Abstract
Despite extensive research on lead (Pb) toxicity’s detrimental effects on male reproductive health, its precise mechanisms remain elusive, and the synergistic protective effects of resveratrol (RSV) and β-glucan (βG) on fertility parameters are underexplored. This study investigates their combined efficacy against Pb-induced reproductive toxicity in male mice. Forty 6-8-week-old mice were randomly divided into five groups: Group I (Control): received normal saline; Group II (Pb): received lead acetate (50 mg/kg); Group III (Pb + RSV): received Pb and RSV (20 mg/kg); Group IV (Pb + βG): received Pb and βG (50 mg/kg); Group V (Pb + RSV + βG): received Pb, RSV, and βG. Mice received treatments for 35 days. Sperm parameters (count, motility, viability, DNA damage), oxidative stress markers (TAC, SOD, GPx, MDA), hormone levels (testosterone, LH, FSH), testicular histopathology, and apoptosis-related gene expression (Bax, Bcl-2, caspase-3) were evaluated. The Pb + RSV + βG group, compared to the Pb-only group, showed a 61.5% increase in sperm motility, 68.1% reduction in sperm DNA damage, and 87.2% increase in sperm count. It also exhibited a 63.9% reduction in MDA levels and increased TAC, SOD, and GPx levels. Hormone levels and anti-apoptotic Bcl-2 expression increased, while Bax and caspase-3 decreased significantly. These preclinical findings suggest that RSV and βG may mitigate Pb-induced reproductive toxicity in mice via antioxidant and anti-apoptotic mechanisms.
Similar content being viewed by others
Introduction
Among the most common environmental pollutants, lead (Pb) is the main cause of serious ecological problems worldwide1. The manufacturing of chemicals, printing, and pigments for paints and cosmetics is highly dependent on this employment-related element2. Lead can enter the body via inhalation of contaminated air or consumption of contaminated food and water3. Lead has been shown to cause chronic toxicity and physiological, behavioral, and biochemical dysfunction in humans and animals upon introduction into the body1. Previous research indicates that lead poisoning may worsen male reproductive disorders2. It has been found to reduce sperm quality, decrease testicular weight, and cause damage to the testes in rats and mice4,5.
In addition, one of the adverse effects of lead on testicular tissue is increased oxidative stress, characterized by excess reactive oxygen species (ROS) and reduced activity of antioxidant enzymes6,7. In addition, changes in the expression of key genes related to antioxidant protection mechanisms were observed8,9. Male infertility is ultimately caused by oxidative stress, which also reduces the weight of the reproductive organs and impairs the quality of the sperm4. Natural antioxidants are considered to be a potential protection against oxidative damage caused by heavy metals and to maintain the balance between free radicals and antioxidants10. In particular, several antioxidant compounds have been shown to have protective properties against reproductive toxicity induced by lead7,9.
Beta-glucans are polysaccharide compounds that are naturally occurring in yeast, fungi and cereal species11. In previous studies, the biological activity of beta-glucans, including immunomodulation and antioxidation, has been demonstrated12. Beta-glucans protect against oxidative stress by scavenging reactive oxygen species and enhancing antioxidant enzyme activity, which is critical for mitigating lead-induced testicular damage13,14. These properties are particularly relevant to male reproductive health, as oxidative stress disrupts spermatogenesis and sperm function15. Studies have shown that beta-glucans reduce lipid peroxidation and DNA damage in tissues exposed to heavy metal toxicity, supporting their potential protective role in the testes16.
At least 27 plant species contain the natural substance phytotoxan resveratrol (RSV; 3,5,4’-trihydroxy-trans-stilbene). Numerous studies have demonstrated the anti-inflammatory, antidiabetic, and antioxidant properties of resveratrol17. Reportedly, it inhibits lipid peroxidation more strongly than other phenolic compounds18. In addition, Xiao19 found that resveratrol is more effective than melatonin, vitamin E, and acetyl-N-tert-butyl nitrate in reducing oxidative DNA damage. In addition, the ability of resveratrol to neutralize hydroxyl radicals has been shown to reduce DNA damage. In another study, resveratrol dramatically reduced apoptosis in testicular germ cells20. Because of its many medicinal benefits, particularly its antioxidant properties, resveratrol can protect against the oxidative toxicity of the testis caused by anesthetics such as isoflurane21. According to recent in vitro and in vivo studies, resveratrol protects spermatozoocytes against lipid peroxidation and improves sperm motility, viability, and mitochondrial membrane potential22,23,24.
However, despite the known protective roles of β-glucan and resveratrol against a variety of oxidative and inflammatory disorders, their potential protective effect against lead-induced reproductive toxicity is yet to be well understood. The synergistic action of β-glucan and resveratrol may enhance antioxidant defenses and reduce apoptosis in testicular tissue, offering a novel approach to counteract lead’s reproductive toxicity13. To bridge this gap, the present study aims to evaluate whether resveratrol and β‐glucan reverse testicular injury, improve sperm quality, and normalize antioxidant status in male mice exposed to lead toxicity. Through the elucidation of their putative therapeutic benefits, this study may pioneer novel therapeutic strategies to counteract heavy metal-caused reproductive dysfunction (Tables 1, 2, 3).
Results
Resveratrol and/or β-Glucan improved lead Acetate-Evoked alterations in semen analyses in mice
Lead exposure reduced sperm concentration by 51.4% (15.47 ± 3.32 vs. 31.82 ± 5.95 million/mL in control), total motility by 41.7% (46.32 ± 1.18 vs. 79.45 ± 2.21% in control), and progressive motility by 77.5% (9.03 ± 1.22 vs. 40.12 ± 1.85% in control; p < 0.001; Table 4). Sperm kinematic parameters (VCL, VSL, VAP, ALH, BCF) were also impaired (p < 0.01; Table 4). Treatment with RSV, βG, or their combination improved these parameters. Compared to Pb-only, Pb + RSV increased sperm concentration by 67.4% (25.89 ± 6.42 million/mL), total motility by 47.1% (68.12 ± 2.75%), and progressive motility by 237.2% (30.45 ± 1.80%; p < 0.001). Pb + βG increased these by 53.6% (23.75 ± 4.98 million/mL), 35.1% (62.58 ± 2.49%), and 220.8% (28.97 ± 1.41%; p < 0.001). Pb + RSV + βG showed the greatest improvement: 87.2% in sperm concentration (28.96 ± 5.87 million/mL), 61.5% in total motility (74.83 ± 2.05%), and 277.9% in progressive motility (34.12 ± 1.59%) compared to Pb-only, though reduced by 9.0%, 5.8%, and 15.0% vs. control, respectively (p < 0.05; Table 4). Sperm viability increased by 49.1% (81.95 ± 2.49 vs. 54.98 ± 2.39% in Pb-only), plasma membrane functionality increased by 61.3% (77.35 ± 2.14 vs. 47.98 ± 3.61% in Pb-only), DNA damage decreased by 68.1% (9.11 ± 1.19 vs. 28.52 ± 1.45% in Pb-only), and abnormal morphology decreased by 65.8% (11.75 ± 0.65 vs. 34.32 ± 1.25% in Pb-only) in Pb + RSV + βG compared to Pb-only, but viability and plasma membrane functionality were reduced by 5.2% and 6.3%, while DNA damage and abnormal morphology increased by 80.4% and 64.3% vs. control, respectively (p < 0.001; Table 5).
