Abstract
Tobacco metabolites play a crucial role in shaping the sensory experience of tobacco products, such as cigarettes. Bitter metabolites in tobacco not only serve as protective agents in plants, but also significantly influence the overall flavor profile of cigarettes. In this study, we employed an integrated approach combining chemical profiling, cell-based calcium imaging assays, and molecular docking studies to identify key bitter compounds in cigarette smoke and explore the molecular mechanisms underlying their bitterness. We analyzed 22 commercial cigarette brands, revealing notable variability in bitterness perception. GC-MS analysis identified 88 compounds in mainstream cigarette smoke (MCS), with pyridines, esters, and phenols being the most prevalent. Using a TAS2R14-Gα16gust44-HEK293T cell-based assay, we objectively measured bitterness and identified nicotine, succinimide, and scopoletin as key bitter compounds through correlation analysis. Molecular docking studies provided detailed insights into the interactions of these compounds with the TAS2R14 receptor, highlighting significant hydrogen bonding and hydrophobic interactions. This integrated methodology not only identifies key contributors to cigarette bitterness but also enhances our understanding of the molecular basis of bitterness perception in tobacco. These findings pave the way for future studies to further elucidate the complex interactions of bitter compounds. This understanding of the links between tobacco bitterness and consumption holds significant implications for public health and tobacco control initiatives.
Similar content being viewed by others
Introduction
Tobacco, a member of the Solanaceae family, produces specialized metabolites such as nicotine that are critical for plant defense and significantly influence the sensory properties of tobacco products, particularly bitterness. Bitterness is a fundamental taste attribute that defines the sensory experience of products derived from tobacco, such as cigarette1,2. While its aversive nature may initially deter some individuals from smoking, this effect is frequently counterbalanced by nicotine’s addictive properties. Therefore, understanding the interplay between bitterness and nicotine addiction is crucial. Gaining insights into the molecular basis of tobacco bitterness, and its dynamics with nicotine addiction is essential for unraveling the complex nature of taste perception in tobacco products consumption. This understanding not only contributes to the field of flavor science but also holds important implications for public health and tobacco control strategies.
Traditionally, the taste profile of cigarette smoke, which includes the basic tastes of sour, sweet, bitter, salty, and umami, as well as tactile sensations like astringency and irritancy, has been characterized using sensory evaluation methods. Although these evaluations have provided valuable insights into the sensory experience of cigarette smoke, they have notable limitations. Sensory evaluation demands well-trained evaluators, has limited analytical throughput, and is costly and time-consuming3. Furthermore, variations among evaluators in sensory thresholds, evaluation scales, sensitivities, and inherent subjectivity must be taken into consideration, which significantly complicates this method.
In contrast, chemical analysis offers a more objective and scalable approach to understanding the basis of tobacco bitterness. For example, Soares et al. employed flow-injection coupled with high-resolution mass spectrometry to profile the chemical fingerprint of cigarette mainstream smoke samples, aiming to predict their sensory attributes3. Wang et al., using liquid chromatography coupled with high-resolution mass spectrometry (LC-HRMS), identified 16 bitter compounds in cigarette smoke, including nicotine, nornicotine, myosmine, and cotinine4. Similarly, Arndt et al. developed an LC-HRMS-based method that integrated multiple chromatographic and ionization techniques with compound identification strategies to comprehensively characterize the chemical composition of tobacco smoke5. Furthermore, headspace solid-phase microextraction combined with gas chromatography-mass spectrometry (HS-SPME/GC–MS) has also been utilized to establish and compare the volatile fingerprints of traditional tobacco and e-cigarettes6. Although these chemical analyses can identify and quantify specific compounds in cigarette smoke, the direct correlations between these compounds and human perception of bitterness are often unclear. Human taste perception results from the combined action of multiple compounds through complex physiological mechanisms7 and simple chemical analysis cannot fully reflect this complexity. Moreover, there may be complex interactions among compounds in cigarette smoke, which could enhance or inhibit the perception of certain tastes. Analysis of individual chemical components may overlook these complex interactions.
Recent advances in cell-based assays offer a more mechanistic understanding of bitterness perception8,9. The perception of bitterness mainly involves the recognition of bitter substances by type 2 bitter taste receptors (TAS2Rs), a family of G protein-coupled receptors (GPCRs) located in tongue taste buds10. When bitter agonists bind to TAS2Rs, they activate G protein, triggering intracellular calcium ion mobilization and downstream neural signaling7. Based on the perception principles of bitterness, cell-based calcium imaging assays have been established for the investigation of bitterness of food and beverages, such as hops11 coffee12 soybeans13 and tea14. These assays utilize cell models, which have been previously transfected with cDNA of TAS2Rs and G protein, to monitor the response of the cell model to bitter substances via calcium imaging technique. Furthermore, molecular docking studies allow for a detailed examination of the interactions between bitter compounds and TAS2Rs, offering insights into the structural basis of receptor activation and bitterness perception. By combining cell-based assays with molecular docking techniques, researchers can elucidate the specific components that contribute to bitterness and enhance our understanding of the mechanisms underlying bitter taste in tobacco.
This study aims to conduct a detailed exploration of the bitterness of individual tobacco components and the underlying molecular mechanisms by employing a combination of chemical profiling, cell-based calcium imaging assays, and molecular docking. Firstly, GC-MS was used to identify the main components of cigarette smoke. Subsequently, a cell model heterogeneously expressing bitter receptor and G protein (TAS2R14/Gα16gust44-HEK293T) was established for the assessment of the bitterness of cigarette smoke. Pearson’s correlation analysis was then employed to identify key bitter compounds whose concentrations were positively correlated with bitterness. Finally, the interactions of the key bitter compounds with TAS2R14 were examined through molecular docking to elucidate the molecular mechanisms underlying bitterness perception.
Materials and methods
Samples and reagents
Twenty-two different brands of commercial cigarette products were collected for analysis in this study. These 22 brands of cigarettes include 16 flue-cured Virginia type cigarettes (VTC01–VTC16) and 6 air-cured Burley type cigarettes (BTC01–BTC06).
