NADPH per minute at 25°C.
Non-enzymatic antioxidants
Around 100 mg of fresh tissue were ground in a MagNA Lyser (Roche, Vilvoorde, Belgium), and extracted in ice-cold 6% (v/v) phosphoric acid. Reduced AsA and GSH contents were determined by HPLC analysis. The identity of the peaks was confirmed using an in-line diode array detector (DAD, SPD-M10AVP, Shimadzu). Total AsA and GSH concentration were determined after reducing the samples with 40 mM DTT and the AsA and GSH redox status are calculated as the ratio between reduced and total amount of AsA and GSH respectively.
Ion analysis
Oven dried plant materials were converted to ash in a muffle furnace at 550°C for 5 h. Ash samples were then digested in 1 mL of 20% HCl at 60°C on a hot plate for 15 min. Samples were allowed to cool down at room temperature, dissolved in distilled water and passed through a Whatman No. 42 filter paper. Cations (Na⁺ and K+) were then measured with the help of a flame photometer (Jenway PFP 7), while Chloride (Cl−) was estimated by using chloride analyzer (Corning-920, Germany).
Relative water content
Relative water content (RWC) was calculated by determining the weight of fresh leaf discs and turgid leaf disc after immersing in water fir few hours. After that, the tissue was dried in oven to a constant weight and RWC was determined from the equation given below:
\({\text{RWC }}\left( \% \right)=\left[ {\left( {{\text{FW}} - {\text{DW}}} \right)/\left( {{\text{TW}} - {\text{DW}}} \right)} \right]*{\text{1}}00\)
Where, FW: sample fresh weight; TW: sample turgid weight; DW: sample dry weight.
Salinity tolerance index
Salt Tolerance Index was determined by the following formula:
\({\text{STI}}=\left( {{\text{Tsalt}}/{\text{Tcont}}} \right) * {\text{1}}00\)
In the equation, Tsalt is the mean value of the dry weight under the highest salinity level, and Tcont is the mean value of the dry weight under the control treatment.
Statistical analysis
All experiments were performed in four replicates. The standard error and arithmetic mean were computed by using the software SPSS Inc (16.0) (SPSS, 2007). A paired comparison using two-way factorial analysis was executed. The treatment means were compared by using Tukey test with P < 0.05 used as a significant level. Origin 2021 Pro software was used for making graphs.
Results
Characterization of ZnOSt-NPs
For UV-vis spectroscopic analysis, pure Algal extract and aqueous solution of ZnOSt-NPs were used to spot their production and optical features. Both samples showed no absorption in the visible region. The wide bandgap ZnO material at nanoscale usually shows absorption in the UV region (below 300 nm), and such typical behavior of ZnOSt-NPs is shown in Fig. 1A.
(A) The UV-visible absorption spectra of Algal extract (red line) and Algal based ZnO NPs (black line), (B) Scanning electron microscopy of ZnOSt-NPs, (C) EDX map of ZnOSt-NPs, (D) FTIR analyses of Algal extract and ZnOSt-NPs samples to confirm presence of various active functional groups in marine Algae (Sargassum tenerrimum) and (E) Zeta potential of ZnOSt-NPs (colored lines represent replicates of the same sample).
The SEM image of ZnOSt-NPs in Fig. 1B exhibits 3D nanostructures with average size seems to be of few hundred nanometers. Some of the NPs are cubic-like, while others appear as rectangular and multi-dimensional spread throughout the Algal mass. In SEM image, the coalescence factor of the NPs might be due to trapping behavior of the Algal extract. For elemental analysis of ZnOSt-NPs, Fig. 1C shows the corresponding EDX analysis revealing the occurrence of various contents of multivalent metal elements in Algae i.e. Ca and Mg elements.
Various bioactive compounds of marine Algae can contribute significantly to stabilize and functionalize ZnO-NPs, confirmed by FTIR spectra of Algal extract and ZnOSt-NPs in Fig. 1D. After ZnOSt-NPs formation, most of the shifting in the IR peaks appeared between 400 and 1650 cm− 1, which could be related to the existence of polyphenols, carbohydrates, proteins, phenolics, and aromatic amines with slight deviations in the positions of these previously identified bands of Algae seaweed. Presence of aromatic C–H bend (out of plane) and halogenated organic compounds can be observed below 900 cm− 1 in both spectra. However, the reduced band signals of O–Zn–O stretching around 450 cm–1 are out of the provided frequency range that is why they could not be detected.
Pure Algal extract shows a sharp IR peak at 1030 cm–1, which can be assigned to C–N amines and C–O integrated esters, ethers, alcohols, anhydrides and carboxyl groups. This peak suffered a slight shift to higher frequencies in case of ZnOSt-NPs spectrum. Evidence of a weak IR peak at about 1238.7 cm–1 also supports presence of these carbon-integrated compounds in both samples. Signals of aliphatic fluorocarbon occur at ~ 1406 cm–1. Further organic chemical groups are clear from a sharp IR peak at 1633 cm–1 corresponding to C = C alkene, N–H bend, and C = O amides. Such cyclic peptides could help in the stabilization of ZnO-NPs44. Endorsement of aldehyde is evident by a weak IR signal at 2915.7 cm–1. The observed broad band ranging from 3200 to 3500 cm− 1 implies the stretching vibration of N–H and O–H bonds within various compounds of Algal extract structure, such as proteins, alginates, lipids, and cellulose45. This IR analysis indicates a clear interaction of complexes between the bioactive groups of the Algae chemical structure and the functionalized ZnOSt-NPs. The zeta potential analysis was performed to observe the charge on the surface of biologically synthesized ZnOSt-NPs46. The results unequivocally showed that the zeta potential value for S. tenerrimum based ZnO NPs was detected at 35.71 ± 1.87 mV (Fig. 1.E). Our results matched with the previously published work47. S. tenerrimum extract is composed of a balance of positively and negatively charged molecules, due to which ZnO NPs have high positive values for zeta potential48. Because of the large positive zeta potential, ZnO will repel each other and not tend to agglomerate49.
