Abstract
The purpose was to evaluate aqueous carbonic anhydrase-1(CA-1) expression and explore its correlation with macular structural changes and other cytokines in patients with macular edema (ME) secondary to retinal vein occlusion (RVO). This was a retrospective cross-sectional study in which patients with RVO (n = 109) and control subjects (n = 37) were included. The concentrations of cytokines, including CA-1, PPK/PK, ICAM, bFGF, VEGF and MCP-1, in the aqueous humor were measured before intravitreal injection of anti-VEGF drugs or DEX drugs. We collected optical coherence tomography (OCT) images before obtaining aqueous humor. CA-1 levels in the aqueous humor were greater in the RVO group than in the control group, and CA-1 levels were lower in treated RVO patients than in treatment-naïve patients. In addition, the levels of other cytokines (PPK/PK, ICAM, bFGF, VEGF and MCP-1) tended to be similar to those of CA-1. CA-1 was positively correlated with visual acuity (VA) logMAR (r = 0.215; p = 0.03), PPK/PK (r = 0.58, p < 0.001), ICAM (r = 0.70, p < 0.001), bFGF (r = 0.48, p < 0.001), VEGF (r = 0.55, p < 0.001), MCP (r = 0.67, p < 0.001) and retinal hemorrhage (r = 0.309, p = 0.003) in RVO patients. In conclusion, CA-1 was overexpressed in RVO patients, especially in treatment-naïve patients. Additionally, increased CA-1 levels are associated with other aqueous cytokines, VA logMAR values and retinal hemorrhage in RVO patients.
Similar content being viewed by others

Introduction
Macular edema (ME) secondary to retinal vein occlusion (RVO) is a serious eye disease with a high incidence worldwide. RVOs involve a variety of systemic and local factors, including hypertension, diabetes, cardiovascular diseases, and increased blood viscosity1,2,3,4resulting in the obstruction of retinal veins and leading to pathological changes such as retinal tissue ischemia, hemorrhage, and macular edema, ultimately significantly affecting patients’ vision and quality of life5. Currently, the treatment of ME secondary to RVO mainly includes antineovascular drugs and steroid implant agents; however, a considerable number of patients still have no significant improvement in vision after treatment, macular edema is prone to relapse, and frequent intravitreal injections impose economic and social burdens on patients6,7. The main reasons for the poor response may be that anti-vascular endothelial growth factor (VEGF) treatment mainly targets one pathway but does not affect other possible pathological pathways. Although steroid drug treatment has a broad-spectrum anti-inflammatory effect, it lacks specificity and cannot accurately treat different etiological and pathological mechanisms. In response to these challenges, future treatment strategies may need to explore new treatment targets and pathways to address various pathological mechanisms of RVO.
Carbonic anhydrase (CA) is an enzyme widely distributed throughout the body8. It catalyzes the hydration of carbon dioxide, forming bicarbonate, and promotes the exchange of plasma chloride and sodium ions, providing metabolic support for tissues and maintaining the homeostasis of the ionic environment in tissues. CA-1 is the predominant cytosolic CA isoform9. In addition to red blood cells, CA-1 was detected in the interstitial space of myocardial tissue and increased in capillary endothelial cells in the peri-infarct area10. A previous study reported that CA-1 was detected in the vitreous of individuals with diabetic retinopathy11. Our study investigated the expression of CA-1 and explored its correlation with macular structural changes and other cytokines in ME secondary to RVO.