Trypsin-EDTA, phosphate buffer solution (PBS, 100 mM, pH 7.4), and penicillin-streptomycin were purchased from Biosharp (Shanghai, China). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Hyclone (Logan, USA). Fluo-4AM dye was purchased from Beyotime (Shanghai, China). Fetal bovine serum (FBS) was purchased from Biocell (Hamilton, New Zealand). Lipofectamine™ 3000 and opti-MEM were purchased from Thermo Fisher Scientific (Bremen, Germany). Deionized water was prepared from a Milli-Q system (Billerica, USA).
Sensory evaluation of cigarettes
The sensory evaluation of cigarettes was performed by a five-member expert panel (four male, one female, aged 25 to 43), each possessing over three years of experience in cigarette sensory assessment. The experiment employed the magnitude estimation method of descriptive testing to assess the bitterness intensity of the cigarettes. The entire evaluation process was conducted in three rounds, with each round assessing an equal number of cigarette samples. To prevent the potential influence of the evaluation order on the results, the sequence of sample evaluation was varied in each round, ensuring that each cigarette sample was independently evaluated three times. A 10-point scale was used to quantify the panelists’ perception of bitterness intensity, with BTC06 serving as the reference standard, whose bitterness score was set to 5. If a sample’s bitterness exceeded the standard, it was scored between 5 and 10; if it was less bitter, it was scored between 0 and 5. A score of “0” indicated no bitterness, while “10” represented intense bitterness.
At the start of the sensory evaluation, the panelists rinsed their mouths with warm water to eliminate any residual flavors. During the cigarette evaluation, a local circulation smoking method was employed, wherein the smoke was briefly retained in the mouth before being exhaled through the nose. To prevent taste fatigue, a 5-minute break was arranged after evaluating each sample, and a 20-minute break was arranged between each round of sensory evaluation to ensure the panelists could fully restore their taste sensitivity.
Preparation of mainstream cigarette smoke
The mainstream cigarette smoke (MCS) was prepared according to the procedures described in the Chinese standard GB/T 19,609 − 2004. MCS was extracted using a 20-hole linear smoking machine (SM450, Cerulean, UK) from the cigarette samples. A 44 mm Cambridge filter pad was utilized to capture the MCS, with each filter pad collecting smoke from five cigarettes. After collection, the Cambridge filter pads were weighed both before and after adsorption to determine the amount of MCS captured. Subsequently, the Cambridge filter pads containing the MCS were placed in conical flasks, and a 50% DMSO solvent at a 250-fold volume was added for the extraction of MCS over 30 min, with the assistance of sonication. The resulting extraction solution was then filtered to obtain an MCS solution with a concentration of 4 mg/mL.
GC-MS analysis of MCS
Agilent 7890 A gas chromatograph (Agilent, Palo Alto, CA, USA) equipped with Agilent DB-5MS column (60.0 m × 0.25 mm × 0.25 μm, Agilent, USA), and coupled to Agilent 5977 C mass spectrometer (Agilent, Palo Alto, CA, USA) was used for the chemical composition of MCS analysis. The temperature of the injection port was set to 250 °C. The carrier gas was high purity He (purity > 99.999%) with flow rate of 1.5 mL/min. The injection volume was 2 mL with pulsed splitless mode. The temperature program was as follows: initial column temperature 60 °C for 2 min; temperature was increased to 240 °C at a rate of 2 °C/min and was held at 240 °C for 2 min; then was increased to 280 °C at a rate of 10 °C/min and was held at 280 °C for 10 min. The temperatures of the ion source and quadrupole were 230 °C and 150 °C, respectively. The ion energy for electron impact was 70 eV, and the mass spectra were obtained in full scan mode from 35 to 450 amu. Each chromatographic peak’s mass spectrum was compared against the NIST11 standard spectral library for qualitative analysis. Each chromatographic peak’s mass spectrum was compared against the NIST11 standard spectral library for qualitative analysis. Compound identification was based on the mass spectral matching score. Matches with matching score above 70% were considered reliable identifications. The identified chemical compounds were semi-quantified using phenethyl acetate as the internal standard.
Cell-based calcium imaging analysis of MCS
The cell-based calcium imaging assay was carried out based on the previously described method with some modifications8,14. To construct the expression plasmids for TAS2Rs, one of the five human bitter taste receptors, including TAS2R10 (NCBI accession number: NM_023921.2), TAS2R14 (NM_023922.2), TAS2R39 (NM_176881.2), TAS2R43 (NM_176884.2), and TAS2R46 (NM_176887.2), was fused with the initial 45 amino acid sequence of the rat somatostatin receptor type 3 (ssr3). This fusion helped enhance the localization of the receptor proteins to the cell membrane13. The expression plasmid for G-protein was constructed by combining the C-terminal 44 amino acid sequence of gustducin (NM_001102386.3) with the amino acid sequence of Gα16 protein (NM_002068.4)15 and introducing an RFP red fluorescent tag. All expression plasmids were constructed using the plasmid vector pcDNA3.1(+) and a T4 ligase approach. Human embryonic kidney (HEK)−293T cells, purchased from BeNa Culture Collection (Beijing, China), were employed for the heterologous expression of the five bitter taste receptors and G-protein. These cells were cultured under standard conditions (DMEM, 10% FBS, 1% penicillin/streptomycin; 37 °C, 5% CO2, 95% humidity). Transient transfection using Lipofectamine 3000 was conducted when the cell confluence reached 80–90%.