Soil analysis
The results revealed that foliar spray of ZnOSt-NPs significantly improved soil properties. Electric conductivity increased in 0mM salinity (4.67 mS cm− 1, 5.33 mS cm− 1, 5.80 mS cm− 1) and in 200mM salinity (20.85 mS cm− 1, 13.05 mS cm− 1,18.38 mS cm− 1,20.08 mS cm− 1). Moreover, the decrease in pH was recorded in 0mM salt (7.33,7.31,7.24) and 200 mm salt (7.14,7.30,7.13). Soil organic matter increased in no salt stress (13.04%,19.81%, 52.94%) and in salt stress (10.52%, 6,16%, 11.94%). Similarly, an increase in available phosphorous was observed in 0 mM salinity (8.36%,27.46%,31.35%) and in200 mM salinity (4.92%,17.93%,21.01%). Additionally, available potassium increased under both 0 and 200mM salinity (3.70%,16.71%,38.98%), (5.67%, 42.97%,46.92%). Available Nitrogen increased in both salinity concentrations (7.22%,13.96%,20.22%) and (11.67%, 19.71%, 24.64%) while available sodium decreased in 0mM salt (23.10%,48.88%, 79.91%) and in 200mM salt (13.26%,50.31%, 65.75%) respectively (Table 1).
Growth parameters
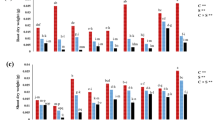
In the absence of salinity stress (-SS), all doses of ZnOSt-NPs significantly increased in root length (10–15%), shoot length (10 to 20%), and number of leaves (10 to 25%) as compared to the without NPs control. Whereas, in the presence of salinity stress (+ SS), ZnOSt-NPs treatment showed a significant increase in root length (10–20%), shoot length (12–20%), and number of leaves (33%), in contrast to unsprayed plants of 200 mM salinity. The application of ZnOSt-NPs exhibited an increase around 10–20% in root fresh weight, 25–40% in shoot fresh weight, 7–16% in root dry weight, and 20–35% in shoot dry weight under –SS condition. Whereas, The ZnOSt-NPs treatments increased 15 to 21% root fresh weight, 23–40% in shoot fresh weight, 12–30% in root dry weight, and 12–25% in shoot dry weight in comparison of unsprayed plants of 200 mM salinity (Fig. 2).
Effect of different concentrations of ZNOSt-NP on (A) root length, (B) shoot length, (C) number of leaves, (D) fresh weight root, (E) fresh weight shoot, (F) dry weight root, and (G) dry weight shoot of Zea mays cultivated without salinity stress (No SS; pink bars) and with salinity stress (SS; yellow bars). The Tukey test showed significant changes at P < 0.05, as indicated by the different letters on the bars representing the mean of four replicates.
Ion analysis, relative water content and salinity tolerance index
Foliar application of ZnOSt-NPs at concentrations of 30, 60, and 80 ppm significantly reduced Na⁺ and Cl⁻ uptake in both salt-stressed (-SS) and non-stressed (No SS) plants (20.83%, 25.53%, 34.25%) and (21.82%, 29.03%, 38.65%). Similarly, in the No SS group, Na⁺ uptake decreased by 23.25%, 25.00%, and 32.26%, and Cl⁻ uptake was reduced by 66.60%, 70.96%, and 85.81%. Conversely, K⁺ uptake increased significantly in response to ZnOSt-NPs application, with increases of 61.53%, 69.87%, and 77.27% in the -SS group and 40.00%, 48.84%, and 53.57% in the No SS group. Relative water content (RWC) also improved with treatment, showing increase of 53.78%, 65.81%, and 74.63% in the -SS group and 10.61%, 23.05%, and 46.38% in the No SS group. Additionally, the salinity tolerance index was enhanced under salt stress conditions, with ZnOSt-NPs leading to increases of 11.54%, 12.30%, and 17.21% (Table 2).
Chlorophyll, carotenoids and lycopene content
In the absence of salinity stress (No SS), chlorophyll a, chlorophyll b, total chlorophyll and carotenoids contents increased up to 20–25%, 20–38%, 20–30% and 12–20%, respectively, when sprayed with ZnOSt-NPs. In contrast, lycopene content was decreased around 35–55%. In the present of salinity stress (SS), chlorophyll a, chlorophyll b, total chlorophyll and carotenoids contents increased up to 25–40%, 40–55%, 25–43% and 15–38%, respectively, when sprayed with ZnOSt-NPs. In contrast, lycopene content was decreased around 15–38% (Fig. 3).
Effect of different concentrations of ZNOSt-NP on (A) chlorophyll a, (B) chlorophyll b, (C) total chlorophyll, (D) carotenoids, and (E) lycopene of Zea mays cultivated without salinity stress (No SS; pink bars) and with salinity stress (SS; yellow bars). The Tukey test showed significant changes at P < 0.05, as indicated by the different letters on the bars representing the mean of four replicates.
Chlorophyll fluorescence parameters
Among chlorophyll fluorescent parameters, Phi2 (31.57%,50%,56.71%), qL (22.50%, 25.54%, 32.21%), PhiNO (6.96%, 12.56%, 24.43%) and Fv′/Fm′(11.24%,15.75%,21.91%) increased while PhiNPQ (16.66%, 30.08%, 52.42%) and NPQt (26.75%, 41.71%, 46.54%) declined when plants were exposed to ZnOSt-NPs in the absence of salinity (No SS). Whereas the application of ZnOSt-NP improved Phi2(2.7%,22.2%, 26.50%) PhiNO(8.69%,24%, 38.36%) and Fv′/Fm′(4.69%, 5.76%, 16.92%) in plant of saline condition (SS) while reduced qL (8,45%, 17.64%, 4.13%) PhiNPQ (9.5%, 51%, 40%) and NPQt (26.36%, 36.14%,70.93%) chlorophyll fluorescent attributes (Table 3).