Method
Study subjects
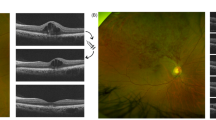
We included RVO patients who were treated with anti-VEGF (ranibizumab, aflibercept or conbercept) or dexamethasone (DEX) implants (Ozurdex) from November 2022 to December 2023 for ME secondary to RVO (including BRVO and CRVO) and control patients who underwent cataract surgery/simple vitrectomy combined with epiretinal membrane or internal membrane peeling. The protocol used in this study was approved by the Institutional Review Board of West China Hospital (No. 2023 − 1375). Anti-VEGF or DEX intravitreal injection was used as therapy for ME secondary to RVO, and a laser was used if needed according to the EURETINA Guidelines for the diagnosis and treatment of RVO. Informed consent was obtained from the patients in accordance with the tenets of the Declaration of Helsinki. Complete ophthalmic examinations, including a visual acuity (VA) test, slit lamp examination, fundus examination, and measurement of intraocular pressure (IOP) via noncontact tonometry, were performed before intravitreal injection. The BCVA was measured via a Snellen chart and converted to the logarithm of the minimum angle of resolution for comparison. Optical coherent tomography (Zeiss Cirrus HD-OCT 6000, CA, USA) was also performed. Retinal hemorrhages in the fundus were classified as dot-blot or flame-shaped hemorrhages. For OCT biomarkers, central subfoveal thickness (CST) was measured using the value in the center circle of the ETDRS subfields. Intraretinal cysts were identified as hyporeflective, round or oval spaces within retinal layers, distinctly separate from vessels. Subretinal fluid (SRF) was characterized as a hyporeflective space situated between the retina and the retinal pigment epithelium (RPE). Hyperreflective foci (HRF) were defined as dot-like hyperreflective structures. Ellipsoid zone and interdigitation zone (EZ/IZ) disruptions were noted as the loss of continuous hyperreflective bands corresponding to photoreceptor mitochondria (EZ) and RPE microvilli (IZ). Disorganization of retinal inner layers (DRIL) was identified by the inability to distinguish boundaries between the ganglion cell layer (GCL), inner plexiform layer (IPL), and inner nuclear layer (INL) within 500 μm of the fovea. Retinal hemorrhages, intraretinal cysts, SRF, HRF, EZ/IZ disruption, and DRIL were graded as either absent or present (Fig. 1). These macular structural changes were analyzed by graders (JXS and LJ), in cases where there were discrepancies in the results, a third researcher (FL) was consulted to make a final determination.
The retinal vein occlusion characteristics observed in fundus and optical coherence tomography (OCT) images include various key indicators. In images (A) and (D), the fundus displays hemorrhagic spots, as denoted by yellow arrowheads. Images (B) and (E) present the average thickness measurements of the ETDRS subfields, corresponding to the region highlighted by the yellow square in the fundus view, with the central subfoveal thickness (CST) represented by the value in the center circle. Images (C) and (F) illustrate OCT biomarkers such as cysts (marked by yellow arrows), disruptions in the ellipsoid zone and interdigitation zone (EZ/IZ) (marked by asterisk), disorganization of the retinal inner layers (DRIL) (marked by pentagram), hyperreflective foci (HRFs) (marked by red arrowheads), and subretinal fluid (SRF) (marked by hash mark).
Sample collection
Aqueous humor samples were collected each time before intravitreal injection or surgery was conducted. A mean volume of 0.1 mL of aqueous humor was collected through anterior chamber limbal paracentesis via a 30-gauge needle attached to an insulin syringe. Immediately after collection, aqueous humor samples were transferred to sterile plastic tubes and stored at -80 °C until analysis.
Production of CA-1 and prekallikrein beads
CA-1 (carbonic anhydrase 1) capture beads were produced via functional beads (BD CBA functional bead E4 (558584)) and monoclonal antibodies against carbonic CA1 (Abcam, Ab281221). The preparation of the prekallikrein (PK) antibody was performed at Kanghong Bio Company via hybridoma technologies12. PK capture beads were produced via the use of functional beads (BD CBA Functional Bead A6 (560032)) and a monoclonal antibody against PK. The binding capacity, specificity and stability of CA-1 capture beads and PPK capture beads were tested to ensure that they are functional and specific.
Measurement of cytokines
A cytometric bead array (CBA) was used to determine the concentrations of CA-1, PK, ICAM, MCP-1, bFGF, and VEGF (BD, CA, US) following the manufacturer’s instructions. Data were acquired via a BD FACSCelesta™ Flow Cytometer (BD, CA, US) and analyzed via FCAP Array TM Software Version 3.0 (BD, CA, US). Standard curves for each cytokine were generated using the reference cytokine concentrations supplied in the kit. The cytokine concentration was calculated from a standard curve for each cytokine.