Forty-eight hours after transfection, the cells were seeded into 35 mm confocal culture dishes with a density ranging from 3 × 105 to 4 × 105 cells per dish and cultured for an additional 12 h. The medium in the confocal culture dishes was then aspirated, and the cells were rinsed twice with PBS buffer. Subsequently, 150 µL of 2 µM Fluo-4 AM in serum-free DMEM medium was introduced, and the cells were incubated in darkness at 37 °C for 60 min. After incubation, the medium containing Fluo-4 AM was discarded, and the cells were again rinsed twice with PBS buffer. Next, 150 µL of DAPI stain was introduced, and the cells underwent another incubation session in darkness at 37 °C for 20 min. After DAPI staining, the cells were rinsed twice with PBS buffer before adding 900 µL of complete medium. Treated cells were then placed under a Confocal Laser Scanning Microscope (CLSM). A 100 µL of MCS sample was introduced, and the cell observation was conducted using a 20x objective lens. FITC, DAPI, and TRITC channels were utilized, and images were captured both before and after MCS sample introduction. The peak fluorescence value within 3 min following the sample introduction was recorded.
The fluorescence intensity in the CLSM images of the FITC channel before and after MCS introduction was analyzed by using ImageJ software (version, 1.53 K). The change in intracellular calcium ion concentration was represented by ∆F, calculated using the following formula:
where F0 represents the fluorescence intensity in the CLSM image of the FITC channel before the sample addition, and Ft represents the maximum fluorescence intensity in the CLSM image of the FITC channel obtained within 3 min after the sample addition.
Untransfected cells were used as the control. It has been reported that cigarette smoke can induce the release of calcium ions from lysosomes in HEK293T cells16 while bitter substances can induce the release of calcium ions from the endoplasmic reticulum17 and/or mitochondria18 in TAS2Rs containing cells. Based on the changes in fluorescence intensity, the cigarette smoke bitter taste intensity (BTV) was calculated using the following formula:
where ∆FS represents the change in fluorescence intensity before and after sample addition in TAS2R14-Gα16gust44-HEK293T cells, and ∆FB represents the change in fluorescence intensity before and after sample addition in untransfected HEK293T cells. Samples with BTV > 0 were considered to exhibit a bitter taste, while samples with BTV ≤ 0 were considered non-bitter. All determinations were performed in triplicate.
Molecular docking analysis
To investigate the interaction between key bitter compounds in MCS and TAS2R14, molecular docking analysis was conducted. Firstly, the stable spatial conformation of TAS2R14 was obtained using the AlphaFold server. Subsequently, the binding pockets of TAS2R14 were predicted using the DoGSiteScorer function in the ProteinsPlus server. The depth, hydrophobicity, surface area, volume, and surface area to volume ratio of the binding pocket were then calculated. Next, the spatial structures of key bitter compounds in MCS were optimized. The SDF file of the 3D structure of bitter compounds was obtained from the PubChem server. The geometry optimization of the bitter compounds was based on density functional theory (DFT) calculations using Gaussian 09 software at the DFT//B3LYP/6-31G(d, p) theoretical level, incorporating the conductor-like polarizable continuum model (CPCM) in a water environment at 298.15 K. Finally, molecular docking of bitter components with TAS2R14 was conducted using AutoDock Vina software (V1.2.0). Based on the analysis results, the optimal docking poses of bitter components with TAS2R14 were selected, and visualization processing was performed using PyMol software (version 2.5.4). The analysis included examining the binding mode and interaction forces between bitter compounds and TAS2R14, such as hydrogen bonds, van der Waals forces, hydrophobic interactions, and π-π stacking.
Statistical analysis
Statistical significance analysis was performed employing IBM SPSS software (version 22.0), utilizing student’s t-test or one-way analysis of variance (ANOVA). The significance threshold was set at a level of p < 0.05.
Results and discussion
Sensory evaluation of the cigarette samples
As shown in Fig. 1, the sensory evaluation results, with BTC06 as the reference sample standardized to a bitterness score of 5, exhibit a wide range of bitterness scores across the 22 cigarette samples. Several samples show large interquartile ranges, indicating diverse perceptions of bitterness among the panelists. Notably, certain samples, such as VTC03, BTC03, and BTC04, show higher median bitterness scores with narrower interquartile ranges, indicating more consistent assessments among the panelists. Conversely, samples like VTC04, VTC11, and VTC12 show broader interquartile ranges, indicating greater differences in the panelists’ assessments. Overall, the median bitterness scores reported by the panelists did not show significant differences among the samples. This result indicates substantial individual variability among the sensory evaluation panelists. This variability may be attributed to individual differences in bitter taste receptor sensitivity and potential psychological biases. The inherent subjectivity of sensory evaluations suggests that individual differences significantly impact the assessment of bitterness intensity in cigarette smoke. This pronounced variability indicates the need to integrate objective analytical methods with sensory evaluations, providing a more comprehensive understanding of the bitterness profile in cigarette smoke. The following sections will discuss the chemical and molecular analyses used to identify the key contributors to bitterness, addressing the limitations of sensory evaluation.
GC-MS analysis of chemical components in MCS
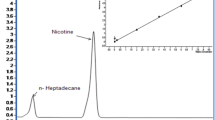
To elucidate the chemical basis underlying the bitterness of cigarette smoke, chemical profiling of MCS was conducted using GC-MS. A total of 88 compounds were identified across the 22 MCS samples. These compounds consisted of 11 alcohols, 10 esters, 4 fatty acids, 7 furans, 10 hydrocarbons, 13 ketones, 19 phenols, 5 pyridines, and 9 other compounds. Quantitative analysis revealed that pyridines, esters, and phenols are the predominant classes in MCS, accounting for 46.09%, 20.63%, and 10.29% of the total detected compounds, respectively. Pyridine alkaloids, primarily nicotine, were the most abundant class identified. Nicotine alone accounted for a significant portion of the pyridine class and was characterized by its distinct pungent and bitter taste19. Extensive studies have documented nicotine as the most abundant compound in tobacco, influencing both its addictive properties and sensory perception2. The prominent presence of nicotine in the chemical profile of MCS highlights its significance in the flavor profile of cigarette smoke, making it a critical compound for further mechanistic studies on bitterness perception. Esters, comprising 20.63% of the detected compounds, are typically associated with fruity and floral aromas. These compounds likely play a crucial role in modulating the sensory attributes of cigarette smoke by masking harsh and bitter flavors. The presence of esters suggests a complex interplay between bitter compounds and those that may mitigate bitterness, contributing to the overall flavor balance of cigarette smoke. Phenols, comprising 10.29% of the detected compounds, are recognized for their bitter and astringent taste attributes. The high concentration of phenols suggests their potential contribution to the bitter taste perceived in cigarette smoke. The general chemical profile of MCS is illustrated in Fig. 2A, while detailed information on these compounds can be found in Supplementary Material Table S1. To further explore the chemical differences between different cigarette types, PCA was conducted based on the quantitative data of the detected compounds. The PCA plot, depicted in Fig. 2B, distinctly separated the chemical profiles of VTC samples from BTC samples, highlighting the variation in chemical composition between these two types of cigarettes. The chemical differences between VTC and BTC also suggest their distinct flavor profiles and bitterness perceptions.
