Abstract
Human immunity involves both innate and adaptive defence mechanisms, with inflammation playing a central role in responding to cellular injury, pathogenic infections, and allergic stimuli. Reactive oxygen species (ROS) are closely associated with the onset and progression of inflammation. While moderate ROS levels function as crucial signalling molecules, excessive ROS can damage cellular components. This study aimed to evaluate the anti-inflammatory and antioxidant potential of plant-derived bioactive compounds including chlorogenic acid, oleuropein, tomatine, and tyrosol using human monocytic cell models (U-937 and THP-1). Differentiation of U-937 and THP-1 cells was induced prior to treatment with the selected bioactive compounds. Cell morphology and integrity were examined utilizing confocal microscopy. Gene expression stability was evaluated using reference genes β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Protein expression levels of key inflammatory markers were determined by Western blot analysis. In addition, molecular docking studies were conducted to assess the binding affinity of the compounds to human target proteins [Interleukin-4 (IL-4), 5-Lipoxygenase (LOX-5), Myeloperoxidase (MPO), and Tumor necrosis factor-alpha ( TNF-α)]. No cytotoxic effects were observed in treated cells, and GAPDH was confirmed as a stable reference gene under all experimental conditions. In U-937 cells, treatment with the bioactive compounds led to increased expression of the anti-inflammatory cytokine IL-4 and decreased expression of MPO. Notably, exposure to chlorogenic acid and tyrosol reduced MPO activity. Oleuropein and tyrosol demonstrated a strong suppressive effect on the expression of LOX-5, an enzyme responsible for leukotriene production. All tested bioactive compounds significantly reduced the phorbol 12-myristate 13-acetate (PMA) induced increase in LOX-5 activity. Molecular docking supported the potential of these compounds to interact with key inflammatory proteins, contributing to reduced oxidative stress. The plant-derived compounds, particularly oleuropein and tyrosol from olives, exhibit promising anti-inflammatory and antioxidant effects by modulating ROS-associated signalling pathways and downregulating inflammatory markers. These findings support the therapeutic potential of agricultural waste-derived bioactive in inflammation management and oxidative stress regulation.
Similar content being viewed by others
Introduction
Oxidative stress arises when the production of free radicals in the body exceeds the availability of antioxidants to neutralize them, leading to cellular damage and inflammation1. Activated macrophages play a key role in this inflammatory process by generating reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are linked to the development of chronic diseases. Since a typical diet may lack sufficient antioxidants, bioactive compounds have gained attention for their potential to fill this gap1,2. Research indicates that these compounds, often classified as nutraceuticals, are rich in antioxidants and can help mitigate inflammation. Notably, bioactive compounds can also be derived from biological waste materials2,3. Biological waste, particularly from plants, constitutes a major portion of organic waste generated through agriculture and food processing. Agricultural residues such as straw, husks, stems, and leaves, along with by-products from food processing like peels, pulp, shells, and seeds, are often discarded despite being rich sources of bioactive compounds. These plant-derived compounds such as polyphenols, flavonoids, terpenoids, and alkaloids are known for their health-promoting properties, including anti-inflammatory, anti-cancer, and cardioprotective effects.
Chlorogenic acid, an intermediate in plant lignin biosynthesis, belongs to the family of polyphenols, present abundantly in fruits, vegetables, tea, coffee and can act as an anti-inflammatory and anti-obesity agent2,4. A study investigating the anti-inflammatory effect of chlorogenic acid in lipopolysaccharide (LPS) stimulated RAW 264.7 macrophages and microglial cells suggests that chlorogenic acid can inhibit the production of nitric oxide (NO) and suppression of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukins (IL-1β, IL-6) without causing cytotoxicity. This suggests that it can be used as a therapeutic agent for anti-inflammatory diseases including sepsis4. Oleuropein is one of the most prevalent phenolic compounds extracted from olive leaves known to have anti-inflammatory and antioxidant properties which can reduce oxidative stress2,5. Various studies suggest that oleuropein can downregulate M1 pro regulatory cytokines IL-12, Interferon-γ (IFN-γ), TNF-α while enhancing the M2 anti-inflammatory cytokines IL-10, transforming growth factor-β (TGF-β). These findings suggest that it can act as a potential therapeutic agent against inflammation5.
α-tomatine, is a naturally occurring bioactive substance extracted from tomato leaves. It possesses strong anti-inflammatory, antioxidant, and anti-tumoral properties6. Studies have demonstrated that α-tomatine can inhibit the production of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 in LPS-stimulated macrophages3. Findings suggest that α-tomatine may serve as a valuable therapeutic agent for treating inflammation-related diseases. Tyrosol is a naturally occurring phytochemical primarily found in olives, olive oil, wine, and certain herbal preparations. It exhibits various beneficial properties, including antioxidant, anti-inflammatory, anticancer, antistress, cardioprotective, and neuroprotective effects2,7. Several studies have shown that tyrosol and its derivatives can significantly reduce ROS in human endothelial cells and help restore glutathione levels7. These findings indicate that tyrosol may play a key role in alleviating oxidative stress and inflammation. Research to date has shown that many bioactive compounds have strong antioxidant and anti-inflammatory effects, leading to growing scientific interest in how they interact with specific molecular targets and enzymes involved in inflammation. One such target is myeloperoxidase (MPO), an enzyme predominantly found in neutrophils and known to contribute significantly to oxidative stress and inflammation. Another key protein is lipoxygenase (LOX) which is involved in the production of ROS and induction of oxidative stress.
Myeloperoxidase (MPO) is a heme-containing peroxidase enzyme encoded by the MPO gene on chromosome 17, accounting for up to 5% of the dry weight of neutrophils and primarily localized in azurophilic granules8. It is also present in monocytes and certain macrophage subpopulations, contributing to ROS generation during immune responses. Elevated MPO levels are associated with neutrophil recruitment and oxidative bursts9. MPO contributes to oxidative stress by catalyzing the formation of hypochlorous acid (HOCl), a cytotoxic agent that damages proteins, lipids, and DNA. It also influences neutrophil polarization and inflammation-related signaling pathways, including PI3K/AKT. Several plant-derived bioactive compounds have demonstrated the ability to inhibit MPO activity and reduce inflammation. For example, bioflavonoids such as quercetin, rutin, eriodictyol, as well as polyphenols like curcumin exhibit antioxidant, anti-inflammatory, and cardioprotective properties by modulating MPO activity9. These findings support the therapeutic potential of natural compounds in managing MPO-related inflammation.