Total soluble protein and oxidative stress markers
The foliar treatment of ZnOSt-NPs significantly increased in total soluble proteins up to 72% in plants, while decreased lipid peroxidation and H2O2 around 96% and 40%, respectively from the plants present in control condition of –SS groups. Whereas the application of ZnOSt-NPs considerably increases in total soluble proteins up to 88% and declined lipid peroxidation and H2O2 ~ 46% and 33%, respectively than the response of plant exposed with control condition of + SS groups. (Fig. 4).
Effect of different concentrations of ZNOSt-NP on (A) total soluble proteins, (B) lipid peroxidation, and (C) hydrogen peroxide (H₂O₂) of Zea mays cultivated without salinity stress (No SS; pink bars) and with salinity stress (SS; yellow bars). The Tukey test showed significant changes at P < 0.05, as indicated by the different letters on the bars representing the mean of four replicates.
Enzymatic antioxidants
Salinity increased the enzymes activity of POD, CAT and APX upto 80% and SOD upto 25% in comparison of non-salinity treated (-SS) control plant. However, all antioxidant enzymes were reduced due to the application of ZnOSt-NPs on plant irrespective of salinity treatment and this decreased was up to 37% in POD and SOD, while 49% in CAT and 92% in APX of –SS group of plants. Whereas, the treatment of ZnOSt-NPs, decreased the activity of POD, SOD, CAT and APX around 26%, 25%, 60%, and 54% respectively in the presence of salinity stress (+ SS) (Fig. 5).
Effect of different concentrations of ZNOSt-NP on (A) POD (Peroxidase), (B) SOD (Superoxide dismutase), (C) CAT (Catalase), and (D) APX (Ascorbate peroxidase) enzymes activity in Zea mays cultivated without salinity stress (No SS; pink bars) and with salinity stress (SS; yellow bars). The Tukey test showed significant changes at P < 0.05, as indicated by the different letters on the bars representing the mean of four replicates.
Non-enzymatic antioxidants
All non-enzymatic antioxidants (GSH, AsA and GR) increased in plants under salt stress. However, ZnOSt-NPs treatment decreased in GSH (37%), AsA (28%), and GR (90%) in plant present in –SS group. Moreover, in the presence of salinity stress (+ SS), ZnOSt-NPs treatment decreased GSH (35%), AsA (10%) and GR (53%) in comparison of respective control condition (Fig. 6).
Effect of different concentrations of ZNOSt-NP on (A) GSH (Glutathione), (B) AsA (Ascorbic acid), and (C) GR (Glutathione reductase) of Zea mays cultivated without salinity stress (No SS; pink bars) and with salinity stress (SS; yellow bars). The Tukey test showed significant changes at P < 0.05, as indicated by the different letters on the bars representing the mean of four replicates.
Convex hull and hierarchical cluster analysis
The Convex Hull cluster analysis results show a distinct grouping of data points across four labeled clusters: Control, 30 ZnOSt-NPs, 60 ZnOSt-NPs, and 80 ZnOSt-NPs. The two principal components, PC1 and PC2, account for 94.65% and 2.09% of the variance, respectively. This analysis suggests that PC1 predominantly captures the variability within the dataset, while PC2 contributes minimally. Examining the clusters reveals distinct patterns: the control group covers points with negative PC1 values and varying PC2 values, 30 ZnOSt-NPs includes points with positive values in both PC1 and PC2, 60 ZnOSt-NPs consists of points with positive PC1 and negative PC2 values, and 80 ZnOSt-NPs encompasses points exhibiting higher positive values in both PC1 and PC2 (Fig. 7A).
The Convex Hull Cluster Analysis results outline two discernible clusters, -SS and + SS. These clusters are characterized by distinct coordinates representing the points forming the convex hulls for each group. The SS cluster spans coordinate from (-1.84, 1.54) to (-0.23, -1.31), summarizing a specific range within the PC 1 and 2 space. Conversely, the No SS cluster consists of coordinates ranging from (-0.20, 0.34) to (1.70, 1.60), showing a different region within the analyzed space (Fig. 7B).
The analysis has clustered the variables into groups showing similar characteristics or relationships. These clusters highlight various patterns within the dataset: One cluster consists of variables related to oxidative stress and enzymatic activity, such as H2O2 content, lipid peroxidation, and antioxidant enzyme levels. Another cluster encompasses measurements related to chlorophyll content and shoot and leaf characteristics, suggesting a correlation between plant pigments and morphological traits. Additionally, a group of variables, including protein levels, enzymatic activity, and root characteristics, form a separate cluster, indicating a possible relationship between root health, protein levels, and enzymatic activity. Carotenoid content appears as an individual cluster, signifying its distinct nature compared to other measured attributes. Furthermore, distinct clusters also emerge for parameters related to leaf physiological traits, such as NPQ-related measures, while other clusters represent specific traits like chlorophyll fluorescence parameters and leaf counts. Notably, variables such as relative chlorophyll content and the number of leaves exhibit individual clusters, indicating their unique characteristics within the dataset (Fig. 7C).
Pearson correlation analysis
The analyses indicate strong positive correlations between factors such as root and shoot characteristics. For instance, variables like root length, shoot length, and the number of leaves exhibit strong positive correlations ranging from approximately 0.8 to 0.95, indicating that as one of these factors increases, the others tend to increase proportionally. Additionally, notable positive correlations are observed among plant physiology and biochemistry, including strong positive relationships between various biochemical components like chlorophyll a, chlorophyll b, total chlorophyll, carotenoids, total soluble protein, and several enzymatic activities. Conversely, there are negative correlations observed between certain variables, such as lycopene content and most other measured attributes, including enzymatic activities like superoxide dismutase (SOD) and peroxidase (POD) activities. Furthermore, negative correlations are found between lipid peroxidation, hydrogen peroxide (H2O2) levels, and antioxidant enzymatic activities, suggesting an inverse relationship between these oxidative stress-related factors and the activities of antioxidant enzymes (Fig. 8).