Resveratrol and/or β-Glucan restored lead Acetate-Induced hormonal imbalances
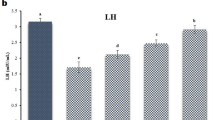
Pb exposure decreased serum follicle-stimulating hormone (FSH) by 67.2% (1.34 ± 0.21 vs. 4.09 ± 0.16 IU/L in control), luteinizing hormone (LH) by 55.1% (1.32 ± 0.14 vs. 2.94 ± 0.13 IU/L), and testosterone 48.8% (2.78 ± 0.08 vs. 5.43 ± 0.11 ng/mL in control; p < 0.01; Fig. 1). Treatment with RSV, βG, or their combination restored these hormones. Pb + RSV increased FSH by 149.3% (3.34 ± 0.18 IU/L), LH by 77.3% (2.34 ± 0.09 IU/L), and testosterone by 66.2% (4.62 ± 0.10 ng/mL) vs. Pb-only (p < 0.01). Pb + βG increased these by 126.9% (3.04 ± 0.17 IU/L), 65.2% (2.18 ± 0.08 IU/L), and 52.5% (4.24 ± 0.09 ng/mL) vs. Pb-only (p < 0.01). Pb + RSV + βG showed increases of 171.6% (3.64 ± 0.19 IU/L), 91.7% (2.53 ± 0.07 IU/L), and 82.0% (5.06 ± 0.09 ng/mL) compared to Pb-only, reduced by 11.0%, 13.9%, and 6.8% vs. control, respectively (p < 0.05; Fig. 1).
Resveratrol and/or β-Glucan ameliorated lead Acetate-Induced histological lesions in testicular tissue
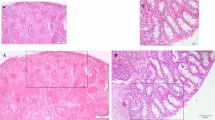
Pb exposure reduced body weight by 30.1% (18.52 ± 0.02 vs. 26.48 ± 0.03 g in control) and testicular weight by 40.2% (0.067 ± 0.001 vs. 0.112 ± 0.002 g in control; p < 0.001; Table 6). Testicular length and testis/body weight ratio also decreased (p < 0.01). Histopathological analysis revealed seminiferous tubule atrophy, intraepithelial vacuolization, and germ cell loss in the Pb-only group, with a Johnsen score of 5.51 ± 0.23 vs. 9.42 ± 0.21 in control (p < 0.001; Fig. 2). Seminiferous tubule diameter decreased by 42.9% (31.11 ± 1.29 vs. 54.45 ± 1.29 μm in control; p < 0.01; Table 6). Treatment with RSV, βG, or their combination restored body weight, testicular weight, and histological parameters. Pb + RSV increased body weight by 20.4% (22.29 ± 0.02 g), testicular weight by 52.2% (0.102 ± 0.002 g), and Johnsen score by 33.9% (7.38 ± 0.16) vs. Pb-only (p < 0.001). Pb + βG increased these by 17.8% (21.81 ± 0.03 g), 40.3% (0.094 ± 0.002 g), and 29.2% (7.12 ± 0.31) vs. Pb-only (p < 0.001). Pb + RSV + βG achieved a body weight increase of 32.8% (24.59 ± 0.02 g), testicular weight increase of 61.2% (0.108 ± 0.002 g), Johnsen score increase of 53.5% (8.46 ± 0.19), and tubule diameter increase of 50.7% (46.89 ± 1.35 μm) compared to Pb-only, though reduced by 10.2% and 13.9% vs. control, respectively (p < 0.05; Table 6).
Testicular histo-architecture in different experimental groups. (A) Control; (B) Lead (Pb); (C) Pb + β-glucan; (D) Pb + Resveratrol; (E) Pb + β-glucan + Resveratrol (hematoxylin and eosin staining, 400×). Group A (Control) showed normal seminiferous tubules with active spermatogenesis and high sperm density in the lumen. Group B (Pb) exhibited severe histopathological aberrations, including seminiferous tubule atrophy, intraepithelial vacuolization, germ cell loss, and basal membrane irregularities, with reduced viable sperm in the lumen (white arrows) and a low Johnsen score (5.51 ± 0.23). Groups C, D, and E showed improved histology with partial restoration of tubule structure, increased viable sperm density (white arrows), and higher Johnsen scores (7.12–8.46), though mild disorganization persisted (asterisks). Different superscripts demonstrate significant differences (p ≤ 0.05; Mean ± SD). n = 8 per group.
Resveratrol and/or β-Glucan enhanced antioxidant defenses against lead acetate toxicity
Pb exposure reduced total antioxidant capacity (TAC) by 62.2% (0.34 ± 0.03 vs. 0.90 ± 0.02 mmol/g in control), superoxide dismutase (SOD) by 52.0% (3.64 ± 0.12 vs. 7.59 ± 0.11 U/g in control), and glutathione peroxidase (GPx) by 77.8% (0.06 ± 0.0061 vs. 0.27 ± 0.0073 U/g in control), while increasing malondialdehyde (MDA) by 292.3% (11.26 ± 0.19 vs. 2.87 ± 0.21 nmol/g in control; p < 0.001; Fig. 3; Table 6). Treatment with RSV, βG, or their combination enhanced TAC, SOD, and GPx activities and reduced MDA. Pb + RSV increased TAC by 94.1% (0.66 ± 0.03 mmol/g), SOD by 60.4% (5.84 ± 0.14 U/g), GPx by 200.0% (0.18 ± 0.0065 U/g), and reduced MDA by 43.7% (6.34 ± 0.18 nmol/g) vs. Pb-only (p < 0.001). Pb + βG increased TAC by 76.5% (0.60 ± 0.03 mmol/g), SOD by 48.9% (5.42 ± 0.13 U/g), GPx by 166.7% (0.16 ± 0.0062 U/g), and reduced MDA by 38.2% (6.96 ± 0.19 nmol/g) vs. Pb-only (p < 0.001). Pb + RSV + βG increased TAC by 129.4% (0.78 ± 0.03 mmol/g), SOD by 82.4% (6.64 ± 0.16 U/g), GPx by 283.3% (0.23 ± 0.0079 U/g), and reduced MDA by 54.2% (5.16 ± 0.17 nmol/g) compared to Pb-only, though reduced by 13.3%, 12.5%, 14.8%, and increased by 79.8% vs. control, respectively (p < 0.001; Fig. 3; Table 6).