Arachidonate 5-lipoxygenase (LOX-5) is a non-heme iron containing enzyme encoded by the ALOX5 gene and is primarily expressed in inflammatory and immune cells such as neutrophils, monocytes, and macrophages10,11. It catalyzes the conversion of arachidonic acid into leukotrienes, potent lipid mediators that contribute to chronic inflammation even at nanomolar concentrations. LOX-5 plays a central role in the inflammatory cascade, alongside cyclooxygenases (COX-1 and COX-2), which also metabolize arachidonic acid to produce eicosanoids12. Although pharmacological inhibitors of inflammation exist, many are associated with adverse effects, prompting interest in natural bioactive compounds with anti-inflammatory potential. These compounds have been shown to modulate cytokine production, inhibit inducible nitric oxide synthases (iNOS), and downregulate COX-2 expression12. However, while COX-2 inhibition is well studied, the therapeutic potential of LOX-5 inhibition remains comparatively underexplored, highlighting a need for further research in this area.
In our study, we focused on four specific bioactive compounds: chlorogenic acid, oleuropein, tomatine, and tyrosol. Among these, tomatine and oleuropein were extracted from their natural sources-tomato and olive leaves, respectively-while chlorogenic acid and tyrosol were obtained as commercial products. We evaluated in-vitro anti-inflammatory and antioxidant activities using biochemical and biophysical techniques. Additionally, we explored the underlying mechanisms of MPO and LOX regulation to understand how these enzymes are modulated during supplementation with the selected bioactive compounds.
Materials and methods
Reagents and antibodies
Oleuropein was extracted from olive leaves (Olea europaea L.) and tomatine from tomato leaves (Solanum lycopersicum L.) following the protocols described by Pardini et al., and Tamasi et al.13,14,15. For oleuropein, the average concentration and purity were 0.602 mg/g and 60.8 ± 0.27% w/w, respectively, while for α-tomatine, the average concentration and purity were 0.935 mg/g and 94.2 ± 0.49% w/w2. Chlorogenic acid (purity > 97%) and tyrosol (purity > 98%) were commercial products (Sigma-Aldrich GmbH, Mannheim, Germany) and were used without extra purification. Cell culture medium (RPMI-1640), fetal bovine serum (FBS), and antibiotics (antibiotic–antimycotic solution) were obtained from Biosera (Nuaille, France). Phorbol 12-myristate 13-acetate (PMA) was purchased from Sigma-Aldrich (St. Louis, Missouri, USA). A rabbit polyclonal anti-IL-4 antibody was procured from Abcam (Cambridge, UK). Mouse monoclonal antibodies, including those against LOX-5, TNF-α, GAPDH, β-actin, and MPO, were purchased from Proteintech (GmbH, Germany). Additionally, HRP-conjugated anti-rabbit and anti-mouse secondary antibodies were also obtained from Proteintech (GmbH, Germany). Protease and phosphatase inhibitor cocktails were purchased from Roche (Mannheim, Germany). Detailed information on antibodies used in this study is provided in Supplementary data 1.
Cell lines and culture conditions
U-937 and THP-1 monocytic leukemia cell lines were purchased from the American Type Culture Collection (ATCC; Rockville, Maryland, USA). The cells were maintained in RPMI-1640 medium supplemented with 0.05 mM L-glutamine and 2.0 g/L sodium bicarbonate. This basal medium was further enriched with 10% FBS and 1% (v/v) antibiotic–antimycotic solution to prepare the complete growth medium. U-937 and THP-1 cells were cultured under optimal conditions and passaged upon reaching 80–90% confluency. Cell density and viability were assessed using the TC20 automated cell counter (Bio-Rad Laboratories, Hercules, CA, USA) with 0.2% trypan blue staining. Only cell suspensions with ≥ 75% viability were used for experimental procedures.
Cell differentiation
For the experiments, U-937 and THP-1 cells were seeded in 6-well plates at a density of 1 × 10⁶ cells/mL in complete medium and treated with phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St. Louis, Missouri, USA) at concentrations of 250 nM for U-937 and 150 nM for THP-1 cells for 72 h. PMA is a well-established inducer of cell differentiation, transforming round monocytic cells into macrophage-like cells. Following differentiation, the cells were incubated with the selected bioactive compounds (chlorogenic acid, oleuropein, tomatine, and tyrosol) at a final concentration of 1.6667 µM for 24 h.
Cell viability assay
The trypan blue exclusion assay was used to determine total, viable, and dead cell counts under the influence of bioactive compounds. Trypan blue is a vital stain that penetrates cells with compromised membranes but is excluded by intact, viable cells. As a result, dead cells appear darkly stained, whereas live cells remain lightly stained or unstained16. U-937 and THP-1 cell suspensions were mixed with 0.4% trypan blue in a 1:1 ratio and incubated at room temperature for 2 min. The stained samples were then loaded into a counting chamber slide, and cell numbers were assessed using the TC20 automated cell counter (Bio-Rad). In this study, U-937 and THP-1 cells were first treated with differentiation inducers for 72 h, followed by incubation with bioactive compounds for 24 h. Subsequently, the total and viable cell numbers were quantified.
Microscopy
The cellular morphology of the monocytic cell lines was visualized using a confocal unit attached to an IX80 microscope (Olympus Czech Group, Prague, Czech Republic). For all experimental samples, staining was performed using FM4-64 (Sigma-Aldrich GmbH, Mannheim, Germany), a water-soluble dye used to assess cell membrane integrity, and Hoechst 33342 to visualize the nucleus. During sample preparation, a final of 15 µM for FM4-64 and 2 µM for Hoechst 33342 were used. Samples were incubated at room temperature for 5 min, then placed on glass slides and covered with coverslips prior to imaging.
FM4-64 was excited using a He–Ne laser at 543 nm, and its emission was captured in the 655–755 nm range. Hoechst 33342, a nuclear counterstain that specifically binds to AT-rich regions in the minor groove of DNA, was excited using a 405 nm diode laser, with emission recorded between 430 and 470 nm.
Protein isolation
In our experiments, U-937 and THP-1 cells were treated with a differentiation inducer for 72 h, followed by incubation with bioactive compounds for 24 h. After treatment, the cells were pelleted by centrifugation, washed three times with 1 × PBS, and resuspended in ice-cold Radioimmunoprecipitation assay (RIPA) buffer [150 mM NaCl, 50 mM Tris (pH 8.0), 0.5% sodium deoxycholate, 0.1% SDS, 1% NP-40] supplemented with 1% (v/v) protease and phosphatase inhibitors. The cell suspensions were then sonicated and centrifuged at 14,000 rpm for 30 min at 4 °C. The resulting supernatant, containing the total protein, was collected into fresh Eppendorf tubes. Protein quantification was performed using the Pierce Bicinchoninic Acid (BCA) Protein Assay Kit (Thermo Fisher Scientific, Paisley, UK), following the manufacturer’s instructions.
Western blotting analysis
Total purified protein from cell pellets of different experimental samples was subjected to immunoblot analysis to evaluate the expression of target proteins: β-actin, GAPDH, TNF-α, LOX-5, MPO and IL-4. For each sample, 10–40 µg of protein was mixed with 2 × loading dye buffer, heated at 70 °C for 10 min, and loaded onto a 6–10% Tricine SDS-PAGE gel. A pre-stained molecular weight marker (PL00001, Proteintech GmbH Germany) was loaded into one lane as a reference. Following electrophoresis, proteins were transferred onto a nitrocellulose membrane (Bio-Rad, California, USA) using the Trans-Blot Turbo transfer system (Bio-Rad, California, USA). The membranes were rinsed with distilled water and blocked with 5% Bovine Serum Albumin (BSA) or skimmed milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 2 h at room temperature (RT). Membranes were then incubated overnight at 4 °C with primary antibodies (Supplementary Table S1).