Discussion
Salinity stress exerts a profound negative impact on plant productivity by reducing water availability and disrupting nutrient uptake, ultimately leading to poor soil health and a decline in crop yield50. This abiotic stress condition promotes the accumulation of toxic ions, particularly sodium (Na⁺), which causes ionic imbalance, osmotic stress, and oxidative damage. In our current investigation, foliar application of Sargassum tenerrimum-mediated zinc oxide nanoparticles (ZnOSt-NPs) at varying concentrations (30, 60, and 80 ppm) significantly improved soil quality by enhancing organic matter content and increasing the availability of essential macronutrients such as phosphorus (P) and potassium (K), while effectively reducing Na⁺ accumulation. These results align with the findings of Mohammed et al.51who also reported improved nutrient dynamics upon nanoparticle treatment under saline conditions.
The application of ZnOSt-NPs led to notable improvements in plant growth under both normal and salt stress conditions. This suggests that the treatment not only alleviates stress-induced damages but also promotes physiological vigor in unstressed plants. Salinity has been well-documented to cause a reduction in growth due to smaller cell sizes and inhibition of mitotic activity, largely caused by the excessive accumulation of Na⁺ ions in the rhizosphere, which disrupts mineral uptake and causes nutritional deficiencies52. However, the negative impact on growth was less pronounced in maize seedlings treated with ZnOSt-NPs. Morphological attributes such as shoot length, root length, shoot dry weight, and root dry weight all exhibited significant improvement under ZnOSt-NP treatment, indicating a protective and growth-promoting effect.
These observations are in agreement with those of Itroutwar et al.53who reported enhanced morphological features in Oryza sativa L. following the application of Turbinaria ornata-based ZnO nanoparticles. The observed growth improvement in our study can be attributed to the rich phytochemical profile of Sargassum tenerrimum, which includes cytokinins, auxins and auxin-like compounds, polyamines, amino acids, alginates, gibberellins, and essential nutrients. These bioactive constituents are known to modulate cellular metabolism, enhance mitotic division, and facilitate cell expansion, thereby promoting overall plant vigor and seedling establishment50.
Moreover, one of the contributing factors to the observed safety and efficacy of ZnOSt-NPs could be the metal ion reducing capacity of S. tenerrimum, which likely minimizes the toxicity associated with free zinc ions54. This unique property may enable a controlled and sustained release of zinc in a plant-available form, avoiding the adverse effects typically associated with excessive zinc exposure.
Salt stress is also known to detrimentally affect pigment composition and photosynthetic activity in plants. Several reports have highlighted a decline in chlorophyll a, chlorophyll b, total chlorophyll, carotenoids, and photosynthetic fluorescence parameters under salt stress55. This is often due to the inhibition of key enzymes involved in chlorophyll biosynthesis, such as ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), or the activation of degradative enzymes such as chlorophyllase, leading to the breakdown of chlorophyll pigments and impaired functioning of photosystem II (PSII)56.
In the current study, salt stress substantially decreased photosynthetic pigments and chlorophyll fluorescence parameters (including ΦII, qL, ΦNO, and Fv′/Fm′), indicating compromised light energy utilization and electron transport. Additionally, we observed a marked increase in lycopene content under salt stress. Lycopene is a known oxidative stress marker that accumulates as a secondary metabolite in response to environmental stresses57. Interestingly, a significant reduction in lycopene levels was noted in plants treated with ZnOSt-NPs, suggesting mitigation of oxidative stress and restoration of metabolic homeostasis. Although Sharifan et al.45 reported zinc-induced enhancement of lycopene in tomato plants, our findings imply that the bio-reduction and modulation of stress pathways by Sargassum constituents counteracted the lycopene accumulation under salinity. Crucially, these physiological and biochemical shifts are further corroborated by Fig. 7C (Hierarchical Cluster Analysis), where photosynthetic efficiency trait ΦII (Phi2) emerged as the most representative variable, closely clustering with other critical photosynthetic parameters. This strongly supports the hypothesis that restoration of photosynthetic machinery is central to the salt tolerance conferred by ZnOSt-NPs. The inclusion of ΦII as the dominant trait in the clustering analysis confirms its relevance as a key indicator of improved physiological status in treated plants. Therefore, this parameter can serve as a sensitive and integrative marker to evaluate the effectiveness of ZnOSt-NPs in future stress physiology studies.
A substantial improvement in photosynthetic pigments and fluorescence parameters was recorded in plants treated with ZnOSt-NPs, particularly at the 80 ppm concentration. Specifically, chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids showed significant enhancement in both non-stressed and salt-stressed Zea mays plants. Similarly, chlorophyll fluorescence parameters, Phi2 (ΦII), qL, PhiNO, and Fv′/Fm′, were markedly elevated, indicating improved efficiency of Photosystem II (PSII) and better photochemical quenching under salinity conditions. This improved performance can be attributed to the bioavailable nitrogen (1.0%) and iron (0.20%) present in Sargassum tenerrimum, which are key structural components of chlorophyll molecules. Additionally, betaines present in Sargassum may play a protective role in stabilizing photosynthetic membranes and maintaining pigment integrity58.
These findings are consistent with those reported by Ramya et al.59who observed an increase in photosynthetic pigments in Cyamopsis tetragonoloba following the application of Sargassum wightii extract under stress. Their results emphasized the role of strong magnesium ion absorption, another essential element of chlorophyll biosynthesis, by seaweed extracts, which likely explains similar enhancements in our study. Furthermore, the observed reduction in lycopene levels in ZnOSt-NP-treated plants under salinity suggests mitigation of stress-induced oxidative metabolism. Lycopene, a carotenoid and potent antioxidant, generally accumulates in response to stress-induced ROS production57. The decline in lycopene here may reflect reduced oxidative load due to the antioxidant potential of algal-derived ZnO nanoparticles. Similar stress-alleviating effects of algal components were previously reported by Elansary et al.60. The integrative nature of these changes is clearly visualized in Fig. 7C, where ΦII (Phi2), a photosynthetic efficiency marker, was identified as the most representative variable in the entire dataset, clustering closely with other photosynthetic parameters. This indicates that photosynthetic recovery is central to the mechanism of salt tolerance conferred by ZnOSt-NPs. Such a result not only confirms the biochemical relevance of the measured traits but also provides strong support for using chlorophyll fluorescence indices like ΦII as key diagnostic indicators for evaluating the efficacy of biogenic nanomaterials under abiotic stress.