Biochemical findings in different experimental groups. Pb: lead; βG: β-glucan; RSV: resveratrol. (A) Total antioxidant capacity (TAC); (B) glutathione peroxidase (GPx); (C) superoxide dismutase (SOD); (D) Malondialdehyde (MDA). Different superscripts demonstrate significant differences (p ≤ 0.05; Mean ± SD). n = 8 per group.
Resveratrol and/or β-Glucan curtailed testicular apoptotic pathway in lead Acetate-Intoxicated mice
Pb exposure upregulated pro-apoptotic Bax and caspase-3 mRNA levels and downregulated anti-apoptotic Bcl-2 compared to controls (p < 0.01; Fig. 4). Treatment with RSV, βG, or their combination suppressed Bax and caspase-3 expression and increased Bcl-2 levels. Pb + RSV reduced Bax and caspase-3 by 35.2% and 41.7%, and increased Bcl-2 by 45.8% vs. Pb-only (p < 0.01). Pb + βG reduced Bax and caspase-3 by 28.6% and 33.3%, and increased Bcl-2 by 37.5% vs. Pb-only (p < 0.01). Pb + RSV + βG showed the greatest modulation, reducing Bax and caspase-3 by 48.9% and 55.0%, and increasing Bcl-2 by 58.3% vs. Pb-only, approaching control levels (p < 0.01 vs. controls; Fig. 4).
Reverse transcription-polymerase chain reaction findings in different experimental groups. Pb: lead; βG: β-glucan; RSV: resveratrol. The densities of Bcl-2 (A), Bax (B), and Caspase-3 (C) mRNA levels in testicular tissue were measured by densitometry and normalized to the 18SrRNA mRNA expression level. Significant differences between groups are indicated by different superscripts (p ≤ 0.05; Mean ± SD). n = 8 per group.
Resveratrol and/or β-Glucan improved fertility indices in lead Acetate-Exposed mice
Pb exposure reduced the male fertility index by 80% (20% vs. 100% in control) and pregnancy index by 85% (15% vs. 100% in control; p < 0.001; Table 7). Treatment with RSV, βG, or their combination improved these indices. Pb + RSV increased male fertility by 200% (60%) and pregnancy index by 166.7% (40%) vs. Pb-only (p < 0.001). Pb + βG increased these by 150% (50%) and 100% (30%) vs. Pb-only (p < 0.001). Pb + RSV + βG achieved a male fertility index of 90% (350% increase from Pb-only, 10% lower than control, p < 0.05) and a pregnancy index of 80% (433.3% increase from Pb-only, 20% lower than control, p < 0.05; Table 7).
Discussion
Lead (Pb) exposure is a well-documented cause of male infertility, primarily through oxidative stress, hormonal imbalances, testicular damage, and increased apoptosis5. Our study demonstrates that Pb significantly increased oxidative stress, with elevated malondialdehyde (MDA) levels and reduced total antioxidant capacity (TAC), superoxide oxidase (SOD), and glutathione peroxidase (GPx) activities25. Oxidative stress is central to Pb’s toxicity, with Pb’s induction of ROS overwhelming antioxidant defenses, triggering testicular injury26,27,28. Pb’s detrimental effects on sperm parameters align with studies reporting reduced motility and count in Pb-exposed rodents and humans29,30,31. Chronic Pb exposure disrupts spermatogenesis by inducing ROS-mediated lipid peroxidation, damaging sperm membranes, and impairing ATP synthesis, which reduces motility32,33. Moreover, Pb’s interference with zinc homeostasis in testicular tissue exacerbates oxidative stress, further compromising sperm quality4,34. Testicular histopathology revealed severe Pb-induced damage, including seminiferous tubule atrophy, vacuolization, and germ cell loss, reducing testicular weight25. This aligns with reports of Pb disrupting Sertoli cell function and blood-testis barrier integrity, leading to germ cell apoptosis35,36. Pb exposure reduced serum FSH, LH, and testosterone, disrupting the hypothalamic-pituitary-gonadal axis37. This mirrors findings of Pb suppressing gonadotropin-releasing hormone secretion, impairing Leydig cell steroidogenesis38. Furthermore, Pb upregulated pro-apoptotic Bax and caspase-3 expression and downregulated anti-apoptotic Bcl-2 expression, contributing to germ cell apoptosis and reduced fertility39. Fertility indices dropped significantly in the Pb group, reflecting impaired sperm function and hormonal deficits40.
Treatment with resveratrol (RSV), β-glucan (βG), or their combination mitigates these effects, with the Pb + RSV + βG group showing significant recovery in sperm quality, testicular weight, and histological integrity5,25. These improvements likely stem from RSV and βG’s synergistic antioxidant and anti-apoptotic properties, which counteract Pb-induced reactive oxygen species (ROS)41,42,43. Specifically, RSV enhances mitochondrial function and reduces oxidative damage in spermatogenic cells, while βG’s polysaccharide structure scavenges ROS and upregulates antioxidant enzymes14,44. This dual mechanism explains the superior efficacy of the combined treatment, restoring sperm parameters to near-control levels41. RSV and βG’s ability to restore sperm viability and reduce DNA damage in the Pb + RSV + βG group suggests a protective role in DNA repair and membrane stabilization45,46. These results are consistent with studies on other antioxidants, such as naringenin, which mitigate Pb-induced sperm damage47but our study highlights the unique synergy of RSV and βG25. Compared to cadmium-induced testicular damage, Pb’s effects are more pronounced on tubule architecture, underscoring its potency as a reproductive toxicant48. The restoration of hormonal levels by RSV and βG likely supports Leydig cell function and pituitary signaling, contributing to improved sperm parameters and fertility44,49. The Pb + RSV + βG group’s recovery in testicular histology suggests that RSV and βG restore Sertoli cell support and spermatogenesis, possibly by upregulating Bcl-241,50. Collectively, our findings demonstrate that RSV and βG counteract Pb’s multi-faceted toxicity through antioxidant and anti-apoptotic mechanisms, with implications for human occupational exposure to lead51. Future studies should explore optimal dosing, chronic exposure models, and clinical translation to validate these preclinical findings51.