Statistical analysis
The data were processed and statistically analyzed via IBM SPSS Statistics v26.0 (IBM Corp., Armonk, USA). All the data are presented as the means ± standard deviations (SDs). Categorical data were compared between groups via the chi-square test. The Mann–Whitney U test was used to detect differences between the RVO and control groups. Spearman’s correlation analysis was adopted to analyze the relationships among the cytokines and logMAR/OCT characteristics in our study. Values of p < 0.05 were considered statistically significant.
Results
A total of 109 eyes from 109 patients (66 men and 80 women) were ultimately included in this study, and 37 eyes from 37 patients who underwent cataract surgery/simple vitrectomy combined with epiretinal membrane or internal membrane peeling were used as controls. The mean ages of the patients in the RVO group and the control group were 62.3 ± 10.3 (mean ± SD) and 65.0 ± 11.3 years, respectively (P > 0.05, Table 1). There were 50 men and 59 women, 51 right eyes and 58 left eyes, in the RVO group. Sixteen men and 21 women, 21 right eyes and 16 left eyes, were included in the control group, and there were no significant differences in age, sex or eyes between these groups (P > 0.05, Table 1).
The concentrations of aqueous humor cytokines (CA-1, PK, ICAM, bFGF, VEGF, and MCP-1) in the RVO group were significantly greater than those in the control group (P < 0.001, Table 2; Fig. 2). The concentrations of aqueous humor cytokines in the RVO subgroups are listed in Table 3. The levels of all measured cytokines were significantly greater in treatment-naïve patients than in treated subjects (P < 0.001) (Fig. 3).
In the RVO group, 36 RVO treatment-naïve patients and 73 RVO-treated patients were included, and the disease duration was 6.7 ± 2.2 months for the treatment-naïve patients and 22.6 ± 3.5 months for the treated patients. Overall, the mean visual acuity (VA) was 1.09 ± 0.07 logMAR (logarithm of the minimum angle of resolution), and the baseline central subfoveal thickness (CST) was 414.58 ± 23. 4 μm. The visual acuity was 1.4 ± 0.16 LogMAR in the treatment-naïve patients and 1.0 ± 0.08 LogMAR in the treated patients (P = 0.03). The CST was 531.7 ± 61.1 μm in size in the treatment-naïve patients and 383.6 ± 23.6 μm in size in the treated patients (P = 0.01). For the OCT parameters, intraretinal cysts were noted in 88.9% of the treatment-naïve patients, whereas in 80.9% of the treated patients, ellipsoid zone and interdigitation zone (EZ/IZ) disruption was noted in 94.4% of the treatment-naïve patients, whereas in 79.4% of the treated patients, disorganization of the retinal inner layers (DRIL) was noted in 88.9% of the treatment-naïve patients, whereas in 76.5% of the treated patients. There were no statistically significant differences in the number of intraretinal cysts, EZ/IZ disruption, or DRIL between the two subgroups. Hyperreflective foci (HRFs) were noted in 88.9% of the treatment-naïve patients, whereas in 60.3% of the treated patients, subretinal fluid (SRF) was noted in 38.9% of the treatment-naïve patients and in 11.8% of the treated patients. There was a significant difference in the CST (p = 0.01), HRF (p = 0.02), and SRF (p = 0.01) between the two subgroups (Table 4). CST, HRF and SRF were lower in treated patients than in treatment-naïve patients.
In RVO patients, CA-1 was positively correlated with VA logMAR (r = 0.215; p = 0.03), PPK/PK (r = 0.58, p < 0.001), ICAM (r = 0.70, p < 0.001), bFGF (r = 0.48, p < 0.001), VEGF (r = 0.55; p < 0.001) and MCP (r = 0.67, p < 0.001). In the treatment-naïve RVO groups, CA-1 was positively correlated with bFGF, VEGF and MCP. In the RVO-treated groups, CA-1 was positively correlated with VA logMAR, ICAM, bFGF, VEGF and MCP. In all RVO patients, CA-1 was analyzed with OCT parameters and was not correlated with CST, cysts, HRF, EZ/IZ disruption, DRIL, or HRF but was correlated with retinal hemorrhage (r = 0.309, p = 0.003) (Table 5).