Screening the bitter taste receptor and optimizing MCS concentration
The recombinant plasmids of Gα16gust44, TAS2R10, TAS2R14, TAS2R39, TAS2R43, and TAS2R46 were transiently transfected into HEK293T cells using Lipofectamine 3000. The transfection efficiency was assessed by observing the RFP sequence carried by the plasmids using CLSM. As shown in supplementary material Fig. S1, all five bitter taste receptors and the G protein were successfully expressed in the HEK293T cells, confirming effective transfection.
To investigate whether the cells transfected with TAS2Rs respond to MCS, we compared the calcium mobilization responses of untransfected HEK293T cells and TAS2R14-Gα16gust44-HEK293T cells upon exposure to MCS from VTC01. A 100 µL aliquot of MCS solution (4 mg/L in 50% DMSO) was added to the cell culture medium, and calcium mobilization was observed using CLSM. As shown in supplementary material Fig. S2, untransfected HEK293T cells exhibited a weak fluorescent signal (Fig. S2A & B), whereas TAS2R14-Gα16gust44-HEK293T cells displayed a significantly stronger fluorescent signal (Fig. S2C & D). Previous studies have shown that nicotine in cigarette smoke induces the release of calcium ions from lysosomes in various mammalian cells16,20. The released calcium ions bind to Fluo-4 fluorescent dye, resulting in green fluorescence. This explains the calcium mobilization observed in the untransfected HEK293T cells. The enhanced response observed in TAS2R14-Gα16gust44-HEK293T cells suggests that, beyond lysosomal calcium release, the activation of TAS2Rs by bitter compounds in MCS further elevates intracellular calcium levels. Upon binding of the bitter compounds to TAS2R14, the receptor activates Gα16gust44, which in turn triggers downstream signaling pathways, opening calcium ion channels on the cell membrane. This influx of calcium ions increases intracellular calcium concentration, binding to Fluo-4 dye and producing green fluorescence. Control experiments with 50% DMSO alone showed no significant fluorescence signal, confirming that the solvent did not interfere with the experimental results (data not shown).
Furthermore, we compared the response of cells transfected with the five TAS2Rs (TAS2R10, TAS2R14, TAS2R39, TAS2R43, and TAS2R46) to the same MCS sample, using untransfected HEK293T cell as a control. As shown in supplementary material Figure S3, all five receptor-transfected cells exhibited elevated fluorescence signals compared to the control following MCS exposure. Notably, TAS2R14-Gα16gust44-HEK293T cells demonstrated the strongest fluorescence response, suggesting the highest sensitivity to MCS. Previous study has shown that TAS2R14 can recognize about 150 natural and synthetic bitter compounds, making it the most broadly tuned human bitter taste receptor with the most diverse set of ligands21. This may be due to its large ligand binding pocket, offering diverse contact sites for ligand interactions and sufficient space for accommodating diverse agonists21. Therefore, in this study, TAS2R14-Gα16gust44-HEK293T cells were selected for subsequent experiments.
To optimize the concentration of MCS for use in the assays, we evaluated the fluorescence response of TAS2R14-Gα16gust44-HEK293T cells at varying MCS concentrations, using untransfected HEK293T cells as a control. As shown in supplementary material Fig. S4, an increase in MCS concentration correlated with enhanced fluorescence intensity in both transfected and untransfected cells, indicating a dose-dependent effect. Notably, when the concentration of MCS solution exceeded 4 mg/mL (final concentration of 400 µg/mL), the intracellular calcium ion fluorescence signal in the transfected cells was significantly higher than that in the control (p < 0.001). Conversely, at MCS concentrations below 2 mg/mL (final concentration of 200 µg/mL), no significant difference in intracellular fluorescence signal was observed between the transfected and untransfected cells (p > 0.05). Therefore, to avoid strong fluorescence background interference while still detecting the bitter substances in the MCS solution, a concentration of 4 mg/mL was selected for subsequent experiments.
Cigarette bitterness intensity assessment
After selecting the optimal bitter taste receptor cell model (TAS2R14-Gα16gust44-HEK293T cells) and the concentration of MCS (4 mg/mL), we further evaluated the bitterness intensities of the 22 cigarette samples. The fluorescence imaging results of these MCS solutions are shown in Fig. 3 (A, 20x magnification; B, 200x magnification). As illustrated in the images, the TAS2R14-Gα16gust44-HEK293T cells exhibited negligible green fluorescence signals before sample addition, but exhibited intense green fluorescence signals after sample addition. This increase in fluorescence indicates a substantial rise in intracellular calcium ion concentration, reflecting calcium mobilization. At 200x magnification, the green fluorescence signals of the cells were uniformly distributed within the cell area, with no significant fluorescence signals detected in the extracellular area. This uniformity confirms that the observed signals originated from intracellular calcium flux rather than interference from the culture medium or cigarette samples. More importantly, it was demonstrated that different cigarette samples induced varying degrees of calcium mobilization in the TAS2R14-Gα16gust44-HEK293T cells, demonstrating differential bitterness intensities.