Total soluble proteins (TSP) are often elevated under salt stress as a result of enhanced antioxidant enzyme synthesis and other stress-responsive proteins61. In the present study, TSP levels increased significantly in both control and salt-stressed maize plants treated with ZnOSt-NPs, with the highest increase observed at 80 ppm. This may reflect the induction of stress tolerance pathways and metabolic adjustments facilitated by the bioactive components of Sargassum, such as amino acids, phytohormones, and macro- and micronutrients. Alharbi et al.62 similarly reported that algal treatments promoted soluble protein content under salinity due to activation of protective biochemical mechanisms.
Abiotic stress, including salinity, enhances the generation of reactive oxygen species (ROS), leading to lipid peroxidation and membrane damage. Malondialdehyde (MDA), a byproduct of lipid peroxidation, is widely recognized as a reliable biomarker for oxidative damage63. Our findings corroborate those of Kasim et al.64 and Hameed et al.65who reported elevated MDA and H₂O₂ levels in stress-exposed plants. In the current study, treatment with ZnOSt-NPs, especially at 80 ppm, resulted in a marked decline in MDA and H₂O₂ levels in salt-stressed maize plants. This suggests a strong reduction in oxidative damage and improved membrane stability, likely due to the enhanced antioxidant defense system activated by the algal-based nanoparticles. These results are in agreement with Shanmugam et al.66who also demonstrated enhanced antioxidant activity in plants treated with algal-derived nanoparticles.
Interestingly, although salt stress typically induces antioxidant activity as a defensive response, the foliar application of ZnOSt-NPs in our study led to a reduction in the levels of both enzymatic antioxidants, peroxidase (POD), catalase (CAT), superoxide dismutase (SOD), and ascorbate peroxidase (APX), and non-enzymatic antioxidants, glutathione (GSH), glutathione reductase (GR), and ascorbic acid (AsA). This decrease was particularly evident at 80 ppm for enzymatic antioxidants and 30 ppm for non-enzymatic antioxidants. The lowered antioxidant activity could be attributed to a reduced oxidative burden in ZnOSt-NP-treated plants, which in turn diminished the need for stress-induced upregulation of defense systems. This interpretation is further supported by the low clustering of AsA in Fig. 7C, where it was identified as the least representative variable. Its distal clustering from core physiological and biochemical traits suggests that AsA levels were relatively independent and did not significantly contribute to the observed treatment effects, especially under ZnOSt-NP-mediated mitigation.
The enhancement in antioxidant defense, both enzymatic and non-enzymatic, is likely linked to the osmoprotective and redox-balancing compounds present in Sargassum tenerrimum, including proline, phenolic compounds, vitamins, and fatty acids67. The role of ZnO in activating antioxidant enzyme systems is well documented, and our observations are consistent with the reports of Hezaveh et al.68 and Adhikari et al.69who demonstrated improved stress resilience upon ZnO nanoparticle application. However, in the present context, the presence of Sargassum-derived bioactive constituents likely enhanced the efficiency of ZnOSt-NPs, resulting in a combined effect of reduced ROS generation and efficient ROS scavenging, which collectively contributed to improved physiological resilience.
Salt stress severely impairs plant water relations by reducing turgor pressure and limiting water availability in roots and shoots. This water deficit has a direct impact on key physiological traits such as relative water content (RWC) and the salinity tolerance index (STI), which collectively determine the plant’s capacity to withstand osmotic stress70. In our study, Zea mays seedlings exposed to 200 mM NaCl exhibited a significant decline in both RWC and STI, indicating impaired cellular hydration and stress resilience. However, foliar application of ZnOSt-NPs, particularly at the highest concentration of 80 ppm, markedly restored RWC and improved STI values under salinity conditions. This recovery suggests that ZnOSt-NPs help maintain internal water balance and improve water uptake capacity under stress.
The beneficial effects of ZnOSt-NPs on water status may be partially attributed to the exopolysaccharides present in Sargassum tenerrimum, which are known to retain moisture in plant tissues and support turgor maintenance71. These polysaccharides likely function by forming a hydrophilic matrix that facilitates water absorption and reduces transpiration loss. Additionally, Rajivgandhi et al.72 reported that bioactive compounds in Sargassum, such as amino acids, vitamins, and osmolytes, can enhance membrane permeability and promote efficient transport of water and ions, thereby improving cellular hydration and mitigating the osmotic effects of salinity. These bioactive molecules may also support osmotic adjustment by stabilizing proteins and preserving cell membrane integrity, which are crucial for maintaining growth and metabolic activity under salt stress.
One of the defining features of salt stress is the excessive accumulation of sodium (Na⁺) and chloride (Cl⁻) ions, which disrupts ionic balance, inhibits nutrient uptake (particularly potassium), and causes severe physiological dysfunction23. In our experiment, salt-stressed plants showed high levels of Na⁺ and Cl⁻ in tissues. However, ZnOSt-NP application significantly reduced Na⁺ and Cl⁻ accumulation, with the greatest reductions observed at the 80 ppm treatment. This suggests a strong ion exclusion or compartmentalization effect, likely mediated by the proline and glycine betaine content in Sargassum. These compounds are known osmoprotectants that stabilize cellular osmotic potential and prevent excessive Na⁺ uptake.
Our findings are consistent with those of Jafarlou et al.73who reported enhanced ionic equilibrium and reduced Na⁺ toxicity in plants treated with algal extracts. Glycine betaine, in particular, plays a dual role: it protects against salinity-induced damage by maintaining membrane structure and simultaneously preserves a high K⁺/Na⁺ ratio, which is critical for enzyme activation, stomatal regulation, and turgor pressure maintenance. In line with this, our data revealed that the K⁺/Na⁺ ratio significantly increased in ZnOSt-NP-treated plants, coinciding with improved RWC and STI, particularly at the optimal 80 ppm concentration. This restoration of ionic balance and water homeostasis is particularly important because it underpins multiple physiological functions including photosynthesis, nutrient transport, and growth recovery. Notably, the hierarchical cluster analysis (Fig. 7C) underscores this point: key traits such as STI and RWC cluster closely with major physiological indicators, highlighting their central role in defining the plant’s overall response to ZnOSt-NP treatment. Their close clustering with representative variables such as Phi2 also suggests that water status and photosynthetic efficiency are interlinked core responses driving salt tolerance in Zea mays under nanoparticle treatment.