Discussion
The pathogenesis of retinal vascular damage and macular edema in RVO is complex, and vascular damage is accompanied by complex reactions, which include damage to the blood‒retinal barrier and the release of various cytokines into the vitreous and aqueous humor. Many studies have reported that angiogenic and inflammatory mediators are disrupted in the ocular fluid in RVO13,14,15,16,17. In our study, we present evidence of disturbances in various cytokines, including CA-1, PK, VEGF, ICAM and MCP-1, in the eyes of RVO patients compared with those in the eyes of control subjects. These cytokines were significantly increased in RVO eyes compared with control eyes. The trends of increasing VEGF, MCP-1 and ICAM levels were similar to those reported in previous studies18,19,20.
CA-1 is a zinc-containing metalloenzyme that catalyzes the interconversion between carbon dioxide and bicarbonate and plays key roles in a variety of physiological processes, such as pH regulation, metabolic processes and chemical sensing of environmental changes21. CA1 was found to participate in ischemia-induced cardiac fibroblast development, hyperglycemia-induced endothelial cell death, cell proliferation, proinflammatory cytokine production, and vascular permeability alterations10,22. With mass spectrometry-based label-free quantitative analysis, Sagnik et al. reported that CA-1 protein expression was upregulated 10-fold in vitreous proteomes in patients with proliferative diabetic retinopathy11. An animal study demonstrated that increased exogenous CA-1 could cause vascular injury in the retina via activation of the plasma kallikrein system23which is similar to our results showing that CA-1 is associated with PK in the humor of RVO patients. Our study explored CA-1 in the aqueous humor of RVO patients for the first time and compared its concentration in treatment-naïve and treated RVO patients. CA-1 was significantly increased in RVO patients, and it was lower in treated patients than in treatment-naïve patients. These results indicate that CA-1 could be reduced by administering treatment to RVO patients but was still unbalanced in the eye.
We also retrospectively compared treatment-naïve and treated RVO patients and reviewed their retinal fundus and OCT images. Compared with treatment-naïve patients, treated RVO patients have a decreased CST and lower HRF and SRF. CA-1 was correlated with retinal hemorrhage but not with the CST, HRF or SRF, which are parameters that reflect blood–retinal barrier (BRB) leakage and local inflammation. CA-1 has been shown to lead to blood‒brain barrier permeability, and previous studies have shown that CA-1 can be released from red blood cells (RBCs) to induce cerebral edema following hemorrhage; CA-1 has also been shown to be injected into the subdural space, inducing blue leakage on the surface of the brain23. Our study revealed CA-1 was not correlated with CST but have a positive correlation with hemorrhage, a higher CA-1 level was significantly correlated with retinal hemorrhage in RVO patients. CST measures fluid leakage in subfoveal, where hemorrhage reflects acute vascular damage. This suggests CA-1 is more sensitive to RVO-triggered endothelial injury.
We found that CA-1 was correlated with PK, ICAM, bFGF, VEGF and MCP-1 in RVO eyes, whereas in treatment-naïve RVO eyes, CA-1 was correlated with bFGF, VEGF and MCP-1. In treated RVO eyes, CA-1 was correlated with ICAM, bFGF, VEGF and MCP-1. The plasma protease kallikrein (PK) and its precursor, prekallikrein (PPK), can help release the vasoactive peptide bradykinin, which can regulate vascular tone and permeability24,25,26. PK was increased after RVO, and CA-1 was moderately strongly correlated with PK in all RVO eyes in our study. Gao et al. reported that exogenous CA-1-induced retinal edema could be decreased by the inhibition of the PK system23. Intercellular adhesion molecule-1 (ICAM-1), which is expressed mainly on endothelial cells, facilitates the adhesion of leukocytes to retinal vascular endothelial cells and exacerbates endothelial injury and breakdown of the blood‒retinal barrier. Our study revealed that ICAM-1 was upregulated in RVO eyes and was greater in treatment-naïve eyes than in treated RVO eyes. When the central or branch rental vein is occluded, ischemia and hypoxia trigger the upregulation of ICAM-1, and anti-VEGF or DEX treatment can downregulate ICAM-1 after RVO. Our results are similar to those of Noma et al., who reported that ICAM-1 levels are elevated in the vitreous fluid of patients with CRVO and BRVO15,20 and that ICAM-1 levels are also correlated with the severity of macular edema. Basic fibroblast growth factor (bFGF), a member of the fibroblast growth factor family27can promote retinal epithelial cell and vascular endothelial cell mitosis to induce massive hyperplasia28. bFGF can also promote leukocyte recruitment to inflammatory sites29. In our study, we found that the bFGF concentration increased significantly in RVO patients, especially in treatment-naïve patients, and that it could be downregulated in treated RVO patients after anti-VEGF or DEX intravitreal injection. Monocyte chemoattractant protein-1 (MCP-1), also known as chemokine (ligand 2 (CCL2)), is a chemokine that plays a central role in recruiting monocytes and macrophages to sites of inflammation. MCP-1, which is produced by various retinal cell types, is upregulated in response to ischemia due to RVO and can attract immune cells to participate in pathological processes and macular edema progression. We found that CA-1 was strongly correlated with MCP-1 in RVO eyes, including those in the treatment-naïve and treated RVO groups.