While our study provides robust evidence of RSV and βG’s protective effects against Pb-induced reproductive toxicity, several limitations should be acknowledged. First, the use of a murine model limits direct extrapolation to humans, as species differences in metabolism and reproductive physiology may influence outcomes52. Second, the 35-day Pb exposure represents an acute model, which may not fully capture the effects of chronic human exposure typical in occupational settings. Third, the absence of RSV-only and βG-only control groups precludes assessing their independent effects, though their combined efficacy was the study’s focus. Finally, the doses used (Pb: 50 mg/kg, RSV: 20 mg/kg, βG: 50 mg/kg) may not directly translate to human therapeutic levels, requiring further pharmacokinetic studies. These limitations do not undermine our findings but highlight the need for future research, including human studies, chronic exposure models, and dose optimization, to validate and extend our results.
Conclusion
The results of this study indicate that RSV, βG, and their combination (RSV + βG) may provide protective effects on the male reproductive system, particularly the testes, against damage caused by Pb. This protective action is demonstrated by significant improvements in oxidative stress markers, which include both pro-oxidants and enzymatic antioxidants. Additionally, RSV, βG, and their combination have been shown to repair histopathological changes and restore the morphological structure of the testes to normal histological conditions. It is hypothesized that the beneficial effects of RSV, βG, and their combination arise from their natural nutrient properties and high antioxidant content, which help mitigate testicular damage caused by lead acetate exposure. Therefore, RSV, βG, and their combination have potential as therapeutic options for addressing testicular toxicity and oxidative stress, warranting further research and potential pharmaceutical applications.
Methods
Chemicals
All chemical reagents were sourced from reliable suppliers to ensure high quality and reproducibility. Lead acetate trihydrate [(C₂H₃O₂)₂Pb·3 H₂O] (Cat. No. 4676-66-2) and resveratrol (Cat. No. R5010) were obtained from Sigma-Aldrich, 3050 Spruce Street, St. Louis, MO 63,103, USA. β-Glucan (Cat. No. 9041-22-9) was sourced from Merck KGaA, Frankfurter Str. 250, Darmstadt 64,293, Germany. Ketamine (Cat. No. 6740-88-1) and xylazine (Cat. No. 7361-61-7) were procured from Alfasan International B.V., Kuipersweg 9, 3449 JA Woerden, Netherlands. Normal saline (0.9% NaCl) was obtained from Merck KGaA, Frankfurter Str. 250, Darmstadt 64,293, Germany. Hematoxylin (Cat. No. H3136) and eosin (Cat. No. E4009) for histological staining were sourced from Sigma-Aldrich, 3050 Spruce Street, St. Louis, MO 63,103, USA. SinaSyer Blue HF-qPCR mix was obtained from CinnaGen Co., No. 34, Sepehr Street, Farahzadi Blvd, Shahrak Gharb, Tehran, Iran. Biochemical assay kits (Naxifer, Nagpax, Nasdox, Nalondi) were procured from Navand Salamat Co., Unit 111, Science and Technology Park, Serow Road, Urmia, West Azerbaijan, Iran. These reagents were selected to ensure reliability and consistency in experimental outcomes.
Animal
Forty male mice (6 to 8 weeks old) weighing 26.0 ± 2.0 g were housed in the Urmia University Animal Breeding Center in Iran. The mice were housed in wire mesh cages with adequate ventilation and had free access to fresh water and standard pellets. The temperature, light-dark cycle, and humidity were maintained at 21 ± 2 °C and 50 ± 10%, respectively. The study, which was approved by the Animal Ethics Committee of the Islamic University of Azad, IR-IAU-2/27/35, was conducted following ethical guidelines.
Experimental protocol
This study evaluated the protective effects of RSV and βG against Pb-induced reproductive toxicity, with groups designed to isolate these effects against Pb exposure while using a control group to establish baseline parameters7,53,54. After one week of acclimatization, mice were randomly allocated to five groups (n = 8 per group):
-
Group 1 (Control): Daily intragastric gavage of oral normal saline was administered55.
-
Group 2 (Pb): Received an intragastric gavage of 50 mg/kg (2% solution in saline) of lead acetate trihydrate [(C₂H₃O₂)₂Pb·3 H₂O] orally every day55.
-
Group 3 (βG + Pb): As previously mentioned, they received Pb in combination with a daily oral dose of 50 mg of βG by gavage56.
-
Group 4 (RSV + Pb): received Pb treatment as prescribed, in addition to the daily oral RSV dose of 20 mg gavage57.
-
Group 5 (βG + RSV + Pb): A daily oral dose of βG (50 mg/kg) and RSV (20 mg/kg) were given by gavage in addition to Pb treatment.
Following the 35-day trial duration, the male mice were euthanized three days after mating to maximize the chances of sperm maturity in the caudal epididymis. Their euthanasia was conducted through an IP injection of ketamine at 80 mg/kg alongside xylazine at 10 mg/70 kg, both procured from the Alfasan (Netherlands)58,59,60.
Levels of plasma reproductive hormone
Blood samples were obtained on Day 35 by cardiology puncture and stored in a standardized test tube for hormone analysis. The radioimmunoassay (RIA) method (DIA Source) was used to measure serum testosterone (T), Latinizing hormone (LH), and follicle-stimulating hormone (FSH) concentrations61,62,63,64.
Sperm collection
After the experiment and before euthanizing the mouse, body weights were recorded using the method described by Zolfaghari et al.65. Weight gain was calculated by determining the difference between the starting weight and the ending weight. Following the euthanization of the mouse, the epididymis and testes were carefully removed, and both absolute and relative weights were measured, in accordance with the procedures outlined by Sadeghi Rad et al.66.
The cauda epididymis was manually fragmented to retrieve sperm, which was then placed in a Petri dish containing 1 mL of human tubal fluid (HTF) medium. The sperm suspension was incubated at 37 °C in a 5% CO2 for 30 min. The procedures for obtaining the sperm were conducted following the methods described by Kashiwazaki et al.67 and Soleimanzadeh et al.43.
Sperm analysis
Count of sperm
After a 1:5 dilution with distilled water for devitalization, the concentration of sperm was measured using a Neubauer hemocytometer (Brand, Germany)66,68,69.
Motility of sperm
Sperm motility and its characteristics were assessed at room temperature using Test Sperm 3.2 software (Videotest, Russia) as shown in Table 1. A 10 µL sample was analyzed under an Olympus BX41 microscope (Japan)61,70,71.
Viability and morphology of sperm
Eosin-nigrosin staining was used to assess sperm viability and morphology following the World Health Organization (WHO) protocol72. Non-viable sperm were identified based on staining observed in the head, neck, or tail regions. Additionally, sperm with abnormal morphology, such as those with cytoplasmic residues, were categorized61,73,74.