After incubation, the membranes were washed three times with 1 × TBST buffer for 10 min each to remove unbound primary antibody. They were then incubated with HRP-conjugated secondary antibodies (Supplementary Table S1) for 1 h at RT, followed by another three washes with 1 × TBST. Blot development was performed using Immobilon Western Chemiluminescent HRP Substrate (Sigma-Aldrich GmbH, Germany), and band intensities were captured using the Amersham Imager 600. For statistical analysis, the band intensity was quantified using ImageJ software and densitogram are presented.
Determination of enzyme activities
Myeloperoxidase
Myeloperoxidase (MPO) activity was assessed by the method of Suzuki et al.17. Briefly, cells were harvested, washed twice with PBS, and homogenized using 150 mM sodium phosphate buffer. After centrifugation at 14,000 rpm for 15 min, the supernatant was transferred to an Eppendorf tube and processed for MPO enzyme activity. For the assay, 100 µL of cell lysate was mixed with 1.6 mM 3,3,5,5-Tetramethylbenzidine (TMB), 0.3 mM hydrogen peroxide (H2O2), 80 mM sodium phosphate buffer (pH 5.4), 8% N, N-Dimethylformamide (DMF), and 40% PBS at final reaction volume of 0.5 mL. The reaction mixture was incubated at 37 °C for 3 min and then placed on ice for a few seconds. Subsequently, 1.75 ml of 200 mM sodium acetate (pH 3.0) was added to terminate the reaction. The absorbance at 655 nm was recorded at 10s intervals for up to 5 min. Enzyme activity was calculated using the following formula and expressed as μmol/min/mg
5-Lipoxygenase (LOX-5)
5-Lipoxygenase (LOX-5) activity was measured based on the increase in absorbance at 234 nm, which corresponds to the formation of 5-hydroperoxyeicosatetraenoic acid (5-HPETE) from linoleic acid, as previously described18,19. Cell lysates for the LOX-5 assay were prepared as described above. Briefly, the reaction mixture in a cuvette consisted of 2.8 mL of 50 mM phosphate buffer (pH 6.5) and 0.1 mL of linoleic acid substrate solution (prepared by mixing 78 µL of linoleic acid with 90 µL of Tween 20 as an emulsifier, then diluted in 25 mL of water). After zeroing the spectrophotometer, 0.1 mL of cell lysate was added to initiate the reaction. The increase in absorbance at 234 nm was recorded at 10s intervals for 2 min. The enzyme activity was calculated using the following formula and expressed as μmol/min/mg.
Protein–ligand docking
Protein–ligand docking was conducted as previously described20. Briefly, the 3D structures of bioactive compounds such as chlorogenic acid, oleuropein, tomatine, and tyrosol were prepared by adding polar hydrogens and assigning Gasteiger charges. Energy minimization of these compounds was then performed using UCSF Chimera 1.1621. The three-dimensional crystal structures of the target proteins LOX-5, MPO, IL-4, and TNF-α were retrieved from the Protein Data Bank (PDB) database22. The active site of IL-4 was predicted using the Computed Atlas of Surface Topography of Proteins (CASTp) server23.
CASTp quantitatively analyzes the topographical features of proteins and measures the area and volume of each pocket and cavity. It also identifies the amino acids crucial for docking studies. The target proteins were prepared for docking by removing all water molecules and non-protein residues, followed by structure optimization and energy minimization using Chimera21,24. Using AutoDock, all missing atoms in the target proteins were repaired. Subsequently, only polar hydrogens and Kollman charges were added, and the target proteins were converted into PDBQT format for docking. Docking was performed using AutoDock Vina, as described by Krishnaswamy et al.25. The grid box dimensions were set to 30 Å × 30 Å × 30 Å, which was found to be optimal for the default exhaustiveness value of 8. The ligand binding site was positioned at the center of the grid box. The spatial dimensions (XYZ axes) and grid box size were specified in a configuration file. Using AutoDock vina version 1.1.2’s command line interface, docking was accomplished. The results were limited to nine binding modes. The generated log file included a list of binding modes in increasing order of binding energy. BIOVIA Discovery Studio Visualizer 2025 was used to visualize the binding modes and all non-bonded interactions.
Results and discussion
Cell proliferation and effect of bioactive compounds
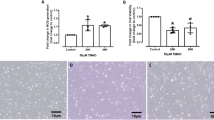
Our primary goal was to assess the effects of biologically derived compounds on cell viability and differentiation. In Fig. 1A,B, light gray bars represent viable cell counts, while dark gray bars indicate non-viable cells for THP-1 and U-937 cell lines, respectively shown as the proportion of total cell population. The results show that the percentage of viable cells remained approximately 75% or higher across all experimental conditions for both cell lines, while non-viable cells made up less than or around 25% of the population. Furthermore, cells treated with the differentiation inducer and bioactive compounds showed no significant reduction in viability compared to control cells. These findings show that neither the differentiation inducer nor the bioactive compounds affected cell viability, and the cells stayed metabolically active during the experiment. This is consistent with our previous research, confirming that bioactive compounds derived from biological waste do not adversely impact cell viability, even after 24 h of exposure2. The effects of PMA, a differentiation inducer, were also observed by Kuno et al. (2020), who found that treatment with PMA at concentrations up to 200 nM had no significant impact on cell proliferation or viability26. Chanput et al. states that around 100 ng/mL concentration is able to induce differentiation27. Browne et al. (2022) reported that concentrations up to 1000 ng/mL maintained cell viability above 80% after 72 h of incubation. However, increasing the concentration of bioactive compounds to between 50 and 150 µM was found to negatively affect cell proliferation and viability in cancer cell line models28,29. Ultimately, these bioactive compounds have significant implications for research in two distinct ways: while low concentrations are safe for cellular viability, higher doses may be utilized for therapeutic purposes.
Cell viability of monocytic cell lines. Cell differentiation was induced using PMA at a final concentration of (A) 150 nM for THP-1 cells and (B) 250 nM for U-937 cells for 72 h, followed by incubation with bioactive compounds for 24 h. Cell viability was assessed using the trypan blue exclusion method. Bar graphs show the ratio of live to non-viable cells, as determined by a Bio-Rad automated cell counter. In the upper panel (A), the bar graph represents the percentage of viable (light grey) and non-viable (dark grey) cells in the THP-1 cell line. In the lower panel (B), the bar graph shows the percentage of viable (light grey) and non-viable (dark grey) cells in the U-937 cell line. Values are presented as mean ± standard deviation, based on three replicates (n = 3).