Our study has several limitations. The study was not prospective. We correlated CA-1 levels with OCT parameters in RVO patients but did not find a strong correlation, but the small sample size may have prevented us from obtaining significant results. A future prospective study with a large number of patients is necessary to determine the associations between CA-1 concentration and retinal imaging biomarkers in RVO patients.
In conclusion, this study is the first to address CA-1 upregulation and revealed that CA-1 is correlated with retinal hemorrhage in RVO patients. CA-1 is also correlated with many other cytokines, such as PK, ICAM, bFGF, VEGF and MCP-1. In addition to the VEGF family and inflammation, further research into the roles of CA-1 and its interaction in the ocular microenvironment is warranted. Understanding these mechanisms could lead to more targeted and effective treatments for RVO eyes, ultimately improving the visual prognosis of affected patients.
Data availability
The datasets generated and analysed during the current study are not publicly available due to very strict data protection regulations but are available from the corresponding author on reasonable request.
References
Ponto, K. A. et al. Hypertension and multiple cardiovascular risk factors increase the risk for retinal vein occlusions: results from the Gutenberg retinal vein occlusion study. J. Hypertens. 37 (7), 1372–1383 (2019).
Lisa Gracia, M., Vieitez Santiago, M., Salmón Gonzalez, Z., Qiu Liu, S. & Hernández Hernández, J. L. Napal lecumberri JJ. [Hypertension and Framingham general vascular risk score in retinal vein occlusion]. Hipertens Riesgo Vasc. 36 (4), 193–198 (2019).
Muraoka, Y. & Tsujikawa, A. Arteriovenous crossing associated with branch retinal vein occlusion. Jpn J. Ophthalmol. 63 (5), 353–364 (2019).
Opremcak, E. M. et al. Radial optic neurotomy for central retinal vein occlusion: a retrospective pilot study of 11 consecutive cases. Retina 21 (5), 408–415 (2001).
Ho, M., Liu, D. T., Lam, D. S. & Jonas, J. B. Retinal vein occlusions, from basics to the latest treatment. Retina 36 (3), 432–448 (2016).
Busch, C. et al. Long-term visual outcome and its predictors in macular oedema secondary to retinal vein occlusion treated with dexamethasone implant. Br. J. Ophthalmol. 103 (4), 463–468 (2019).
Hogg, H. D. J., Talks, S. J., Pearce, M. & Di Simplicio, S. Real-World visual and neovascularisation outcomes from anti-VEGF in central retinal vein occlusion. Ophthalmic Epidemiol. 28 (1), 70–76 (2021).
Kupriyanova, E. Carbonic anhydrase – a universal enzyme of the carbon-based life. Photosynthetica 55, 3–19 (2017).
Supuran, C. T. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 7 (2), 168–181 (2008).
Torella, D. et al. Carbonic anhydrase activation is associated with worsened pathological remodeling in human ischemic diabetic cardiomyopathy. J. Am. Heart Assoc. 3 (2), e000434 (2014).
Sen, S. et al. Comparative proteomics of proliferative diabetic retinopathy in people with type 2 diabetes highlights the role of inflammation, visual transduction, and extracellular matrix pathways. Indian J. Ophthalmol. 71 (8), 3069–3079 (2023).