However, it is important to note that although zinc is an essential micronutrient that enhances antioxidant defense and stabilizes biomembranes, excessive zinc levels can become phytotoxic. Our observations indicate that while 80 ppm ZnOSt-NPs improved physiological performance, higher or prolonged exposure may risk ionic imbalance, reduced RWC, and suppressed stomatal function. Shao et al.74 previously reported that zinc toxicity at elevated concentrations can lead to oxidative stress and disrupted ion homeostasis. Therefore, careful optimization of ZnOSt-NP dosage is essential to ensure beneficial outcomes without adverse effects. ZnOSt-NPs enhanced water relations and ion balance in salt-stressed Zea mays by promoting RWC, STI, and K⁺/Na⁺ ratios, largely through the synergistic effects of zinc and bioactive compounds derived from Sargassum tenerrimum. These findings highlight the multifaceted role of ZnOSt-NPs in conferring salt tolerance, integrating osmoprotection, ionic regulation, and photosynthetic recovery as key mechanisms of stress alleviation.
Conclusion
Our findings confirmed that the foliar application of ZnOSt-NP enhanced K+/Na⁺ ratio and improved nutrient availability, uptake of the plant and attributing to effective osmotic adjustment and facilitating water movement in salt stress. In addition, our results provide a novel perception about the mechanism of the salinity response of Zea mays as mediated by priming with Algal based ZnO-NPs and the use of 80 ppm ZnOSt-NP is recommended. This study highlights the potential of ecofriendly green Nano-fertilizers in mitigating salt stress and increasing overall agricultural yield of Zea mays.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Parihar, M. & Rakshit, A. Arbuscular mycorrhiza: A versatile component for alleviation of salt stress. Nat. Environ. Pollution Technol. 15, 417–428 (2016).
Hussain, S. et al. Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. J. Integr. Agric. 16, 2357–2374 (2017).
Akram, S. et al. Exogenous glutathione modulates salinity tolerance of soybean [Glycine max (L.) Merrill] at Reproductive Stage. J. Plant. Growth Regul. 36, 877–888 (2017).
Sayed, M. A. et al. Genome-Wide association study of salt Tolerance-Related traits during germination and seedling development in an Intermedium-Spike barley collection. Int. J. Mol. Sci. 23, 11060 (2022).
Kataria, S. & Verma, S. K. Salinity stress responses and adaptive mechanisms in major glycophytic crops: The story so far. in Salinity Responses and Tolerance in Plants, Volume 1: Targeting Sensory, Transport and Signaling Mechanisms (eds. Kumar, V., Wani, S. H., Suprasanna, P. & Tran, L. S. P.) vol. 1 1–39Springer, (2018).
Marriboina, S. & Attipalli, R. R. Hydrophobic cell-wall barriers and vacuolar sequestration of Na + ions are among the key mechanisms conferring high salinity tolerance in a biofuel tree species, Pongamia pinnata L. pierre. Environ. Exp. Bot. 171, 103949 (2020).
Zhao, S. et al. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 22, 6455 (2021).
Uzair, B. et al. Green and cost-effective synthesis of metallic nanoparticles by algae: safe methods for translational medicine. Bioengineering 7, 1–22 (2020).
Ragavan, P., Ananth, A. & Rajan, M. R. Impact of selenium nanoparticles on growth, biochemical characteristics and yield of cluster bean cyamopsis Tetragonoloba. Int. J. Environ. Agric. Biotechnol. 2, 2917–2926 (2017).
Hussein, M. M. & AbouBaker, N. H. The contribution of Nanozinc to alleviate salinity stress on cotton plants. R Soc. Open. Sci. 5, 171809 (2018).
Zafari, S. & others. Greensynthesized zinc oxide nanoparticles mitigate salt stress in Sorghum bicolor. Agriculture 12, 597 (2022).
Alabdallah, N. M. & Alzahrani, H. S. The potential mitigation effect of ZnO nanoparticles on Abelmoschus esculentus L. Moench metabolism under salt stress conditions. Saudi J. Biol. Sci. 27, 3132–3137 (2020).
Aziz, S., Khan, M. I. R. & Farooq, M. Zinc oxide nanoparticles mitigate salt stress by improving antioxidant defense in brassica Napus L. Sci. Rep. 11, 12231 (2021).
Adrees, M. & others. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) under combined cd and water-deficit stress. Ecotoxicol. Environ. Saf. 208, 111627 (2021).
Sun, L. & others. NanoZnO alleviates drought stress via modulating water use and carbohydrate metabolism in maize. Arch. Agron. Soil. Sci. 67, 245–259 (2020).
Yadav, R. K., Singh, V. P. & Sharma, S. Foliar application of ZnO nanoparticles improves salinity tolerance and antioxidant enzyme activities in Helianthus annuus. J. Plant. Growth Regul. 39, 1255–1266 (2020).
Khan, M. I. R., Yadav, R. K. & Singh, A. Nano zinc oxide application enhances salinity tolerance via upregulating antioxidant systems in spinacia Oleracea. Plant. Physiol. Biochem. 150, 1–10 (2020).
Gupta, A. et al. Zinc oxide nanoparticles application alleviates salinity stress by modulating plant growth, biochemical attributes and nutrient homeostasis in phaseolus vulgaris L. Front Plant. Sci 15, (2024).
Oscar, F. L., Bakkiyaraj, D., Nithya, C. & Thajuddin, N. Deciphering the diversity of microalgal bloom in wastewater-an attempt to construct potential consortia for bioremediation. J. Cur Per Appl. Microbiol. 2278, 92 (2014).
El-Katony, T. M., Ward, F. M., Deyab, M. A. & El-Adl, M. F. Algal amendment improved yield and grain quality of rice with alleviation of the impacts of salt stress and water stress. Heliyon 7, (2021).