We further quantitatively analyzed the fluorescence intensity in the CLSM images of the FITC channel before and after MCS addition. Subsequently, the values of ∆FS and ∆FB, representing the maximum intracellular calcium response of TAS2R14-Gα16gust44-HEK293T cells and untransfected HEK293T cells to MCS, respectively, were calculated using Formula (1) Next, the BTV values, representing the bitterness intensity of each sample, were calculated using Formula (2) The results are demonstrated in Fig. 4. All tested samples had BTV values exceeding 0, indicating that cells transfected with bitter taste receptors responded to the bitter compounds in MCS. Of the 22 cigarette samples, 13 samples (BTC05, VTC08, BTC04, VTC03, BTC03, VTC06, VTC04, BTC01, BTC02, VTC02, VTC05, BTC06, and VTC07) exhibited relatively higher BTV values (BTV > 1), indicating greater bitterness intensity. In contrast, the remaining nine samples had BTV values below 1, indicating lower bitterness intensity. Notably, BTC05 had the highest BTV value, while VTC11 had the lowest. Overall, flue-cured Virginia cigarettes (VTC) demonstrated relatively lower BTV values compared to air-cured Burley cigarettes (BTC) (0.908 vs. 1.425), suggesting a general trend of lower bitterness in VTC samples.
Pearson correlation analysis was subsequently conducted to examine the relationship between the BTV values obtained from the cell-based assay and the average bitterness scores from sensory evaluation. As depicted in Fig. 5, a positive correlation was observed between BTV and sensory evaluation results (r = 0.67, p < 0.01). It is worth noting that certain samples, such as VTC11, VTC14, VTC16, VTC10, VTC12, and BTC03, displayed a lack of correlation between their sensory evaluation bitterness scores and the BTV values, potentially reflecting the inherent complexity of cigarette bitterness. Nevertheless, the general positive correlation suggests that the results from the cell-based assay are consistent with those from the sensory evaluation, validating the reliability and relevance of the TAS2R14-Gα16gust44-HEK293T cell model for assessing the bitterness of cigarette smoke. Furthermore, this approach provides a quantitative and consistent measure of bitterness, reducing the variability seen in human panel assessments, thereby offering a more reliable understanding of cigarette bitterness intensity.
Identification of key bitter compounds in MCS
The key bitter compounds in MCS were identified using the correlation analysis between the chemical compounds detected by GC-MS and the BTV values derived from the cell-based calcium imaging assay. The full results are demonstrated in Supplementary materials Table S1. This analysis revealed that a total of 12 compounds demonstrated a relatively high positive correlation (r > 0.5 and p < 0.05) with BTV. Among these, three compounds may contribute to bitter sensation in tobacco based on a comprehensive literature review. These compounds include nicotine (r = 0.535, p < 0.01), succinimide (r = 0.551, p < 0.01), and scopoletin (r = 0.684, p < 0.01). These correlations suggest a potential key role of these compounds in shaping the bitter taste perception of cigarettes.
Among these compounds, nicotine was identified as the key bitter compound with a positive correlation with BTV values across the 22 MCS samples (Fig. 6A). Nicotine, a pyridine alkaloid, is a fundamental component in tobacco that profoundly influences the flavor of cigarette smoke. It imparts a distinctive bitter and pungent taste to cigarette smoke2. The bitterness of nicotine arises from its ability to stimulate bitter taste receptors on the tongue22. It has been reported that the concentration of nicotine in cigarette smoke directly correlates with the perceived bitterness23. Additionally, nicotine’s bitterness can be influenced by its synergistic interactions with other compounds. In the complex matrix of cigarette smoke, nicotine’s bitterness can be enhanced by its interactions with flavor compounds like alkaloids, phenolics, and terpenes, which contribute to the overall harshness of the smoke24. Conversely, its bitterness is sometimes masked or balanced by other flavor compounds present in cigarette smoke, such as sugars, aldehydes, and ketones, which provide sweetness, fruitiness, and other sensory notes25,26. Therefore, the combination of nicotine’s inherent bitterness and its interactions with other compounds creates a distinctive bitter flavor profile essential for the characteristic taste of cigarettes. When comparing the nicotine contents between VTC and BTC samples, BTC samples contain higher nicotine concentrations compared to VTC samples, with average concentrations of approximately 58.3 mg/g and 49.1 mg/g in MCS samples, respectively (Fig. 6B). This difference in nicotine content likely contributes to the higher bitterness intensity observed in BTC samples.
Succinimide, another key bitter compound identified (Fig. 6C), is a cyclic imide. Although specific literature on the flavor of succinimide is sparse, its chemical structure suggests a bitter taste. Chemical compounds containing an imide group typically exhibit pronounced bitterness. For example, caffeine, which contains an imidazole ring, is well-known for its bitter taste in coffee and tea27. Similarly, theobromine, which also contains an imide group, contributes to the slightly bitter taste of dark chocolate28. This suggests that succinimide may play a role in the bitterness of cigarette smoke through similar mechanisms. Figure 6D indicates that VTC samples have relatively lower succinimide content compared to BTC samples, suggesting that this compound also contributes to the higher bitterness observed in BTC samples.
Scopoletin, a naturally occurring coumarin derivative found in various plants such as chicory, passion flower, and some species of Artemisia29was also identified as a key bitter compound in MSC (Fig. 6E). Coumarin derivatives, both in aglycone form and as glycosides, impart a bitter taste to the plants in which they are found30. Zhao et al. reported that the content of scopoletin in tobacco leaves is about 81 µg/g and can vary at different growth stages30. Therefore, it is expected that scopoletin contributes to the bitter taste of cigarette smoke. Additionally, scopoletin has been associated with sweet, woody, and nutty aromas in cigars and tobacco products31 adding complexity to the flavor profile of tobacco. As shown in Fig. 6F, VTC samples have relatively lower scopoletin content compared to BTC samples, which correlates with their lower bitterness intensity.