DNA damage of sperm
The integrity of sperm DNA was assessed using Acridine Orange (AO) staining. First, the sperm samples were fixed for two hours in a 1:3 mixture of acetic acid and methanol. After fixation, concentrated sperm smears were air-dried for five minutes before being stained with an AO solution (1 mg of AO in 1000 mL of filtered water) for five minutes at 4 °C, in low light conditions. Fluorescence microscopy was then used to evaluate the integrity of the DNA61,75,76,77.
Plasma membrane functionality (PMF) of sperm
To evaluate sperm plasma membrane function, the hypoosmotic swelling (HOS) test was conducted. In this test, 100 µL of a hypoosmotic solution containing fructose and sodium citrate was mixed with 10 µL of the sperm sample. The mixture was then incubated for one hour at 37 °C. PMF was assessed using an Olympus BX41 microscope at a magnification of 400×; swollen or curled tails indicate functional membranes61,66,78,79,80.
Enzymatic antioxidant activity assessment
Mouse testes were homogenized in 1000 µL of lysis buffer and then centrifuged at 9000 rpm for 15 min. The supernatant was collected for biochemical analyses66,81,82,83,84,85,86. The testicular total antioxidant capacity (TAC), as well as the activities of glutathione peroxidase (GPx) and superoxide dismutase (SOD), were measured using the Naxifer, Nagpix™, and Nasdox kits (Navand Salamat, Iran), respectively. Additionally, malondialdehyde (MDA) levels were quantified using the Nalondi™ kit (Navand Salamat, Iran) at a wavelength of 535 nm and expressed as nmol/g of protein66.
Testicular histopathology and histomorphometry
Testicular tissues were preserved in 10% formalin, dehydrated through a graded series of ethanol, and embedded in paraffin. Using a microtome, sections measuring 7 μm in thickness were obtained and stained with hematoxylin and eosin (H&E). The spermiogenesis index (SPI) was calculated as the ratio of sperm-containing tubules to those without. Additional histological parameters, including the Sertoli cell index (SCI), meiotic index (MI), tubular differentiation index (TDI), and repopulation index (RI), were evaluated according to standard protocols61. The quality of the seminiferous tubules was assessed using Johnsen’s score (Table 2). Testicular injury was classified based on the Cosentino grading system: Grade 1 (normal), Grade 2 (mild disruption), Grade 3 (moderate disorganization), and Grade 4 (severe damage with necrosis)58,60,66.
Gene expression analysis via qRT-PCR
The expression levels of Bax, caspase-3, and Bcl-2 were analyzed using quantitative real-time polymerase chain reaction (qRT-PCR). The reactions were performed with SinaSyber Blue HF-qPCR mix (CinnaGen, Iran) on a StepOne system (Applied Biosystems, USA), while 18 S rRNA was applied as the reference gene (Table 3). The cycling protocol included 35 cycles of 94 °C, 55 °C, and 72 °C following an initial denaturation step at 95 °C. The relative expression levels of the gene were calculated by the 2−ΔCt method and then log-transformed for further statistical analysis61.
Fertility indexes
Male mice that were treated were paired with untreated females at a ratio of 1 male to 2 females for a maximum of 72 h. Vaginal smears were conducted at 24, 48, and 72 h after pairing to confirm the presence of sperm, which established gestational day 0 (GD0)87. On gestational day 17 (GD17), pregnant females were euthanized using xylazine (10 mg/kg, intraperitoneally) and ketamine (80 mg/kg, intraperitoneally) from Alfasan, Netherlands. Pregnancy was confirmed by the presence of fetuses. Indices were calculated as follows: Female mating index = (number of females mated / number of females) × 100; Male mating index = (number of males mated / number of males) × 100; Pregnancy index = (number of females pregnant / number of females mated) × 100; Male fertility index = (number of males impregnating females/number of males mated) × 100 66.
Statistical analysis
Forty male mice were assigned to five groups (n = 8 per group) using a random number generator to ensure unbiased allocation88. Normality and homogeneity of variances were confirmed using Shapiro-Wilk and Levene’s tests, respectively, before analysis89. Data were analyzed using SPSS (version 27.0; IBM, USA). Continuous variables, including reproductive parameters (e.g., sperm motility, concentration), histological scores (e.g., Johnsen score), oxidative stress markers (e.g., MDA, TAC), and gene expression levels, were expressed as mean ± standard deviation (SD), while fertility indices were reported as percentages89. A one-way ANOVA with Tukey’s post hoc test was performed for comparisons (p ≤ 0.05). Analyses, including sperm motility and gene expression, employed objective methods (e.g., Test Sperm 3.2 software, qRT-PCR) to minimize bias, while histopathological assessments adhered to standardized protocols under the supervision of trained researchers. Blinding was not explicitly implemented, but future studies could incorporate it for subjective analyses like histopathology to further reduce bias88.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- RSV:
-
Resveratrol
- βG:
-
β-glucan
- TM:
-
Total motility
- PM:
-
Progressive motility
- VCL:
-
Curvilinear velocity
- VSL:
-
Straight-line velocity
- VAP:
-
Average path velocity
- STR:
-
Straightness
- LIN:
-
Linearity
- ALH:
-
Amplitude of lateral head displacement
- BCF:
-
Beat-cross frequency
- PMF:
-
Plasma membrane functionality
- TAC:
-
Total antioxidant capacity
- MDA:
-
Malondialdehyde
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- SOD:
-
Superoxide dismutase
- NO:
-
Nitric oxide
- ROS:
-
Reactive oxygen species
References
Jin, X., Xu, Z., Zhao, X., Chen, M. & Xu, S. The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney. Chemosphere 180, 259–266 (2017).
Wen, L. et al. Cyanidin-3-O-glucoside promotes the biosynthesis of progesterone through the protection of mitochondrial function in Pb-exposed rat Leydig cells. Food Chem. Toxicol. 112, 427–434 (2018).
Caito, S., Carolina, B. A. L. A., Monica, M. B. P. & Aschner, M. Lead–Its Effects on Environment and Health. (2017).
Anjum, M. R., Madhu, P., Reddy, K. P. & Reddy, P. S. The protective effects of zinc in lead-induced testicular and epididymal toxicity in Wistar rats. Toxicol. Ind. Health. 33, 265–276 (2017).
Li, C. et al. Lead exposure reduces sperm quality and DNA integrity in mice. Environ. Toxicol. 33, 594–602 (2018).
El-Magd, M. A. et al. A potential mechanism associated with lead‐induced testicular toxicity in rats. Andrologia 49, e12750 (2017).