Cell differentiation under chemical inducers
In our study, the effects of the differentiation inducer and bioactive compounds were assessed using confocal microscopy. Morphological changes in U-937 and THP-1 cells were captured after 96 h of treatment [72 h with PMA followed by 24 h with bioactive compounds]. Our results show that treatment with PMA altered cell surface morphology, changing from a spherical shape to a pseudopodia-like structure compared to untreated cells. Cells treated with chlorogenic acid and α-tomatine during the final 24 h exhibited a more pronounced amoeboid morphology in both cell lines, whereas pseudopodia-like protrusions were less prominent in cells treated with oleuropein and tyrosol (Figs. 2 and 3). The observed differentiation patterns and morphological changes are believed to result primarily from the action of the differentiation inducer rather than from the presence or absence of bioactive compounds. PMA, a known differentiation inducer, activates protein kinase C, which subsequently promotes NF-κB expression and stimulates the expression of proteins associated with macrophage maturation. It also inhibits cell proliferation and induces cellular differentiation30,31. Additionally, PMA-treated cells exhibit an increased cytoplasm-to-nucleus (C/N) ratio, along with changes in cell surface area, cytoplasmic protrusions, and the formation of pseudopodia- and filopodia-like structures, which enhance cell adhesion, as confirmed by previous studies26,32,33.
THP-1 cell morphology under the influence of bioactive compounds. THP-1 cells were double-stained with FM4-64 and Hoechst 33342 following treatment with PMA for 72 h, followed by incubation with bioactive compounds for an additional 24 h. Multiple images were captured, and representative images are presented (arranged from top to bottom) for each experimental group: negative control (− PMA), positive control (+ PMA), chlorogenic acid, oleuropein, tomatine, and tyrosol.
U-937 cell morphology under the influence of bioactive compounds. U-937 cells were double-stained with FM4-64 and Hoechst 33342 following treatment with PMA for 72 h, followed by incubation with bioactive compounds for 24 h. Multiple images were captured, and representative images are presented (arranged from top to bottom) for each experimental group: negative control (− PMA), positive control (+ PMA), chlorogenic acid, oleuropein, tomatine, and tyrosol.
The objective of this experiment was to determine whether PMA treatment and the resulting alterations in cell morphology had any negative effects on cell membrane integrity. To assess this, FM4-64, a water-soluble dye that specifically binds to the cell membrane and Hoechst 33342, which stains the nucleus was used. Imaging results confirmed that U-937 and THP-1 cells treated with bioactive compounds showed no signs of cellular damage (Figs. 2 and 3). Notably, nuclear and membrane structural integrity was preserved under all experimental conditions. In our previous study, we also found that PMA and selected bioactive compounds had no adverse effects on cell membrane integrity2,34. The observed cell integrity implies that these selective bioactive compounds are cyto-compatible without any cytolysis and apoptotic effects.
Housekeeping gene vs cell differentiation
In protein expression studies, it is crucial to identify internal reference genes whose expression remains relatively stable under various experimental conditions. Housekeeping genes are essential for maintaining the structural and functional integrity of cells and are generally expected to exhibit consistent expression in both normal and altered cellular states. Commonly used reference proteins include tubulin, GAPDH, and β-actin.
Housekeeping genes are generally defined as those that are stably expressed across different tissue types, developmental stages, cell cycle phases, and in response to external stimuli. They are considered essential for basic cellular functions, regardless of their specific roles in particular tissues or organisms35. However, some studies have shown that the expression of β-actin can increase during induced differentiation processes, making it an unreliable loading control under such conditions. Furthermore, β-actin expression has been observed to fluctuate in various conditions, including certain diseases, hormonal imbalances, infections caused by viruses and bacteria, and during neuronal differentiation36. The ambiguous expression levels of β-actin are thought to reflect the cellular and morphological changes that occur under these diverse conditions. Due to its dynamic expression and plasticity, β-actin has been reported to be unsuitable as a reliable reference or internal loading control in experimental setups37. In contrast, GAPDH expression has been found to remain stable under differentiation conditions, as reported by Murphy and Polak37. Additionally, GAPDH has been shown to maintain consistent expression levels across various experimental conditions, further supporting its use as a more stable reference gene38.
In our current study, we selected β-actin and GAPDH as reference proteins and examined their expression in both non-differentiated and differentiated cells across all experimental conditions. As part of the experimental design, U-937 and THP-1 cells were treated with PMA for 72 h to induce differentiation, followed by 24 h of incubation with selected bioactive compounds: chlorogenic acid, tomatine, oleuropein, or tyrosol. Across all conditions, β-actin expression varied between differentiated and non-differentiated cells. Specifically, non-differentiated cells showed lower levels of β-actin expression, whereas differentiated cells demonstrated higher expression levels (Figs. 4A and 5A). This variability in β-actin expression is likely due to cytoskeletal remodelling that occurs during cellular differentiation to support structural and functional demands. GAPDH expression remained largely stable in both monocytic cell lines (Figs. 4B and 5B). Based on these observations and corroborating previous reports, we conclude that GAPDH is a more appropriate reference gene for studies of cell differentiation.
Western blot analysis of housekeeping proteins in THP-1 cells. A. Western blotting performed using an anti-β-actin antibody (molecular weight: 42 kDa) in undifferentiated and differentiated THP-1 cells. Lane assignments are as follows: Lane 1- molecular weight marker; Lane 2- negative control (− PMA); Lane 3- positive control (+ PMA); Lane 4- tomatine; Lane 5- chlorogenic acid; Lane 6- oleuropein; Lane 7- tyrosol. B. Western blot analysis of GAPDH expression (molecular weight: 36 kDa) in undifferentiated and differentiated THP-1 cells. Lane assignments: Lane 1- molecular weight marker; Lane 2-negative control (− PMA); Lane 3- positive control (+ PMA); Lane 4- tomatine; Lane 5- chlorogenic acid; Lane 6- oleuropein; Lane 7- tyrosol.
Western blot analysis of housekeeping proteins in U-937 cells. (A) Western blotting performed using an anti-β-actin antibody (molecular weight: 42 kDa) in undifferentiated and differentiated U-937 cells. Lane assignments are as follows: Lane 1- molecular weight marker; Lane 2- negative control (− PMA); Lane 3- positive control (+ PMA); Lane 4- tomatine; Lane 5- chlorogenic acid; Lane 6- oleuropein; Lane 7- tyrosol. B. Western blot analysis of GAPDH expression (molecular weight: 36 kDa) in undifferentiated and differentiated U-937 cells. Lane assignments: Lane 1- molecular weight marker; Lane 2- negative control (− PMA); Lane 3- positive control (+ PMA); Lane 4- tomatine; Lane 5- chlorogenic acid; Lane 6- oleuropein; Lane 7- tyrosol.