It is crucial to recognize the compositional complexity of cigarette smoke. While our analysis identified three compounds with strong positive correlations to BTV, many other constituents in MCS likely contribute to its overall bitterness, potentially through interactions with TAS2R14 or other taste receptors. Thus, the overall bitterness profile of cigarette smoke is a collective effect of multiple compounds, underscoring the multifaceted nature of taste perception in cigarette smoke. Nonetheless, through the cell-based calcium imaging assay, nicotine, succinimide, and scopoletin exhibited strong positive correlations with BTV values, suggesting their potential significant roles in the bitterness perception of cigarette smoke. Additionally, the comparative analysis between flue-cured Virginia cigarettes and air-cured Burley cigarettes showed that BTC generally had higher concentrations of these three bitter compounds, correlating with the higher BTV values, and thus greater bitterness intensity. To further elucidate the mechanisms of bitterness perception of these three compounds, the following section presents the results of a molecular docking study on these three compounds with the TAS2R14.
Molecular docking studies of bitter compounds with TAS2R14
Given the potential roles of nicotine, succinimide, and scopoletin in the bitterness perception of cigarette smoke, we further explored their binding mechanisms with TAS2R14 through molecular docking studies. Molecular docking is a computational chemistry method used to predict the interaction potential between molecules, assess binding capabilities, and determine binding modes. This technology is widely applied in molecular sensory science, particularly in elucidating the taste mechanisms of bitter substances and their interactions with taste receptors32,33.
The protein structure of the human TAS2R14 was obtained from the AlphaFold sever, which is regarded as one of the most effective methods for protein structure prediction. AlphaFold utilizes deep learning and neural networks to accurately generate 3D protein structures with near-experimental accuracy. This model provides a reliable starting point for our docking studies, as it provides the intricate structural details of TAS2R14, including the transmembrane helices and potential ligand binding pockets. Next, using the DoGSiteScorer function in the ProteinsPlus server, two main binding pockets were predicted for TAS2R14, as shown in Fig. 7A. The meshes colored in green represent the first binding pocket, while the meshes colored in blue represent the second binding pocket. Both pockets are located in the central cavity within the transmembrane bundle of TAS2R14, consistent with the typical ligand-binding region of class A GPCRs. The first binding pocket has a volume of 667.65 Å3a surface area of 875.05 Å2and a depth of 23.23 Å; it contains 7 hydrogen donors, 28 hydrogen acceptors, and 48 hydrophobic interaction sites, with a hydrophobicity ratio of 0.56. The second binding pocket has a volume of 436.38 Å3a surface area of 466.70 Å2and a depth of 18.03 Å; it contains 6 hydrogen donors, 22 hydrogen acceptors, and 23 hydrophobic interaction sites, with a hydrophobicity ratio of 0.45. These characteristics suggest that both pockets have relatively large ligand binding areas and numerous ligand binding sites, which could potentially accommodate a variety of ligands.
Molecular docking of key bitter compounds in MCS with TAS2R14. (A) Predicted binding pockets of TAS2R14. The two main binding pockets colored in green and blue respectively, were predicted using the DoGSiteScorer function in the ProteinsPlus server (https://proteins.plus/). (B & C) Docking pose of nicotine with TAS2R14. (D & E) Docking pose of succinimide with TAS2R14. (F & G) Docking pose of scopoletin with TAS2R14. All structures were visualized using PyMol software (version 2.5.4).
The optimal geometrical conformations of nicotine, succinimide, and scopoletin were calculated using Gaussian 09 software, employing DFT with the B3LYP/6-31G(d, p) basis set. This level of theory provides a good balance between accuracy and computational cost for small organic molecules. Subsequently, Autodock Vina software was employed for the molecular docking studies. Interestingly, all three compounds were found to bind to the second binding pocket of TAS2R14, located in the blue meshes as illustrated in Fig. 7A. For nicotine, the lowest binding energy with TAS2R14 is −6.3 kcal/mol, indicating a moderately strong interaction. The docking pose with the lowest binding energy is shown in Fig. 7B and C. It is demonstrated that nicotine penetrates deeply into the central cavity, with its pyridine ring forming a hydrogen bond with the carboxyl group on the amino acid side chain of ASN933.36 on the third transmembrane helix (TM3) of TAS2R14. This interaction likely contributes significantly to the binding affinity and specificity. It also forms hydrophobic interactions with TRP893.32 on TM3 and PHE2476.55 on the TM6; the other hydrogenated pyrrole ring forms hydrophobic interactions with VAL973.40 on TM3 and ILE1875.47 on TM5. These hydrophobic interactions stabilize the ligand within the binding pocket and may contribute to the overall binding energy. For succinimide, the lowest binding energy is −4.8 kcal/mol, suggesting a weaker interaction compared to nicotine. The pyrrolidine ring of succinimide forms hydrophobic interactions with PHE2476.52 on TM6 and TYR2727.45 on TM7 (Fig. 7D and E). Additionally, its imide group forms a hydrogen bond with SER2446.49 on TM6. The lower binding energy of succinimide compared to nicotine may be attributed to its smaller size and fewer interaction points with the receptor. For scopoletin, the lowest binding energy is −7.4 kcal/mol, indicating the strongest interaction among the three compounds. As shown in Fig. 7F and G, the lactone ring of scopoletin forms hydrophobic interactions with TYR2406.45 on TM6 and TYR2727.45 on TM7. The benzene ring forms hydrophobic interactions with ILE1875.47 on TM5. The methoxy group at the 6-position forms a hydrogen bond with ASN933.36 on TM3, and the carbonyl oxygen atom of scopoletin forms a hydrogen bond with TYR2406.45 on TM6. Additionally, the benzene ring forms a π-π stack with PHE2476.52 on TM6. The multiple interaction points and the π-π stacking likely contribute to scopoletin’s higher binding affinity. Due to the similar binding sites of these compounds, they are found to bind with several identical amino acid residues, including PHE2476.52, ILE1875.47, TYR2727.45, and ASN933.36. Notably, PHE2476.52 and ASN933.36 have been identified as the key residues for TAS2R14 to recognize the agonists, such as flufenamic acid34 hop-derived bitter compounds33. This convergence on key residues suggests a common mechanism for bitter taste recognition in TAS2R14, despite the structural diversity of the ligands. These molecular docking results provide detailed information on the potential binding modes of nicotine, succinimide, and scopoletin with TAS2R14. The predicted interactions align with known structure-activity relationships for bitter taste receptors, where both hydrogen bonding and hydrophobic interactions play crucial roles. The involvement of residues from multiple transmembrane helices (TM3, TM5, TM6, and TM7) in ligand binding is consistent with the activation mechanism of class A GPCRs35.