Rao, F., Zhai, Y. & Sun, F. Punicalagin mollifies lead acetate-induced oxidative imbalance in male reproductive system. Int. J. Mol. Sci. 17, 1269 (2016).
Rodríguez-Estival, J., de la Lastra, J. M. P., Ortiz‐Santaliestra, M. E., Vidal, D. & Mateo, R. Expression of immunoregulatory genes and its relationship to lead exposure and lead‐mediated oxidative stress in wild ungulates from an abandoned mining area. Environ. Toxicol. Chem. 32, 876–883 (2013).
Wang, X. et al. Subchronic exposure to lead acetate inhibits spermatogenesis and downregulates the expression of Ddx3y in testis of mice. Reprod. Toxicol. 42, 242–250 (2013).
Hagar, H. & Al Malki, W. Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: Role of oxidative stress and caspase-3. Environ. Toxicol. Pharmacol. 37, 803–811 (2014).
Kaya, K., Ciftci, O., Cetin, A., Tecellioğlu, M. & Başak, N. Beneficial effects of β-glucan against cisplatin side effects on the nervous system in rats 1. Acta Cir. Bras. 31, 198–205 (2016).
Dietrich-Muszalska, A., Olas, B. & Kontek, B. Rabe-Jabłońska, J. Beta-glucan from Saccharomyces cerevisiae reduces plasma lipid peroxidation induced by haloperidol. Int. J. Biol. Macromol. 49, 113–116 (2011).
Bashir, K. M. I. & Choi, J. S. Clinical and physiological perspectives of β-glucans: The past, present, and future. Int. J. Mol. Sci. 18, 1906 (2017).
Vetvicka, V. & Vetvickova, J. Glucans and cancer: Comparison of commercially available β-glucans–part IV. Anticancer Res. 38, 1327–1333 (2018).
Agarwal, A., Virk, G., Ong, C. & Du Plessis, S. S. Effect of oxidative stress on male reproduction. World J. Mens Health. 32, 1 (2014).
Vetvicka, V. Effects of β-glucan on some environmental toxins: An overview. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 158, 1–4 (2014).
Oktem, G. et al. Resveratrol attenuates doxorubicin-induced cellular damage by modulating nitric oxide and apoptosis. Exp. Toxicol. Pathol. 64, 471–479 (2012).
Collodel, G. et al. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod. Toxicol. 31, 239–246 (2011).
Xiao, N. N. Effects of Resveratrol supplementation on oxidative damage and lipid peroxidation induced by strenuous exercise in rats. Biomol. Ther. (Seoul). 23, 374 (2015).
Uguralp, S., Usta, U. & Mizrak, B. Resveratrol May reduce apoptosis of rat testicular germ cells after experimental testicular torsion. Eur. J. Pediatr. Surg. 15, 333–336 (2005).
Smoliga, J. M., Baur, J. A. & Hausenblas, H. A. Resveratrol and health–a comprehensive review of human clinical trials. Mol. Nutr. Food Res. 55, 1129–1141 (2011).
Mojica-Villegas, M. A., Izquierdo-Vega, J. A., Chamorro-Cevallos, G. & Sánchez-Gutiérrez, M. Protective effect of Resveratrol on biomarkers of oxidative stress induced by iron/ascorbate in mouse spermatozoa. Nutrients 6, 489–503 (2014).
Sun, L. et al. Resveratrol protects Boar sperm in vitro via its antioxidant capacity. Zygote 28, 417–424 (2020).
Bang, S., Qamar, A. Y., Tanga, B. M., Fang, X. & Cho, J. Resveratrol supplementation into extender protects against Cryodamage in dog post-thaw sperm. J. Vet. Med. Sci. 83, 973–980 (2021).
Akacha, A., Badraoui, R., Rebai, T. & Zourgui, L. Effect of opuntia ficus indica extract on methotrexate-induced testicular injury: A biochemical, Docking and histological study. J. Biomol. Struct. Dyn. 40, 4341–4351 (2022).
Chen, X. W. et al. Protective mechanism of selenium on mercuric chloride-induced testis injury in chicken via p38 MAPK/ATF2/iNOS signaling pathway. Theriogenology 187, 188–194 (2022).
Li, J. et al. Nrf2 pathway activation contributes to anti-fibrosis effects of ginsenoside Rg1 in a rat model of alcohol-and CCl4-induced hepatic fibrosis. Acta Pharmacol. Sin. 35, 1031–1044 (2014).
Elhemiely, A. A., Yahia, R. & Gad, A. M. Naringenin alleviate reproductive toxicity evoked by lead acetate via Attenuation of sperm profile and biochemical alterations in male Wistar rat: Involvement of TGFβ/AKT/mTOR pathway. J. Biochem. Mol. Toxicol. 37, e23335 (2023).
AD, S. Protective effect of abutilon indicum against lead-induced reproductive toxicity in male Wistar rats. J. Cell. Biochem. 120, 11196–11205 (2019).
Pizent, A., Tariba, B. & Živković, T. Reproductive toxicity of metals in men. Arh Hig Rada Toksikol. 63, 35–45 (2012).
Arab, H. H. et al. Targeting JAK2/STAT3, NLRP3/Caspase-1, and PK2/PKR2 pathways with arbutin ameliorates lead Acetate-Induced testicular injury in rats. Pharmaceuticals 17, 909 (2024).
Ileriturk, M. et al. Chrysin protects against testicular toxicity caused by lead acetate in rats with its antioxidant, anti-inflammatory, and antiapoptotic properties. J. Food Biochem. 45, e13593 (2021).
Fan, Y. et al. Lead-induced oxidative damage in rats/mice: A meta-analysis. J. Trace Elem. Med. Biol. 58, 126443 (2020).
Martin, K. K. et al. Effects of chronic lead exposure on zinc concentration and spermatic parameters in Wistar rats. Ann. Med. Biomed. Sci. 3, 51–58 (2017).
Ramos-Trevino, J. et al. In vitro evaluation of damage by heavy metals in tight and gap junctions of Sertoli cells. DNA Cell. Biol. 36, 829–836 (2017).
Soleimanzadeh, A., Kian, M., Moradi, S. & Malekifard, F. Protective effects of hydro-alcoholic extract of Quercus brantii against lead-induced oxidative stress in the reproductive system of male mice. Original Res. Article 8.
Doumouchtsis, K. K., Doumouchtsis, S. K., Doumouchtsis, E. K. & Perrea, D. N. The effect of lead intoxication on endocrine functions. J. Endocrinol. Invest. 32, 175–183 (2009).
Sikka, S. C. & Wang, R. Endocrine disruptors and estrogenic effects on male reproductive axis. Asian J. Androl. 10, 134–145 (2008).