Effect of bioactive compounds on inflammatory cytokine expression
Upon activation, macrophages are known to produce inflammatory cytokines such as IL-6, TNF-α, and IL-1239. Our research focused on evaluating the impact of selected bioactive compounds on inflammatory and anti-inflammatory responses based on previously described methodologies2,40. IL-4, an anti-inflammatory cytokine, showed upregulated expression in response to tomatine and chlorogenic acid treatment compared to PMA-treated controls predominatly in U-937 cells (Fig. 6). In U-937 cells, IL-4 expression was markedly elevated following treatment with chlorogenic acid and tomatine, while in THP-1 cells, a partial increase in IL-4 expression was observed under the same treatments (Figs. 6A,B). The elevation of IL-4 expression is known to suppress pro-inflammatory cytokines such as TNF-α and IL-12 through both STAT6-dependent and STAT6-independent mechanisms41. Consistent with our findings, a study by Zhou et al. (1994) demonstrated that IL-4 expression inhibits the transcription of inflammatory cytokines, including IL-1α, IL-1β, IL-8, and TNF-α42. Furthermore, exogenous IL-4 has been shown to resolve pro-inflammatory states in neutrophils, promoting a shift toward an anti-inflammatory phenotype in macrophages and thereby facilitating the clearance of apoptotic neutrophils43. Additionally, the localized and controlled application of IL-4 within the periodontium has been shown to skew macrophage polarization toward the M2 phenotype, consequently reducing inflammation-mediated bone degradation and facilitating bone regeneration44.
Anti-inflammatory effects of bioactive compounds. The anti-inflammatory effects of the bioactive compounds were evaluated by measuring IL-4 expression in THP-1 and U-937 cell samples. Western blot analysis targeting IL-4 was performed on THP-1 cells (A) and U-937 cells (B). Protein samples were loaded according to the lane assignments detailed in Fig. 4.
Myeloperoxidase (MPO) and lipoxygenase-5 (LOX-5) expressions
In our previous study, we found that the four selected bioactive compounds possess anti-MDA activity2. The formation of malondialdehyde (MDA) adducts is indicative of ROS generation and lipid peroxidation processes closely associated with the activity of key enzymes such as LOX-5 and MPO. LOX-5 contributes to the oxidation of polyunsaturated fatty acids, while MPO facilitates the formation of HOCl, thereby promoting oxidative stress and inflammatory responses in monocytes45,46.
Our experimental findings revealed that MPO expression was significantly downregulated in the presence of chlorogenic acid, oleuropein, and tyrosol, whereas upregulated in presence of tomatine (Fig. 7A). Concurrently, MPO enzyme activity elevated after the addition of PMA relative to untreated U-937 cells. Furthermore, among the four bioactive compounds, chlorogenic acid and tyrosol induced a decrease in the MPO activity. MPO activity enhanced in the presence of the tomatine correlating their expression at the protein level. The MPO activity declined by 0.82- and 0.09-fold upon incubation with chlorogenic acid and tyrosol respectively, in the comparison with PMA treated cells (Fig. 7B). Similarly, LOX-5 expression was suppressed by oleuropein and tyrosol, with no substantial changes observed under the influence of tomatine or chlorogenic acid (Fig. 8A). As seen in Fig. 8B, treatment with PMA led to a significant increase in LOX-5 enzyme activity compared to undifferentiated control THP-1 cells. However, treatment with the bioactive compounds resulted in a significant reduction in LOX-5 activity. Notably, tyrosol treatment showed the most pronounced effect among the compounds tested. Specifically, LOX-5 activity was reduced by approximately 0.42-, 0.68-, 0.79-, and 0.47-fold following treatment with chlorogenic acid, oleuropein, tyrosol, and tomatine, respectively, when compared to PMA-treated differentiated THP-1 cells. LOX-5 activity was also measured in a biological replicate, and the data are presented in Supplementary Data 2.
Analysis of MPO expression via western blotting and enzymatic activity. (A) Total protein was isolated from undifferentiated, differentiated, and bioactive compound-treated U-937 cells, separated by SDS-PAGE, transferred onto a nitrocellulose membrane, and probed with an anti-MPO antibody to detect MPO expression. Protein samples were loaded as follows: Lane 1- molecular weight marker; Lane 2- control; Lane 3- tomatine; Lane 4- chlorogenic acid; Lane 5- oleuropein; Lane 6- tyrosol. The MPO heavy chain was detected at approximately 55 kDa. All other experimental conditions are as described in Fig. 4. (B) MPO enzyme activity was measured in U-937 cells treated with a bioactive compound, as well as in untreated control cells. MPO activity was expressed in μmol/min/mg.
Investigation of LOX-5 expression and enzyme catalytic efficiency. (A) Western blot analysis to detect LOX-5 expression. Lane 1 contained the molecular weight marker; Lane 2 corresponded to the protein sample from the undifferentiated cell; and Lane 3 represented the differentiated control sample. Samples treated with bioactive compounds were loaded as follows: Lane 4- tomatine, Lane 5- chlorogenic acid, Lane 6- oleuropein, and Lane 7- tyrosol. (B) LOX-5 catalytic efficiency was investigated in control and samples treated with bioactive compounds. LOX-5 activity was measured spectrophotometrically, and data are presented as enzyme activity expressed in μmol/min/mg.
MPO not only directly contributes to oxidative stress and lipid peroxidation but also indirectly promotes the activation of LOX-5. The breakdown of arachidonic acid catalyzed by LOX-5 leads to the production of pro-inflammatory mediators such as leukotrienes47. Moreover, upregulation of lipoxygenases has been linked to the progression of chronic diseases, including asthma, atherosclerosis, rheumatoid arthritis, and cancer48. Elevated levels of MPO protein have also been implicated in the development and progression of various inflammatory chronic diseases49. Altogether, targeting LOX-5 and MPO expression with bioactive compounds could represent a promising therapeutic strategy for treating chronic and inflammatory diseases. Expression of TNF-α was also measured, and a significant difference was observed in the bioactive-treated samples. Although the expected molecular weight is approximately 26 kDa, a band was observed at around 60 kDa (Supplementary Data 3). The complete uncropped images of the western blots are available in Supplementary Data 4 and 5.
Overall, our study highlights the beneficial potential of these bioactive compounds in managing inflammatory conditions and modulating ROS generation. Among the compounds tested, chlorogenic acid, oleuropein and tyrosol exhibited strong antioxidant and ROS scavenging properties, potentially by regulating the expression of key enzymes involved in oxidative stress and inflammatory pathways.