Alharbi, K. & Alaklabi, A. Alleviation of salinity induced growth and photosynthetic decline in wheat due to Biochar and jasmonic acid application involves up-regulation of ascorbate-glutathione pathway, glyoxylase system and secondary metabolite accumulation. Rhizosphere 24, 100603 (2022).
Khan, Z. et al. Sargassum Wightii aqueous extract improved salt stress tolerance in Abelmoschus esculentus by mediating metabolic and ionic rebalance. Front. Mar. Sci. 9, 853272 (2022).
Lamlom, S. F., Abdelghany, A. M., Ren, H. & Ali, H. M. Revitalizing maize growth and yield in waterlimited environments through silicon and zinc foliar applications. Heliyon 10, e35118 (2024).
Tahir, R. & Yasin, M. Proximate composition of maize (Zea Mays L.) seeds: carbohydrate, protein, sugar, oil and fiber content analysis. J. Agric. Food Chem. 64, 1234–1238 (2016).
Khan, N. A., Alam, M., Khan, R., Khan, K. & Rahman, S. U. Evaluating the nutritional value of the newly developed quality protein maize in pakistan: impact on broiler performance and profitability. Pakistan J. Zool. 52, 585–592 (2020).
Farooq, M., Hussain, M., Wakeel, A. & Siddique, K. H. M. Salt stress in maize: effects, resistance mechanisms and management. Agron. Sustain. Dev. 35, 461–481 (2015).
Sabagh, A. E. et al. Salinity stress in maize: effects of stress and recent developments of tolerance for improvement. in El Sabagh, A (ed. in Maize (ed, S. S.) (et al.), Cereal Grains, 1, 213, (2021).
Srivastav, S., Singh, R. K. & Singh, V. P. Impact of ZnO nanoparticles on physiological and antioxidant parameters in maize under salt stress. Plant. Physiol. Biochem. 175, 123–132 (2022).
Kulshreshtha, G. & others. Red seaweeds sarcodiotheca gaudichaudii and chondrus Crispus downregulate virulence factors of Salmonella enteritidis and induce immune responses in caenorhabditis elegans. Front. Microbiol. 7, 1–12 (2016).
Mahendiran, D. et al. Biosynthesis of zinc oxide nanoparticles using plant extracts of Aloe Vera and hibiscus sabdariffa: phytochemical, antibacterial, antioxidant and Anti-proliferative studies. Bionanoscience 7, 530–545 (2017).
Saad, R. et al. Fabrication of zno/cnts for application in CO2 sensor at room temperature. Nanomaterials 11, 3087 (2021).
Hardie, M. & Doyle, R. Measuring soil salinity. Methods Mol. Biol. 913, 415–425 (2012).
Mazhar, R., Ilyas, N., Arshad, M., Khalid, A. & Hussain, M. Isolation of heavy Metal-Tolerant PGPR strains and amelioration of chromium effect in wheat in combination with Biochar. Iran. J. Sci. Technol. Trans. Sci. 44, 1–12 (2020).
Brougham, R. K. The relationship between the critical leaf area, total chlorophyll content, and maximum growth-rate of some pasture and crop Planst. Ann. Bot. 24, 463–474 (1960).
Arnon, D. I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant. Physiol. 24, 1–15 (1949).
Amiri-Rigi, A. & Abbasi, S. Microemulsion-based lycopene extraction: effect of surfactants, co-surfactants and pretreatments. Food Chem. 197, 1002–1007 (2016).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Cavalcanti, F. R., Oliveira, J. T. A., Martins-Miranda, A. S., Viégas, R. A. & Silveira, J. A. G. Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed Cowpea leaves. New Phytol. 163, 563–571 (2004).
Velikova, V., Yordanov, I. & Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant. Sci. 151, 59–66 (2000).
Chance, B. & Maehly, A. C. [136] assay of catalases and peroxidases. {black small square}. Methods Enzymol. 2, 764–775 (1955).
Giannopolitis, C. N. & Ries, S. K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 59, 309 (1977).
Foyer, C. H. & Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 133, 21–25 (1976).
Diacono, M. & Montemurro, F. Effectiveness of organic wastes as fertilizers and amendments in Salt-Affected soils. Agric. (Switzerland). 5, 221–230 (2015).
Solanki, A. D. & Patel, I. C. Sargassum tenerrimum-mediated green synthesis of silver nanoparticles along with antimicrobial activity. Appl. Nanosci. (Switzerland). 13, 4415–4425 (2023).
Sharifan, H., Noori, A., Bagheri, M. & Moore, J. M. Postharvest spraying of zinc oxide nanoparticles enhances shelf life qualities and zinc concentration of tomato fruits. Crop Pasture Sci. 73, 22–31 (2022).
Saif, M. S. & others. Potential of CME@ZIF-8 MOF nanoformulation: smart delivery of Silymarin for enhanced performance and mechanism in albino rats. ACS Appl. Bio Mater. 7, 6919–6931 (2024).
José, B. J. A. & Shinde, M. D. Colloidal stability and dielectric behavior of eco-friendly synthesized zinc oxide nanostructures from Moringa seeds. Sci. Rep. 14, 2310 (2024).
Abdi, G. & others. Pharmacological potential of Sargassum sp. of west coast of Maharashtra Kunkeshwar, India. Front. 9, (2022).
Saif, M. S. Improving the bioavailability of three-dimensional ZIF-8 MOFs against carbon tetrachloride-induced brain and spleen toxicity in rats. Mater. Chem. Phys. 328, 129997 (2024).
Chourasia, K. N. et al. Salinity stress in potato: Understanding physiological, biochemical and molecular responses. Life 11, 545 (2021).
Mohammed, S. et al. Inductive role of the brown Alga sargassum polycystum on growth and biosynthesis of imperative metabolites and antioxidants of two crop plants. Front Plant. Sci 14, (2023).
Sanwal, S. K. et al. Salinity stress tolerance in potato cultivars: evidence from physiological and biochemical traits. Plants 11, 1842 (2022).