Tomita, M. & Tsumoto, K. Hybridoma technologies for antibody production. Immunotherapy 3 (3), 371–380 (2011).
Scholl, S., Kirchhof, J. & Augustin, A. J. Pathophysiology of macular edema. Ophthalmologica 224 (Suppl 1), 8–15 (2010).
Pe’er, J. et al. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology 105 (3), 412–416 (1998).
Noma, H. et al. Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye (Lond). 22 (1), 42–48 (2008).
Fonollosa, A. et al. Vitreous levels of interleukine-8 and monocyte chemoattractant protein-1 in macular oedema with branch retinal vein occlusion. Eye (Lond). 24 (7), 1284–1290 (2010).
Koss, M. J. et al. Comparison of cytokine levels from undiluted vitreous of untreated patients with retinal vein occlusion. Acta Ophthalmol. 90 (2), e98–e103 (2012).
Funk, M. et al. Intraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumab. Invest. Ophthalmol. Vis. Sci. 50 (3), 1025–1032 (2009).
Koss, M. et al. Correlation from undiluted vitreous cytokines of untreated central retinal vein occlusion with spectral domain optical coherence tomography. Open. Ophthalmol. J. 7, 11–17 (2013).
Noma, H., Mimura, T. & Eguchi, S. Association of inflammatory factors with macular edema in branch retinal vein occlusion. JAMA Ophthalmol. 131 (2), 160–165 (2013).
Supuran, C. T. Carbonic anhydrase versatility: from pH regulation to CO(2) sensing and metabolism. Front. Mol. Biosci. 10, 1326633 (2023).
Noma, N. et al. Impact of acetazolamide, a carbonic anhydrase inhibitor, on the development of intestinal polyps in min mice. Int. J. Mol. Sci 18(4) (2017).
Gao, B. B. et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat. Med. 13 (2), 181–188 (2007).
Ivanov, I. et al. Protease activity in single-chain prekallikrein. Blood 135 (8), 558–567 (2020).
Dobó, J. et al. Cleavage of kininogen and subsequent Bradykinin release by the complement component: mannose-binding lectin-associated Serine protease (MASP)-1. PLoS One. 6 (5), e20036 (2011).
Langhauser, F. et al. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood-brain barrier damage, and inflammation. Blood 120 (19), 4082–4092 (2012).
Martin, T. A., Mansel, R. & Jiang, W. G. Hepatocyte growth factor modulates vascular endothelial-cadherin expression in human endothelial cells. Clin. Cancer Res. 7 (3), 734–737 (2001).
Rosenthal, R. et al. The fibroblast growth factor receptors, FGFR-1 and FGFR-2, mediate two independent signalling pathways in human retinal pigment epithelial cells. Biochem. Biophys. Res. Commun. 337 (1), 241–247 (2005).
Zittermann, S. I. & Issekutz, A. C. Basic fibroblast growth factor (bFGF, FGF-2) potentiates leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression. Am. J. Pathol. 168 (3), 835–846 (2006).
Acknowledgements
The datasets generated and analysed during the current study are not publicly available due to very strict data protection regulations but are available from the corresponding author on reasonable request.
Funding
This research received Natural Science Foundation of Sichuan Provincial Science and Technology Department (2023NSFSC1666).
Author information
Authors and Affiliations
Contributions
Conception and design, XSJ, XK, YHZ and FL; analysis and interpretation, XSJ and PFR; acquisition of data, PFR, JXZ, GNL, JL, WWS; writing—original draft preparation, XSJ; writing—review and editing, PFR, JXZ, GNL, JL, WWS, XK, YHZ and FL; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
The protocol used in this study was approved by the Institutional Review Board of West China Hospital (2023 − 1375), and the procedures conformed to the tenets of the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, X., Ren, P., Ke, X. et al. Aqueous carbonic anhydrase-1 overexpression and its association with cytokine profiles and macular structural changes in retinal vein occlusion-associated macular edema. Sci Rep 15, 30780 (2025). https://doi.org/10.1038/s41598-025-16487-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16487-3