Molecular docking of bioactive compounds with IL4, MPO, LOX-5 and TNF-α
Molecular docking was done to estimate the free energy of binding between target human proteins (IL-4, LOX-5, MPO, and TNF-α) and bioactive compounds, as well as with their respective native ligands. Since the tested bioactive compounds in this study were of plant origin, the target proteins were also docked with curcumin, a well-known natural, plant-derived anti-inflammatory compound. The binding energies of the best-docked ligands compared to their native ligands, along with their binding interactions with the active site residues of the target proteins are presented in Table 1. Additionally, the 3D and 2D structures of the best-docked complexes of target proteins with bioactive compounds are presented in Figs. 9, 10, 11 and 12. TNF-α docking results revealed that Tomatine (TOM) was the only bioactive compound showing slightly lower binding energy (− 6.67 kcal/mol) than the native ligand (− 6.49 kcal/mol) but higher binding energy than the control ligand, curcumin (− 5.48 kcal/mol) (Table 1). Tomatine (TOM) formed one hydrogen bond with Tyr151 and four hydrophobic contacts with Tyr119 (× 3) and Leu57 residues of human TNF-α (hTNF-α) (Fig. 9). Interestingly, spotted residues were notably identical to the co-crystallised native ligand which formed eight hydrophobic contacts with amino acids Tyr59, Tyr119, Tyr59, Tyr59, Leu57, Ile155, Leu57, and Ile155 of hTNF-α, but did not form hydrogen bonding (Fig. 9). On the other hand, curcumin formed two hydrogen bonds with Tyr59 and Ser60, and one hydrophobic interaction with Tyr151 (Table 1). It has been reported that Tyr119 plays a key role in the formation of π–π stacking with the native ligand. This interaction helps anchor the inhibitor to hTNF-α50. On the other hand, Ley57 is important for the formation of the hydrophobic pocket in the hTNF-α stabilization50. Previous studies also showed that plant-derived natural compounds exhibit strong binding affinity, predominantly interacting with Leu57 and Tyr119, resulting in complete burial within the binding pocket without affecting the conformational structure of hTNF-α51.
Figure 10 shows the 3D and 2D structures of the best-docked complexes of LOX-5 with bioactive compounds. As evident from Table 1, three bioactive compounds—chlorogenic acid, oleuropein, and tomatine showed high binding affinity towards LOX-5. The binding affinity of the ligands was in the order: tomatine (− 9.45 kcal/mol) > oleuropein (− 8.01 kcal/mol) > curcumin (− 7.97 kcal/mol) > chlorogenic acid (− 7.62 kcal/mol) > native ligand (arachidonic acid; ACD) (− 5.69 kcal/mol). As presented, ACD formed three hydrogen bonds with amino acids Gln557, Tyr558, and Val604 and seven hydrophobic contacts with Val671, Phe555 (× 3), and Phe610 (× 3). Tomatine formed three hydrogen bonds with Asn180, Gln413, and Ala603, along with nine hydrophobic interactions with Phe555 (× 3), Leu368 (× 2), Ala410, Val671, and Phe610 (× 2) (Fig. 10). Oleuropein formed nine hydrogen bonds with Phe555, Gln557, Ser608 (× 2), Val604, Ser547, Ala546, Glu614, and Ala603. It also formed six hydrophobic contacts with Ala551, Val671, Phe610, and Phe555 (× 2). Chlorogenic acid formed four hydrogen bonds with Gln557, Ser608, Val604, and Gln549. However, it formed only two hydrophobic contacts with Phe555 and Phe610 (Fig. 10). Many previous studies reported that Asn554 and Tyr558 play key role in the inhibition of LOX-5 protein52,53. Although the tested bioactive compounds in this study did not specifically interact with Tyr558, they formed multiple hydrophobic contacts with the neighbouring amino acids Phe555 and Gln557. These interactions suggest that the best-docked compounds may occupy a similar spatial active site pocket and potentially inhibit LOX-5 activity. Therefore, these top-docked compounds might serve as potential lead compounds to inhibit LOX-5 and its associated inflammation.
The best-docked complexes of human MPO (PDB ID: 1DNU) with bioactive compounds are depicted in Fig. 11. Docking results showed that only oleuropein (− 8.66 kcal/mol) and chlorogenic acid (− 8.69 kcal/mol) exhibited slightly lower binding energies than the native ligand, HEME (− 8.4 kcal/mol). HEME formed two hydrogen bonds with His95 and Arg239 and eight hydrophobic contacts with amino acids Arg424, Glu102 (× 3), Phe147, Ala104 (× 2), and Arg239 (Table 1). Chlorogenic acid formed five hydrogen bonds with Phe147, Arg424 (× 2), Glu102, and Phe146, but only one hydrophobic contact with Arg239. Oleuropein also formed one hydrophobic contact with Arg239 and three hydrogen bonds with His95, Arg239, and Pro220 (Fig. 11). Arg239 and Phe99 are key amino acids located in the binding cavity of MPO. The observed binding affinities of chlorogenic acid and oleuropein toward MPO can be attributed to their interaction with Arg239, suggesting their potential involvement in the inhibition of MPO activity54. The best-docked complexes of IL-4 with bioactive compounds are shown in Fig. 12. Oleuropein (− 6.34 kcal/mol) and chlorogenic acid (− 6.43 kcal/mol) showed slightly lower binding energy values compared to the control ligand, curcumin (− 6.28 kcal/mol) indicating moderate binding affinity towards IL-4. Chlorogenic acid formed one hydrogen bond with Asn19 and two hydrophobic contacts with Phe86 and Lys16 (Fig. 12). Oleuropein formed seven hydrogen bonds with Arg89, Gln82, Glu23, Ser20, Glu13, Thr17, and Gln82, but only two hydrophobic contacts with Phe86 and Lys16. Curcumin formed five hydrogen bonds and five hydrophobic bonds with the active site residues of IL-4 (Table 1).
Conclusion
The effects of biologically derived waste compounds on the modulation of oxidative stress and their influence on inflammation were evaluated. Following confirmation of low cytotoxicity, cell morphology and integrity were found preserved under the influence of these compounds, as confirmed via confocal microscopy. The interplay between anti-inflammatory and inflammatory responses was assessed through western blot analysis of marker proteins such as IL-4, MPO, and LOX-5. An elevation in the anti-inflammatory response was observed through the upregulation of IL-4 expression in the presence of tomatine and chlorogenic acid. The anti-inflammatory effect was further evidenced by a marked downregulation of MPO expression in the presence of chlorogenic acid, oleuropein, and tyrosol. Similarly, LOX-5 expression was also found to be downregulated following treatment with oleuropein and tyrosol. In summary, our study indicates that all tested compounds may exhibit anti-inflammatory effects by reducing PMA-induced increases in the activity and protein expression levels of the inflammation associated enzymes (MPO and LOX-5). These findings suggest that these compounds could be promising candidates for clinical applications and as dietary supplements.
This study offers meaningful insights into the anti-inflammatory potential of plant-derived bioactive compounds, yet there are several areas that could be explored further. Using in vitro models like U-937 and THP-1 cells allowed us to examine cellular responses in a controlled environment, but future research in animal models or clinical settings would help bridge the gap toward real-world applications. Although the study focused on key inflammatory markers such as IL-4, MPO, and LOX-5, looking at a broader range of cytokines and signalling pathways could provide a more comprehensive picture on the mechanism of action. The molecular docking results gave predictions about how the compounds might interact with their protein targets, and we see this as a valuable starting point for future experiments to confirm these effects. Comparing the activity of these natural compounds with standard anti-inflammatory drugs and understanding how they behave in the body in terms of absorption and metabolism, will also be important next steps. Overall, we believe this study lays a strong foundation for further preclinical research into the therapeutic promise of bioactive compounds.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Castaneda, O., Lee, S., Ho, C. & Huang, T. Macrophages in oxidative stress and models to evaluate the antioxidant function of dietary natural compounds. J. Food Drug Anal. 25(1), 111–118 (2017).