It’s important to note that this study only explored the interactions of three key bitter compounds with a single bitter taste receptor, TAS2R14, leaving the roles of complex bitter compounds in cigarette smoke and other bitter taste receptors largely unexplored. Furthermore, the potential synergistic or antagonistic effects among diverse smoke compounds were not accounted for. This simplification may only represent part of the bigger picture of cigarette bitterness perception. Despite these limitations, our findings provide a foundation for understanding the molecular basis of cigarette bitterness. Future studies should aim to expand this approach to include more bitter compounds found in cigarette smoke and explore their interactions with other bitter taste receptors. Moreover, the integration of these findings with experimental validation, such as site-directed mutagenesis and functional assays36 will be crucial for a more comprehensive understanding of the bitterness mechanism of cigarette smoke.
Conclusion
By combining GC-MS chemical profiling, cell-based calcium imaging assays, and molecular docking studies, we identified nicotine, succinimide, and scopoletin as key molecular contributors to the bitterness of cigarette smoke. The TAS2R14-Gα16gust44-HEK293T cell model proved to be a reliable and objective method for measuring bitterness. Moreover, molecular docking studies provided insights into the specific interactions between these compounds and the TAS2R14 receptor, offering a detailed understanding of their bitterness perception mechanisms. These findings deepen our understanding of the sensory and chemical aspects of tobacco bitterness.
Data availability
The sequences of the five human bitter taste receptors are available in the NCBI and the accession numbers are TAS2R10 (NM_023921.2), TAS2R14 (NM_023922.2), TAS2R39 (NM_176881.2), TAS2R43 (NM_176884.2), and TAS2R46 (NM_176887.2). Other datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Aroulmoji, V., Mathlouthi, M. & Portmann-Richardson, M. Physico-Chemical properties of nicotine and nicotine-Bitterness inhibitor mixtures in aqueous solution and the role of water in their taste. Int. J. Adv. Sci. Eng. 8, 2136. https://doi.org/10.29294/IJASE.8.2.2021.2136-2146 (2021).
Carstens, E. & Carstens, M. I. Sensory effects of nicotine and tobacco. Nicotine Tob. Res. 24, 306–315. https://doi.org/10.1093/ntr/ntab086 (2022).
Soares, F. L. et al. Chemosensory aerosol assessment of key attributes for tobacco products. J. Chemom. 34, e3297. https://doi.org/10.1002/cem.3297 (2020).
Wang, D. et al. Sensory-Oriented identification and LC-HRMS analysis of bitter compounds in cigarette smoke. J. Chin. Mass. Spectrom. Soc. 37, 8. https://doi.org/10.7538/zpxb.2016.37.05.0414 (2016).
Arndt, D., Wachsmuth, C., Buchholz, C. & Bentley, M. A complex matrix characterization approach, applied to cigarette smoke, that integrates multiple analytical methods and compound identification strategies for non-targeted liquid chromatography with high‐resolution mass spectrometry. Rapid Commun. Mass. Spectrom. 34, e8571. https://doi.org/10.1002/rcm.8571 (2020).
Berenguer, C., Pereira, J. A. & Câmara, J. S. Fingerprinting the volatile profile of traditional tobacco and e-cigarettes: A comparative study. Microchem J. 166, 106196. https://doi.org/10.1016/j.microc.2021.106196 (2021).
Banik, D. D. & Medler, K. F. Bitter, sweet, and Umami signaling in taste cells: it’s not as simple as we thought. Curr. Opin. Physiol. 20, 159–164. https://doi.org/10.1016/j.cophys.2021.01.010 (2021).
Su, W. et al. Multidimensional exploration of the bitterness amelioration effect of roasting on Wuyi rock tea. Food Chem. 437, 137954. https://doi.org/10.1016/j.foodchem.2023.137954 (2024).
Ke, J. et al. Identification of two bitter components in Zanthoxylum bungeanum maxim. And exploration of their bitter taste mechanism through receptor hTAS2R14. Food Chem. 338, 127816. https://doi.org/10.1016/j.foodchem.2020.127816 (2021).
Behrens, M. & Meyerhof, W. Bitter taste receptor research comes of age: from characterization to modulation of TAS2Rs. Semin Cell. Dev. Biol. 24, 215–221. https://doi.org/10.1016/j.semcdb.2012.08.006 (2013).
Intelmann, D. et al. Three TAS2R bitter taste receptors mediate the psychophysical responses to bitter compounds of hops (Humulus lupulus L.) and beer. Chemosens. Percept. 2, 118–132. https://doi.org/10.1007/s12078-009-9049-1 (2009).
Pirastu, N. et al. Association analysis of bitter receptor genes in five isolated populations identifies a significant correlation between TAS2R43 variants and coffee liking. PLoS ONE. 9, e92065. https://doi.org/10.1371/journal.pone.0092065 (2014).
Roland, W. S. et al. Soy isoflavones and other isoflavonoids activate the human bitter taste receptors hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 59, 11764–11771. https://doi.org/10.1021/jf202816u (2011).
Lu, Y. et al. Sensory and molecular insights into the bitterness of Wuyi rock tea. Food Biosci. 59, 103966. https://doi.org/10.1016/j.fbio.2024.103966 (2024).
Takashi, U., Shinya, U., Hisao, Y., Yuji, I. & Shoichi, S. Functional interaction between T2R taste receptors and G-Protein α subunits expressed in taste receptor cells. J. Neurosci. 23, 7376–7380. https://doi.org/10.1523/JNEUROSCI.23-19-07376.2003 (2003).
Sassano, M. F., Ghosh, A. & Tarran, R. Tobacco smoke constituents trigger cytoplasmic calcium release. Appl. Vitro Toxicol. 3, 193–198. https://doi.org/10.1089/aivt.2016.0039 (2017).