Zhao, Y. et al. Resveratrol attenuates testicular apoptosis in type 1 diabetic mice: Role of Akt-mediated Nrf2 activation and p62-dependent Keap1 degradation. Redox Biol. 14, 609–617 (2018).
De Rensis, F. & Scaramuzzi, R. J. Heat stress and seasonal effects on reproduction in the dairy cow—a review. Theriogenology 60, 1139–1151 (2003).
Xia Ning, X. N., Daiber, A. & Förstermann, U. & Li huige, L. H. Antioxidant effects of Resveratrol in the cardiovascular system. (2017).
Baur, J. A. & Sinclair, D. A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 5, 493–506 (2006).
Soleimanzadeh, A., Kian, M., Moradi, S. & Mahmoudi, S. Carob (Ceratonia siliqua L.) fruit hydro-alcoholic extract alleviates reproductive toxicity of lead in male mice: Evidence on sperm parameters, sex hormones, oxidative stress biomarkers and expression of Nrf2 and iNOS. Avicenna J. Phytomed. 10, 35 (2020).
Zhong, X. et al. Immunomodulatory effect and biological significance of β-glucans. Pharmaceutics 15, 1615 (2023).
Li, L. et al. Typical phthalic acid esters induce apoptosis by regulating the PI3K/Akt/Bcl-2 signaling pathway in rat Insulinoma cells. Ecotoxicol. Environ. Saf. 208, 111461 (2021).
Dhulqarnain, A. O. et al. Pentoxifylline improves the survival of spermatogenic cells via oxidative stress suppression and upregulation of PI3K/AKT pathway in mouse model of testicular torsion-detorsion. Heliyon 7 (2021).
Javorac, D. et al. Exploring the endocrine disrupting potential of lead through benchmark modelling–study in humans. Environ. Pollut. 316, 120428 (2023).
Massányi, P., Massányi, M., Madeddu, R., Stawarz, R. & Lukáč, N. Effects of cadmium, lead, and mercury on the structure and function of reproductive organs. Toxics 8, 94 (2020).
Morita, Y. et al. Resveratrol promotes expression of SIRT1 and star in rat ovarian granulosa cells: An implicative role of SIRT1 in the ovary. Reprod. Biol. Endocrinol. 10, 1–10 (2012).
Byun, E., Park, S., Jang, B., Sung, N. & Byun, E. Gamma-irradiated β‐glucan induces Immunomodulation and anticancer activity through MAPK and NF‐κ B pathways. J. Sci. Food Agric. 96, 695–702 (2016).
Kumar, S. Occupational and environmental exposure to lead and reproductive health impairment: An overview. Indian J. Occup. Environ. Med. 22, 128–137 (2018).
Burton, G. J. Human and animal models: Limitations and comparisons. in The First Twelve Weeks of Gestation 469–485 (Springer, 1992).
Russell Wms, B. & Burch, R. L. The principles of humane experimental technique. Special ed. South Mimms, Potters Bar, Herts, England: Universities Federation for Animal Welfare 165 (1959).
Soleimanzadeh, A., Mohammadnejad, L., Ahmadi, A. & Dvm, A. S. Ameliorative effect of allium sativum extract on busulfan-induced oxidative stress in mice sperm. ARTICLE Vet. Res. Forum. 9, 265–271 (2018).
Abdel-Emam, R. A. & Ahmed, E. A. Ameliorative effect of L‐carnitine on chronic lead‐induced reproductive toxicity in male rats. Vet. Med. Sci. 7, 1426–1435 (2021).
Metin, T. O., Turk, A., Yalcın, A. & Adanır, I. Anti-inflammatory and anti-apoptotic potential of beta-glucan on chemotherapy-induced nephrotoxicity in rats. J. Surg. Med. (JOSAM) 7 (2023).
Mitra, S. et al. Sub-chronic cadmium and lead compound exposure induces reproductive toxicity and development of testicular germ cell neoplasia in situ in murine model: Attenuative effects of Resveratrol. J. Biochem. Mol. Toxicol. 36, e23058 (2022).
Zeynali, E., Soleimanzadeh, A. & Azizi, S. Synergistic Protection of Alpha-Glucosyl Hesperidin and Procyanidin Against Testicular Ischemia‐Reperfusion Injury. J. Food Biochem. 5634210 (2025).
Soleimanzadeh, A. & Saberivand, A. Effect of Curcumin on rat sperm morphology after the freeze-thawing process. Vet. Res. Forum. 4, 185–189 (2013).
Baqerkhani, M., Soleimanzadeh, A. & Mohammadi, R. Effects of intratesticular injection of hypertonic mannitol and saline on the quality of Donkey sperm, indicators of oxidative stress and testicular tissue pathology. BMC Vet. Res. 20, 99 (2024).
Soleimanzadeh, A., Karvani, N., Davoodi, F., Molaie, R. & Raisi, A. Efficacy of silver-doped carbon Dots in chemical castration: A rat model study. Sci. Rep. 14, 24132 (2024).
Soleimanzadeh, A., Saberivand, A., Ahmadi, A., Effect of α-tocopherol & on spermatozoa of rat semen after the freeze-thawing process. URMIA Med. J. 25, 826–834 (2014).
Malekifard, F., Dalirezh, N. & Soleimanzadeh, A. Modulatory effect of Pioglitazone on sperm parameters and oxidative stress, apoptotic and inflammatory biomarkers in testes of Streptozotocin-Induced diabetic rats. 19–34 (2018).
Soleimanzadeh, A., Malekifard, F. & Kabirian, A. R. Protective effects of hydro-alcoholic Garlic extract on spermatogenic disorders in streptozotocin-induced diabetic C57BL/6 mice. Sci. J. Kurdistan Univ. Med. Sci. 22, 8–17 (2017).
Zolfaghari, S., Soleimanzadeh, A. & Baqerkhani, M. The synergistic activity of fisetin on quercetin improves testicular recover in ischemia-reperfusion injury in rats. Sci. Rep. 15 (1), 12053 (2025).
Rad, M. S., Soleimanzadeh, A., Shalizar-Jalali, A. & Behfar, M. Synergistic protective effects of 3, 4-dihydroxyphenylglycol and Hydroxytyrosol in male rats against induced heat stress-induced reproduction damage. Food Chem. Toxicol. 114818 (2024).
Kashiwazaki, N. et al. Techniques for in vitro and in vivo fertilization in the rat. Rat Genom. Methods Protoc. 311–322 (2010).
Soleimanzadeh, A., Pourebrahim, M., Delirezh, N. & Kian, M. Ginger ameliorates reproductive toxicity of formaldehyde in male mice: Evidences for Bcl-2 and Bax. J. Herbmed Pharmacol. 7, 259–266 (2018).