A. Prasad, C. Rossi, R.R. Manoharan, M. Sedlářová, L. Cangeloni, D. Rathi, G. Tamasi, P. Pospíšil, M. Consumi. Bioactive compounds and their impact on protein modification in human cells. Int. J. Mol. Sci. 23(13) (2022).
Zhao, B., Zhou, B., Bao, L., Yang, Y. & Guo, K. Alpha-tomatine exhibits anti-inflammatory activity in lipopolysaccharide-activated macrophages. Inflammation 38(5), 1769–1776 (2015).
S. Hwang, Y. Kim, Y. Park, H. Lee, K. Kim. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 63(1), 81–90.
Z. Mirsanei, N. Heidari, A. Hazrati, Y. Asemani, B. Niknam, Z. Yousefi, R. Jafari. Oleuropein reduces LPS-induced inflammation via stimulating M2 macrophage polarization. Biomed. Pharmacother. 163 (2023).
S. Serrati, L. Porcelli, S. Guida, A. Ferretta, R.M. Iacobazzi, T. Cocco, I. Maida, G. Tamasi, C. Rossi, M. Manganelli, S. Tommasi, A. Azzariti, G. Guida. Tomatine displays antitumor potential in in vitro models of metastatic melanoma. Int. J. Mol. Sci. 21(15) (2020).
F. Ali, K. Badran, M. Baraka, H. Althagafy, E. Hassanein. Mechanism and impact of heavy metal–aluminum (Al) toxicity on male reproduction: Therapeutic approaches with some phytochemicals. Life Sci. 340 (2024).
P. Valadez-Cosmes, S. Raftopoulou, Z. Mihalic, G. Marsche, J. Kargl. Myeloperoxidase: Growing importance in cancer pathogenesis and potential drug target. Pharmacol. Therap. 236 (2022).
S. Chen, H. Chen, Q. Du, J. Shen. Targeting myeloperoxidase (MPO) mediated oxidative stress and inflammation for reducing brain ischemia injury: Potential application of natural compounds. Front. Physiol. 11 (2020).
Rådmark, O., Werz, O., Steinhilber, D. & Samuelsson, B. 5-Lipoxygenase: Regulation of expression and enzyme activity. Trends Biochem. Sci. 32(7), 332–341 (2007).
Brash, A. R. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 274(34), 23679–23682 (1999).
J. Giménez-Bastida, A. González-Sarrías, J. Laparra-Llopis, C. Schneider, J. Espín. Targeting mammalian 5-lipoxygenase by dietary phenolics as an anti-inflammatory mechanism: A systematic review. Int. J. Mol. Sci. 22(15) (2021).
A. Pardini, M. Consumi, G. Leone, C. Bonechi, G. Tamasi, P. Sangiorgio, A. Verardi, C. Rossi, A. Magnani. Effect of different post-harvest storage conditions and heat treatment on tomatine content in commercial varieties of green tomatoes. J. Food Compos. Anal. 96 (2021).
Tamasi, G. et al. Chemical characterization and antioxidant properties of products and by-products from Olea europaea L. Food Sci. Nutr. 7(9), 2907–2920 (2019).
Tamasi, G. et al. Characterization of nutraceutical components in tomato pulp, skin and locular gel. Eur. Food Res. Technol. 245(4), 907–918 (2019).
W. Strober. Trypan blue exclusion test of cell viability. Curr. Protocols Immunol. 111, A3.B.1-A3.B.3 (2015).
Suzuki, K., Ota, H., Sasagawa, S., Sakatani, T. & Fujikura, T. Assay-method for myeloperoxidase in human polymorphonuclear leukocytes. Anal. Biochem. 132(2), 345–352 (1983).
Baylac, S. & Racine, P. Inhibition of 5-lipoxygenase by essential oils and other natural fragrant extracts. Int. J. Aromather. 13(2), 138–142 (2003).
Gökmen, V., Bahçeci, S. & Acar, J. Characterization of crude lipoxygenase extract from green pea using a modified spectrophotometric method. Eur. Food Res. Technol. 215(1), 42–45 (2002).
P. Alugoju, V. Vishnu Bhandare, V. S Patil, K.S. V K D, P.K. Borugadda, T. Tencomnao. In silico molecular docking and molecular dynamic simulation of agarwood compounds with molecular targets of Alzheimer’s disease. F1000RESEARCH 12 (2023) 230.
A.B. Gurung, M.A. Ali, J. Lee, M.A. Farah, K.M. Al-Anazi. Molecular docking and dynamics simulation study of bioactive compounds from Ficus carica L. with important anticancer drug targets. PLOS ONE 16(7), e0254035 (2021).
A. Gurung, M. Ali, J. Lee, M. Abul Farah, K. Al-Anazi, F. Al-Hemaid. Identification of SARS-CoV-2 inhibitors from extracts of Houttuynia cordata Thunb. Saudi J. Biol. Sci. (2021) 28(12), 7517–7527.
W. Tian, C. Chen, X. Lei, J. Zhao, J. Liang, CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 46(W1), W363–W367 (2018).
Isa, M. et al. In silico molecular docking and molecular dynamic simulation of potential inhibitors of 3C-like main proteinase (3CLpro) from severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) using selected African medicinal plants. Adv. Traditional Med. 22(1), 107–123 (2022).
V. Krishnaswamy, P. Alugoju, L. Periyasamy. Multifaceted targeting of neurodegeneration with bioactive molecules of saffron (Crocus sativus): An insilco evidence-based hypothesis. Med. Hypotheses. 143 (2020).
Kuno, S., Srinoun, K. & Penglong, T. The effects of Phorbol 12-myristate 13-acetate concentration on the expression of miR-155 and miR-125b and their macrophage function-related genes in the U937 cell line. J. Toxicol. Sci. 45(12), 751–761 (2020).
Chanput, W., Mes, J. J. & Wichers, H. J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 23(1), 37–45 (2014).
Lee, K., Do, H., Kim, D. & Kim, W. Impact of chlorogenic acid on modulation of significant genes in dermal fibroblasts and epidermal keratinocytes. Biochem. Biophys. Res. Commun. 583, 22–28 (2021).
C. Goldsmith, D. Bond, H. Jankowski, J. Weidenhofer, C. Stathopoulos, P. Roach, C. Scarlett. The olive biophenols oleuropein and hydroxytyrosol selectively reduce proliferation, influence the cell cycle, and induce apoptosis in pancreatic cancer cells. Int. J. Mol. Sci. 19(7) (2018).