Iguchi, N., Ohkuri, T., Slack, J. P., Zhong, P. & Huang, L. Sarco/Endoplasmic reticulum Ca2+-ATPases (SERCA) contribute to GPCR-mediated taste perception. PLoS ONE. 6, e23165. https://doi.org/10.1371/journal.pone.0023165 (2011).
Pan, S., Sharma, P., Shah, S. D. & Deshpande, D. A. Bitter taste receptor agonists alter mitochondrial function and induce autophagy in airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 313, L154–L165. https://doi.org/10.1152/ajplung.00106.2017 (2017).
Koleva, I. I., van Beek, T. A., Soffers, A. E., Dusemund, B. & Rietjens, I. M. Alkaloids in the human food chain–natural occurrence and possible adverse effects. Mol. Nutr. Food Res. 56, 30–52. https://doi.org/10.1002/mnfr.201100165 (2012).
Rasmussen, J. E., Sheridan, J. T., Polk, W., Davies, C. M. & Tarran, R. Cigarette smoke-induced Ca2 + release leads to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. J. Biol. Chem. 289, 7671–7681. https://doi.org/10.1074/jbc.M113.545137 (2014).
Karaman, R. et al. Probing the binding pocket of the broadly tuned human bitter taste receptor TAS2R14 by chemical modification of cognate agonists. Chem. Biol. Drug Des. 88, 66–75. https://doi.org/10.1111/cbdd.12734 (2016).
Meyerhof, W. et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses. 35, 157–170. https://doi.org/10.1093/chemse/bjp092 (2010).
Mo, D. et al. Establishment of bitterness index model for tobacco smoke based on taste active values. Tob. Sci. Technol. 48, 31–39. https://doi.org/10.16135/j.issn1002-0861.20150106 (2015).
Perfetti, T. A. & Rodgman, A. The complexity of tobacco and tobacco smoke. Contrib. Tob. Nicotine Res. 24, 215–232. https://doi.org/10.2478/cttr-2013-0902 (2011).
Talhout, R., Opperhuizen, A. & Van Amsterdam, J. G. Sugars as tobacco ingredient: effects on mainstream smoke composition. Food Chem. Toxicol. 44, 1789–1798. https://doi.org/10.1016/j.fct.2006.06.016 (2006).
Talhout, R., Opperhuizen, A. & van Amsterdam, J. G. Role of acetaldehyde in tobacco smoke addiction. Eur. Neuropsychopharmacol. 17, 627–636. https://doi.org/10.1016/j.euroneuro.2007.02.013 (2007).
Poole, R. L. & Tordoff, M. G. The taste of caffeine. J. Caffeine Res. 7, 39–52. https://doi.org/10.1089/jcr.2016.0030 (2017).
McClure, A. P., Spinka, C. M. & Grün, I. U. Quantitative analysis and response surface modeling of important bitter compounds in chocolate made from cocoa beans with eight roast profiles across three origins. J. Food Sci. 86, 4901–4913. https://doi.org/10.1111/1750-3841.15924 (2021).
Gnonlonfin, G. B., Sanni, A. & Brimer, L. Review scopoletin–a coumarin phytoalexin with medicinal properties. Crit. Rev. Plant. Sci. 31, 47–56. https://doi.org/10.1080/07352689.2011.616039 (2012).
Oliveira-Alves, S., Lourenço, S., Fernandes, T. A. & Canas, S. Coumarins in spirit beverages: sources, quantification, and their involvement in quality, authenticity and food safety. Appl. Sci. 14, 1010. https://doi.org/10.3390/app14031010 (2024).
Geng, Z. et al. Aroma precursors of cigars from different tobacco parts and origins, and their correlations with sensory characteristics. Front. Plant. Sci. 14, 1264739. https://doi.org/10.3389/fpls.2023.1264739 (2023).
Lang, T. et al. Numerous compounds orchestrate coffee’s bitterness. J. Agric. Food Chem. 68, 6692–6700. https://doi.org/10.1021/acs.jafc.0c01373 (2020).
Dunkel, A., Hofmann, T. & Di Pizio, A. In Silico investigation of bitter hop-derived compounds and their cognate bitter taste receptors. J. Agric. Food Chem. 68, 10414–10423. https://doi.org/10.1021/acs.jafc.9b07863 (2020).
Di Pizio, A. et al. Rational design of agonists for bitter taste receptor TAS2R14: from modeling to bench and back. Cell. Mol. Life Sci. 77, 531–542. https://doi.org/10.1007/s00018-019-03194-2 (2020).
Venkatakrishnan, A. J. et al. Diverse activation pathways in class A GPCRs converge near the G-protein-coupling region. Nature 536, 484–487. https://doi.org/10.1038/nature19107 (2016).
Sakurai, T. et al. Characterization of the β-d-glucopyranoside binding site of the human bitter taste receptor hTAS2R16. 285, 28373–28378, (2010). https://doi.org/10.1074/jbc.M110.144444
Acknowledgements
This study was financially supported by Technology Center, China Tobacco Fujian Industrial Co., Ltd. (No. 2022350000340579).
Author information
Authors and Affiliations
Contributions
conceptualization, D.W., C.H., L.N., and Z.L.;methodology, X.C., X.L., T.Z., and Z.L.;validation, X.C., X.L., and T.Z.;formal analysis, X.C. and X.L.;investigation, X.C., X.L., and T.Z.;resources, X.C. and C.H.;data curation, X.C., X.L., T.Z., and Z.L.;writing—original draft preparation, X.C. and X.L.;writing—review and editing, L.N., D.W., C.H. and Z.L.;visualization, X.C., X.L., and Z.L.;supervision, C.H., and Z.L.;project administration, C.H. and Z.L.All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
X.C., D.W., T.Z., and C.H. are employed by Technology Center, China Tobacco Fujian Industrial Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, X., Liu, X., Wang, D. et al. Unraveling molecular mechanisms of cigarette smoke bitterness via chemical profiling and bitter taste receptor activation analysis. Sci Rep 15, 30670 (2025). https://doi.org/10.1038/s41598-025-16488-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16488-2