Itroutwar, P. D. et al. Seaweed-Based biogenic ZnO nanoparticles for improving Agro-morphological characteristics of rice (Oryza sativa L). J. Plant. Growth Regul. 39, 717–728 (2020).
Saldarriaga-Hernandez, S., Hernandez-Vargas, G., Iqbal, H. M. N., Barceló, D. & Parra-Saldívar, R. Bioremediation potential of sargassum sp. biomass to tackle pollution in coastal ecosystems: circular economy approach. Sci. Total Environ. 715, 136978 (2020).
ALKahtani, M. D. F. et al. Chlorophyll fluorescence parameters and antioxidant defense system can display salt tolerance of salt acclimated sweet pepper plants treated with Chitosan and plant growth promoting rhizobacteria. Agronomy 10, 1180 (2020).
Hnilickova, H., Kraus, K., Vachova, P. & Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in portulaca Oleracea l. Plants 10, 845 (2021).
Riffat, A. & Ahmad, M. S. A. Regulation of antioxidant activity in maize (Zea Mays L.) by exogenous application of sulfur under saline conditions. Turk. J. Bot. 44, 62–75 (2020).
Abd El-Gawad, H. & Osman, H. S. Effect of exogenous application of boric acid and seaweed extract on growth, biochemical content and yield of eggplant. J. Hortic. Sci. Ornam. Plants. 6, 133–143 (2014).
Ramya, S. S., Vijayanand, N. & Rathinavel, S. Influence of seaweed liquid fertilizers on growth, biochemical and yield parameters of cluster bean plant. J. Green. Bioenergy. 1, 19–32 (2012).
Elansary, H. O. et al. Seaweed extracts enhance Salam turfgrass performance during prolonged irrigation intervals and saline shock. Front. Plant. Sci. 8, 830 (2017).
Weng, Q. et al. Identification of salt stress-responsive proteins in maize (Zea may) seedlings using itraq-based proteomic technique. Iran. J. Biotechnol. 19, 106–120 (2021).
Alharbi, K. et al. Potassium humate and plant Growth-Promoting microbes jointly mitigate water deficit stress in soybean cultivated in Salt-Affected soil. Plants 11, 3016 (2022).
Miller, G., Suzuki, N., Ciftci-Yilmaz, S. & Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant. Cell. Environ. 33, 453–467 (2010).
Kasim, W., Hamada, E., … N. E.-D.-Int. J. Agron. A. & 2015, undefined. Influence of seaweed extracts on the growth, some metabolic activities and yield of wheat grown under drought stress. CiteseerWA Kasim, EAM Hamada, NGS El-Din, S EskanderInt. J. Agron. Agric. Res, 2015•Citeseer7, 173–189 (2015).
Hameed, A., Bibi, N., Akhter, J. & Iqbal, N. Differential changes in antioxidants, proteases, and lipid peroxidation in flag leaves of wheat genotypes under different levels of water deficit conditions. Plant Physiol. Biochem. 49, 178–185 (2011).
Shanmugam, R., Chelladurai, M. & Vanaja, M. Antibacterial activity of algaemediated synthesis of gold nanoparticles from turbinaria Conoides. Der Pharm. Chem. 5, 224–229 (2013).
Safaat, M., Tursiloadi, S., Perisha, B. & Zulpikar, F. Nanoparticles green synthesis macroalgae-based and its application and distribution in Indonesia - An overview. in IOP Conference Series: Earth and Environmental Science vol. 744 (2021).
Hezaveh, T. A., Pourakbar, L., Rahmani, F. & Alipour, H. Effects of ZnO NPs on phenolic compounds of rapeseed seeds under salinity stress. J. Plant. Process. Function. 8, 1–10 (2020).
Adhikari, S. et al. Assessment of ZnO-NPs toxicity in maize: an integrative MicroRNAomic approach. Chemosphere 249, 126–197 (2020).
Shahzad, A. S. et al. Acidified Biochar improves lead tolerance and enhances morphological and biochemical attributes of mint in saline soil. Sci. Rep. 13, 8720 (2023).
Senousy, H. H. et al. Algal Bio-Stimulants enhance salt tolerance in common bean: dissecting morphological, physiological, and genetic mechanisms for stress adaptation. Plants 12, 3714 (2023).
Rajivgandhi, G. N. et al. Phytochemical screening and anti-oxidant activity of sargassum Wightii enhances the anti-bacterial activity against Pseudomonas aeruginosa. Saudi J. Biol. Sci. 28, 1763–1769 (2021).
Jafarlou, M. B., Pilehvar, B., Modaresi, M. & Mohammadi, M. Interactive effects of seaweed and microalga extract priming as a biostimulant agent on the seed germination indices and primary growth of milkweed (Calotropis procera Ait). Biol. (Bratisl). 77, 1283–1293 (2022).
Shao, J. et al. How does zinc improve salinity tolerance?? Mechanisms and future prospects. Plants 12, 3207 (2023).
Acknowledgements
The authors express their gratitude for the financial support received from Ongoing Research Funding program, (ORF-2025-1245), King Saud University, Riyadh, Saudi Arabia. The authors also acknowledge Dr. Aisha Khan, Associate Professor, Department of Botany, University of Karachi, for her assistance in the taxonomic identification of the plant material used in this study.
Funding
This research was financed by Ongoing Research Funding program, (ORF-2025-1245), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
HA & MR; Experimentation and Methodology, MZA & GN; Conceptualization, Supervision and Validation, SU; writing-original draft preparation and Statistical analysis, AAS; Resource acquisition, Statistical Analysis and Investigation, SS & AA; Data curation and Formal analysis, and HOE: writing-revised draft preparation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
We declare that the manuscript reporting studies do not involve any human participants, human data or human tissues. So, it is not applicable. Our experiment follows the relevant institutional, national, and international guidelines and legislation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ashraf, H., Ramzan, M., Ahmad, M.Z. et al. Sargassum-synthesized ZnO nanoparticles induce salt tolerance in maize plants through enhanced physiological and biochemical mechanisms. Sci Rep 15, 30633 (2025). https://doi.org/10.1038/s41598-025-16397-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16397-4