S.M. Pinto, H. Kim, Y. Subbannayya, M.S. Giambelluca, K. Bosl, L. Ryan, A. Sharma, R.K. Kandasamy. Comparative proteomic analysis reveals varying impact on immune responses in phorbol 12-myristate-13-acetate-mediated THP-1 monocyte-to-macrophage differentiation. Front. Immunol. 12 (2021).
S. Lanone, F. Rogerieux, J. Geys, A. Dupont, E. Maillot-Marechal, J. Boczkowski, G. Lacroix, P. Hoet. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Particle Fibre Toxicol. 6 (2009).
Hong, O., Mohamed, N., Kalaichelvam, R., Lim, V. & Ismail, I. Effects of Clinacanthus nutans extracts on cytokine secretion in PMA-induced U937 macrophage cells. Res. J. Pharmacognosy 8(2), 27–35 (2021).
S. Mukherjee, J. Graber, C. Moore. Macrophage differentiation is marked by increased abundance of the mRNA 3’ end processing machinery, altered poly(A) site usage, and sensitivity to the level of CstF64. Front. Immunol. 14 (2023).
A. Prasad, M. Sedlářová, A. Balukova, A. Ovsii, M. Rác, M. Krupka, S. Kasai, P. Pospíšil. Reactive oxygen species imaging in U937 cells. Front. Physiol. 11 (2020).
C. Joshi, W. Ke, A. Drangowska-Way, E. O'Rourke, N. Lewis. What are housekeeping genes? Plos Comput. Biol. 18(7) (2022).
Ruan, W. & Lai, M. Actin, a reliable marker of internal control?. Clin. Chim. Acta 385(1–2), 1–5 (2007).
Murphy, C. & Polak, J. Differentiating embryonic stem cells: GAPDH, but neither HPRT nor β-tubulin is suitable as an internal standard for measuring RNA levels. Tissue Eng. 8(4), 551–559 (2002).
A. Zainuddin, K. Chua, N. Rahim, S. Makpol. Effect of experimental treatment on GAPDH mRNA expression as a housekeeping gene in human diploid fibroblasts. BMC Mol. Biol. 11 (2010).
Bashir, S., Sharma, Y., Elahi, A. & Khan, F. Macrophage polarization: The link between inflammation and related diseases. Inflamm. Res. 65(1), 1–11 (2016).
Manoharan, R. R., Sedlářová, M., Pospíšil, P. & Prasad, A. Detection and characterization of free oxygen radicals induced protein adduct formation in differentiating macrophages. Biochim. Biophys. Acta 1867(5), 130324–130324 (2023).
Levings, M. & Schrader, J. IL-4 inhibits the production of TNF-α and IL-12 by STAT6-dependent and -independent mechanisms. J. Immunol. 162(9), 5224–5229 (1999).
Zhou, Y., Lin, G., Baarsch, M., Scamurra, R. & Murtaugh, M. Interleukin-4 suppresses inflammatory cytokine gene-transcription in porcine macrophages. J. Leukoc. Biol. 56(4), 507–513 (1994).
Daseke, M. I. et al. Exogenous IL-4 shuts off pro-inflammation in neutrophils while stimulating anti-inflammation in macrophages to induce neutrophil phagocytosis following myocardial infarction. J. Mol. Cell. Cardiol. 145, 112–121 (2020).
M. Shehabeldin, J. Kobyra, Y. Cho, J. Gao, R. Chong, T. Tabib, R. Lafyatis, S. Little, C. Sfeir. Local controlled delivery of IL-4 decreases inflammatory bone loss in a murine model of periodontal disease. J. Immunol. 213(11) (2024).
Mashima, R. & Okuyama, T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 6, 297–310 (2015).
W. Lin, H. Chen, X. Chen, C. Guo. The roles of neutrophil-derived myeloperoxidase (MPO) in diseases: The new progress. Antioxidants. 13(1) (2024).
Ii, M., Yamamoto, H., Adachi, Y., Maruyama, Y. & Shinomura, Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp. Biol. Med. 231(1), 20–27 (2006).
Wisastra, R. & Dekker, F. Inflammation cancer and oxidative lipoxygenase activity are intimately linked. Cancers 6(3), 1500–1521 (2014).
Vanhamme, L., Boudjeltia, K., Van Antwerpen, P. & Delporte, C. The other myeloperoxidase: Emerging functions. Arch. Biochem. Biophys. 649, 1–14 (2018).
J. O'Connell, J. Porter, B. Kroeplien, T. Norman, S. Rapecki, R. Davis, D. McMillan, T. Arakaki, A. Burgin, D. Fox Iii, T. Ceska, F. Lecomte, A. Maloney, A. Vugler, B. Carrington, B.P. Cossins, T. Bourne, A. Lawson. Small molecules that inhibit TNF signalling by stabilising an asymmetric form of the trimer. Nat. Commun. 10(1), 5795 (2019).
M. Manne, G. Goudar, S.R. Varikasuvu, M.C. Khetagoudar, H. Kanipakam, P. Natarajan, M.D. Ummiti, V.A. Yenagi, S. Chinthakindi, P. Dharani, D.S.S. Thota, S. Patil, V. Patil. Cordifolioside: Potent inhibitor against M(pro) of SARS-CoV-2 and immunomodulatory through human TGF-β and TNF-α. 3 BIOTECH. 11(3), 136 (2021).
S. Muzaffar, W. Shahid, N. Riaz, M. Saleem, M. Ashraf, R. Aziz Ur, B. Bashir, A. Kaleem, M. Al-Rashida, B. Baral, K. Bhattarai, H. Gross, probing phenylcarbamoylazinane-1,2,4-triazole amides derivatives as lipoxygenase inhibitors along with cytotoxic, ADME and molecular docking studies. Bioorg. Chem. 107, 104525 (2021).
M. Belaiba, S. Aldulaijan, S. Messaoudi, M. Abedrabba, A. Dhouib, J. Bouajila. Evaluation of biological activities of twenty flavones and in silico docking study. Molecules. (2023).
P. Solo, M. Arockia doss, D. Prasanna. Designing and docking studies of imidazole-based drugs as potential inhibitors of myeloperoxidase (MPO) mediated inflammation and oxidative stress. Biocatal. Agric. Biotechnol. 43, 102421 (2022).
Funding
This work was funded by Grant no. IGA_PrF_2025_028 entitled "Current research topics in molecular and general biophysics" of Palacký University.
Author information
Authors and Affiliations
Contributions
AP and CR conceived and designed the project. RK, RS, MS, and AP performed the measurements. PA and NVAA carried out the molecular docking studies. GT and CB isolated and prepared the bioactive compounds. AP and RK wrote the manuscript; RS and PA contributed to manuscript writing. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kushwaha, R., Alugoju, P., Anthikapalli, N.V.A. et al. Bioactive compounds in the modulation of oxidative stress in monocytes and macrophages. Sci Rep 15, 32012 (2025). https://doi.org/10.1038/s41598-025-16505-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16505-4