Alan, A. P., Ayen, E., Khaki, A. & Soleimanzadeh, A. Epigallocatechin-3-gallate affects the quality of fresh and frozen-thawed semen of simmental bull by two different cryopreservation methods. in Vet. Res. Forum 15 (2024).
Sheikholeslami, S. A., Soleimanzadeh, A., Rakhshanpour, A. & Shirani, D. The evaluation of lycopene and cysteamine supplementation effects on sperm and oxidative stress parameters during chilled storage of canine semen. Reprod. Domest. Anim. 55, 1229–1239 (2020).
Shakouri, N., Soleimanzadeh, A., Rakhshanpour, A. & Bucak, M. N. Antioxidant effects of supplementation of 3, 4-dihydroxyphenyl glycol on sperm parameters and oxidative markers following cryopreservation in canine semen. Reprod. Domest. Anim. 56, 1004–1014 (2021).
Organization, W. H. Department of reproductive health and research. WHO Lab. Man. Exam. Process. Hum. Semen. 5, 21–22 (2010).
Mostahsan, Z., Azizi, S., Soleimanzadeh, A. & Shalizar-Jalali, A. The protective roles of Mito-TEMPO on testicular ischemiareperfusion injury based on biochemical and histopathological evidences in mice. Iran J. Vet. Surg. 97–105 (2024).
Salehi, S., Soleimanzadeh, A., Goericke-Pesch, S. & Ayen, E. Evaluation of the effects of Crocin addition on canine semen Dilution during refrigerated storage. Iran. J. Anim. Sci. 54, 303–315 (2023).
Jahangiri Asl, E., Soleimanzadeh, A., Javadi, S. & Asri Rezaei, S. The effect of intratesticular injection of zinc oxide nanoparticles on plasma concentrations of LH, FSH, testosterone and corticosterone in rat. Vet. Res. Biol. Prod. 34, 103–194 (2021).
Soleimanzadeh, A., Talavi, N., Yourdshahi, V. S. & Bucak, M. N. Caffeic acid improves microscopic sperm parameters and antioxidant status of Buffalo (Bubalus bubalis) bull semen following freeze-thawing process. Cryobiology 95, 29–35 (2020).
Sabzeie, M. M., Ayen, E., Soleimanzadeh, A. & Bucak, M. N. Tribulus terrestris aqueous extract supplementation effects on sperm characteristics and anti-oxidant status during chilled storage of canine semen. Vet. Res. Forum. 14, 71 (2023).
Jalali, S. S., Talebi, J., Allymehr, M., Soleimanzadeh, A. & Razi, M. Effects of nano-selenium on mRNA expression of markers for spermatogonial stem cells in the testis of broiler breeder males. in Veterinary Research Forum vol. 10 139 (Faculty of Veterinary Medicine, Urmia University, Urmia, Iran, 2019).
Izanloo, H., Soleimanzadeh, A., Bucak, M. N., Imani, M. & Zhandi, M. The effects of glutathione supplementation on post-thawed Turkey semen quality and oxidative stress parameters and fertilization, and hatching potential. Theriogenology 179, 32–38 (2022).
Izanloo, H., Soleimanzadeh, A., N Bucak, M., Imani, M. & Zhandi, M. The effects of varying concentrations of glutathione and Trehalose in improving microscopic and oxidative stress parameters in Turkey semen during liquid storage at 5° C. Cryobiology 101, 12–19 (2021).
Kabirian, A., Batavani, R. A., Asri-Rezaei, S. & Soleimanzadeh, A. Comparative study of the protective effects of chicken embryo amniotic fluid, vitamin C and coenzyme Q10 on cyclophosphamide-induced oxidative stress in mice ovaries. Vet. Res. Forum. 9, 217–224 (2018).
Panahi, M., Soleimanzadeh, A., Shalizar-Jalali, A., Ayen, E. & Molaie, R. Effect of selenium nanoparticles on quantitative and qualitative parameters of dog sperm after storage in the refrigerator. Iran. J. Anim. Sci. 54, 379–390 (2023).
Nourian, A. & Soleimanzadeh, A. Ali Shalizar Jalali & Gholamreza Najafi. Effects of bisphenol-S low concentrations on oxidative stress status and in vitro fertilization potential in mature female mice. in Veterinary Research Forum vol. 8 341–345 (Faculty of Veterinary Medicine, Urmia University, Urmia, Iran, 2017).
Mohammadnejad, K., Mohammadi, R., Soleimanzadeh, A., Shalizar-Jalali, A. & Sarrafzadeh-Rezaei, F. The effect of β-Cryptoxanthin on testicular Ischemia-Reperfusion injury in a rat model: Evidence from testicular histology. Iran J. Vet. Surg. (2024).
Ramazani, N. et al. The influence of L-proline and fulvic acid on oxidative stress and semen quality of Buffalo bull semen following cryopreservation. Vet. Med. Sci. 9, 1791–1802 (2023).
Ramazani, N. et al. Reducing oxidative stress by κ-carrageenan and C60HyFn: The post-thaw quality and antioxidant status of Azari water Buffalo bull semen. Cryobiology 111, 104–112 (2023).
Elhemiely, A. A. & Elesawy, W. H. Modulation of VEGF/eNOS/TGF-β axis by Piracetam as a new avenue to ameliorate valproic Acid‐Induced placental toxicity and teratogenicity in rats. J. Biochem. Mol. Toxicol. 39, e70266 (2025).
Karanicolas, P. J., Farrokhyar, F. & Bhandari, M. Blinding: Who, what, when, why, how? Can. J. Surg. 53, 345 (2010).
Field, A. Discovering Statistics Using IBM SPSS Statistics (Sage Publications Limited, 2024).
Acknowledgements
The authors would like to sincerely thank the members of the Faculty of Veterinary Medicine, Islamic Azad University Urmia Branch Research Council, for the approval and support of this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
TM and AMR contributed to the conception, design, data collection, statistical analysis, and drafting of the manuscript. TM and AMR contributed to the conception, design, supervision of the study, and drafting of the manuscript. All authors approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Animal Ethics Committee of Urmia University approved the experiments, and all methods were carried out in accordance with the relevant guidelines and regulations (IR-UU-2361/PD/3). Additionally, the study adhered to the ARRIVE guidelines (Animals in Research: Reporting In Vivo Experiments) and complied with institutional and national regulations for the care and use of laboratory animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rohanii, A.M., Mohammadi, T. β-Glucan and resveratrol mitigate lead induced reproductive toxicity in male mice. Sci Rep 15, 31277 (2025). https://doi.org/10.1038/s41598-025-16510-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16510-7